Introduction
Diabetes mellitus (DM) is a significant risk factor
for carotid arterial injury by carotid intima-media thickness
(1). Compared with patients
without diabetes, patients with diabetes often exhibit severe
neointimal hyperplasia (2).
Hyperglycemia can also induce neointimal hyperplasia following
vascular injury in a rat carotid artery injury model (3,4).
Inflammation is important in this process, provoking vascular
smooth muscle cells (VSMCs) to migrate to the intima, which results
in neointimal formation (5).
Increasing evidence indicates that inflammatory cytokines are
implicated in peripheral arterial disease and carotid artery
disease (6). Nucleotide-binding
domain, leucine-rich-containing family, pyrin domain-containing-3
(NLRP3) inflammasome components are abundantly expressed in
microvascular endothelial cells, and the activation of NLRP3
inflammasome signaling enhances neointimal formation in mice
(7). The NLRP3 inflammasome, as
intracellular inflammatory machinery, has been reported to be
involved in obesity-associated coronary atherosclerotic injury and
endothelial dysfunction (7).
Clinical studies have shown that NLRP3 is overexpressed in the
aorta of patients with coronary atherosclerosis and carotid
atherosclerotic plaques (8,9).
These findings suggest that the NLRP3 inflammasome mechanism may be
involved in hyperglycemia-induced carotid arterial injury.
Oleanolic acid is a triterpenoid compound, which
exists widely in food and Chinese herbal medicine (10). It has a variety of biological
effects, including antioxidant (10), renoprotective (11), hepatoprotective (12), and anticancer effects (13). In addition, oleanolic acid, as a
synergistic therapeutic drug, improves glucose and insulin
homeostasis in db/db diabetic mice (14). Oleanolic acid, or its isomer, can
improve type 2 diabetes-associated complications, particularly
inflammation, through inhibition of the nuclear transcription
factor-κB (NF-κB) signaling pathway (15). Oleanolic acid suppresses NF-κB
signaling by inhibiting the lipopolysaccharide (LPS)-induced
phosphorylation of inhibitor of NF-κB (15,16).
The administration of ursolic acid, an isomer of oleanolic acid, in
mice fed a high fat diet (HFD) also inhibits the expression of
inflammatory cytokines tumor necrosis factor (TNF)-α and
interleukin (IL)-1 through NF-κB signaling (16). The anti-atherogenic effects of
oleanolic acid in apolipoprotein E-knockout mice are mediated by
reducing the expression of inducible nitric oxide synthase
(17). However, the arterial
protection of oleanolic acid in diabetic rats by NLRP3 inflammasome
signaling remains to be fully elucidated.
In the present study, whether the long-term
administration of oleanolic acid can protect against
hyperglycemia-induced carotid artery injury was investigated in a
diabetic rat animal model. The expression levels of NLRP3
inflammasome components were also measured in the carotid arteries
of diabetic rats.
Materials and methods
Animal experiments
Male Sprague-Dawley (SD) rats (n=18; 8-week-old)
were purchased from the Animal Center of Zhengzhou University
(Zhengzhou, China) and fed in an SPF laboratory. The rats were fed
under controlled temperature (23±2°C) and humidity (55±5%) with an
artificial 12-h light/dark cycle, and were given free access to
food and tap water. The rats were allowed to acclimate to the
environment for 1 week. All experimental procedures were performed
in accordance with the guidelines on Animal Care of the Fifth
Affiliated Hospital of Zhengzhou University (Zhengzhou, China).
Rats weighing 200–220 g were used for the experiments in the
present study, which were randomized into three groups: i) control
group (n=6); ii) diabetic rats with carotid artery injury (model
group, n=6); and iii) diabetic rats with carotid artery injury +
oleanolic acid (HPLC ≥98%; Aladdin Chemical Co., Ltd., Shanghai,
China) treatment (oleanolic acid group, n=6). The establishment of
a streptozotocin (STZ)-induced diabetic rat model with carotid
artery injury was performed as described previously (3). All other chemicals were of analytical
grade and purchased from Sigma-Aldrich (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany).
Histological examination
The normal or injured carotid arteries from the rats
were fixed in 4% paraformaldehyde for 1 week and then embedded in
paraffin. Sections measuring 5 µm were cut and stained with
hematoxylin & eosin (H&E). For morphologic analysis of
neointimal formation, Image-Pro Plus 5.0 image analysis software
(Media Cybernetics Inc., Rockville, MD, USA) was used. The medial
and intimal cross-sectional areas were measured, and the
intima/media ratios were calculated.
Serum levels of inflammatory
cytokines
Following sacrifice of the rats, blood was collected
using Vacutainer tubes (BD Biosciences, Franklin Lakes, NJ, USA)
and centrifuged immediately at 3,000 × g for 10 min at 4°C. The
supernatant was then collected and stored at −80°C until used for
the subsequent assay. The levels of TNF-α, IL-1β, IL-6 and IL-18
were analyzed using enzyme-linked immunosorbent assay (ELISA) kits
(Biosource, Camarillo, CA, USA) according to the manufacturer's
instructions. ELISA kits were used to measure the levels of
endothelin 1 (ET-1; cat. no. E-EL-R0167; Elabscience Biotechnology
Co., Ltd., Wuhan, China), nitric oxide (NO; cat. no. A012-1;
Nanjing Jiancheng Biology Engineering Institute, Nanjing, China)
and von Willebrand factor (vWF; cat. no. E-EL-R1079; Elabscience
Biotechnology) in the supernatant on an ELISA reader (BioTek
Instruments, Inc., Winooski, VT, USA) according to the
manufacturers' instructions.
