Introduction
Reperfusion therapy performed as early as possible
is a key strategy for the treatment of ischemic heart disease;
however, it may cause myocardial ischemia/reperfusion (I/R) injury,
leading to myocardial dysfunction, and even cardiomyocytes
apoptosis (1,2). Effective measures for the prevention
of myocardial I/R injury have come under heated debate (3), including various ischemic
pre-conditionings or post-conditionings from pharmacological and
mechanical methods (4,5). However, the efficiency of these
therapeutic agents for limiting myocardial I/R injury has been
questioned due to the complicated mechanisms involved in I/R injury
(6,7). At present, increasing studies have
suggested that endoplasmic reticulum (ER) stress has an important
role in the pathological process of I/R injury (8).
ER stress refers to a pathological process in which
ER homeostasis is affected and physical function is disordered due
to exposure to various harmful stimuli, such as intracellular
Ca2+ overload, ATP deprivation and hypoxia; which may
cause an accumulation of misfolded or unfolded proteins in the ER
lumen (9). ER stress results in an
increased expression of the glucose-regulated protein 78 (GRP78),
and increased rate of activation of inositol-requiring protein-1,
protein kinase RNA-like ER kinase (PERK), and activating
transcription factor-6 (ATF6) pathways that regulate accumulation
of unfolded proteins in order to protect the cell from damage
(10,11). In cases of prolonged or excessive
ER stress, ER-associated apoptosis occurs following the activation
of the C/EBP-homologous protein (CHOP), c-Jun-N-terminal protein
kinase (JNK) and caspase-12 apoptosis pathways (12,13).
The CHOP pathway is an important branch of ER stress apoptosis
pathways. When ER stress occurs, increased PERK activation may
phosphorylate eukaryotic translation initiation factor-2 (eIF2α)
for the upregulation of the PERK/eIF2α signaling pathway in order
to increase the expression of the downstream target, CHOP.
Proanthocyanidins are a series of bioavailable
polyphenol flavonoids compounds derived from vegetables and fruits,
containing dimers, trimers and other oligomers of catechin and
epicatechin and their glallic acid esters. Grape seed
proanthocyanidins (GSPs) are potent antioxidants that possess a
greater ability in the scavenging free radicals than that of
vitamin C, vitamin E or β-carotene (14). GSPs provide various protective
effects, such as anti-inflammatory (15), anti-oxidant, anti-apoptotic
(16), antitumor (17) and cardioprotective activities
(18). However, the effect of GSPs
on hypoxia/reoxygenation (H/R) injury involving PERK-CHOP-mediated
ER stress-associated apoptosis has not been investigated thus
far.
Therefore, in this study, the protective effects of
GSPs subjected to H/R injury were investigated as well as the role
of ER stress-associated apoptosis in H9C2 cardiomyocytes through
the PERK/eIF2α signaling pathway under varying administered
concentrations of GSPs.
Materials and methods
Reagents
The following reagents were used: GSPs (Aladdin Co.,
Ltd., Shanghai, China); H9C2 cardiomyocytes (Chinese Academy of
Sciences Cell Bank, Shanghai, China); Dulbecco's modified Eagle's
medium (DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA); fetal bovine serum (FBS; HyClone, Logan, UT, USA); dimethyl
sulfoxide (DMSO), 4-phenyl butyric acid (4-PBA) and MTT
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany); lactate
dehydrogenase (LDH) Release assay kit (Nanjing Jiancheng
Bioengineering Institute, Nanjing, China). The Annexin V/Propidium
Iodide Apoptosis Detection kit; Total Protein Extraction kit
[WLA019, including radioimmunoprecipitation assay (RIPA) lysis
buffer and phenylmethylsulfonyl fluoride], enhanced
chemiluminescence solution (WLA003) and antibodies against GRP78
(1:500; WL00621), CHOP (1:500; WL00621), eIF2α (1:500; WL01909) and
β-actin (1:1,000; WL01845) were all from Wanleibio Co., Ltd.
(Shenyang, China). Antibodies against PERK (1:500; bs-2469R),
phosphorylated PERK (p-PERK; 1:1,000, bs-3330R) and, phosphorylated
eIF2α (p-eIF2α; 1:500; bs-4842R) were all from BIOSS (Beijing,
China). The RNA Extraction kit (RP1201), Super M-MLV reverse
transcriptase (PR6502) and 2X Power Taq PCR MasterMix (PR1702) were
from BioTeke Corporation (Beijing, China). SYBR-Green (SY1020) was
from Beijing Solarbio Science and Technology Co., Ltd. (Beijing,
China) and the primers were from Sangon Biotech Co., Ltd.
(Shanghai, China).
H9C2 cardiomyocyte culture and
H/R
H9C2 cardiomyocytes were cultured in DMEM containing
10% FBS in 6-well plates and maintained at a constant humidity in
an incubator containing 5% CO2 at 37°C for 24 h. The
cardiomyocyte status was then observed and the cells continued to
undergo culture post-cleaning with 2 ml PBS and replacement of 2 ml
fresh culture fluid. When cultured to 90% confluence, H9C2
cardiomyocytes were incubated in 96-well plates at a density of
1×104 cells/well for further experiments.
The H/R process was modified from a previously
described method (19). Medium was
replaced with DMEM without glucose and transferred into a tri-gas
incubator containing 95% N2 and 5% CO2 at
37°C to mimic hypoxia for 3 h. The medium was then changed to
normal DMEM and cells were cultured in a regular incubator (37°C,
5% CO2) for 3 h to mimic reoxygenation.
Experimental design
The cardiomyocytes were divided into six groups and
cultured in 6-well or 96-well culture plates at a density of
1×104/ml. The groups were as follows: i) Control group,
cardiomyocytes cultured with normal DMEM medium under normoxic
conditions throughout the duration of the experiments; ii) H/R
group, cardiomyocytes cultured for 3 h in hypoxic conditions and
then 3 h in under normal conditions (H3 h/R3 h); iii-v) H/R +
GSPL/M/H groups, cardiomyocytes were cultured in DMEM medium
containing GSPs of different concentrations (50, 100 and 200 µg/ml,
respectively) under normal conditions for 30 min followed by H3
h/R3 h; vi) H/R+4-PBA group, cardiomyocytes were pretreated with 1
mM 4-PBA for 30 min followed by H3 h/R3 h.
