Introduction
Dendritic cells (DCs) are the most potent
professional antigen-presenting cells (APC). As we know, DCs can
capture, process and present antigens to T cells and play a key
role in the induction of Ag-specific immune responses to viruses,
bacteria, allergens and tumor antigens (1). DCs have been commonly used in cancer
immunotherapy in recent years. However, there are only a low
frequency of DCs (<2%) in human peripheral blood mononuclear
cells (PBMCs) and peripheral organs. Moreover, this population
cannot be expanded in vitro (2,3). As
a result, DCs separated from human PBMCs or organs directly have
been seldom applied to clinical trials. Instead the
monocyte-derived DCs have been widely studied in clinical trials as
these DCs subsets could sustain most of DCs function and be
cultured much easier compared with the DCs in vivo.
Traditionally, investigators use
granulocyte-macrophage colony-stimulating factor (GM-CSF) and
interleukin (IL)-4 to stimulate monocyte to differentiate into
IL-4-DC. Reports have showed that IL-4-DC derived from both rat
bone marrow and PBMCs can present and cross-present antigens in
vitro (4,5). Moreover, IL-4-DC has been utilized in
immunotherapy of cancer and HIV infection. Recent studies have
showed that interferon (IFN)-α is an important cytokine belonging
to the type I IFN family, which is endowed with potent antiviral,
antitumor, and immunoregulatory activities (6). Paquette et al (7) firstly revealed that IFN-α and GM-CSF
could induce the differentiation of monocytes into IFN-DC. Some
reports have showed that IFN-DC could be more effective than IL-4
DC to induce cluster of differentiation (CD)4+ T cell
and CD8+ T cell response in different models (8–11).
Lapenta et al (8) found
that IFN-DC loaded with HIV-1 antigen could induce the
cross-priming of CD8+ T cells against HIV in the
hu-PBL-SCID mouse more effectively than IL-4 DC. Moreover, IFN-DC
could cross-present low amounts of nonstructural-3 protein (NS3) of
hepatitis C virus (HCV) and activate HCV-specific CD8+ T
cells efficiently (12). However,
the mechanisms of the effect of IFN-DC remain to be determined and
the details of the phenotypes and function of these DCs still need
to be explored.
In this study, we cultured both IFN-DC and IL-4-DC
and investigated the difference between these two DCs subsets in
the aspects of cell morphology, cell phenotypes and secretion of
cytokines. The function of IFN-DC and IL-4-DC in the presentation
and cross-presentation of virus antigen also was explored.
Materials and methods
Human blood donors and preparation of
PBMCs
PBMCs were obtained from healthy volunteers. Written
informed consents were obtained from all donors in accordance with
the Declaration of Helsinki. PBMCs were isolated using Ficoll
density gradient centrifugation (TBD, Tianjin, China) and cultured
in RPMI-1640 medium containing 10% fetal bovine serum (FBS; Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA), 100 U/ml
penicillin and 100 ng/ml streptomycin. All studies were approved by
the Institutional Review Board (IRB) of the Second Hospital of
Nanjing.
Cell separation and DC generation
Monocytes were isolated by immunomagnetic cell
sorting (MACS Cell Isolation kits; Miltenyi Biotec, Bergisch
Gladbach, Germany). Positive selected CD14+ cells were
analyzed by flow cytometry. Purity of the CD14+ cells
was >98%. Purified CD14+ monocytes were cultured in
RPMI-1640 medium containing 10% FBS, 100 U/ml penicillin and 100
ng/ml streptomycin at the concentration of 1×106/ml,
supplemented with 1,000 U/ml IFN-α2b (Anterferon;, Anhui, China)
and 40 ng/ml GM-CSF for IFN-DC or 20 ng/ml IL-4 (both from R&D
Systems, Minneapolis, MN, USA) and 40 ng/ml GM-CSF for IL-4-DC. The
cells were incubated at 37°C and 5% CO2 for 5 days. Half
of the supernatants were moved and fresh cytokines and mediums were
added every 3 days. DCs were matured by adding 20 ng/ml tumor
necrosis factor-α (TNF-α; R&D Systems) and culturing for
another 48 h.
Electron microscopy
For ultrastructural analysis, electron microscopy
was performed using standard procedures (13). Briefly, all samples were washed and
fixed in 2.5% glutaraldehyde in 85 mM phosphate buffer (pH 7.2) and
post-fixed in OsO4 solution. Then the cells were
dehydrated in graded alcohol solutions and embedded in epoxy resin.
Mature IFN-DC and IL-4-DC were examined at 80 kV under Hitachi
electron microscope H-7650.
Immunophenotypic analysis
Cultured DCs were washed and resuspended in PBS
containing 1% FBS and incubated with a series of monoclonal
antibodies (mAbs) including anti-HLA-DR, CD11c, CD80, CD83 and CD86
(BD Pharmingen, San Diego, CA, USA) for 30 min at 4°C. All mAbs
were conjugated with PerCp-, APC, or PE-. Then the samples were
analyzed by a fluorescence-activated cell sorting (FACS)Canto II
flow cytometer (BD Biosciences, San Jose, CA, USA). Data were
collected with BD FACSDiva software and analyzed with TreeStar
FlowJo software.
Cytokine secretion analysis
Supernatants from immature and mature DCs were
harvested at day 5 and day 7 separately. Cytokine concentrations of
supernatants were determined by ELISA. IL-10, IL-18, IL-23, IL-1β
and IL-12p70 were measured using the ELISA kits according to the
manufacturer's protocol (Multi Sciences, Hangzhou, China).
