Introduction
MicroRNAs (miRNAs) are endogenous, non-coding,
single-stranded RNAs consisting of fewer than 22 nucleotides, and
are encoded by short inverted repeats within the genome (1,2).
miRNAs perform their physiological and pathological functions by
regulating the expressions of target genes (3). Previous studies have suggested that a
number of miRNAs are involved in the pathogenesis of cardiovascular
disease (4–6). One of these is miRNA-21.
miRNA-21 was first identified as a tumor growth
enhancer (7,8), however was later observed to be
involved in mediating the homeostasis of the cardiovascular system
(9–11). Abnormal levels of miRNA-21
contribute to the development of a number of cardiovascular
diseases including coronary heart disease, cardiac fibrosis and
hypertrophy (12,13). For example, Dong et al
(14) identified abundant miRNA-21
in rat hypertrophic cardiac cells, and Thum et al (15) reported that miRNA-21 was
significantly upregulated in fibrotic failing hearts, and involved
in activating extracellular signal-related kinase-mitogen-activated
protein kinase signaling that led to fibroblast proliferation and
fibrosis. Conversely, the antagonist of miRNA-21 prevented cardiac
hypertrophy and reversed cardiac remodeling (15).
Heart failure (HF) is an end stage of numerous
cardiac muscle disorders including cardiac hypertrophy, and is the
leading cause of hospitalization in the elderly and death worldwide
(16,17). Histologically, the failing heart is
accompanied by cardiomyocyte death and fibrosis (18).
miRNA-21 in human HF has been studied previously, in
human failing heart tissues, miRNA-21 levels were elevated
(19). In a rat HF model, elevated
miRNA-21 levels facilitated the development of HF, at least in part
by promoting cardiac fibrosis (20). Thus, miRNA-21 in the heart
contributes to the pathogenesis of HF. However, while miRNA-21 is
produced predominantly in the heart, it is unclear whether
circulating miRNA-21 can serve as an indicator for HF in human
patients.
Identification of specific, reliable and sensitive
biomarkers for human diseases is a field of active research. In the
cardiovascular field, several molecules have been identified as
putative biomarkers for different cardiovascular diseases or
different stages of a particular cardiovascular disease. For
example, it is well documented that high levels of low-density
lipoprotein cholesterol are closely associated with increased risk
of coronary heart disease (21).
Circulating brain natriuretic peptide (BNP), which is predominantly
produced in heart ventricles in response to stress, serves as a
sensitive predictor for cardiac dysfunction and for vascular
patients with major adverse cardiac events (22,23).
The identification of circulating miRNAs as specific
biomarkers for cardiovascular disorders has been researched
previously. For example, circulating miRNA-208 and miRNA-150 have
been identified as potentially promising biomarkers for both
agonist-induced cardiac hypertrophy and cardiac remodeling
following acute myocardial infarction (24,25).
A number of miRNAs, including miRNA-21, have been observed to be
elevated in the serum of elderly patients with non-ST segment
myocardial infarction (26),
suggesting that miRNA-21 may serve as a biomarker for coronary
heart diseases.
Given the pathogenic role that miRNA-21 has in the
development of HF, and that increased levels of miRNA-21 have been
demonstrated in failing hearts in humans and animal models, the
present study explored an association between the levels of
circulating miRNA-21 and human HF. The observations of the current
study provided evidence that miRNA-21 could be a promising
biomarker for diagnosis and prognosis of human HF.
Materials and methods
Study subjects
The Institutional Review Board of First Affiliated
Hospital of Xinjiang Medical University approved the study
protocol. A total of 120 patients among those hospitalized between
March 2013 and October 2013 met the inclusion criteria of the
study, signed an informed consent form, and were subsequently
recruited into the study. All patients underwent coronary
angiogram, radiofrequency ablation or cardiac resynchronization
therapy (CRT). Due to the fact that previous studies indicated that
serum miRNA-21 levels were high in patients with malignant tumors
(7,27,28),
the current study excluded patients with malignant tumors.
Among the patients recruited, 40 had normal cardiac
function and were placed in the control group, and 80 had abnormal
cardiac function and were placed in the HF group. The diagnosis of
HF met the guidelines of the 2012 European Society of Cardiology
for heat failure (29). The
patients in the control group had arrhythmia or angina. The
inclusion criteria for HF were: Cardiomyopathy; HF as the
first-listed diagnosis; a left ventricular ejection fraction
(LVEF)<50%; and a history of HF for >6 months. The exclusion
criterion for the HF group was the presence of a malignant tumor.
The inclusion criteria of the control group were: LVEF≥50%; and no
symptoms of HF. The exclusion criteria for the control group were:
A history of myocardial infarction; or presence of a malignant
tumor.
Blood sample collection
All subjects underwent either coronary angiography
or radiofrequency catheter ablation. Sheathes were placed in the
femoral vein and blood samples were extracted from the coronary
sinus (CS) and femoral vein using a coronary angiographic catheter.
The patients in the HF group were further stratified into 4
subgroups based on the New York Heart Association (NYHA) Functional
Classification. All patients with HF were treated with anti-HF
drugs, and their cardiac functions were improved to NYHA II. When
the blood was being collected from the CS the X-ray position was at
30 degrees, relative to the left anterior oblique position.