MTT assay of cardiomyocyte viability
analysis
Cell viability was determined by the MTT assay. MTT
(5 mg/ml) was added to cardiomyocytes from each group after
designated disposal and incubated at 37°C for 4 h. The supernatant
extract was then removed and 200 µl DMSO was then added in order to
dissolve the formazan crystals. The absorbance value at 490 nm
using a microplate reader (BioTek Instruments, Inc., Winooski, VT,
USA) was then determined.
Detection of LDH activity
LDH activity was detected using the LDH Release
assay kit. Supernatant (20 µl) was obtained from each experimental
group and mixed with 25 µl 2,4-dinitrobenzene hydrazine in a 37°C
bath for 15 min, and then mixed with 250 µl 0.4 M NaOH at room
temperature for 5 min before determining the absorbance value at
490 nm on the aforementioned microplate reader.
Flow cytometric measurement of
apoptosis
The cell apoptosis and necrosis ratio was measured
using the Annexin V/Propidium Iodide kit for flow cytometric
analysis. Cardiomyocytes were collected, centrifuged and washed
with PBS, and then resuspended in binding buffer. Annexin
V-Fluorescein isothiocyanate (FITC) (5 µl) and propidium iodide (10
µl) were then added to each sample and the samples were then
incubated for 15 min in the dark at room temperature. Results were
analyzed using BD Accuri C6 (BD Biosciences, Franklin Lakes, NJ,
USA) and presented as scatterplots, with the lower right quadrant
representing early apoptotic cells and upper right quadrant
representing late apoptotic and necrotic cells.
Morphologic observation under electron
microscope
Cardiomyocytes were collected from each group for
analysis by electron microscopy. Each sample was immersed in 2.5%
glutaraldehyde for >2 h. Following serial dehydration, samples
were embedded and polymerized in Epon-812 sequentially at 35°C,
40°C and 60°C (24 h each), for a total of 72 h. Following this,
50–70 nm microsections of the samples were cut using a LEICA EM UC7
ultramicrotome (Leica Microsystems, Inc., Buffalo Grove, IL, USA)
followed by staining in acetic acid oil and lead citrate (5 drops
for 30 min respectively). The ultrastructure was observed under
magnification ×10,000 to ×20,000.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The RNA samples were extracted using the RNA
Extraction kit, and RT of the extracted RNA samples was performed
on an Exicycler™ 96 (Bioneer Corporation, Dajeon, Korea) to obtain
appropriate cDNA as follows: RNA samples were heated at 70°C for 5
min before cooling rapidly for 2 min; M-MLV reverse transcriptase
was added and the samples were placed in a water bath at 25°C for
10 min, 42°C for 50 min and then 95°C for 5 min to terminate the
reaction. The qPCR reaction comprised of 1 µl cDNA sample, 0.5 µl
5′ and 3′ primers (10 µM), 9.7 µl PCR Mastermix, 0.3 µl SYBR-Green
and 8 µl ddH2O in total volume of 20 µl. The cDNA was
amplified under the following conditions: 95°C for 10 min, 40
cycles of 95°C for 10 sec, 60°C for 20 sec, 72°C for 30 sec and 4°C
for 5 min. The oligonucleotide primer sets were as follows: GRP78
forward, 5′-GATAATCAGCCCACCGTAA-3′ and reverse,
5′-TTGTTTCCTGTCCCTTTGT-3′; CHOP forward, 5′-CTCTGCCTTTCGCCTTTGA-3′
and reverse, 5′-GCTTTGGGAGGTGCTTGTG-3′; PERK forward,
5′-TTAGCAAGCCAGAGGTGTT and reverse, 5′-GAGCCCGTATGTGGTCAG-3′; eIF2α
forward, 5′-TCCACCCAGGTATGTGAT-3′ and reverse,
5′-TTTGGCTTCCATTTCTTC-3′; β-actin forward,
5′-GGAGATTACTGCCCTGGCTCCTAGC-3′ and reverse,
5′-GGCCGGACTCATCGTACTCCTGCTT-3′. Data obtained were analyzed using
the relative gene expression 2−∆∆Cq method (20).
Western blot
Cardiomyocyte protein was extracted with RIPA lysis
buffer containing 1% phenylmethylsulfonyl fluoride. Bicinchoninic
acid assay was used to measure protein concentration. Protein (40
µg) from each sample was separated using SDS-PAGE (8–13%
polyacrylamide), transferred to a polyvinylidene fluoride membrane
and blocked with 5% non-fat milk in Tris-buffered saline containing
0.05% Tween-20. Following this, the membranes were incubated with
either primary or β-actin antibodies overnight at 4°C. The
membranes were then washed and incubated with secondary horseradish
peroxidase-conjugated IgG antibodies for 45 min at 37°C. Enhanced
chemiluminescence was then carried out after a 30 min wash. The
films were then scanned and the densities of the protein bands were
analyzed using Image J software (GraphPad Software, Inc., La Jolla,
CA, USA).
Statistical analysis
All data are presented as the mean ± standard error.
One-way analysis of variance with Student-Newman-Keuls post hoc
test was used for analysis using SPSS version 17.0 software.
P<0.05 was considered to indicate a statistically significant
difference.
Results
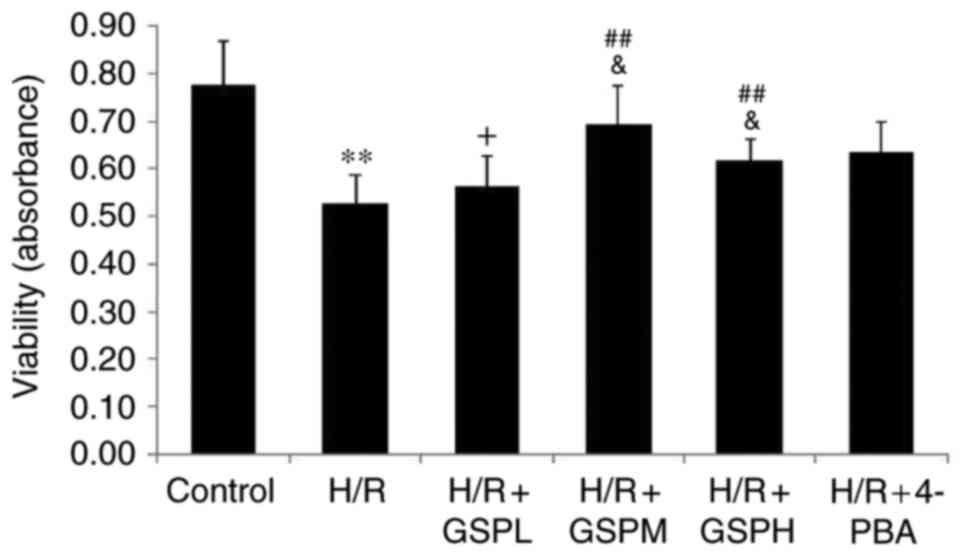
GSPs improves cell viability in
cardiomyocytes subjected to H/R
Compared with the control group, the levels of cell
viability were dramatically decreased in the H/R group. Compared
with the H/R group, the levels of cell viability were increased in
the H/R + GSPM/H group, whereas the levels of cell viability in the
H/R + GSPL group showed no significant change compared with the H/R
group (P=0.408). Cell viability in the GSPs (100 µg/ml)
pretreatment group was higher than that in the GSPs (50 and 200
µg/ml) pretreatment groups. Compared with the group pretreated with
4-PBA, the groups pretreated with GSPs (100 and 200 µg/ml) had no
differences in cell viability (Fig.