Vascular permeability assay
Aortic blood vessel leakage was quantitated using
Evans blue dye, which binds non-covalently to plasma albumin in the
blood stream, as described previously (18). Aortic blood vessels were visualized
under a microscope (Leica DM 2500; Leica Microsystems GmbH,
Wetzlar, Germany).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
RNA was extracted using TRIzol®
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
according to the manufacturer's instructions. The synthesis of cDNA
was performed with 2 µg total RNA using Moloney murine leukemia
virus reverse transcriptase (Promega Corporation, Madison, WI, USA)
and oligo(dT)15 primers (Thermo Fisher Scientific, Inc.) according
to the manufacturer's instructions. The qPCR was performed using an
Applied Biosystems 7300 Real-Time PCR system (Thermo Fisher
Scientific, Inc.). Reaction mixtures (25 µl) were prepared as
follows: 12.5 µl SYBR-Green Supermix (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA), 1 µl cDNA, 300 nM of each primer, and DEPC
H2O to a final volume of 25 µl. Thermocycling procedure
was performed as follows: 94°C for 1 min, 40 cycles of 94°C for 30
sec, 50°C for 30 sec and 72°C for 30 sec. The quantification cycle
fluorescence value (Cq) was calculated using SDS software, version
2.1 (Applied Biosystems; Thermo Fisher Scientific, Inc.), and the
relative mRNA expression levels of RANK were calculated using the
2−ΔΔCq method (19) and
normalized to the internal control, glyceraldehyde 3-phosphate
dehydrogenase (GAPDH). The following primer sequences were used:
ET-1 forward, 5′-AAGCGCTGTTCCTGTTCTTCA-3′ and reverse,
5′-CTTGATGCTATTGCTGATGG-3′; NLRP3 forward,
5′-AAAGCCAAGAATCCACAGTGTAAC-3′ and reverse,
5′-TTGCCTCGCAGGTAAAGGT-3′; capsase-1 forward,
5′-AGGCATGACAATGCTGCTACAA-3′ and reverse,
5′-TGTGCAAATGCCTCCAGCTC-3′; IL-1β forward,
5′-TCGCCAGTGAAATGATGGCTTA-3′ and reverse,
5′-GTCCATGGCCACAACAACTGA-3′; GAPDH forward,
5′-ACAGGGGAGGTGATAGCATT-3′ and reverse,
5′-GACCAAAAGCCTTCATACATCTC-3′.
Western blot analysis
The normal and injured carotid arteries of the rats
were homogenized and lysed in NP-40 buffer (Beyotime Institute of
Biotechnology, Haimen, China). Following 5–10 min of boiling, the
cells were centrifuged at 10,000 × g at 4°C for 10 min to obtain
the supernatant. Protein concentrations were determined using the
Bicinchoninic Acid kit for Protein Determination (Sigma-Aldrich;
Merck KGaA). Protein samples (50 µg) were separated by 10% sodium
dodecyl sulfate-polyacrylimide gel electrophoresis and transferred
onto polyvinylidene difluoride membranes (EMD Millipore, Billerica,
MA, USA). The membranes were blocked with 5% (w/v) non-fat milk