Analysis of antigen-specific T cells
by intracellular IFN-γ staining
For antigen presentation assays, cultured DCs from
heathy donors were seeded into a 96-well round-bottomed plate at
1×105 cells/well. Then, 10 µg/ml cytomegalovirus
(CMV)-pp65 protein (Miltenyi Biotec) was added into the specified
wells. After 2 h, CD4+ and CD8+ T lymphocytes
obtained by immunomagnetic cells sorting as described above were
co-cultured with CMV-pp65 protein loaded DCs at the DCs/T
lymphocytes ratio of 1:10 in RPMI-1640 containing 10% human AB
serum, 100 U/ml penicillin and 100 ng/ml streptomycin for 12 h at
37°C. Then GolgiPlug protein transport inhibitor (BD Pharmingen)
was added into the wells. After another 6 h, cells were harvested
and washed in washing buffer and stained with live/dead fixable
dead cell staining (Invitrogen; Thermo Fisher Scientific, Inc.),
FITC-conjugated anti-CD4, PE-conjugated anti-CD8 and
PerCp-conjugated anti-CD3 (BD Pharmingen) for 30 min at 4°C. After
washing, the cells were fixed and permeabilized by the
Cytofix/Cytoperm solution (BD Pharmingen) for 20 min at 4°C. Then
the cells were rewashed in perm washing buffer and stained with
APC-conjugated anti-IFN-γ (BD Pharmingen) for 30 min at 4°C. At
last, the cells were analyzed on a BD Canto II flow cytometer.
Statistics
Data were expressed as means ± SEMs and analyzed
with SPSS V20.0 software. The statistical significance of
differences was determined by the Student's t-test and one-way
ANOVA. A value of P<0.05 was considered to indicate a
statistically significant result.
Results
Morphological analysis of IFN-DC and
IL-4-DC
In the past few years, some reports have showed that
IFN-DC and IL-4-DC had some similar characteristics of DCs in
morphology (14). However, McRae
et al (15) showed that
IL-4-DC contained more and longer spikes than IFN-DC. So we
explored the details of these two DCs subsets in morphology. We
cultured purified CD14+ monocytes added with GM-CSF and
IFN-α or GM-CSF and IL-4 to get immature IFN-DC (imIFN-DC) and
immature IL-4 DC (imIL-4-DC), respectively.
TNF-α, LPS, CD40L and polyinosinic:polycytidylic
acid (poly I:C) was reported to be used to promote the mature of
DCs in vitro (16,17). However, disable DCs could be
induced by the stimulation of LPS or poly I:C (17). So TNF-α was used to induce imIFN-DC
and imIL-4-DC to differentiate into mature IFN-DC (mIFN-DC) and
mature IL-4-DC (mIL-4-DC). Then morphological differences between
mIFN-DC and mIL-4-DC were compared by scanning electron micoscopy.
Fig. 1A and B showed that the size
of mIL-4-DC was larger than mIFN-DC. Meanwhile, the outcomes of
FACS analyses were consistent with the electron micoscopy results
and both mIFN-DC and mIL-4-DC appeared to be larger in size
compared with the monocytes (P<0.01) (Fig. 1C and D). Furthermore, although both
mIFN-DC and mIL-4-DC obtained numerous of pseudopodia, the sharp of
the spikes of mIFN-DC was short and thick while the mIL4-DC had