Under fluoroscopy, the catheter reached the upper
right atrium and turned toward the spine. The operator then slowly
pulled back the catheter, jumping of the catheter signified that
the catheter's tip had reached the CS. The operator used a contrast
agent during angiography to ensure that the catheter was localized
in the coronary sinus.
Serum isolation and storage
A total of 2 6–8 ml blood samples were separately
collected from the CS and femoral vein of each subject. The
collection tube (BD 762165 PAXgene Blood RNA tube) was purchased
from BD Biosciences (Franklin Lakes, NJ, USA). All blood samples
were allowed to stand for less than 2 h prior to being centrifuged
at 1,800 × g for 10 min, after which the serum samples were
collected and stored at −80°C.
Measurement of BNP
The level of BNP was measured using a human BNP
enzyme-linked immunosorbent assay kit (cat. no. AK0014JUN09001;
Elabscience Biotechnology Co., Ltd., China). The measurement of
each sample was conducted in triplicate. Testers of the samples
were blinded to the study group.
RNA extraction and cDNA synthesis
Total RNA was extracted from serum using the TRIzol
LS reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) based on the protocol provided by the manufacturer.
Briefly, 0.25-ml serum samples were homogenized in 0.75 ml TRIzol
reagent and 0.2 ml chloroform was added to each sample. All samples
were centrifuged at 12,000 × g for 15 min at 4°C to separate the
mixture. RNA in the aqueous phase was precipitated with 0.5 ml
isopropyl alcohol. Following centrifugation, the pellets (i.e.,
RNA) were washed with 75% ethanol. RNA purity was determined from
the 260/280 nm ratio, and only those samples with a ratio of
between 1.8 and 2.1 were used in the present study.
Reverse transcription (RT) was performed using 4 µg
of total RNA in a total reaction volume of 20 µl, with the
RevertAid First Strand cDNA Synthesis kit (Fermentas; Thermo Fisher
Scientific, Inc.) using hsa-mir-21 RT primer
5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACTCAACATC-3′.
Briefly, subsequent to an initial treatment at 70°C for 5 min, the
RT reaction was conducted at 42°C for 1 h using 1 µl Moloney murine
leukemia virus reverse transcriptase, and terminated by heating to
92°C for 5 min. Following cDNA synthesis, all cDNA samples were
diluted 10 times in molecular grade water and stored at −20°C.
Quantitative polymerase chain reaction
(qPCR)
qPCR was performed using an ABI viia7 Sequence
Detection system (Applied Biosystems; Thermo Fisher Scientific,
Inc.). The hsa-mir-21 PCR primers were: Forward,
5′-TGCGCTAGCTTATCAGACTGA-3′ and reverse,
5′-CCAGTGCAGGGTCCGAGGTATT-3′. U6 small nuclear RNA was used as an
endogenous control for normalization. The qPCR reaction contained
10 µl 2X SYBR-Green/Fluorescein qPCR Master mix (Fermentas; Thermo
Fisher Scientific, Inc.), the forward and reverse primers,
RNase-free water and 2 µl cDNA template per reaction in a final
volume of 20 µl. The thermocycling conditions for quantitative PCR
were 1 cycle of 50°C for 2 min, 95°C for 10 min, 40 cycles of 30
sec at 94°C and 30 sec at 60°C.
The data were processed using the relative
quantification method. The relative values were measured as
2−ΔΔCt (30). Testers
were blinded to the patient group. The expression levels relative
to U6 were 18.88±1.49 in the vein and 28.10±1.49 in the coronary of
the control group, and 20.01±0.82 in the vein and 28.94±1.07 in the
coronary of the HF group.
Data analysis and statistics
Data were analyzed using the statistical software
package SPSS 17.0 (SPSS, Inc., Chicago, IL, USA). Continuous
clinical variables were compared between the 2 groups using a
two-sided unpaired t-test. Analysis of covariance was used to
analysis the effect of confounding factors. The Chi-square test was
applied to compare categorical clinical variables. Receiver
operating characteristic (ROC) curve analysis was used to evaluate
the association between miRNA-21 levels and a diagnosis of HF.
Pearson's correlation coefficient was employed to evaluate the
correlation between clinical features and serum miR-21. Binary
logistic regression was used to evaluate the correlation between
serum miRNA-21 and other factors with the re-hospitalization rate
for HF. A COX regression model was used to evaluate the correlation
between serum miRNA-21 and other factors with the prognosis of HF.
Continuous clinical variables were presented as the mean ± standard
deviation. P<0.05 was considered to indicate a statistically
significant difference.
Results
Demographic and clinical
characteristics of the HF and control groups
The HF and control groups were significantly
different with regard to age, gender, pathogenesis, hemoglobin,
systolic pressure, renal function and levels of BNP and miRNA-21 in
the peripheral vein (PV) and CS (Table
I).
 | Table I.Demographics and clinical
characteristics of the HF and control groups. |
Table I.