powder in Tris-buffered saline and 0.1% (w/v) with Tween-20, and
incubated with the following primary antibodies: NLRP3 (cat. no.
ab210491, 1:500; Abcam), ET-1 (cat. no. sc-57116, 1:10,00)
caspase-1 (cat. no. sc-398715, 1:500), IL-1β (cat. no. sc-515598,
1:10,00) and β-actin (cat. no. sc-130065, 1:2,000) (all from Santa
Cruz Biotechnology, Inc., Dallas, TX, USA) overnight at 4°C.
Following washing with PBS three times (5 min/each), the membranes
were incubated with HRP-conjugated anti-IgG (cat. no. sc-516102,
1:10,000; Santa Cruz Biotechnology, Inc.) at room temperature for 2
h. Signal detection was performed using an ECL system (GE
Healthcare Life Sciences, Chalfont, UK). Quantitative analysis of
protein was assessed using Quantity One software version 4.5 (Bio
Rad Laboratories, Inc.).
Statistical analysis
Data are expressed as the mean ± standard error of
the mean. All statistical analyses were performed using GraphPad
Prism software, version 5.0 (GraphPad Software, Inc., La Jolla, CA,
USA). Groups were compared using one-way analysis of variance,
followed by Tukey's multiple comparison post hoc test to compare
the mean values of each group. P<0.05 was considered to indicate
a statistically significant difference.
Results
Oleanolic acid improves body weight
and glucose levels in rats with hyperglycemia-induced carotid
artery injury
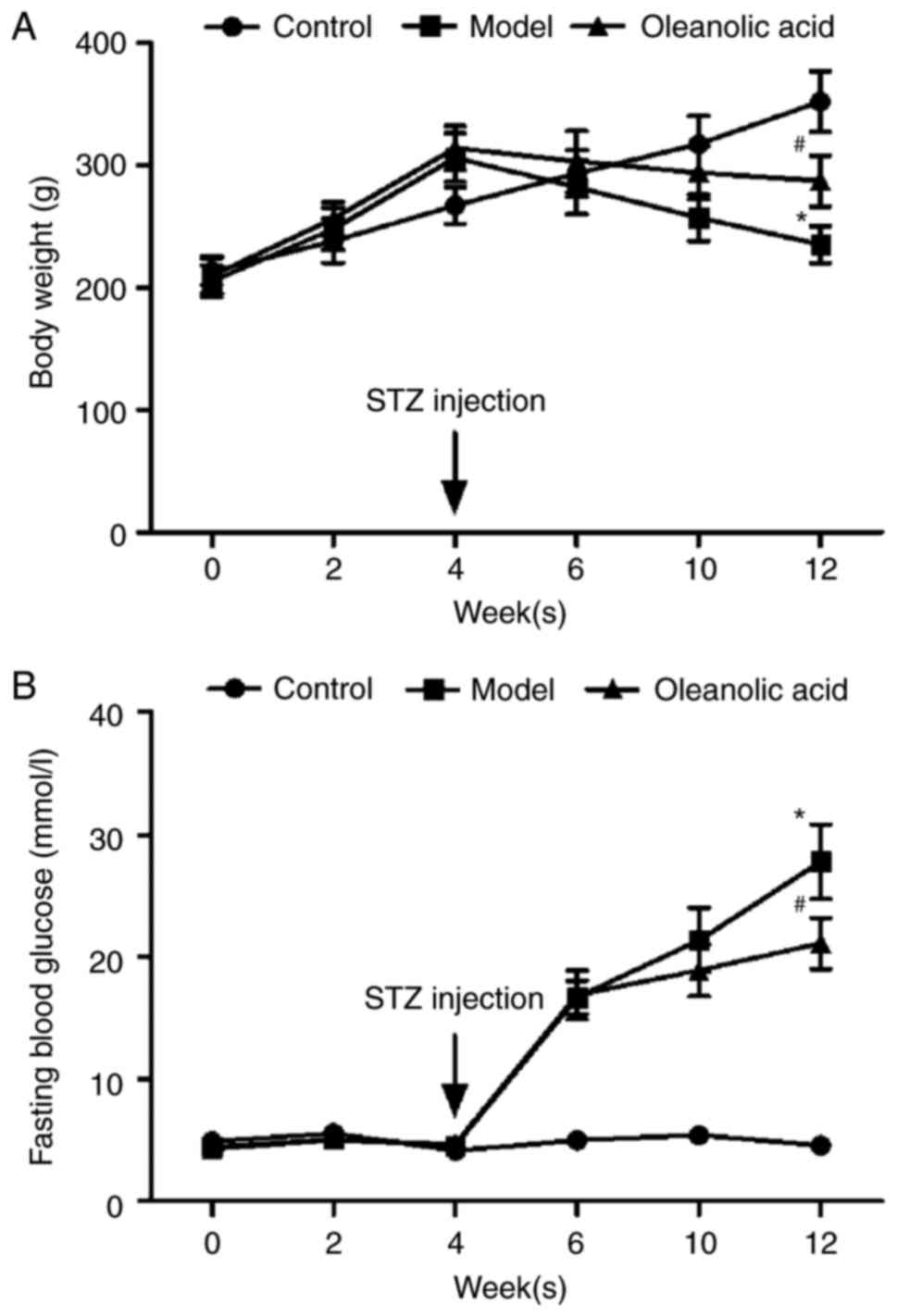
As shown in Fig. 1A and
B, body weights steadily increased, and the fasting blood
glucose (FBG) level was maintained within the normal range of
3.9–6.1 mmol/l in the non-diabetic control rats during the
experimental period. In the rats fed with a HFD 4 weeks prior to
STZ injection, the body weights of the rats significantly
increased, however, injection of STZ markedly decreased the body
weights of the rats (Fig. 1A). At
2 weeks post-STZ injection, the FBG values of the diabetic rats and
oleanolic acid-treated diabetic rats increased from 4.6 mmol/l at
week 4 to 16.7 mmol/l at week 6, and 4.7 mmol/l at week 4 to 16.9
mmol/l at week 6, respectively. These increases were significantly
higher than that of the control group (5.0 mmol/l) at week 6
(Fig. 1B). Oleanolic acid
administration reversed the HFD- and STZ-induced changes in body
weight and glucose metabolic disorder (Fig. 1A and B).
Oleanolic acid alleviates carotid
artery injury in diabetic rats
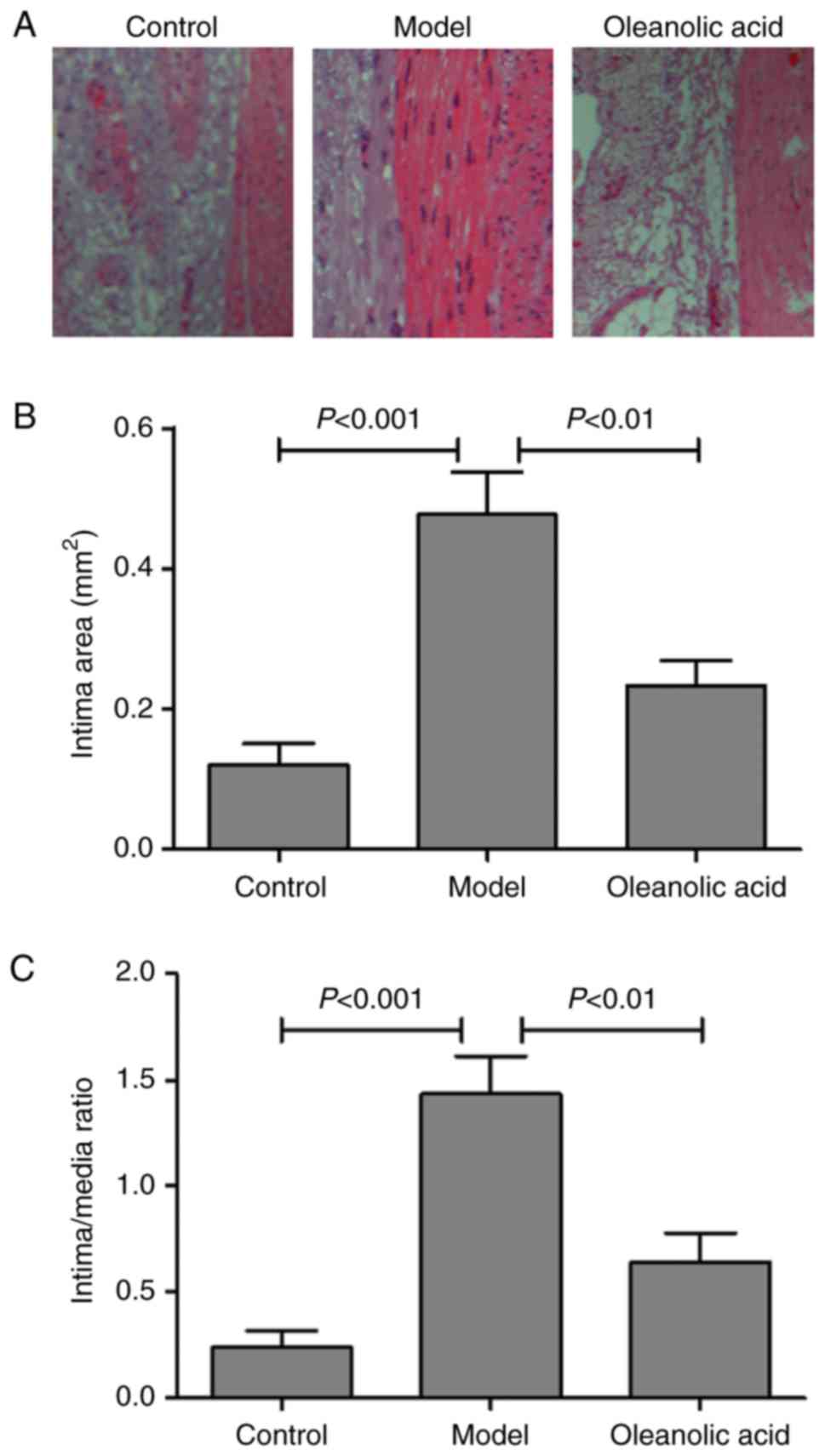
The degree of neointimal hyperplasia was evaluated
by morphologic analysis with H&E staining. The results
demonstrated that the injured carotid arteries of the model group
developed severe stenosis and neointimal hyperplasia, compared with
the non-diabetic normal rats (Fig.