1). These results therefore suggest that treatment with GSPs
leads to improved cell viability and that 100 µg/ml GSPs
pretreatment provided the optimal effect.
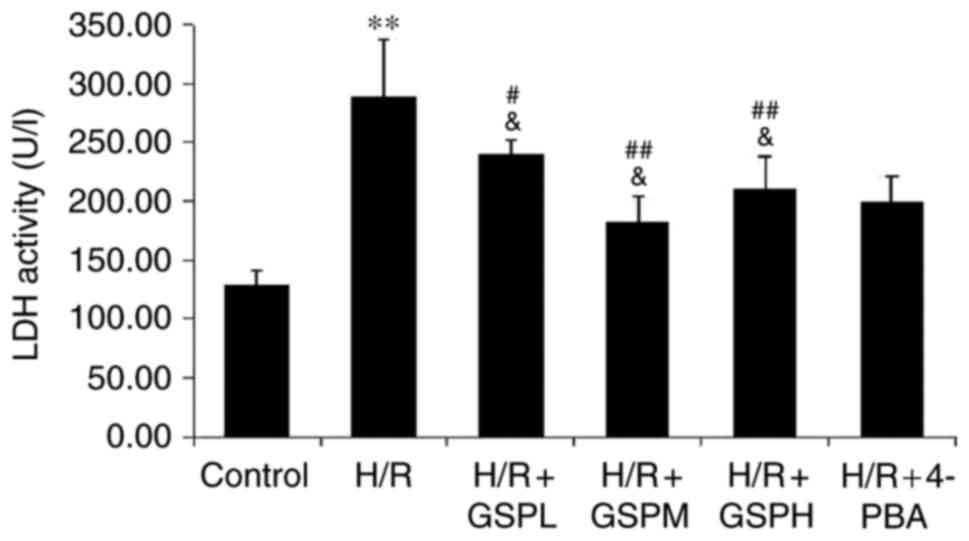
GSPs reduce LDH activity in
cardiomyocytes subjected to H/R
Analysis of the extent of cellular release of LDH is
shown in Fig. 2. A significant
increase of LDH activity was observed in the H/R group compared
with that of the control group (289.34±48.03 vs. 129.04±11.62;
P<0.01). Pretreatment with GSPs was demonstrated to
significantly reduce LDH activity (240.44±11.19, 182.35±21.68,
210.30±27.93 vs. H/R). No significant differences were observed
among the GSPs-treated groups and 4-PBA pretreatment (200.00±21.68
vs. GSPs pretreatment; P>0.05).
GSPs attenuates cell apoptosis ratio
in cardiomyocytes subjected to H/R
Cell apoptosis was also assessed using Annexin-V
FITC/propidium iodide staining (Fig.
3). The apoptosis rate was markedly increased in the H/R group
compared to that of the control group (24.01±1.21 vs. 0.35±0.02;
P<0.01). Additionally, pretreatment with GSPs significantly
decreased levels of apoptotic and necrotic cells caused by H/R
(15.31±1.09, 9.23±0.81, 12.62±1.23 vs. 24.01±1.21; P<0.01). The
GSPs (100 µg/ml) and 4-PBA treatment groups displayed similar
levels of apoptosis (9.23±0.81 vs. 10.76±1.28, P>0.05), while 50