Demographics and clinical
characteristics of the HF and control groups.
| Category | HF | Control | P-value |
|---|
| Subjects | 80 | 40 |
|
| Gender,
male/female | 57/23 | 20/20 | 0.027 |
| Age, years | 59.68±10.24 | 53.45±12.40 | 0.04 |
| Weight, kg | 69.50±12.80 | 70.42±16.08 | 0.752 |
| Dilated
cardiomyopathy | 33 | 0 |
|
| Ischemic
cardiomyopathy | 41 | 0 |
|
| Hypertensive
cardiomyopathy | 4 | 0 |
|
| Valvular heart
disease | 2 | 0 |
|
| PSVT | 0 | 21 |
|
| Premature RVOT | 0 | 3 |
|
| Coronary heart
disease | 41 | 10 | 0.007 |
| Physical
examination | 0 | 5 |
|
| Hypertension | 23 | 12 | 0.877 |
| Diabetes
mellitus | 20 | 5 | 0.001 |
| Atrial
fibrillation | 9 | 1 | 0.089 |
| History of heart
failure, years | 2.72±2.78 | – |
|
| NYHA I | 11 | – |
|
| NYHA II | 22 | – |
|
| NYHA III | 39 | – |
|
| NYHA IV | 8 | – |
|
| ACEI/ARB | 80 | 0 |
|
| Βeta blocker | 77 | 1 |
|
| Aldospirone | 55 | 0 |
|
| Amiodarone | 9 | 1 | 0.139 |
| CRT | 20 | 0 |
|
| Systolic blood
pressure, mmHg | 115.54±18.40 | 125.98±14.40 | 0.02 |
| Diastolic blood
pressure, mmHg | 71.13±12.62 | 75.70±10.70 | 0.052 |
| Hemoglobin,
g/l | 132.53±15.21 | 137.20±15.39 | 0.117 |
| Creatinine,
mmol/l | 79.31±25.93 | 61.55±15.30 | 0 |
| Alanine
aminotransferase, U | 31.16±18.86 | 28.27±4.14 | 0.439 |
| CK-MB, U/l | 14.41±7.41 | 13.09±4.83 | 0.308 |
| LVED, mm | 63.07±7.79 | 47.75±3.13 | 0 |
| Left atrial
diameter, mm | 42.18±4.98 | 33.85±4.14 | 0 |
| LVEF, % | 37.76±4.98 | 61.85±5.43 | 0 |
| BNP-PV, pg/ml | 767.34±362.75 | 199.80±75.43 | 0 |
| BNP-CS, pg/ml |
1,277.43±515.61 | 319.82±122.77 | 0 |
| miRNA-21-PV | 3.98±2.59 | 0.90±0.62 | 0 |
| miRNA-21-CS | 17.25±12.28 | 3.81±3.24 | 0 |
Increased circulating BNP and miRNA-21
in HF patients
A number of studies have indicated a higher level of
BNP in the CS than in other tissues of the body (31,32),
and it has been well documented that the majority of circulating
BNP originates from the heart (33). Consistent with the above
observations, in the present study BNP levels in the CS were higher
than in the PV in patients of either the HF or control group
(Fig. 1A). Notably, the data of
the present study indicated that BNP levels from either the PV or
CS in HF patients were significantly higher than that of the
control group.
The levels of circulating miRNA-21 in the PV were
lower in the CS in both the HF and control groups, and circulating
miRNA-21 in the patients with HF was significantly higher, than
that of the control group (Fig.
1B). Previous studies have indicated that age (34) and diabetes (35) affected the expression of miRNA-21.
Therefore, analysis of covariance was used to analyze the effect of
age and diabetes. It was identified that in the samples
investigated, age and diabetes did not significantly correlate with
the circulating levels of miRNA-21, in either the vein or coronary
(Table II).
 | Table II.Age and diabetes were not correlated
with circulating microRNA-21 levels in patients with heart failure
as analyzed by analysis covariance. |
Table II.
Age and diabetes were not correlated
with circulating microRNA-21 levels in patients with heart failure
as analyzed by analysis covariance.
|
| Vein | Coronary |
|---|
|
|
|
|
|---|
| Category | P-value | 95% CI | P-value | 95% CI |
|---|
| Heart failure | <0.001 |
2.197–3.926 | <0.001 |
8.669–16.815 |
| Age | 0.796 | −0.32–0.42 | 0.484 | −0.112–0.236 |
| Diabetes | 0.993 | −1.007–0.997 | 0.219 | −2.238–7.029 |
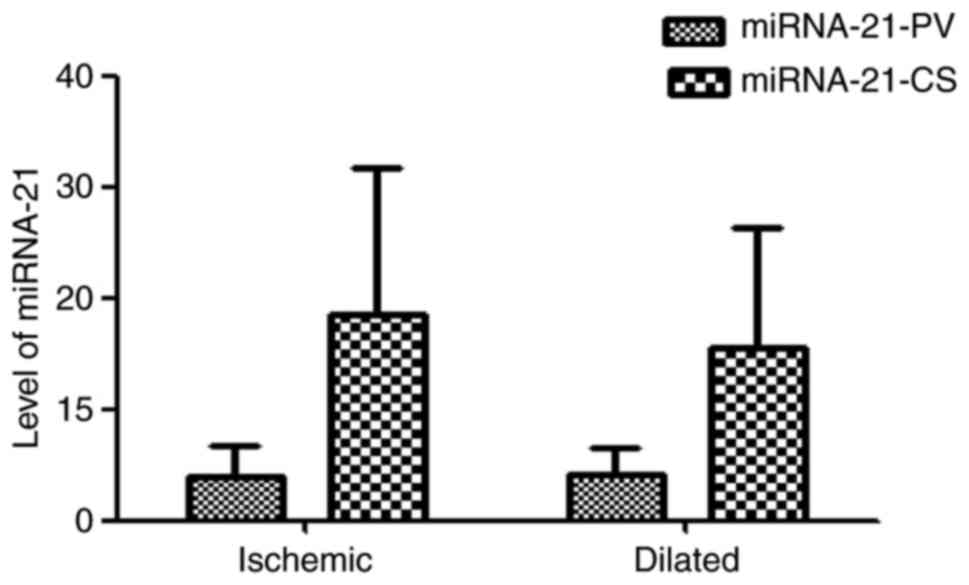
Levels of miRNA-21 in ischemic
cardiomyopathy and dilated cardiomyopathy
Ischemic cardiomyopathy and dilated cardiomyopathy
are two main causes of HF. In the HF group, 41 patients had
ischemic cardiomyopathy and 33 had dilated cardiomyopathy. There
was no significant difference in the EF between the ischemic
cardiomyopathy and dilated cardiomyopathy groups (37.90±4.01 vs.