2A). Oleanolic acid treatment significantly reduced the intimal
area, compared with that in the model group (Fig. 2B). The intima/media ratio was also
lower in the oleanolic acid-treated group, compared with that in
the model group (Fig. 2C).
Oleanolic acid improves endothelial
function in rats with hyperglycemia-induced carotid artery
injury
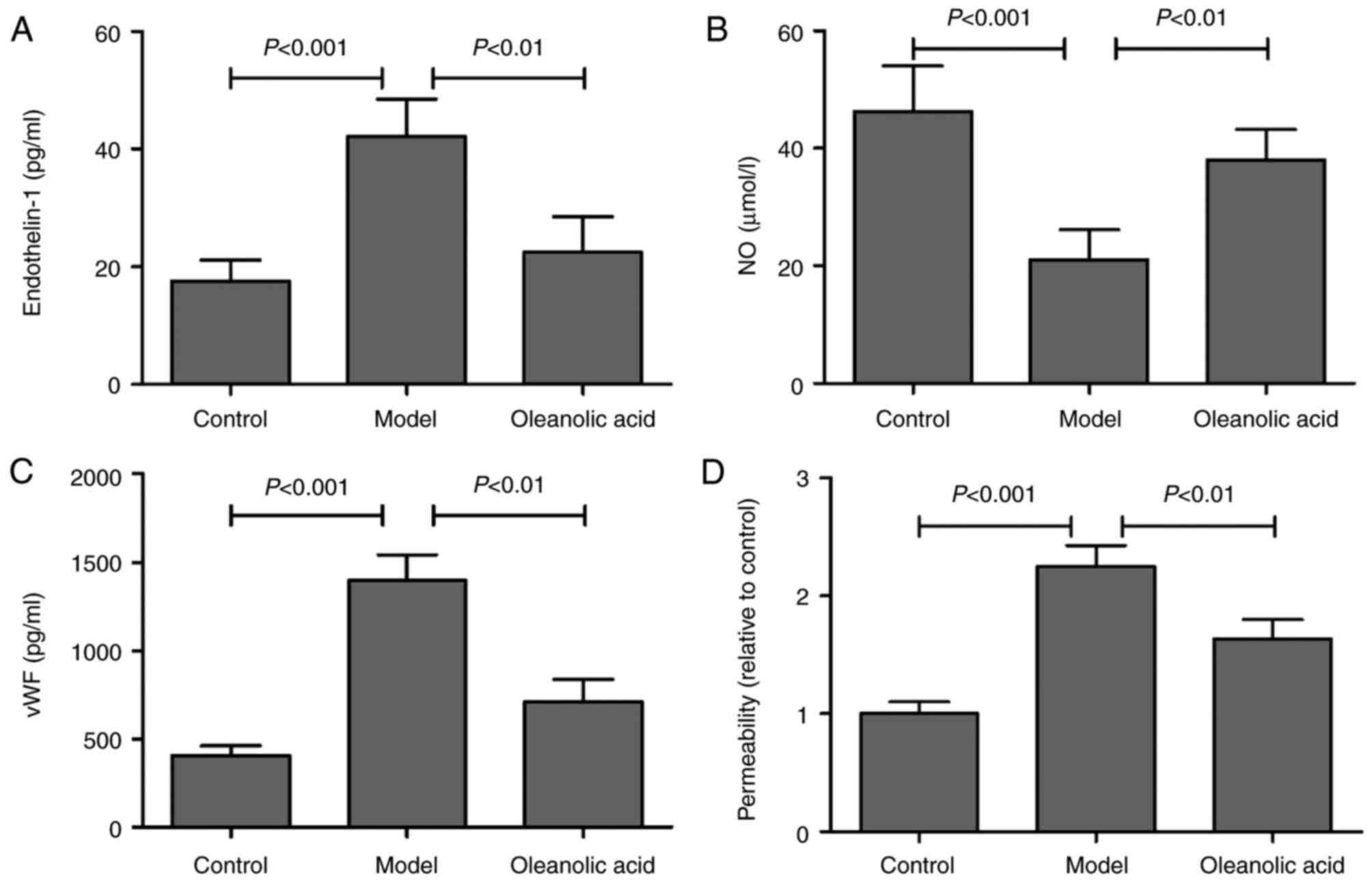
ET-1 is a potent vasoconstrictor, and its increase
in endothelial cells leads to endothelial dysfunction and
cardiovascular disorders (20). NO
is the most important vascular relaxing factor, which is also
regulated in the endothelium, and alterations in the endothelial
production of NO are known to correlate with endothelial
dysfunction (21). Compared with
the normal rats, an increase in the serum level of ET-1 was
observed in the model group, whereas oleanolic acid administration
significantly downregulated the level of ET-1 in the diabetic rats
(Fig. 3A). The results also showed
that the levels of NO were significantly decreased in the serum of
diabetic rats, compared with the non-diabetic control group.
Oleanolic acid administration significantly prevented the
hyperglycemia-induced decrease NO in diabetic rats (Fig. 3B). The serum concentration of vWF,
a well-known marker of endothelial function/injury, was
significantly higher in the diabetic rats, compared with that in
the control group rats (Fig. 3C).
Oleanolic acid treatment significantly reduced the levels of vWF in
the diabetic rats. The findings also demonstrated that oleanolic
acid significantly reversed the hyperglycemia-induced increase in
vascular permeability (Fig. 3D),
which is a characteristic of diabetic vasculopathy (22).