and 200 µg/ml GSP treatment displayed higher apoptosis rates than
that of 4-PBA treatment (P<0.05). These results suggest that
GSPs may have an anti-apoptosis function and work optimally at a
concentration of 100 µg/ml.
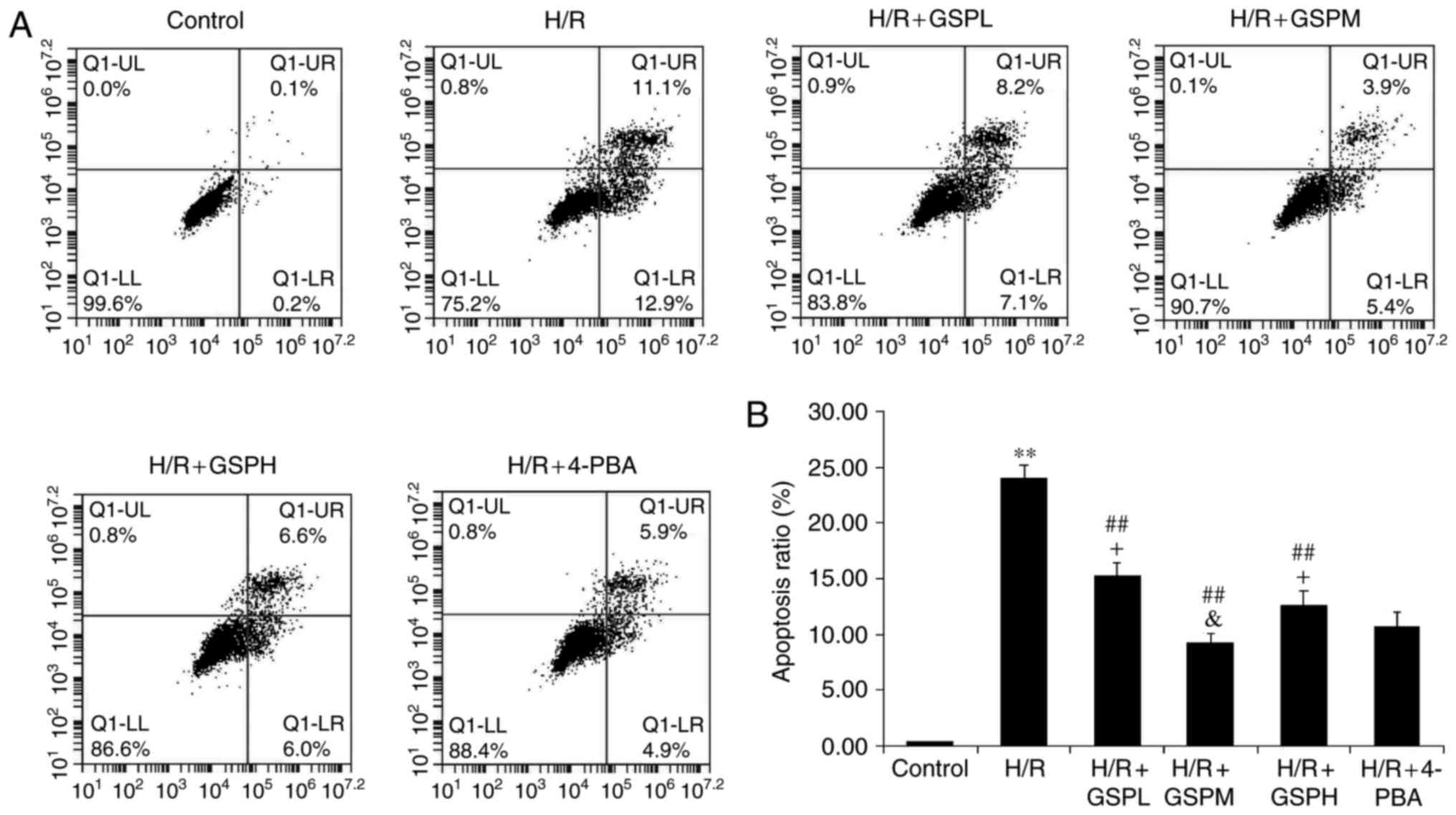 | Figure 3.GSPs attenuated cell apoptosis ratio
in cardiomyocytes subjected to H/R. Flow cytometry results were
displayed as quantitative assessment of cell apoptosis (A) and (B)
quantitative bar graphs. Data were expressed as the mean ± standard
deviation; n=5. **P<0.01 vs. the control group,
##P<0.01 vs. the H/R group, &P>0.05
and +P<0.05 vs. the H/R+4-PBA group. GSP, grape seed
proanthocyanidins; GSPL, GSP low (50 µg/ml); GSPM, GSP medium (100
µg/ml); GSPH, GSP high (200 µg/ml); H/R, hypoxia/reoxygenation; LL,
lower left quadrant; LR, lower right quadrant; UL, upper left
quadrant; UR, upper right quadrant; 4-PBA, 4-phenylbutyrate. |
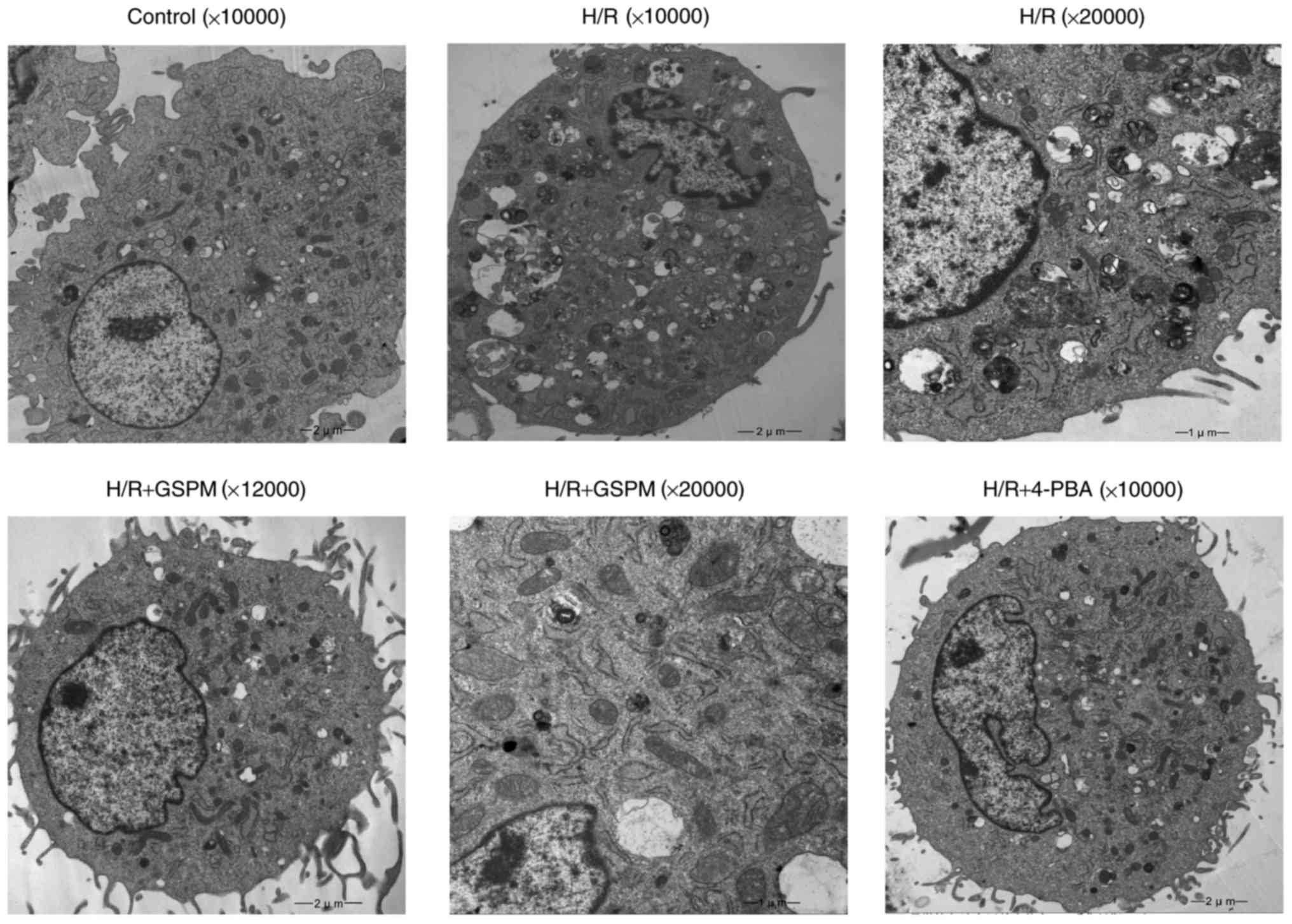
Ultrastructural morphology by electron
microscopy
The control group cells displayed normal
cytomembranes, nuclei, mitochondria, ER and other cellular
structures (Fig. 4). However, the
H/R group cells showed obvious signs of cellular damage
demonstrated by nuclear membrane defects, swelling and
vacuolization of mitochondria, and dilatation of the ER.