long and thin spikes (Fig. 1A and
B). On the other hand, we observed that mIFN-DC contained more
organelles, like endoplasmic reticulum, and myelin figures than
mIL-4-DC while mIL-4-DC contained more vacuoles in the cells.
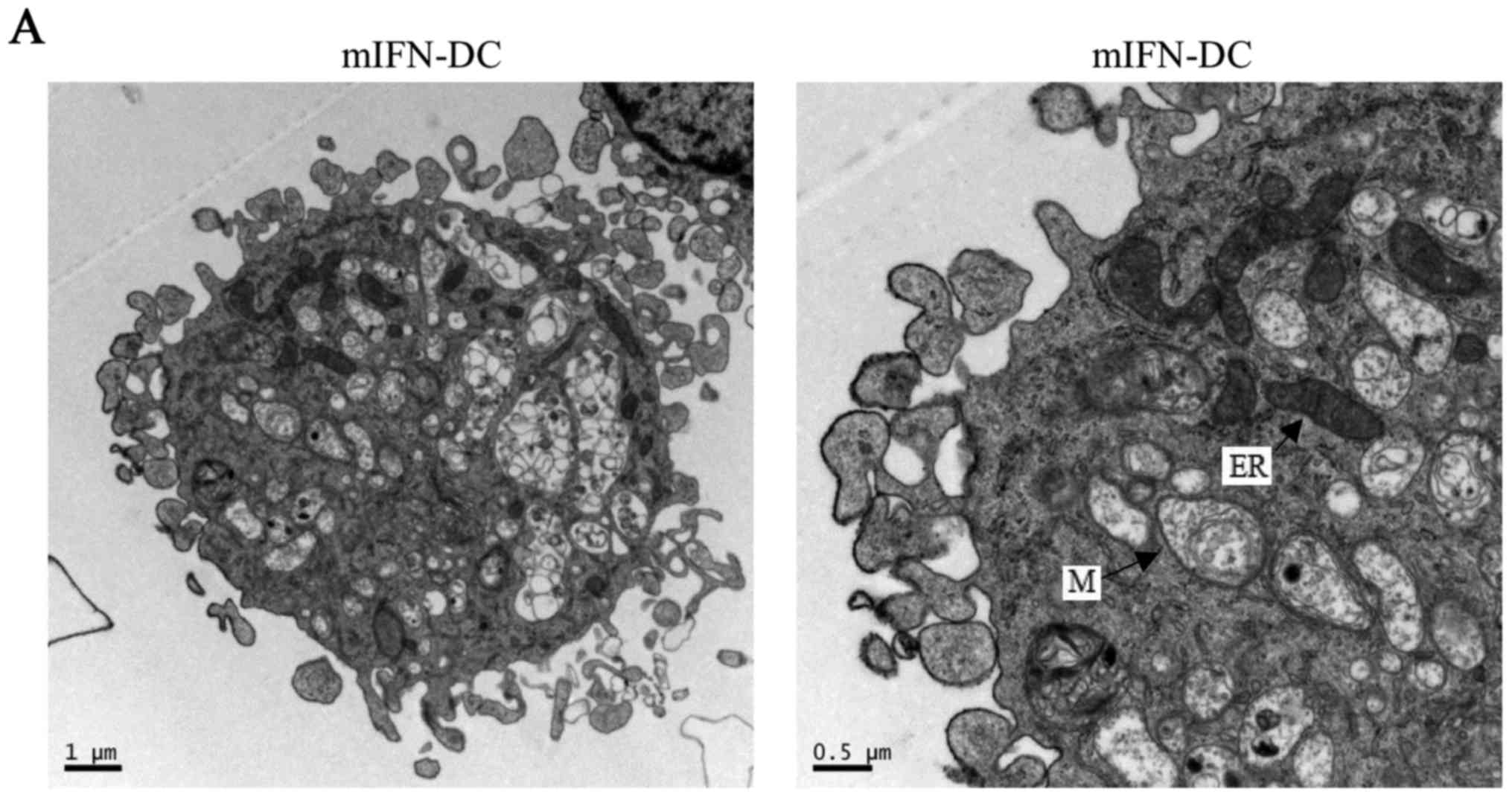 | Figure 1.Morphology of mIFN-DC and mIL-4-DC
derived from CD14+ monocytes. Mature DCs were produced
in vitro by culturing monocytes with IFN-α and GM-CSF or
GM-CSF and IL-4. Then TNF-α was used to promote the maturation. The
scanning electron micoscopy and FACS analysis were conducted to
compare the morphologies of these two DCs. (A and B) Scanning
electron micoscopy photographs of the integral and the local of
mIFN-DC and mIL-4-DC, respectively. (C) FACS analysis of mIFN-DC,
mIL-4-DC and monocytes. Nonviable cells were eliminated from
analysis. (D) Forward scatter values, generated by FACS, revealed
the sizes of mIFN-DC, mIL-4-DC and monocytes. Results were
representative of 5 independent experiments. Statistical analysis
comparing the size of different DCs and monocytes was performed
with the independent-sample t-test (**P<0.01, ***P<0.001).
DCs, dendritic cells; IFN, interferon; TNF-α, tumor necrosis
factor-α; IL, interleukin; FACS, fluorescence-activated cell
sorting; CD, cluster of differentiation; m, mature; M, myelin
figures; ER, endoplasmic reticulum; V, vacuoles; FSC, forward
scatter; SSC, side scatter. |
Comparison of the cell phenotypes
between IFN-DC and IL-4-DC
To investigate the difference of the cell phenotypes
between IFN-DC and IL-4-DC, we detected the expression of CD14 on
the surface of imIFN-DC and imIL-4-DC firstly. Compared with the
monocytes, both of imIFN-DC and imIL-4-DC expressed much lower CD14
as shown in Fig. 2. And there was
no obvious difference between imIFN-DC and imIL-4-DC for the
expression of CD14 (P>0.05).
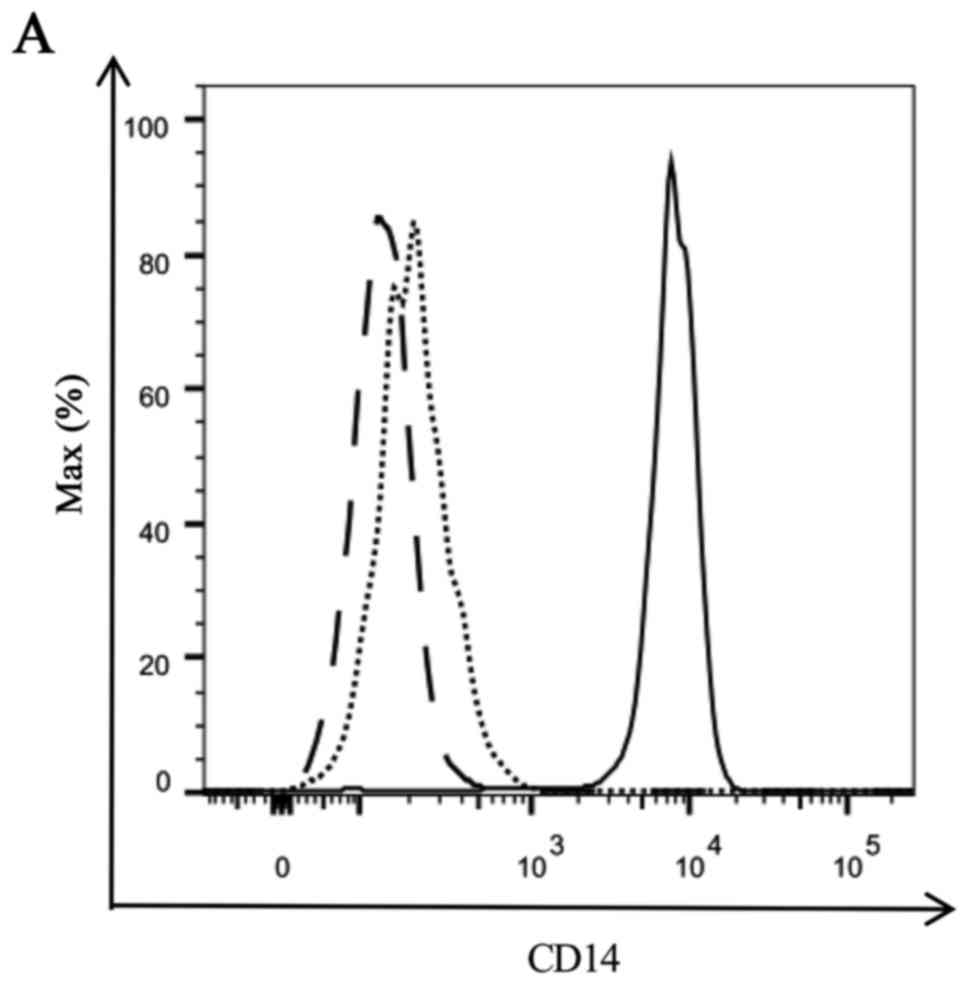 | Figure 2.Expression of CD14 of IFN-DC, IL-4-DC
and monocytes. (A) Representative FACS figure of the CD14
expression on the surface of immature DCs and monocytes. Solid line
represents monocytes, dotted line represents imIFN-DC, dashed line
represents imIL-4-DC. (B) MFI of CD14 detected by FACS. Results
were representative of 5 independent experiments. Statistical
analysis was performed with the independent-sample t-test
(**P<0.01). CD, cluster of differentiation; IFN, interferon; IL,
interleukin; DCs, dendritic cells; FACS, fluorescence-activated
cell sorting; MFI, median fluorescence intensity; im, immature. |
Then, we analyzed the expression of major
histocompatibility complex (MHC) I molecules HLA-DR, mDC marker
CD11c, costimulatory molecules CD80 and CD86 and mature marker CD83
of IFN-DC and IL-4-DC. The results showed that the expression of
HLA-DR, CD11c, CD80, CD83 and CD86 were up-regulated on both
imIFN-DC and imIL-4-DC compared with monocytes (Fig. 3A). After the maturation of DCs
stimulated by TNF-α, we detected the expression of these phenotypic
markers again. As illustrated in Fig.