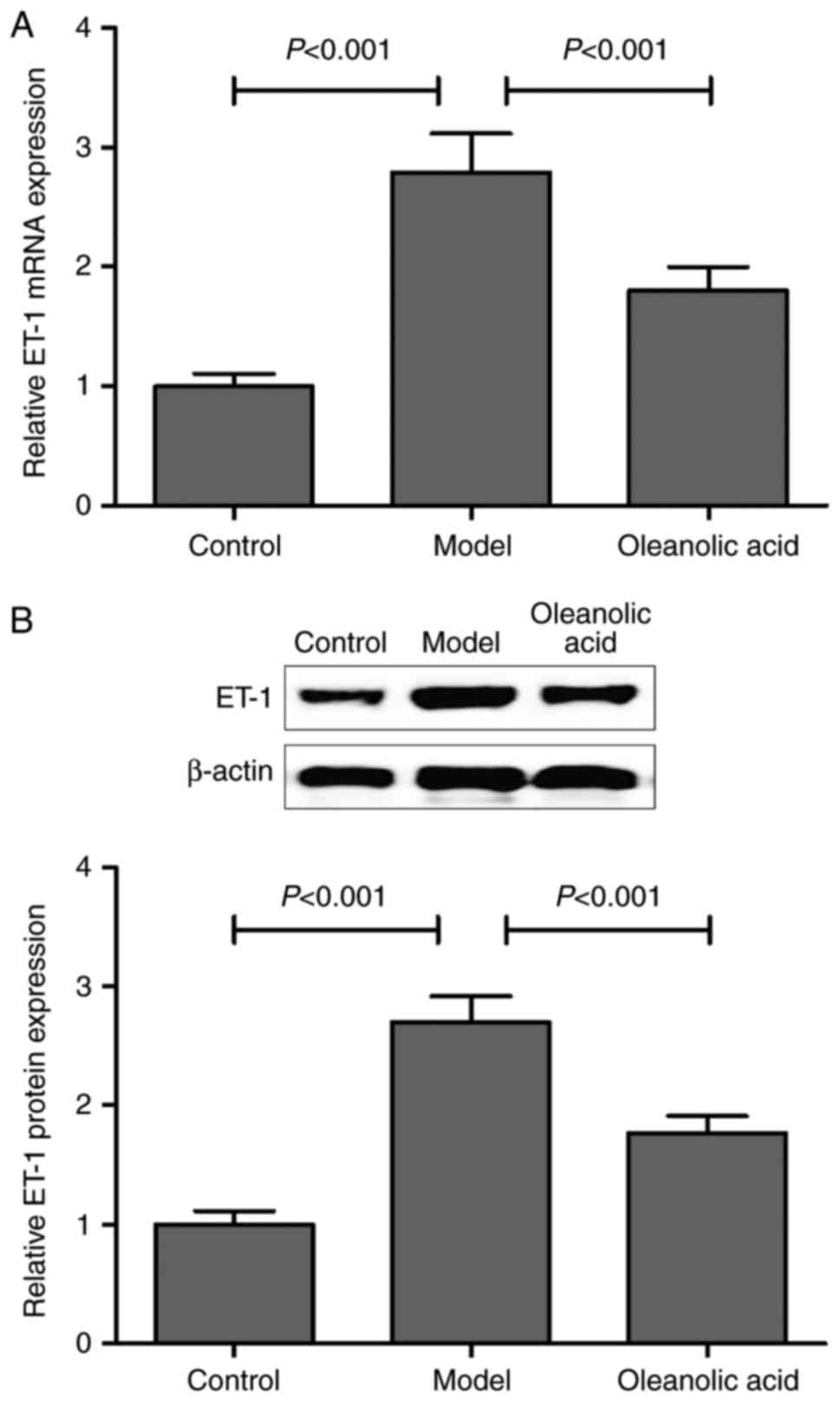
Consistent with the results described above, the
present study confirmed that the mRNA and protein expression levels
of ET-1 were markedly upregulated in the carotid artery of diabetic
rats, compared with those of non-diabetic control rats. Oleanolic
acid administration significantly downregulated the mRNA and
protein expression of ET-1 in the diabetic rats (Fig. 4A and B).
Oleanolic acid inhibits NLRP3
inflammasome signaling in rats with hyperglycemia-induced carotid
artery injury
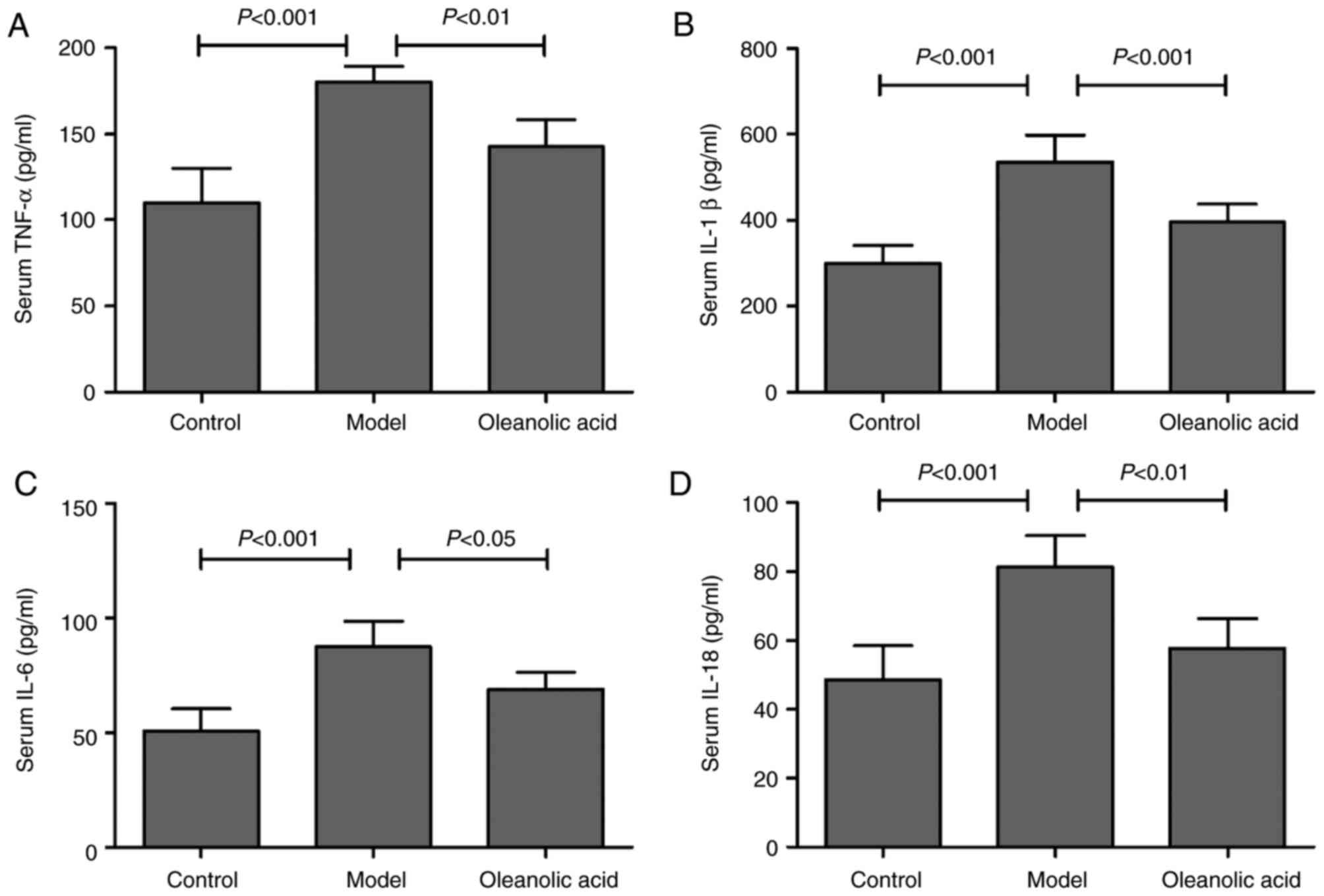
As shown in Fig.