36.39±4.86%; P=0.148). The levels of miRNA-21-PV and miRNA-21-CS in
the two groups were not significantly different (miRNA-21-PV,
3.95±2.84 vs. 4.13±2.42, P=0.773; miRNA-21-CS, 18.48±13.28 vs.
15.46±10.81, P=0.296; Fig. 2).
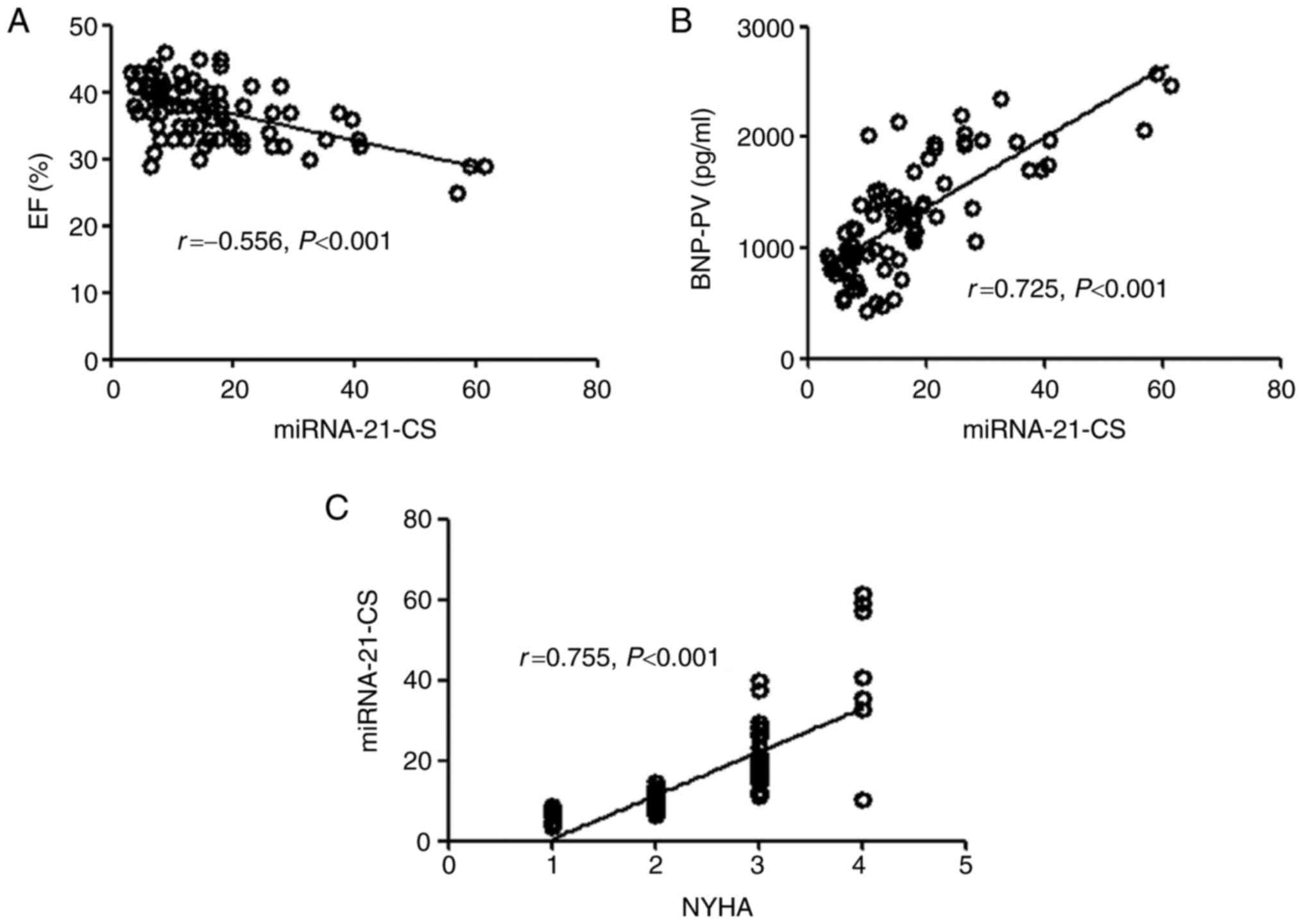
Correlation between miRNA-21 and other
clinical indices
Correlation analyses were conducted to evaluate
associations between serum miRNA-21 and EF, NYHA classification or
BNP in the HF group relative to the control group (Fig. 3). miRNA-21-PV was negatively
correlated with EF (r=−0.540, P<0.001), however positively
correlated with NYHA grade (r=0.580, P<0.001) and BNP-PV
(r=0.760, P<0.001). The associations between miRNA-21-CS and EF
(r=−0.556, P<0.001), NYHA classification (r=0.755, P<0.001)
and BNP-CS (r=0.725, P<0.001) followed the same pattern as that
of miRNA-21-PV to these variables (Fig. 4).