Additionally, a number of apoptotic bodies were visible in the
imaged H/R cells. The groups that underwent pretreatment with GSPs
displayed complete nuclear membranes and increasing numbers of
normal mitochondria and ER, whereas the levels of apoptotic bodies
were decreased. Similar results to those displayed by the GSPs
pretreatment groups were obtained in the 4-PBA group.
Relative quantitative expression of
GRP78, CHOP, PERK and eIF2α mRNA)
The relative expression levels of GRP78, CHOP and
eIF2α mRNA were increased notably following H/R treatment compared
with the control group (P<0.01) and decreased following GSPs
treatment (P<0.01) as demonstrated in Fig. 5. Furthermore, the mRNA expression
levels of GRP78, CHOP and eIF2α were consistent between the H/R +
GSPM group and the H/R + 4-PBA group (P<0.05). Additionally, no
difference in the level of CHOP mRNA was observed between the H/R +
GSPH group and H/R + 4-PBA group. The expression levels of PERK
mRNA remained unchanged among all groups (P>0.05).
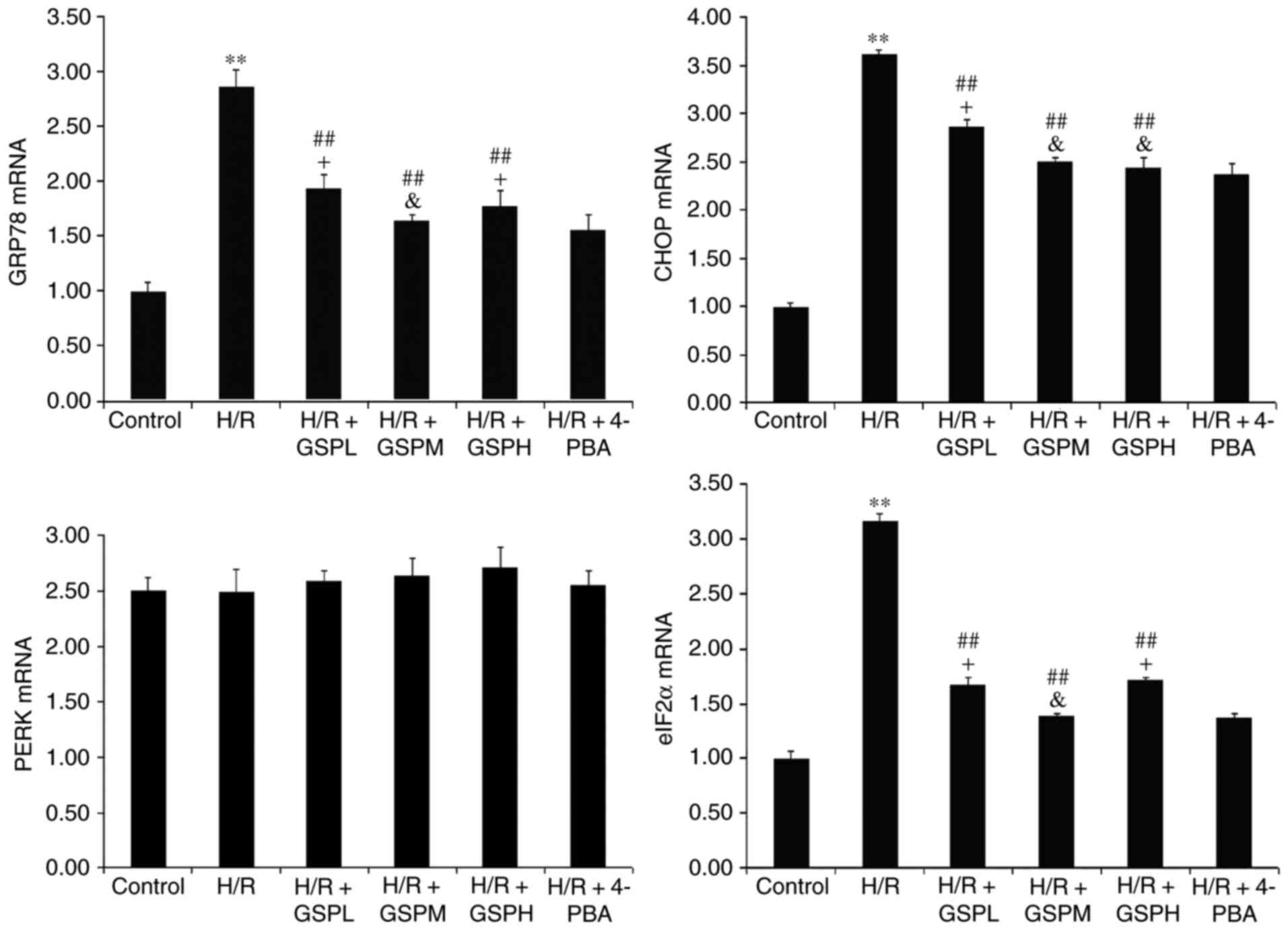 | Figure 5.GSPs inhibited mRNA expression of
GRP78, CHOP and eIF2α in cardiomyocytes subjected to H/R. The
relative quantitative expression levels of GRP78, CHOP, PERK and
eIF2α detected by reverse transcription-quantitative polymerase
chain reaction were displayed as fold change compared with that in
the control group by quantitative bar graphs. Data are expressed as
mean ± standard deviation; n=3. **P<0.01 vs. the control group,
##P<0.01 vs. the H/R group, &P>0.05
and +P<0.05 vs. the H/R + 4-PBA group. GPR78,
glucose-regulated protein 78; CHOP, C/EBP-homologous protein;
eIF2α, eukaryotic translation initiation factor-2 α; GSP, grape
seed proanthocyanidins; GSPL, GSP low (50 µg/ml); GSPM, GSP medium
(100 µg/ml); GSPH, GSP high (200 µg/ml); H/R,
hypoxia/reoxygenation; PERK, protein kinase RNA-like endoplasmic
reticulum kinase; 4-PBA, 4-phenylbutyrate. |
Protein expression of GRP78, CHOP,
PERK and eIF2α
As shown in Fig. 6,
the protein expression levels of GRP78, CHOP, p-PERK and p-eIF2α
were all significantly elevated in cardiomyocytes exposed to H/R
(P<0.01). Concurrently, the protein expression levels were
downregulated in the H/R + GSP groups (P<0.01). There was no
apparent change in protein expression levels between H/R + GSPM
group and H/R + 4-PBA group (P>0.05), whereas the H/R + GSPL/H
groups displayed higher protein expression levels than the H/R +
4-PBA group with the exception of CHOP protein expression. The
results were in accordance with the results of the mRNA expression
levels, suggesting that GSPs inhibit ER stress and the PERK/eIF2α
pathway, and have a similar function to ER stress-specific
inhibitor 4-PBA.