5A, hyperglycemia resulted in upregulation of the serum level
of TNF-α in the rat model of carotid artery injury, compared with
that in the control group. Similarly, the serum levels of IL-1β
(Fig. 5B), IL-6 (Fig. 5C) and IL-18 (Fig. 5D) were significantly increased in
the diabetic rats, compared with those in the control group rats.
However, oleanolic acid administration led to significant decreases
in the levels of TNF-α, IL-1β, IL-6 and IL-18 (Fig. 5A-D). These results suggested a
major role for oleanolic acid in reducing the circulating levels of
pro-inflammatory cytokines. To further investigate the
anti-inflammatory effect of oleanolic acid and its molecular
mechanisms in the progression of hyperglycemia-induced carotid
artery injury, the expression levels of NLRP3 inflammasome
components were examined in the carotid arteries of the rats.
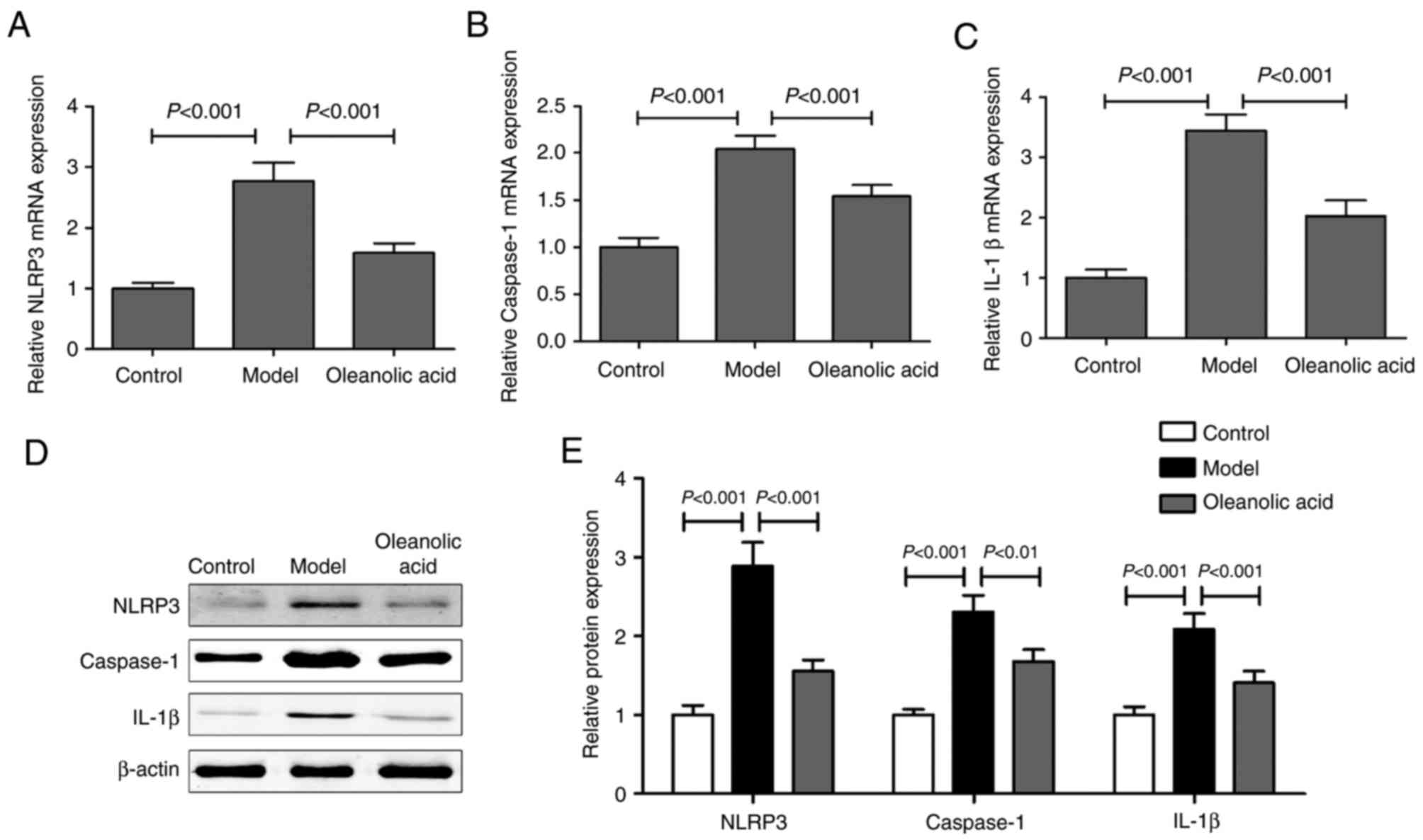
RT-qPCR and western blot analyses of the expression levels of NLRP3
inflammasome components in the carotid arteries revealed that the
mRNA levels of NLRP3 (Fig. 6A),
caspase-1 (Fig. 6B) and IL-1β
(Fig. 6C), and their protein
levels (Fig. 6D and E) were higher
in the diabetic rats, compared with those in the control group
rats. Oleanolic acid administration led to significant decreases in
the levels of these NLRP3 inflammasome components.