Correlation between miRNA-21 and the
severity of HF symptoms
The levels of circulating miRNA-21-PV and
miRNA-21-CS gradually increased with increasing NYHA grade
(Fig. 5). Analysis of variance
indicated that the levels of circulating miRNA-21-PV and
miRNA-21-CS were significantly different in each group, excluding
that between the NYHA I and II groups.
Correlation between circulating
miRNA-21 and diagnosis of HF
The correlation between circulating miRNA-21 and HF
was identified by the ROC curve (Fig.
6). Using a threshold score of 1.98, patients with a score
>1.98 were predicted to have HF. Using miRNA-21-PV, a
sensitivity of 100%, a specificity of 97.5% and area under curve
(AUC)=0.948 was achieved for the identification of patients with
HF. Similar results were obtained for miRNA-21-CS
(sensitivity=100%, specificity=97.5% and AUC=0.940).
Correlation between circulating
miRNA-21 and prognosis of HF
The association between circulating miRNA-21 and the
prognosis of the HF group was analyzed using a Cox regression
model. The patients with HF were followed for 2 years (mean,
18.33±3.882 months). The endpoint of follow-up was death. A total
of 17 patients of the HF group died, 1 as a result of a traffic
accident, 1 from hypoxemia of pulmonary fibrosis and infection, 12
from cardiac sudden death, and 3 from multiple organ failure caused
by HF. EF, BNP-PV, creatinine, CRT and miRNA-21-PV/miRNA-21-CS were
included in the Cox regression model. miRNA-21-PV significantly
correlated with the endpoint [relative risk (RR)=1.936, 95%
confidence interval (CI)=1.310–2.865, P=0.001], as did miRNA-21-CS
(RR=1.125, 95% CI=1.049–1.1206, P=0.001; Table III).
 | Table III.Circulating miRNA-21 levels in
peripheral veins and the coronary sinus were significantly
correlated with the endpoint as analyzed by the Cox regression
model. |
Table III.
Circulating miRNA-21 levels in
peripheral veins and the coronary sinus were significantly
correlated with the endpoint as analyzed by the Cox regression
model.
|
| Vein | Coronary |
|---|
|
|
|
|
|---|
| Category | P-value | RR | 95% CI | P-value | RR | 95% CI |
|---|
| EF | 0.305 | 0.875 | 0.677–1.120 | 0.196 | 0.850 | 0.665–1.088 |
| BNP | 0.103 | 1.002 | 1.000–1.005 | 0.007 | 1.003 | 1.001–1.005 |
| CRT | 0.218 | 0.348 | 0.065–1.807 | 0.237 | 0.384 | 0.078–1.875 |
| miRNA-21 | 0.001 | 1.936 | 1.310–2.862 | 0.001 | 1.125 | 1.049–1.206 |
Correlation between circulating
miRNA-21-CS and re-hospitalization rate for HF
A binary logistic regression model was used to
evaluate a correlation between serum miRNA-21 and other factors and
the re-hospitalization rate for HF. A total of 23 of the 60
patients with HF required re-hospitalization during the follow-up
period.
EF, BNP-PV, creatinine, CK-MB, alanine
aminotransferase, CRT, miRNA-21-PV and miRNA-21-CS were analyzed
using binary logistic regression. Similar to the analysis with the
Cox regression model, the miRNA-21-PV and miRNA-21-CS serum levels
were analyzed to compare their correlations with re-hospitalization
rates for HF. miRNA-21-PV exhibited no significant association with
re-hospitalization rate [overall risk (OR)=1.001, 95%
CI=0.993–1.010, P=0.757], however miRNA-21-CS did (OR=1.160, 95%
CI=1.023–1.315, P=0.021; Table
IV).
 | Table IV.Circulating miR-21 levels in coronary
sinus however not in peripheral veins correlated with
re-hospitalization rate. |
Table IV.