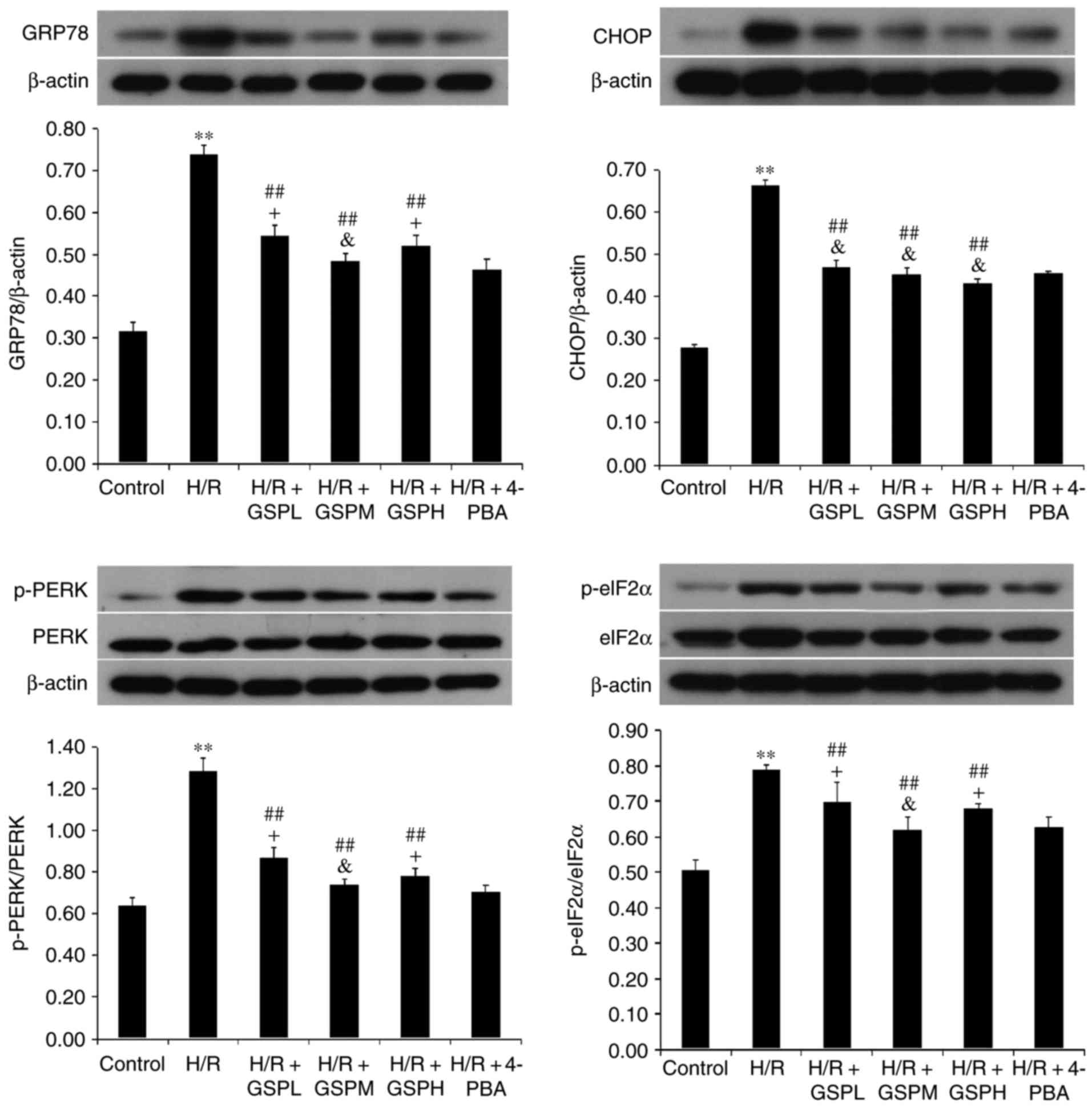 | Figure 6.GSPs downregulated protein expression
of GRP78, CHOP, p-PERK and p-eIF2α in cardiomyocytes subjected to
H/R. Representative immunoblots are represented above the
quantitative bar graphs. p-PERK and p-eIF2α were expressed as the
ratio to total PERK and eIF2α. Data were represented as the mean ±
standard deviation; n=3. **P<0.01 vs. the control group,
##P<0.01 vs. the H/R group, &P>0.05
and +P<0.05 vs. the H/R + 4-PBA group. CHOP,
C/EBP-homologous protein; eIF2α, eukaryotic translation initiation
factor-2 α; p-eIF2α, phosphorylated eIF2α; GPR78, glucose-regulated
protein 78; GSP, grape seed proanthocyanidins; GSPL, GSP low (50
µg/ml); GSPM, GSP medium (100 µg/ml); GSPH, GSP high (200 µg/ml);
H/R, hypoxia/reoxygenation; PERK, protein kinase RNA-like
endoplasmic reticulum kinase; p-PERK, phosphorylated PERK; 4-PBA,
4-phenylbutyrate. |
Discussion
The findings of this study suggest that GSPs
exhibits a significant protective effect against H/R injury in H9C2
cardiomyocytes in a non-dose dependent manner and at an optimal
concentration of 100 µg/ml. A possible mechanism of protection
appears to be dependent on the attenuation of ER stress-mediated
apoptosis as well as downregulation of PERK-eIF2α-CHOP pathway
activation.
Myocardial I/R injury is a complex
pathophysiological process that heavily involves various apoptosis
signaling pathways (21). Previous
studies have reported that mitochondrial-mediated apoptosis and
death receptor pathways contributed to the myocardial I/R injury
process. Previously, ER stress has been verified as the third
largest apoptosis pathway, and has an important role in I/R injury
(22). H/R is an in vitro
cell culture model for simulation of I/R.