Discussion
Inflammation cytokines are crucial in the initiation
and progression of vascular diseases. The NLRP3 inflammasomes have
recently emerged as a pivotal regulator of the chronic inflammatory
response (23), however, the
expression of NLRP3 inflammasomes in carotid artery injury have not
been fully elucidated. In the present study, NLRP3 inflammasome
components were significantly upregulated in the carotid artery of
diabetic rats. Oleanolic acid was closely linked to these factors
and was able to reverse the hyperglycemia-induced upregulation of
NLRP3 inflammasome components in the diabetic rats.
A previous study indicated that NLRP3 inflammasome
components, including NLRP3, ASC, caspase-1, IL-1β and IL-18, are
expressed at high levels in human carotid atherosclerotic plaques
(8). In addition, NLRP3 is
overexpressed in the aorta of patients with coronary
atherosclerosis, and the aortic expression of NLRP3 is correlated
with the severity of coronary artery disease and atherosclerotic
risk factors (9). There is a
positive correlation between the NLRP3 inflammasome and cytokine
levels of IL-1β and IL-18 (23).
NLRP3 forms a multiprotein complex, termed the inflammasome, which
acts as a platform for activation of the cysteine protease
caspase-1. Pro-forms of proinflammatory cytokines IL-1β and IL-18
are then cleaved by active caspase-1 to their active forms
(9). Previous studies suggest a
link between inflammation and glucose metabolic
disturbance-associated diseases, including diabetes and
atherosclerosis (24,25). The results of the present study
revealed a significant increase in the serum levels of TNF-α,
IL-1β, IL-6 and IL-18 in a rat model of carotid artery injury. As
expected, the RT-qPCR and western blot analyses showed a
significant upregulation in the expression levels of NLRP3,
caspase-1 and IL-1β in the carotid artery of diabetic rats. In
apolipoprotein E-knockout (ApoE−/−) or low-density
lipoprotein receptor-knockout mice, the deletion of IL-1β leads to
a decrease in the size of atherosclerotic lesions (9,26).
Similar findings have been observed in
ApoE−/−/caspase-1−/− double knockout or
ApoE−/−/IL-18−/− double knockout mice
(27,28). These results suggest that the NLRP3
inflammasome signaling pathway is important in cardiovascular
diseases.
Several studies have demonstrated that oleanolic
acid has a positive effect on diabetes-associated complications
(13,14), however, the underlying mechanisms
remain to be fully elucidated. Several studies have demonstrated
the ability of oleanolic acid to normalize blood glucose levels in
rodents with diet-induced obesity or diabetes through the
regulation of glucose 6 phosphate and forkhead box protein O1 in
the liver (15,29). Consistent with these results, the
present study showed that oleanolic acid administration improved
hyperglycemia-induced body weight and glucose metabolic disorders
in the rats with carotid artery injury. Feeding an HFD or
hyperglycemia leads to an accentuated proinflammatory state in
local tissues and peripheral circulation (15). The inflammation of local tissues
contributes to hyperglycemia, insulin resistance and
diabetes-associated complications (30). Previous studies have confirmed that
oleanolic acid inhibits inflammation through the suppression of
NF-κB signaling, the inhibition of cytokines, including IL-6 and
TNF-α, and the increased production of antioxidants via the
promotion of Nrf2 signaling (15,30).
In the present study, it was demonstrated that oleanolic acid
improved stenosis and neointimal hyperplasia of the carotid artery
in diabetic rats. In addition, oleanolic acid significantly
prevented hyperglycemia-induced vascular endothelial dysfunction in
the diabetic rats. Oleanolic acid also inhibited the mRNA and
protein expression levels of NLRP3, caspase-1 and IL-1β in the
rates with hyperglycemia-induced carotid artery injury. These data
provide in vivo evidence that oleanolic acid is an effective
NLRP3 inflammasome inhibitor, which leads to the alleviation of
carotid artery injury in diabetic rats.
In conclusion, the present study revealed a novel
triggering mechanism of oleanolic acid in diabetic rats with
carotid artery injury. The underlying mechanism was mediated, at
least partially, through the suppression of NLRP3 inflammasome
signaling pathways, the involvement of which may be an early event
leading to neointimal hyperplasia and endothelial dysfunction.
References
|
1
|
Irie Y, Katakami N, Kaneto H, Kasami R,
Sumitsuji S, Yamasaki K, Tachibana K, Kuroda T, Sakamoto K,
Umayahara Y, et al: Maximum carotid intima-media thickness improves
the prediction ability of coronary artery stenosis in type 2
diabetic patients without history of coronary artery disease.
Atherosclerosis. 221:438–444. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tian F, Chen Y, Liu H, Zhang T, Guo J and
Jin Q: Assessment of characteristics of neointimal hyperplasia
after drug-eluting stent implantation in patients with diabetes
mellitus: An optical coherence tomography analysis. Cardiology.
128:34–40. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang J, Fan Z, Yang J, Ding J, Yang C and
Chen L: MicroRNA-24 attenuates neointimal hyperplasia in the
diabetic rat carotid artery injury model by inhibiting Wnt4
signaling pathway. Int J Mol Sci. 17:E7652016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang K, Zhou Z, Zhang M, Fan L, Forudi F,
Zhou X, Qu W, Lincoff AM, Schmidt AM, Topol EJ and Penn MS:
Peroxisome proliferator-activated receptor gamma down-regulates
receptor for advanced glycation end products and inhibits smooth
muscle cell proliferation in a diabetic and nondiabetic rat carotid
artery injury model. J Pharmacol Exp Ther. 317:37–43. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Simon DI: Inflammation and vascular
injury: Basic discovery to drug development. Circ J. 76:1811–1818.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sirico G, Spadera L, De Laurentis M and
Brevetti G: Carotid artery disease and stroke in patients with
peripheral arterial disease. The role of inflammation. Monaldi Arch
Chest Dis. 72:10–17. 2009.PubMed/NCBI
|
|
7
|
Xia M, Boini KM, Abais JM, Xu M, Zhang Y
and Li PL: Endothelial NLRP3 inflammasome activation and enhanced
neointima formation in mice by adipokine visfatin. Am J Pathol.
