Introduction
Biopersistent fibrous dusts are present in natural
mineral stones and may arise during certain industrial production
processes. Well-known examples of natural minerals are asbestos
fibers. The two most important industrial asbestos minerals
associated with occupational diseases are crocidolite and
chrysotile. Industrially-synthesized inorganic fibers which are
substitutes for asbestos are termed man-made mineral fibers
(1), primary examples of which are
glass fibers [man-made vitreous fibers (MMVF)], ceramic fibers
(including refractory ceramic fibers) and, more recently, carbon
nanotubes. Epidemiological studies have confirmed an increased risk
of lung carcinoma and mesothelioma following exposure to asbestos
(2,3). Due to their geological formations,
asbestos fibers vary in chemical composition, length and diameter.
Inhalable asbestos fibers of critical dimensions were defined as
World Health Organization (WHO; Geneva, Switzerland) fibers: Length
≥5 µm, diameter <3 µm, length:diameter ratio >3:1 (4). However, this convention is not a
robust criterion by which to categorize fibers as toxic. Previous
animal studies revealed that nano-sized fibers, including silver
nano wires (5,6), multiwall carbon nanotubes and rigid
carbon nanotubes (7) may induce
asbestos-like granulomatous inflammation and fibrogenic effects in
the lung tissues and pleura (8,9). In
order to determine the toxicity of fibers, the chemical
composition, as the ultimate cause of biopersistence, in addition
to the surface reactivity of the fibers are as important as fiber
length and diameter (10).
Chemicals, including fibrous dusts, may act in a
number of ways on human and animal cells. They may induce genetic
mutations, influence the transcription and translation rate of
genes or affect protein functions, such as enzyme activities, via
intervention in post-translational regulatory protein kinase
cascades. A number of previous studies have assessed the impact of
different dusts on the expression of genes using genomic,
transcriptomic and proteomic analytical techniques (11–14).
The impact of the chemicals on different genes and their expression
products may potentiate or annul each other. The end product of the
complex regulatory mechanisms and external interventions which take
place at the level of DNA, mRNA and protein are the conversion
rates of the corresponding pathways. In its entirety, the
conversion rates of the different metabolic pathways of cells,
which are summarized as the metabolic signature, mirror the
physiological functions and the general physiological status of the
cells. The present study investigated the effect of different
fibrous dusts on three important metabolic pathways (glycolysis,
glutaminolysis and serine metabolism) of A549 human lung
adenocarcinoma cells in cell culture.
Following inhalation and deposition of dust
particles in the lung, the primary target cells affected are
alveolar macrophages and alveolar epithelial cells. The A549 cells
used in the present study are morphologically assigned to type II
alveolar epithelial cells (15).
Type II alveolar epithelial cells are characterized by their
surfactant synthesis in addition to the proliferation and
differentiation of stem cells following damage to type I epithelial
cells (16). Therefore, alveolar
epithelial type II cells are of particular importance when
inflammatory damage to the lung occurs. A549 cells contain a number
of metabolic features and transport properties characteristic of
type II pneumocytes, including cytochrome P450 1A1 and 2B6, tannic
acid-positive lamellar bodies, concentration-dependent
internalization of cationized ferritin, and uptake of transferrin
(17). A549 is a permanent cell
line developed in 1972 by Giard et al (18) from a human lung carcinoma. An
advantage of A549 cells is their high growth rate and cellular
homogeneity. Due to their ability to perform unlimited cell
division A549 cells are suited to long-term experimentation. In
addition, A549 are capable of internalizing particles (19). According to Castell et al
(20), A549 cells are the most
suitable cell line for the investigation of chemically-induced and
lung-specific toxicity. Foster et al (17) suggested the cell line as a standard
model for the investigation of type II lung epithelial cells and
drug metabolism. A549 cells have been used to investigate the
impact of inhalative chemicals in a high number of publications
(12,20–23).
In comparison to BEAS-2B cells, a bronchoepithelial cell line
genetically modified and immortalized by the adenovirus 12-SV40
virus hybrid (Ad12SV40), A549 cells are more stable in cell culture
and have been extensively characterized regarding cell viability,
transcription factors and signaling molecules (24,25).
The A549 cells used in the present study are classified as a p53
wild type lung cell line in the documentation of one of the
provider (26).