Circulating miR-21 levels in coronary
sinus however not in peripheral veins correlated with
re-hospitalization rate.
|
| Vein | Coronary |
|---|
|
|
|
|
|---|
| Category | P-value | OR | 95% CI | P-value | OR | 95% CI |
|---|
| Creatinine | 0.497 | 0.992 | 0.969–1.016 | 0.508 | 0.991 | 0.965–1.018 |
| ALT | 0.868 | 0.997 | 0.996–1.030 | 0.902 | 1.002 | 0.968–1.038 |
| CK-MB | 0.315 | 1.084 | 0.926–1.270 | 0.575 | 1.048 | 0.890–1.235 |
| EF | 0.073 | 0.896 | 0.745–1.013 | 0.401 | 0.930 | 0.786–1.101 |
| BNP | 0.007 | 1.003 | 1.001–1.005 | 0.384 | 1.001 | 0.999–1.004 |
| CRT | 0.511 | 0.598 | 0.129–2.766 | 0.480 | 1.947 | 0.306–12.379 |
| miR-21 | 0.757 | 1.001 | 0.993–1.010 | 0.021 | 1.160 | 1.023–1.315 |
Discussion
In the present study, it was investigated whether
miRNA-21 could serve as a valuable predicator for HF. The current
study established, to the best of our knowledge for the first time,
that circulating miRNA-21 may not only be a promising biomarker of
human HF, however additionally an efficient predictor of mortality
and re-hospitalization of patients with HF.
Similar to serum BNP, serum miRNA-21 levels taken
from the CS were significantly higher than that from the PV,
regardless of whether the samples were from the control or HF
patients. This suggests that the heart is an important source of
serum miRNA-21 in patients with HF. By contrast, it was identified
that the levels of circulating miRNA-21 in the PVs and CSs of the
HF group were significantly higher than that of the control group,
indicating that serum miRNA-21 may be a predictor of HF. Notably, a
blood sample obtained from one patient in the control group had a
significantly higher level of circulating miRNA-21 relative to the
other control samples, and it was determined that this control
subject had atrial fibrillation. Thus, consistent with a previous
report, miRNA-21 may be involved in the pathogenesis of atrial
fibrillation (36).
For a factor to qualify as a biomarker of HF, the
expression of this factor should not be significantly altered in
the failing hearts caused by different forms of cardiac diseases.
Ischemic cardiomyopathy and dilated cardiomyopathy are the two most
common causes of HF. In the present study, there was no significant
difference in EF between these two groups. Further analysis
identified that the circulating levels of miRNA-21-CS and
miRNA-21-PV additionally exhibited no significant difference
between these two groups, suggesting that the circulating miRNA-21
was not significantly altered due to different causes of HF, i.e.,
ischemic or non-ischemic cardiomyopathy.
Due to the fact that significantly higher
circulating levels of miRNA-21-CS and miRNA-21-PV were identified
in patients with HF compared with the control subjects,
associations between serum miRNA-21 and cardiac function status,
diagnosis, prognosis or re-hospitalization rates were investigated.
It was observed that circulating miRNA-21-PV and miRNA-21-CS
correlated well and linearly with cardiac EF, BNP and NYHA grade;
the latter evidenced by a gradual increase in levels with
increasing NYHA grade. However, the association between miRNA-21-CS
and EF was closer than that of the association between miRNA-21-PV
and EF, indicating that miRNA-21-CS may be a more effective
indicator of cardiac function and HF. miRNA-21-PV and miRNA-21-CS
exhibited similar efficacy in the diagnosis of HF, as similar
sensitivity (100%) and specificity (97.5%) were observed, as
indicated by the ROC curve.
Few clinical studies have investigated the potential
of miRNAs as an indicator of prognosis in HF. In the present study,
the results indicated that serum miRNA-21 was useful for predicting
mortality associated with HF. miRNA-21-PV and miRNA-21-CS were
significantly associated with mortality of HF patients. However,
miRNA-21-CS, but not miRNA-21-PV, was closely associated with
re-hospitalization after normalization of EF, BNP, creatinine,
CK-MB, CRT and alanine aminotransferase.
Previous studies have demonstrated that CRT improved
the quality of life and prognosis of patients with HF (37). However, in the present study, CRT
did not significantly affect prognosis or re-hospitalization. The
discrepancy between previous results and those of the current study
were likely due to the HF group in the present study including
patients with various stages of HF, of which only 25% of those with
severe HF received CRT. Since the heart is the major source of
circulating miRNA-21 in patients with HF, it is understandable that
the level of miRNA-21 in the CS is higher than in the PV, and
therefore is probably a more sensitive predicator. However, it was
suggested that circulating miRNA-21-PV was sufficient for
predicting cardiac function, diagnosis and prognosis of HF. Given
that circulating miRNA-21-PV is more readily available than
miRNA-21-CS, it is anticipated that miRNA-21-PV will have a more
practical application in the clinical setting.
The limitations of the present study were as
follows. The control group did not match the HF group in terms of
age and gender, in addition, the subjects selected for the control
group were not healthy people, which may have led to unpredictable
effects on the results. In addition, the size of the samples used
in the study was small. Thus, further studies with a larger sample
size and an improved control group are required in order to
corroborate the results.
In summary, the present study compared the levels of
circulating miRNA-21 in the PVs and CS of patients with HF and
control subjects. It was determined that both miRNA-21-PV and
miRNA-21-CS were significantly increased in HF patients when
compared with the control group. The correlation analysis indicated
that circulating miRNA-21 correlated with the diagnosis and
prognosis of HF. Therefore, it was suggested that circulating
miRNA-21 has potential to become a novel biomarker of human HF.