3B and C, mIFN-DC expressed higher HLA-DR, CD11c, CD83 and CD86
compared with imIFN-DC (P<0.001), and mIL-4-DC expressed higher
CD11c, CD80 and CD83 compared with imIL-4-DC (P<0.001). Compared
with mIL-4-DC, HLA-DR and CD86 were expressed higher on the surface
of mIFN-DC (P<0.001) while the expression of CD80 and CD83 had
no obvious difference (P>0.05). Intriguingly, mIFN-DC expressed
lower CD11c compared with mIL-4-DC (P<0.01).
 | Figure 3.Phenotypes expressed on the surface
of IFN-DC and IL-4-DC. (A) Both imIFN-DC and imIL-4-DC expressed
HLA-DR, CD11c, CD80, CD83, CD86. Empty histograms showed the
background staining with monocytes, and solid histograms
represented specific staining of the indicated cell surface
markers. (B and C) Median fluorescence intensity of HLA-DR, CD11c,
CD80, CD83 and CD86 expressed by imIFN-DC, imIL-4-DC, mIFN-DC, and
mIL-4-DC. Results were represented as means ± SEMs obtained from 5
independent experiments. Statistical analysis was performed with
one-way ANOVA (**P<0.01, ***P<0.001). CD, cluster of
differentiation; IFN, interferon; IL, interleukin; DCs, dendritic
cells; m, mature; im, immature. |
Cytokines secreted by IFN-DC and
IL-4-DC
A series of cytokines, such as IL-12, IL-27 and
IL-10, could be secreted by DCs and these cytokines played a key
role in immune response (18–20).
So, we evaluated the cytokines secretion in the supernatants from
DCs cultures. Supernatants were quantified for IL-10, IL-18, IL-23,
IL-1β and IL-12p70 by ELISA. When comparing between imIFN-DC and
imIL-4-DC, there was no obvious difference for the secretion of
cytokines IL-10, IL-18 and IL-23 (P>0.05) (Fig. 4A-C). However, IL-1β could be
secreted more effectively by imIFN-DC than imIL-4-DC (P<0.05)
(Fig. 4D). The secretion of
IL-12p70 could not be detected in neither imIFN-DC group nor
imIL-4-DC group (Fig. 4E). In
response to TNF-α, these two mature DCs could secrete amounts of
IL-12p70. Meanwhile, mIFN-DC could secrete more IL-12p70, IL10,
IL-18 and IL-1β compared with mIL-4-DC (P<0.05). In contrast,
there was no difference between mIFN-DC and mIL-4-DC for the
secretion of IL-23 (P>0.05).
 | Figure 4.Cytokines produced by IFN-DC and
IL-4-DC. IL-10 (A), IL-18 (B), IL-23 (C), IL-1β (D) and IL-12p70
(E) secreted by imIFN-DC, mIFN-DC, imIL-4-DC and mIL-4-DC were
tested by ELISA. Results were represented as means ± SEMs obtained
from 3 independent experiments. Statistical analysis was performed
with one-way ANOVA followed by a post-hoc test (*P<0.05,
**P<0.01, ***P<0.001). IFN, interferon; IL, interleukin; DCs,
dendritic cells; m, mature; im, immature. |
Comparison of the presentation ability
between IFN-DC and IL-4-DC to activate CMV-pp65 specific T
lymphocytes
To investigate the presentation ability of IFN-DC
and IL-4-DC, the immature and mature IFN-DC or IL-4-DC loaded with
the CMV-pp65 protein were cultured with autologous CD4+
and CD8+ T cells, respectively. Then the percentages of
IFN-γ+CD4+ and
IFN-γ+CD8+ T lymphocytes were detected by
flow cytometry intracellular staining (Fig. 5A and B). The results showed that
there was no obvious difference for the percentages of
IFN-γ+CD4+ and
IFN-γ+CD8+ T lymphocytes between imIFN-DC
group, imIL-4-DC and negative control group (P>0.05) (Fig. 5C and D). After maturation, both of
mIFN-DC and mIL-4 could induce the secretion of IFN-γ of
CD4+ and CD8+ T lymphocytes compared with the
negative control (P<0.05). Notably, when compared with the
mIL-4-DC, mIFN-DC loaded with CMV-pp65 protein could activate
higher proportion of autologous CD4+ T cells (0.91% vs.