184:1617–1628. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shi X, Xie WL, Kong WW, Chen D and Qu P:
Expression of the NLRP3 Inflammasome in Carotid Atherosclerosis. J
Stroke Cerebrovasc Dis. 24:2455–2466. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zheng F, Xing S, Gong Z and Xing Q: NLRP3
inflammasomes show high expression in aorta of patients with
atherosclerosis. Heart Lung Circ. 22:746–750. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Goyal SN, Mahajan UB, Chandrayan G,
Kumawat VS, Kamble S, Patil P, Agrawal YO, Patil CR and Ojha S:
Protective effect of oleanolic acid on oxidative injury and
cellular abnormalities in doxorubicin induced cardiac toxicity in
rats. Am J Transl Res. 8:60–69. 2016.PubMed/NCBI
|
|
11
|
Madlala HP, Van Heerden FR, Mubagwa K and
Musabayane CT: Changes in renal function and oxidative status
associated with the hypotensive effects of oleanolic acid and
related synthetic derivatives in experimental animals. PLoS One.
10:e01281922015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yu Z, Sun W, Peng W, Yu R, Li G and Jiang
T: Pharmacokinetics in vitro and in vivo of two novel prodrugs of
oleanolic acid in rats and its hepatoprotective effects against
liver injury induced by CCl4. Mol Pharm. 13:1699–1710. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liese J, Abhari BA and Fulda S: Smac
mimetic and oleanolic acid synergize to induce cell death in human
hepatocellular carcinoma cells. Cancer Lett. 365:47–56. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang X, Chen Y, Abdelkader D and Hassan W:
Combination therapy with oleanolic acid and metformin as a
synergistic treatment for diabetes. J Diabetes Res.
2015:9732872015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Camer D, Yu Y, Szabo A and Huang XF: The
molecular mechanisms underpinning the therapeutic properties of
oleanolic acid, its isomer and derivatives for type 2 diabetes and
associated complications. Mol Nutr Food Res. 58:1750–1759. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lu J, Wu DM, Zheng YL, Hu B, Cheng W,
Zhang ZF and Shan Q: Ursolic acid improves high fat diet-induced
cognitive impairments by blocking endoplasmic reticulum stress and
IkappaB kinase beta/nuclear factor-κB-mediated inflammatory
pathways in mice. Brain Behav Immun. 25:1658–1667. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Buus NH, Hansson NC, Rodriguez-Rodriguez
R, Stankevicius E, Andersen MR and Simonsen U: Antiatherogenic
effects of oleanolic acid in apolipoprotein E knockout mice. Eur J
Pharmacol. 670:519–526. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zheng Z, Chen H, Wang H, Ke B, Zheng B, Li
Q, Li P, Su L, Gu Q and Xu X: Improvement of retinal vascular
injury in diabetic rats by statins is associated with the
inhibition of mitochondrial reactive oxygen species pathway
mediated by peroxisome proliferator-activated receptor gamma
coactivator 1alpha. Diabetes. 59:2315–2325. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chai J, He Y, Cai SY, Jiang Z, Wang H, Li
Q, Chen L, Peng Z, He X, Wu X, et al: Elevated hepatic multidrug
resistance-associated protein 3/ATP-binding cassette subfamily C 3
expression in human obstructive cholestasis is mediated through
tumor necrosis factor alpha and c-Jun NH2-terminal
kinase/stress-activated protein kinase-signaling pathway.
Hepatology. 55:1485–1494. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dushpanova A, Agostini S, Ciofini E,
Cabiati M, Casieri V, Matteucci M, Del Ry S, Clerico A, Berti S and
Lionetti V: Gene silencing of endothelial von Willebrand factor
attenuates angiotensin II-induced endothelin-1 expression in
porcine aortic endothelial cells. Sci Rep. 6:300482016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tousoulis D, Kampoli AM, Tentolouris C,
Papageorgiou N and Stefanadis C: The role of nitric oxide on
endothelial function. Curr Vasc Pharmacol. 10:4–18. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wong BW, Wong D, Luo H and McManus BM:
Vascular endothelial growth factor-D is overexpressed in human
cardiac allograft vasculopathy and diabetic atherosclerosis and
induces endothelial permeability to low-density lipoproteins in
vitro. J Heart Lung Transplant. 30:955–962. 2011.PubMed/NCBI
|
|
23
|
Satoh M, Tabuchi T, Itoh T and Nakamura M:
NLRP3 inflammasome activation in coronary artery disease: Results
from prospective and randomized study of treatment with
atorvastatin or rosuvastatin. Clin Sci (Lond). 126:233–241. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Saita D, Ferrarese R, Foglieni C, Esposito
A, Canu T, Perani L, Ceresola ER, Visconti L, Burioni R, Clementi M
and Canducci F: Adaptive immunity against gut microbiota enhances
apoE-mediated immune regulation and reduces atherosclerosis and
western-diet-related inflammation. Sci Rep. 6:293532016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chow BS, Koulis C, Krishnaswamy P,
Steckelings UM, Unger T, Cooper ME, Jandeleit-Dahm KA and Allen TJ:
The angiotensin II type 2 receptor agonist compound 21 is
protective in experimental diabetes-associated atherosclerosis.
Diabetologia. 59:1778–1790. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kirii H, Niwa T, Yamada Y, Wada H, Saito
K, Iwakura Y, Asano M, Moriwaki H and Seishima M: Lack of
interleukin-1beta decreases the severity of atherosclerosis in
ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 23:656–660.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mallat Z, Corbaz A, Scoazec A, Graber P,
Alouani S, Esposito B, Humbert Y, Chvatchko Y and Tedgui A:
Interleukin-18/interleukin-18 binding protein signaling modulates
atherosclerotic lesion development and stability. Circ Res.
89:E41–E45. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gage J, Hasu M, Thabet M and Whitman SC:
Caspase-1 deficiency decreases atherosclerosis in apolipoprotein
E-null mice. Can J Cardiol. 28:222–229. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gao D, Li Q, Li Y, Liu Z, Fan Y, Liu Z,
Zhao H, Li J and Han Z: Antidiabetic and antioxidant effects of
oleanolic acid from Ligustrum lucidum Ait in alloxan-induced
diabetic rats. Phytother Res. 23:1257–1262. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Osborn O and Olefsky JM: The cellular and
signaling networks linking the immune system and metabolism in
disease. Nat Med. 18:363–374. 2012. View
Article : Google Scholar : PubMed/NCBI
|