Acknowledgements
The current study was supported by Youth Project
(grant no. 2013ZRQN19) of the Natural Science Foundation of the
First Affiliated Hospital of Xinjiang Medical University. The
abstract has been previously published in the Proceedings of the
18th South China International Congress of Cardiology April 17-10,
2016.
References
|
1
|
Lee RC, Feinbaum RL and Ambros V: The C.
elegans heterochronic gene lin-4 encodes small RNAs with antisense
complementarity to lin-14. Cell. 75:843–854. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wightman B, Ha I and Ruvkun G:
Posttranscriptional regulation of the heterochronic gene lin-14 by
lin-4 mediates temporal pattern formation in C. elegans. Cell.
75:855–862. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gidlöf O, Smith JG, Miyazu K, Gilje P,
Spencer A, Blomquist S and Erlinge D: Circulating cardio-enriched
microRNAs are associated with long-term prognosis following
myocardial infarction. BMC Cardiovasc Disord. 13:122013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Marfella R, Di Filippo C, Potenza N, Sardu
C, Rizzo MR, Siniscalchi M, Musacchio E, Barbieri M, Mauro C, Mosca
N, et al: Circulating microRNA changes in heart failure patients
treated with cardiac resynchronization therapy: Responders vs.
Non-responders. Eur J Heart Fail. 15:1277–1288. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ellis KL, Cameron VA, Troughton RW,
Frampton CM, Ellmers LJ and Richards AM: Circulating microRNAs as
candidate markers to distinguish heart failure in breathless
patients. Eur J Heart Fail. 15:1138–1147. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Asangani IA, Rasheed SA, Nikolova DA,
Leupold JH, Colburn NH, Post S and Allgayer H: MicroRNA-21 (miR-21)
post-transcriptionally downregulates tumor suppressor Pdcd4 and
stimulates invasion, intravasation and metastasis in colorectal
cancer. Oncogene. 27:2128–2136. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Selcuklu SD, Donoghue MT and Spillane C:
miR-21 as a key regulator of oncogenic processes. Biochem Soc
Trans. 37:918–925. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Suárez Y, Fernández-Hernando C, Pober JS
and Sessa WC: Dicer dependent microRNAs regulate gene expression
and functions in human endothelial cells. Circ Res. 100:1164–1173.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ji R, Cheng Y, Yue J, Yang J, Liu X, Chen
H, Dean DB and Zhang C: MicroRNA expression signature and
antisense-mediated depletion reveal an essential role of MicroRNA
in vascular neointimal lesion formation. Circ Res. 100:1579–1588.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Roy S, Khanna S, Hussain SR, Biswas S,
Azad A, Rink C, Gnyawali S, Shilo S, Nuovo GJ and Sen CK: MicroRNA
expression in response to murine myocardial infarction: miR-21
regulates fibroblast metalloprotease-2 via phosphatase and tensin
homologue. Cardiovasc Res. 82:21–29. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang C: MicroRNomics: A newly emerging
approach for disease biology. Physiol Genomics. 33:139–147. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cheng Y and Zhang C: MicroRNA-21 in
cardiovascular disease. J Cardiovasc Transl Res. 3:251–255. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dong S, Cheng Y, Yang J, Li J, Liu X, Wang
X, Wang D, Krall TJ, Delphin ES and Zhang C: MicroRNA expression
signature and the role of microRNA-21 in the early phase of acute
myocardial infarction. J Biol Chem. 284:29514–29525. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Thum T, Gross C, Fiedler J, Fischer T,
Kissler S, Bussen M, Galuppo P, Just S, Rottbauer W, Frantz S, et
al: MicroRNA-21 contributes to myocardial disease by stimulating
MAP kinase signalling in fibroblasts. Nature. 456:980–984. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shepler SA and Patel AN: Cardiac cell
therapy: A treatment option for cardiomyopathy. Crit Care Nurs Q.
30:74–80. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pruitt AL: Heart failure: It's not just
for men. Crit Care Nurs Clin North Am. 20:327–341. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chiong M, Wang ZV, Pedrozo Z, Cao DJ,
Troncoso R, Ibacache M, Criollo A, Nemchenko A, Hill JA and
Lavandero S: Cardiomyocyte death: Mechanisms and translational
implications. Cell Death Dis. 2:e2442011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Thum T, Galuppo P, Wolf C, Fiedler J,
Kneitz S, van Laake LW, Doevendans PA, Mummery CL, Borlak J,
Haverich A, et al: MicroRNAs in the human heart: A clue to fetal
gene reprogramming in heart failure. Circulation. 116:258–267.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dong S, Ma W, Hao B, Hu F, Yan L, Yan X,
Wang Y, Chen Z and Wang Z: microRNA-21 promotes cardiac fibrosis
and development of heart failure with preserved left ventricular
ejection fraction by up-regulating Bcl-2. Int J Clin Exp Pathol.
7:565–574. 2014.PubMed/NCBI
|
|
21
|
Emerging Risk Factors Collaboration, . Di
Angelantonio E, Sarwar N, Perry P, Kaptoge S, Ray KK, Thompson A,
Wood AM, Lewington S, Sattar N, et al: Major lipids,
apolipoproteins, and risk of vascular disease. JAMA. 302:1993–2000.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
van Holten TC, Waanders LF, de Groot PG,
Vissers J, Hoefer IE, Pasterkamp G, Prins MW and Roest M:
Circulating biomarkers for predicting cardiovascular disease risk;
a systematic review and comprehensive overview of meta-analyses.