Considering that the presence of the LDH enzyme is
specific to myocardial cytoplasm, its increasing expression level
is an important indicator of H/R injury (23). In the current study, increased LDH
activity, apoptosis rate, decreased cell viability as well as
morphological changes in the H/R group indicated severe injury
mediated by H/R. The administration of GSPs reduced the level of
LDH activity and the apoptosis rate, as well as improving cell
viability and morphological changes, which reflected the protective
effect of GSPs on cardiomyocytes subjected to H/R. In addition, the
effect of GSPs was dose-dependent, as the administration of 100
µg/ml GSPs treatment exhibited a greater protective effect than
that of the 50 or 200 µg/ml groups.
Prolonged ER stress may trigger ER stress-associated
apoptosis pathways, including CHOP, JNK and caspase-12 pathways.
Among them, CHOP, a downstream target of the PERK/eIF2α pathway
involved in the unfolded protein response (24), has a vital role in ER
stress-mediated apoptosis (25).
PERK activation responds to ER stress by autophosphorylation and
then by phosphorylating the α-subunit of eIF2α, which may
subsequently trigger the CHOP signaling pathway (26).
To investigate how GSPs resist H/R-mediated injury,
the expression levels of GRP78, CHOP, PERK and eIF2α were measured.
GRP78 is an ER chaperone response protein that is upregulated under
ER stress. The high protein and mRNA expression of GRP78, eIF2α and
CHOP as well as increased protein expression of p-PERK in the H/R
group revealed that ER stress and the activation of the PERK/eIF2α
pathway were induced by H/R. The significantly reduced expression
levels in the GSPs treatment groups demonstrated that GSPs
alleviated ER stress and blocked the PERK/eIF2α pathway.
Furthermore, GSPs were revealed as providing the same effect as
4-PBA in the inhibition of ER stress caused by H/R.
This study demonstrated that there was minimal
change in the mRNA expression of PERK under the different
experimental conditions, however, levels of p-PERK changed
significantly and correlated with the protein expression levels of
eIF2α and CHOP. Therefore, the results indicated that GSPs may
reduce the phosphorylation of PERK.
In response to an early prospective study that
indicated that the polyphenol fraction of proanthocyanidins in red
wine have a beneficial effect with regards to the prevention of
coronary disease (27), GSPs have
become a prevalent research focus worldwide. Although lycopene and
astaxanthin possess a greater anti-oxidative ability compared to
that of proanthocyanidins, their in vivo potential is
limited by their lipid-soluble nature. On the other hand,
proanthocyanidins are water-soluble and act in systemic fluid,
which determines them as the most powerful antioxidant applicable
in medical and nutrition fields.
Several studies have demonstrated various biological
activities of proanthocyanidins, however, there is inadequate
research linking proanthocyanidins to their protective abilities
against myocardial I/R injury. Sato et al (28) reported that GSPs effectively
decreased the extent of myocardial infarction in rats via
scavenging of peroxyl and hydroxyl radicals for the attenuation of
oxidative stress following I/R. Additionally, a previous study
reported that GSPs may prevent ventricular tachycardia and
fibrillation induced by reperfusion through the scavenging of free
radicals in isolated rat hearts (29). Zhao et al (30) further demonstrated that the
upregulation of the Na+/K+-ATPase α-subunit
may be responsible for the protective effect of GSPs on reperfusion
arrhythmias in rats in vivo. Another pathway involving GSPs
for the prevention from myocardial I/R injury is its effect on
reducing intracellular Ca2+ concentrations (29). Shao et al (31) demonstrated that GSPs increased
nitric oxide production, mediated by the Akt-endothelial nitric
oxide synthase signaling pathway, to offer cardioprotection against
H/R injury on cardiomyocytes. The literature, therefore, suggests
that GSPs have an effect of anti-myocardial I/R injury. Previous
studies also reported the protective effect of GSPs against I/R
injury in the liver, brain, intestine and kidney (32–35).
However, its protection against cardiomyocyte apoptosis and
underlying mechanisms associated with ER stress are unknown. This
study, to the best of our knowledge, was the first to demonstrate
that GSPs can attenuate H/R-induced cardiomyocyte injury by
inhibiting ER stress-dependent apoptosis through suppressed
activation of the PERK/eIF2α pathway.
This study explored, for the first time, the
protective effects and inhibitory levels of ER stress among
different administered concentrations of GSPs in H/R
cardiomyocytes. It was confirmed that a higher GSPs concentration
did not provide a greater protective effect and instead may have
been harmful to cardiomyocytes. Additionally, to the best of our
knowledge, the study was the first to propose that GSPs exert
protective effects on H/R cardiomyocytes by blocking both the ER
stress upstream PERK/eIF2α pathway and CHOP apoptosis pathway to
decrease cell apoptosis.
A potential limitation of of this study was that
H9C2 cardiomyocytes do not undergo spontaneous contraction so they
may not fully represent human cardiomyocytes. An animal model
should be established to further support the results of this
study.
Acknowledgements
The present study was supported by the First
Affiliated Hospital of China Medical University (Shenyang,
China).
References
|
1
|
Garcia-Dorado D: Myocardial reperfusion
injury: A new view. Cardiovasc Res. 61:363–364. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sharma V, Bell RM and Yellon DM: Targeting
reperfusion injury in acute myocardial infarction: A review of
reperfusion injury pharmacotherapy. Expert Opin Pharmacother.
13:1153–1175. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bainey KR and Armstrong PW: Clinical
perspectives on reperfusion injury in acute myocardial infarction.
Am Heart J. 167:637–645. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Murry CE, Jennings RB and Reimer KA:
Preconditioning with ischemia: A delay of lethal cell injury in
ischemic myocardium. Circulation. 74:1124–1136. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhao ZQ, Corvera JS, Halkos ME, Kerendi F,
Wang NP, Guyton RA and Vinten-Johansen J: Inhibition of myocardial
injury by ischemic postconditioning during reperfusion: Comparison
with ischemic preconditioning. Am J Physiol Heart Circ Physiol.
285:H579–H588. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kalogeris T, Baines CP, Krenz M and
Korthuis RJ: Cell biology of ischemia/reperfusion injury. Int Rev
Cell Mol Biol. 298:229–317. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stamboul K, Lorin J, Lorgis L, Guenancia
C, Beer JC, Touzery C, Rochette L, Vergely C, Cottin Y and Zeller
M: Atrial fibrillation is associated with a marker of endothelial
function and oxidative stress in patients with acute myocardial
infarction. PLoS One. 10:e01314392015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Logue SE, Cleary P, Saveljeva S and Samali
A: New directions in ER stress-induced cell death. Apoptosis.