0.31%, P<0.001) and CD8+ T cells (0.90% vs. 0.48%,
P<0.001) to secret IFN-γ (Fig. 5C
and D).
 | Figure 5.CD4+ and CD8+ T
cells activation following exposure to the IFN-DC or IL-4-DC loaded
with CMV-pp65 protein. The IFN-DC or IL-4-DC was loaded with
CMV-pp65 protein. After 2 h, autologous lymphocytes were added.
Activation of T cells was assessed by determining the percentage of
IFN-γ+CD4+ cells (A) and
IFN-γ+CD8+ cells (B) detected by
intracellular staining. The representative dot plots from one of
the donors were shown. CD, cluster of differentiation; IFN,
interferon; CMV, cytomegalovirus; IL, interleukin; DCs, dendritic
cells; m, mature; im, immature. CD4+ and CD8+
T cells activation following exposure to the IFN-DC or IL-4-DC
loaded with CMV-pp65 protein. CD4+ (C) and
CD8+ T cell responses (D) from 15 donors were shown.
Statistical analysis was performed with one-way ANOVA followed by a
post-hoc test (*P<0.05, **P<0.01, ***P<0.001). CD, cluster
of differentiation; IFN, interferon; CMV, cytomegalovirus; IL,
interleukin; DCs, dendritic cells; m, mature; im, immature. |
Discussion
Monocytes play diverse roles in human immunity, such
as clearance of senescent cells, pathogen killing and immune
regulation (21,22). In vivo, monocytes can
differentiate into macrophages. In vitro, monocytes from
separated human PBMCs can be induced to differentiate into DC after
the stimulation of numerous cytokines (23). In the past, IL-4 combined with
GM-CSF were widely used to induce the differentiation of monocytes
(17,24). Wang et al (25) discovered that IL-4-DC could express
more phenotypes of mature cells than GM-CSF DC developed by
culturing monocytes with GM-CSF alone. In recent years, many
researchers have focused on the exploit of IFN-DC developed by
culturing monocytes with GM-CSF and IFN-α as this DCs subset could
be more effective than IL-4-DC in the aspect of antigen
cross-presentation (26).
Moreover, some studies revealed that the antigen presentation of
IL-4-DC relied on the signal transducer and activator of
transcription 6 (STAT6) while IFN-DC was not, suggesting that these
two DCs have different presenting ways (25,27,28).
Although IFN-DC and IL-4-DC have been studied for several years,
the details of the morphology, phenotype and function of these DCs
still need to be explored. In this study, we showed that the
morphologies of these two DCs were different in cell size, shape,
spikes and cell internal structure. The phenotypes and secreted
cytokines of IFN-DC and IL-4-DC were diverse. Furthermore, after
loaded with CMV-pp65 protein, IFN-DC could induce the activation of
antigen specific CD4+ and CD8+ T cells more
effectively than IL-4-DC.
Firstly, the scanning electron micoscopy results
showed that mIFN-DC contained abundant organlles compared with
mIL-4-DC. In contrast, mIL-4-DC contained more vacuoles in the
cells. This phenomenon was consistent with the results observed in
BM-derived IL-4-DC from Lewis rats (29). Spadaro et al (10) used FITC conjugated OVA as antigen
to explore the transportation of soluble antigen in IFN-DC and
IL-4-DC. The results showed that IFN-DC took more than 24 h to
digest antigen while IL-4-DC needed 3 h, which suggested that
IL-4-DC possessed a more rapid degradation and endosomal
acidification way than IFN-DC. According to our results, we
speculated that the diversity of morphology of these two DCs might
be the reason of the different route and mechanism of antigen entry
of IFN-DC and IL-4-DC. However, the more details of the different
endocytosis of these two DCs still need to be explored in
future.
Then, we investigated the phenotypes of immature and
mature IFN-DC and IL-4-DC. Of interest, there were no obvious
difference for the expression of CD83 which was the mature marker
of DCs between IFN-DC and IL-4-DC. This result was consistent with
the study conducted by Carbonneil et al (30). However, some reports have revealed
that the level of the expression of CD83 on the surface of imIFN-DC
was higher compared to imIL-4-DC (31,32).
Fujii et al (33)
discovered that the costimulatory molecules CD80 and CD86 were
necessary for the maturation of DCs. Our study showed that the CD80
expression had no obvious difference between these two mature DCs
while the expression of CD86 by mIFN-DC was significantly higher
than mIL-4-DC. Both CD80 and CD86 are prototypical members of the
B7 co-signaling molecule family (34). Some studies showed that CLTA-4 was
the preferential receptor for CD80 while CD28 bound mostly to CD86
(35,36). And the most important function of
CD28 is to induce the proliferation of T cells. Moreover, Lenschow
and co-workers have found that CD86 could be expressed
constitutively following T cell interaction with APCs (37). So we speculated that the different
expression of CD80 and CD86 by IFN-DC and IL-4-DC might be one of
the reasons explaining the higher antigen presenting ability of
IFN-DC compared with IL-4-DC (38). There was no obvious difference for
the expression of Class II MHC antigens HLA-DR and mDC marker CD11c
between the immature IFN-DC and IL-4-DC. However, the expression of
HLA-DR by mIFN-DC was higher than mIL-4-DC, while the CD11c
expression was lower than mIL-4-DC. As HLA-DR is critical for DC to
prime CD4+ cells, higher expression of HLA-DR by IFN-DC
may be another reason to explain its stronger ability of
presentation compared with IL-4-DC (39). The different expression of CD11c by
these two DCs may reflect the different function of cell adhesion
as CD11c is involved in the adhesion of cells (40).