PLoS One. 8:e620802013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rodseth RN, Buse GA Lurati, Bolliger D,
Burkhart CS, Cuthbertson BH, Gibson SC, Mahla E, Leibowitz DW and
Biccard BM: The predictive ability of pre-operative B-type
natriuretic peptide in vascular patients for major adverse cardiac
events: An individual patient data meta-analysis. J Am Coll
Cardiol. 58:522–529. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu L, Aguirre SA, Evering WE, Hirakawa
BP, May JR, Palacio K, Wang J, Zhang Y and Stevens GJ: miR-208a as
a biomarker of isoproterenol-induced cardiac injury in
Sod2+/− and C57BL/6J wild-type mice. Toxicol Pathol.
42:1117–1129. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Devaux Y, Vausort M, McCann GP, Zangrando
J, Kelly D, Razvi N, Zhang L, Ng LL, Wagner DR and Squire IB:
MicroRNA-150: A novel marker of left ventricular remodeling after
acute myocardial infarction. Circ Cardiovasc Genet. 6:290–298.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Olivieri F, Antonicelli R, Lorenzi M,
D'Alessandra Y, Lazzarini R, Santini G, Spazzafumo L, Lisa R, La
Sala L, Galeazzi R, et al: Diagnostic potential of circulating
miR-499-5p in elderly patients with acute non ST-elevation
myocardial infarction. Int J Cardiol. 167:531–536. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang HL, Yang LF, Zhu Y, Yao XD, Zhang
SL, Dai B, Zhu YP, Shen YJ, Shi GH and Ye DW: Serum miRNA-21:
Elevated levels in patients with metastatic hormone-refractory
prostate cancer and potential predictive factor for the efficacy of
docetaxel-based chemotherapy. Prostate. 71:326–331. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang ZX, Bian HB, Wang JR, Cheng ZX, Wang
KM and De W: Prognostic significance of serum miRNA-21 expression
in human non-small cell lung cancer. J Surg Oncol. 104:847–851.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
McMurray JJ, Adamopoulos S, Anker SD,
Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C,
Gomez-Sanchez MA, et al: ESC guidelines for the diagnosis and
treatment of acute and chronic heart failure 2012: The Task Force
for the Diagnosis and Treatment of Acute and Chronic Heart Failure
2012 of the European Society of Cardiology. Developed in
collaboration with the Heart Failure Association (HFA) of the ESC.
Eur J Heart Fail. 14:803–869. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Adnan M, Morton G and Hadi S: Analysis of
rpoS and bolA gene expression under various stress-induced
environments in planktonic and biofilm phase using 2(−ΔΔCT) method.
Mol Cell Biochem. 357:275–282. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Truong QA, Januzzi JL, Szymonifka J, Thai
WE, Wai B, Lavender Z, Sharma U, Sandoval RM, Grunau ZS, Basnet S,
et al: Coronary sinus biomarker sampling compared to peripheral
venous blood for predicting outcomes in patients with severe heart
failure undergoing cardiac resynchronization therapy: The BIOCRT
study. Heart Rhythm. 11:2167–2175. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Phelan D, Watson C, Martos R, Collier P,
Patle A, Donnelly S, Ledwidge M, Baugh J and McDonald K: Modest
elevation in BNP in asymptomatic hypertensive patients reflects
sub-clinical cardiac remodeling, inflammation and extracellular
matrix changes. PLoS One. 7:e492592012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Luchner A, Stevens TL, Borgeson DD,
Redfield M, Wei CM, Porter JG and Burnett JC Jr: Differential
atrial and ventricular expression of myocardial BNP during
evolution of heart failure. Am J Physiol. 274:H1684–H1689.
1998.PubMed/NCBI
|
|
34
|
Olivieri F, Spazzafumo L, Santini G,
Lazzarini R, Albertini MC, Rippo MR, Galeazzi R, Abbatecola AM,
Marcheselli F, Monti D, et al: Age-related differences in the
expression of circulating microRNAs: miR-21 as a new circulating
marker of inflammaging. Mech Ageing Dev. 133:675–685. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Olivieri F, Spazzafumo L, Bonafè M,
Recchioni R, Prattichizzo F, Marcheselli F, Micolucci L, Mensà E,
Giuliani A, Santini G, et al: MiR-21-5p and miR-126a-3p levels in
plasma and circulating angiogenic cells: Relationship with type 2
diabetes complications. Oncotarget. 6:35372–35382. 2015.PubMed/NCBI
|
|
36
|
Cardin S, Guasch E, Luo X, Naud P, Le
Quang K, Shi Y, Tardif JC, Comtois P and Nattel S: Role for
microRNA-21 in atrial profibrillatory fibrotic remodeling
associated with experimental postinfarction heart failure. Circ
Arrhythm Electrophysiol. 5:1027–1035. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Abraham WT, Fisher WG, Smith AL, Delurgio
DB, Leon AR, Loh E, Kocovic DZ, Packer M, Clavell AL, Hayes DL, et
al: Cardiac resynchronization in chronic heart failure. N Engl J
Med. 346:1845–1853. 2002. View Article : Google Scholar : PubMed/NCBI
|