18:537–546. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Doroudgar S and Glembotski CC: New
concepts of endoplasmic reticulum function in the heart: Programmed
to conserve. J Mol Cell Cardiol. 55:85–91. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schroder M and Kaufman RJ: The mammalian
unfolded protein response. Annu Rev Biochem. 74:739–789. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tabas I and Ron D: Integrating the
mechanisms of apoptosis induced by endoplasmic reticulum stress.
Nat Cell Biol. 13:184–190. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Boyce M and Yuan J: Cellular response to
endoplasmic reticulum stress: A matter of life or death. Cell Death
Differ. 13:363–373. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kasseckert SA, Schäfer C, Kluger A,
Gligorievski D, Tillmann J, Schlüter KD, Noll T, Sauer H, Piper HM
and Abdallah Y: Stimulation of cGMP signalling protects coronary
endothelium against reperfusion-induced intercellular gap
formation. Cardiovasc Res. 83:381–387. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bagchi D, Bagchi M, Stohs Sj, Ray SD, Sen
CK and Preuss HG: Cellular protection with proanthocyanidins
derived from grape seeds. Ann N Y Acad Sci. 957:260–270. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim TH, Jeon EJ, Cheung DY, Kim CW, Kim
SS, Park SH, Han SW, Kim MJ, Lee YS, Cho ML, et al:
Gastroprotective effects of grape seed proanthocyanidin extracts
against nonsteroid anti-inflammatory drug-induced gastric injury in
rats. Gut Liver. 7:282–289. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ozkan G, Ulusoy S, Alkanat M, Orem A,
Akcan B, Ersöz S, Yuluğ E, Kaynar K and Al S: Antiapoptotic and
antioxidant effects of GSPE in preventing cyclosporine A-induced
cardiotoxicity. Ren Fail. 34:460–466. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Singh T, Sharma SD and Katiyar SK: Grape
proanthocyanidins induce apoptosis by loss of mitochondrial
membrane potential of human non-small cell lung cancer cells in
vitro and in vivo. PLoS One. 6:e274442011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cheng M, Gao HQ, Xu L, Li BY, Zhang H and
Li XH: Cardioprotective effects of grape seed proanthocyanidins
extracts in streptozocin induced diabetic rats. J Cardiovasc
Pharmacol. 50:503–509. 2007.PubMed/NCBI
|
|
19
|
Gao Y, Jia P, Shu W and Jia D: The
protective effect of lycopene on hypoxia/reoxygenation-induced
endoplasmic reticulum stress in H9C2 cardiomyocytes. Eur J
Pharmacol. 774:71–79. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sun J, Sun G, Meng X, Wang H, Wang M, Qin
M, Ma B, Luo Y, Yu Y, Chen R, et al: Ginsenoside RK3 prevents
hypoxia-reoxygenation induced apoptosis in H9c2 cardiomyocytes via
AKT and MAPK pathway. Evid Based Complement Alternat Med.
2013:6901902013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang Y, Duan W, Jin Z, Yi W, Yan J, Zhang
S, Wang N, Liang Z, Li Y, Chen W, et al: JAK2/STAT3 activation by
melatonin attenuates the mitochondrial oxidative damage induced by
myocardial ischemia/reperfusion injury. J Pineal Res. 55:275–286.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hou X, Han J, Yuan C, Ren H, Zhang Y,
Zhang T, Xu L, Zheng Q and Chen W: Cardioprotective effects of
total flavonoids extracted from xinjiang sprig rosa rugosa against
acute ischemia/reperfusion-induced myocardial injury in isolated
rat heart. Cardiovasc Toxicol. 16:54–66. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Marklund S and Marklund G: Involvement of
the superoxide anion radical in the autoxidation of pyrogallol and
a convenient assay for superoxide dismutase. Eur J Biochem.
47:469–474. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
van Huizen R, Martindale JL, Gorospe M and
Holbrook NJ: P58IPK, a novel endoplasmic reticulum stress-inducible
protein and potential negative regulator of eIF2alpha signaling. J
Biol Chem. 278:15558–15564. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ron D and Walter P: Signal integration in
the endoplasmic reticulum unfolded protein response. Nat Rev Mol
Cell Biol. 8:519–529. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rimm EB, Giovannucci EL, Willett WC,
Colditz GA, Ascherio A, Rosner B and Stampfer MJ: Prospective study
of alcohol consumption and risk of coronary disease in men. Lancet.
338:464–468. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sato M, Maulik G, Ray PS, Bagchi D and Das
DK: Cardioprotective effects of grape seed proanthocyanidin against
ischemic reperfusion injury. J Mol Cell Cardiol. 31:1289–1297.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pataki T, Bak I, Kovacs P, Bagchi D, Das
DK and Tosaki A: Grape seed proanthocyanidins improved cardiac
recovery during reperfusion after ischemia in isolated rat hearts.
Am J Clin Nutr. 75:894–899. 2002.PubMed/NCBI
|
|
30
|
Zhao G, Gao H, Qiu J, Lu W and Wei X: The
molecular mechanism of protective effects of grape seed
proanthocyanidin extract on reperfusion arrhythmias in rats in
vivo. Biol Pharm Bull. 33:759–767. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shao ZH, Wojcik KR, Dossumbekova A, Hsu C,
Mehendale SR, Li CQ, Qin Y, Sharp WW, Chang WT, Hamann KJ, et al:
Grape seed proanthocyanidins protect cardiomyocytes from ischemia
and reperfusion injury via Akt-NOS signaling. J Cell Biochem.
107:697–705. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gao Z, Liu G, Hu Z and Li X, Yang X, Jiang
B and Li X: Grape seed proanthocyanidin extract protects from
cisplatin-induced nephrotoxicity by inhibiting endoplasmic
reticulum stress-induced apoptosis. Mol Med Rep. 9:801–807. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu CM, Ma JQ, Liu SS, Zheng GH, Feng ZJ
and Sun JM: Proanthocyanidins improves lead-induced cognitive
impairments by blocking endoplasmic reticulum stress and nuclear
factor-κB-mediated inflammatory pathways in rats. Food Chem
Toxicol. 72:295–302. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sizlan A, Guven A, Uysal B, Yanarates O,
Atim A, Oztas E, Cosar A and Korkmaz A: Proanthocyanidin protects
intestine and remote organs against mesenteric ischemia/reperfusion
injury. World J Surg. 33:1384–1391. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xu ZC, Yin J, Zhou B, Liu YT, Yu Y and Li
GQ: Grape seed proanthocyanidin protects liver against
ischemia/reperfusion injury by attenuating endoplasmic reticulum
stress. World J Gastroenterol. 21:7468–7477. 2015. View Article : Google Scholar : PubMed/NCBI
|