Next, we analyzed the secretion of cytokines IL-18,
IL-23, IL-12p70, IL-1β and IL-10 by IFN-DC and IL-4-DC. It is known
that IL-18, IL-23, IL-12p70, IL-1β are the T helper cell 1 (Th1)
pro-inflammatory cytokines and IL-10 is the Th2 anti-inflammatory
cytokine (38,41,42).
And for cytokines IL-18, the most important biological activity is
to induce T, B and NK cells to secret IFN-γ (43). Although there was no difference for
the secretion of IL-18 between imIFN-DC and imIL-4-DC, IL-18
secreted by mIFN-DC was significantly higher than mIL-4-DC. This
result is consistent with the study of Mohamad which have showed
that the pro-IL-18 protein existed in IFN-DC but not in IL-4-DC by
western blot analysis (1). IL-23
and IL-12p70, as the members of cytokines IL-12 family, are the
main stimulators of memory T cells proliferation and can induce the
generation of pro-inflammatory Th1 and Th17 cells (44,45).
Moreover, IL-23 has been reported that it could synergize with
IL-12 in promoting the production of cytokines by DC themselves
(46). Our results showed that the
secretions of IL-23 and IL-12p70 by both two types of mature DCs
were increased dramatically compared with the immature DCs, which
was consistent with the strong effect of mature DCs in activing T
cells. As a member of the IL-1 family of cytokines, IL-1β is an
important mediator of inflammatory response and also involved in
proliferation, differentiation, and apoptosis of immune cells. In
our results, IL-1β was secreted more effectively by IFN-DC than
IL-4-DC, which might explain the stronger presenting function of
IFN-DC.
At last we compared the function of presenting
protein antigen between IFN-DC and IL-4-DC by detecting the IFN-γ
secretion by T cells. In accordance with our expectation, both of
mature IFN-DC and IL-4-DC could present and cross-present CMV-pp65
protein more effectively than immature DCs, which was in consistent
with the results of the cytokines secretion above. In consideration
of the low percentage of the specific T cells for CMV-pp65 protein
in PBMC, the IFN-γ producing by CD4+ and CD8+
were relative low and the results were in line with the study by de
Niet et al (47). On the
other hand, we found that mIFN-DC was more effective in the priming
of antigen specific CD4+ and CD8+ T cells
than mIL-4-DC which had been reported by other studies (1,19,48).
As a matter of fact, one of the most critical issues
for DC-based vaccines is to identify the ‘optimal’ DCs subset. This
study revealed the diversity between IFN-DC and IL-4-DC in the
aspect of morphology, phenotypes and cytokines secretion. The data
also suggested that IFN-DC could be more effective than IL-4-DC in
priming and cross-priming T cells. Our results supported the view
that the IFN-DC-based vaccine might be a more attractive and
effective strategy for the immunotherapy.
Acknowledgements
This research was partially supported by grants from
the National Natural Science Foundation of China (no. 81402559 to
W.Y.), the Science and Technology Commission of Nanjing (no.
201605033 to W.Y.), the Project of Six Talent Peaks of Jiangsu
Province (no. WSN-177 to W.Y.) and the Jiangsu Provincial Special
Program of Medical Science (no. BL2014005 to Y.Y.).
Glossary
Abbreviations
Abbreviations:
|
APC
|
antigen-presenting cells
|
|
CLTA-4
|
cytotoxic T lymphocyte-associated
antigen-4
|
|
DCs
|
dendritic cells
|
|
FACS
|
fluorescence-activated cell
sorting
|
|
FBS
|
fetal bovine serum
|
|
GM-CSF
|
granulocyte macrophage-colony
stimulating factor
|
|
HCV
|
hepatitis C virus
|
|
IFN-α
|
interferon-α
|
|
IL-4
|
interleukin-4
|
|
mAbs
|
monoclonal antibodies
|
|
MHC
|
major histocompatibility complex
|
|
PBMCs
|
peripheral blood mononuclear cells
|
|
poly I:C
|
polyinosinic: polycytidylic acid
|
|
STAT6
|
signal transducer and activator of
transcription 6
|
|
Th1
|
T helper cell 1
|
|
Th2
|
T helper cell 2
|
|
Th17
|
T helper cell 17
|
|
TNF-α
|
tumor necrosis factor-α
|
References
|
1
|
Mohty M, Vialle-Castellano A, Nunes JA,
Isnardon D, Olive D and Gaugler B: IFN-alpha skews monocyte
differentiation into Toll-like receptor 7-expressing dendritic
cells with potent functional activities. J Immunol. 171:3385–3393.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
van Montfoort N, van der Aa E and Woltman
AM: Understanding MHC class I presentation of viral antigens by
human dendritic cells as a basis for rational design of therapeutic
vaccines. Front Immunol. 5:1822014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schreibelt G, Klinkenberg LJ, Cruz LJ,
Tacken PJ, Tel J, Kreutz M, Adema GJ, Brown GD, Figdor CG and de
Vries IJ: The C-type lectin receptor CLEC9A mediates antigen uptake
and (cross-)presentation by human blood BDCA3+ myeloid
dendritic cells. Blood. 119:2284–2292. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sugita S, Kawazoe Y, Imai A, Usui Y,
Iwakura Y, Isoda K, Ito M and Mochizuki M: Mature dendritic cell
suppression by IL-1 receptor antagonist on retinal pigment
epithelium cells. Invest Ophthalmol Vis Sci. 54:3240–3249. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim SW, Choi SM, Choo YS, Kim IK, Song BW
and Kim HS: Flt3 ligand induces monocyte proliferation and enhances
the function of monocyte-derived dendritic cells in vitro. J Cell
Physiol. 230:1740–1749. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gabriele L, Borghi P, Rozera C, Sestili P,
Andreotti M, Guarini A, Montefusco E, Foà R and Belardelli F:
IFN-alpha promotes the rapid differentiation of monocytes from
patients with chronic myeloid leukemia into activated dendritic
cells tuned to undergo full maturation after LPS treatment. Blood.
103:980–987. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Paquette RL, Hsu NC, Kiertscher SM, Park
AN, Tran L, Roth MD and Glaspy JA: Interferon-alpha and
granulocyte-macrophage colony-stimulating factor differentiate
peripheral blood monocytes into potent antigen-presenting cells. J
Leukoc Biol. 64:358–367. 1998.PubMed/NCBI
|
|
8
|
Lapenta C, Santini SM, Logozzi M, Spada M,
Andreotti M, Di Pucchio T, Parlato S and Belardelli F: Potent
immune response against HIV-1 and protection from virus challenge
in hu-PBL-SCID mice immunized with inactivated virus-pulsed
dendritic cells generated in the presence of IFN-alpha. J Exp Med.
198:361–367. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rizza P, Moretti F, Capone I and
Belardelli F: Role of type I interferon in inducing a protective
immune response: Perspectives for clinical applications. Cytokine
Growth Factor Rev. 26:195–201. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Spadaro F, Lapenta C, Donati S, Abalsamo
L, Barnaba V, Belardelli F, Santini SM and Ferrantini M: IFN-alpha
enhances cross-presentation in human dendritic cells by modulating
antigen survival, endocytic routing, and processing. Blood.
119:1407–1417. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Carbonneil C, Aouba A, Burgard M,
Cardinaud S, Rouzioux C, Langlade-Demoyen P and Weiss L: Dendritic
cells generated in the presence of granulocyte-macrophage
colony-stimulating factor and IFN-alpha are potent inducers of
HIV-specific CD8 T cells. AIDS. 17:1731–1740. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lapenta C, Santini SM, Spada M, Donati S,
Urbani F, Accapezzato D, Franceschini D, Andreotti M, Barnaba V and
Belardelli F: IFN-alpha-conditioned dendritic cells are highly
efficient in inducing cross-priming CD8(+) T cells against
exogenous viral antigens. Eur J Immunol. 36:2046–2060. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pilon C, Levast B, Meurens F, Le Vern Y,
Kerboeuf D, Salmon H, Velge-Roussel F, Lebranchu Y and Baron C:
CD40 engagement strongly induces CD25 expression on porcine
dendritic cells and polarizes the T cell immune response toward
Th1. Mol Immunol. 46:437–447. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Korthals M, Safaian N, Kronenwett R,
Maihöfer D, Schott M, Papewalis C, Blanco E Diaz, Winter M, Czibere
A, Haas R, et al: Monocyte derived dendritic cells generated by
IFN-alpha acquire mature dendritic and natural killer cell
properties as shown by gene expression analysis. J Transl Med.
5:462007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
McRae BL, Nagai T, Semnani RT, van
Seventer JM and van Seventer GA: Interferon-alpha and -beta inhibit
the in vitro differentiation of immunocompetent human dendritic
cells from CD14(+) precursors. Blood. 96:210–217. 2000.PubMed/NCBI
|
|
16
|
Lapenta C, Donati S, Spadaro F, Castaldo
P, Belardelli F, Cox MC and Santini SM: NK cell activation in the
antitumor response induced by IFN-α dendritic cells loaded with
apoptotic cells from follicular lymphoma patients. J Immunol.
197:795–806. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chabot V, Martin L, Meley D, Sensebé L,
Baron C, Lebranchu Y, Dehaut F and Velge-Roussel F: Unexpected
impairment of TNF-α-induced maturation of human dendritic cells in
vitro by IL-4. J Transl Med. 14:932016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Farkas A, Tonel G and Nestle FO:
Interferon-alpha and viral triggers promote functional maturation
of human monocyte-derived dendritic cells. Br J Dermatol.
158:921–929. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Santini SM, Lapenta C, Donati S, Spadaro
F, Belardelli F and Ferrantini M: Interferon-α-conditioned human
monocytes combine a Th1-orienting attitude with the induction of
autologous Th17 responses: Role of IL-23 and IL-12. PLoS One.
6:e173642011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Trinchieri G: Interleukin-12 and the
regulation of innate resistance and adaptive immunity. Nat Rev
Immunol. 3:133–146. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Askenase MH, Han SJ, Byrd AL, da Fonseca
Morais D, Bouladoux N, Wilhelm C, Konkel JE, Hand TW,
Lacerda-Queiroz N, Su XZ, et al: Bone-marrow-resident NK cells
prime monocytes for regulatory function during infection. Immunity.
42:1130–1142. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Childs BG, Baker DJ, Wijshake T, Conover
CA, Campisi J and van Deursen JM: Senescent intimal foam cells are
deleterious at all stages of atherosclerosis. Science. 354:472–477.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Oehler L, Majdic O, Pickl WF, Stöckl J,
Riedl E, Drach J, Rappersberger K, Geissler K and Knapp W:
Neutrophil granulocyte-committed cells can be driven to acquire
dendritic cell characteristics. J Exp Med. 187:1019–1028. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Farkas A and Kemény L: Interferon-α in the
generation of monocyte-derived dendritic cells: recent advances and
implications for dermatology. Br J Dermatol. 165:247–254. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang S, Sun X, Zhou H, Zhu Z, Zhao W and
Zhu C: Interleukin-4 affects the mature phenotype and function of
rat bone marrow-derived dendritic cells. Mol Med Rep. 12:233–237.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gessani S, Conti L, Del Cornò M and
Belardelli F: Type I interferons as regulators of human antigen
presenting cell functions. Toxins (Basel). 6:1696–1723. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Guenova E, Skabytska Y, Hoetzenecker W,
Weindl G, Sauer K, Tham M, Kim KW, Park JH, Seo JH, Ignatova D, et
al: IL-4 abrogates T(H)17 cell-mediated inflammation by selective
silencing of IL-23 in antigen-presenting cells. Proc Natl Acad Sci
USA. 112:2163–2168. 2015; View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Okada S, Han S, Patel ES, Yang LJ and
Chang LJ: STAT3 signaling contributes to the high effector
activities of interleukin-15-derived dendritic cells. Immunol Cell
Biol. 93:461–471. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Taieb A, Breitinger JJ, Unadkat JV,
Shufesky WJ, Morelli AE, Thomson AW, Lee WP and Feili-Hariri M:
Intrinsic ability of GM+IL-4 but not Flt3L-induced rat dendritic
cells to promote allogeneic T cell hyporesponsiveness. Clin
Immunol. 123:176–189. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Carbonneil C, Saidi H, Donkova-Petrini V
and Weiss L: Dendritic cells generated in the presence of
interferon-alpha stimulate allogeneic CD4+ T-cell
proliferation: Modulation by autocrine IL-10, enhanced T-cell
apoptosis and T regulatory type 1 cells. Int Immunol. 16:1037–1052.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bella S Della, Nicola S, Riva A, Biasin M,
Clerici M and Villa ML: Functional repertoire of dendritic cells
generated in granulocyte macrophage-colony stimulating factor and
interferon-alpha. J Leukoc Biol. 75:106–116. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Papewalis C, Jacobs B, Wuttke M, Ullrich
E, Baehring T, Fenk R, Willenberg HS, Schinner S, Cohnen M,
Seissler J, et al: IFN-alpha skews monocytes into
CD56+-expressing dendritic cells with potent functional
activities in vitro and in vivo. J Immunol. 180:1462–1470. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fujii S, Liu K, Smith C, Bonito AJ and
Steinman RM: The linkage of innate to adaptive immunity via
maturing dendritic cells in vivo requires CD40 ligation in addition
to antigen presentation and CD80/86 costimulation. J Exp Med.
199:1607–1618. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Freeman GJ, Gribben JG, Boussiotis VA, Ng
JW, Restivo VA Jr, Lombard LA, Gray GS and Nadler LM: Cloning of
B7-2: A CTLA-4 counter-receptor that costimulates human T cell
proliferation. Science. 262:909–911. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Evans EJ, Esnouf RM, Manso-Sancho R,
Gilbert RJ, James JR, Yu C, Fennelly JA, Vowles C, Hanke T, Walse
B, et al: Crystal structure of a soluble CD28-Fab complex. Nat
Immunol. 6:271–279. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pentcheva-Hoang T, Egen JG, Wojnoonski K
and Allison JP: B7-1 and B7-2 selectively recruit CTLA-4 and CD28
to the immunological synapse. Immunity. 21:401–413. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lenschow DJ, Walunas TL and Bluestone JA:
CD28/B7 system of T cell costimulation. Annu Rev Immunol.
14:233–258. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Leplina OY, Tyrinova TV, Tikhonova MA,
Ostanin AA and Chernykh ER: Interferon alpha induces generation of
semi-mature dendritic cells with high pro-inflammatory and
cytotoxic potential. Cytokine. 71:1–7. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
van Lummel M, van Veelen PA, de Ru AH,
Janssen GM, Pool J, Laban S, Joosten AM, Nikolic T, Drijfhout JW,
Mearin ML, et al: Dendritic cells guide islet autoimmunity through
a restricted and uniquely processed peptidome presented by
high-risk HLA-DR. J Immunol. 196:3253–3263. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Foster GA, Xu L, Chidambaram AA, Soderberg
SR, Armstrong EJ, Wu H and Simon SI: CD11c/CD18 signals very late
antigen-4 activation to initiate foamy monocyte recruitment during
the onset of hypercholesterolemia. J Immunol. 195:5380–5392. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Arnold IC, Mathisen S, Schulthess J, Danne
C, Hegazy AN and Powrie F: CD11c(+) monocyte/macrophages promote
chronic Helicobacter hepaticus-induced intestinal inflammation
through the production of IL-23. Mucosal Immunol. 9:352–363. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Banchereau J, Pascual V and O'Garra A:
From IL-2 to IL-37: The expanding spectrum of anti-inflammatory
cytokines. Nat Immunol. 13:925–931. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yoshimoto T, Takeda K, Tanaka T, Ohkusu K,
Kashiwamura S, Okamura H, Akira S and Nakanishi K: IL-12
up-regulates IL-18 receptor expression on T cells, Th1 cells, and B
cells: Synergism with IL-18 for IFN-gamma production. J Immunol.
161:3400–3407. 1998.PubMed/NCBI
|
|
44
|
Behzadi P, Behzadi E and Ranjbar R: IL-12
family cytokines: General characteristics, pathogenic
microorganisms, receptors and signalling pathways. Acta Microbiol
Immunol Hung. 63:1–25. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Vignali DA and Kuchroo VK: IL-12 family
cytokines: Immunological playmakers. Nat Immunol. 13:722–728. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Belladonna ML, Renauld JC, Bianchi R,
Vacca C, Fallarino F, Orabona C, Fioretti MC, Grohmann U and
Puccetti P: IL-23 and IL-12 have overlapping, but distinct, effects
on murine dendritic cells. J Immunol. 168:5448–5454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
de Niet A, Stelma F, Jansen L, Sinnige MJ,
Remmerswaal EB, Takkenberg RB, Kootstra NA, Reesink HW, van Lier RA
and van Leeuwen EM: Restoration of T cell function in chronic
hepatitis B patients upon treatment with interferon based
combination therapy. J Hepatol. 64:539–546. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Aarntzen EH, De Vries IJ, Lesterhuis WJ,
Schuurhuis D, Jacobs JF, Bol K, Schreibelt G, Mus R, De Wilt JH,
Haanen JB, et al: Targeting CD4(+) T-helper cells improves the
induction of antitumor responses in dendritic cell-based
vaccination. Cancer Res. 73:19–29. 2013. View Article : Google Scholar : PubMed/NCBI
|



