In proliferating cells, including normal
proliferating cells, embryonic cells, adult stem cells and
immortalized cells, energy is regenerated in two pathways:
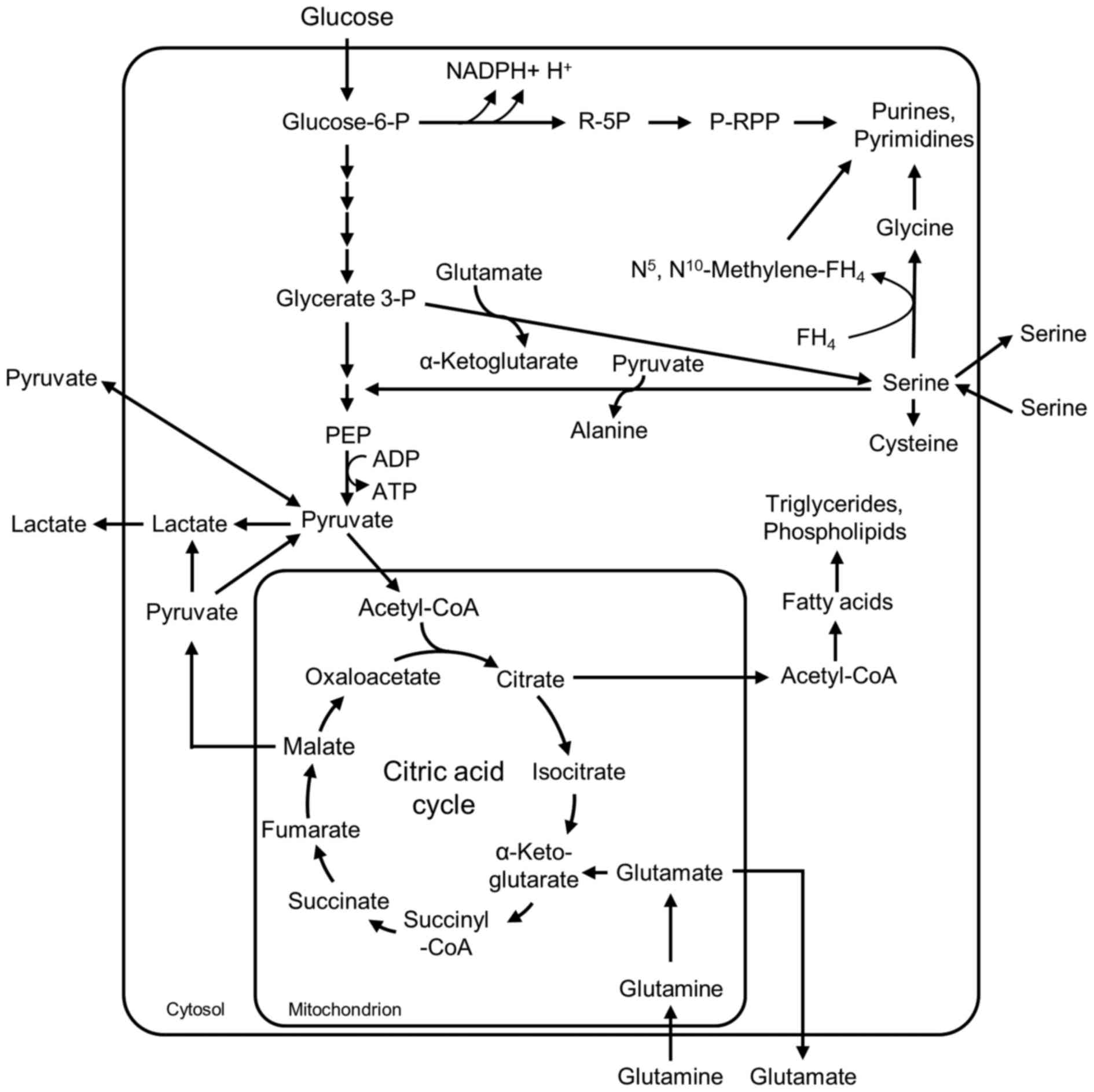
Glycolysis and glutaminolysis (Fig.
1) (27–29). In glycolysis, net ATP production
occurs in the pyruvate kinase reaction. The degradation of
glutamine via glutaminolysis recruits reaction steps of the citric
acid cycle and depends on oxygen. In addition, intermediates of
glycolysis, glutaminolysis and serine metabolism serve as
substrates for the synthesis of important cellular building blocks,
such as nucleic acids, phospholipids, amino acids and C1-building
blocks for folic acids (Fig. 1).
Therefore, cell division of proliferating cells only proceeds when
the cellular metabolism is capable of providing a high enough
concentration of intermediates to ensure energy regeneration, and
the synthesis of cellular building blocks in sufficient amounts
(29,30). In order to obtain an impression of
whether or not chemicals have an impact on the conversion rates of
glycolysis, glutaminolysis and serine metabolism, a rapid and
effective method is to measure the conversion rates of their
corresponding substrates (glucose, glutamine and serine) and
metabolic products (lactate and glutamate) in the culture
supernatants of the cells in cell culture.
The present study compared the impact of the
following fibrous dusts on the glucose, glutamine and serine
conversion rates of A549 cells: i) Crocidolite and chrysotile
asbestos, as biopersistent fibrous dusts with documented
carcinogenic characteristics; ii) glass fibers, as an example of
low-biopersistence MMVF (2); and
iii) biopersistent multi-walled-carbon-nanotubes (MWCN) with two
different lengths, at 1–2 µm and 5–15 µm. In contrast with
crocidolite, chrysotile and glass fibers, short MWCN do not fulfil
the geometric ratios of the WHO fiber criteria. The two types of
MWCN, which were produced under the same reaction conditions, are
characterized by an identical chemical composition and diameter,
although they differ in length. This selection of fibers allowed
for the comparison of different biopersistent WHO fibers.
Additionally, the effect of length was evaluated in the case of
MWCN.
Materials and methods
Fibrous dusts
Crocidolite asbestos was obtained from the Union
Internationale Contre le Cancer (UICC; WHO; South African NB
#4173-111-3). Chrysotile asbestos was obtained from UICC (Rhodesian
NB #4173-11-2). Glass fibers (MMVFs) were removed from commercial
glass wool used for insulation. Biopersistent MWCN (1–2 µm MWCN;
cat. no. CP-0012-SG and 5–15 µm MWCN; cat. no. CP-0009-SG) were
purchased from Ionic Liquids Technologies GmbH (Heilbronn,
Germany).
Techniques used for fibrous dust
characterization
Scanning electron microscopy (SEM; Hitachi S-2700;
Hitachi, Ltd., Tokyo, Japan) was used to identify particle geometry
in addition to the microstructure of the fibers. The element
analysis resulted from energy dispersive X-rays (EDX). To optimize
the conductivity (electron beam), all fiber samples were sputtered
with a fine layer of Au. Transmission electron microscopy analysis
combined with electron diffraction (detection of crystallinity) was
performed using a transmission electron microscope (H-7100;
Hitachi, Ltd.).
Culture conditions and metabolite
measurements
A549 cells were obtained from the European
Collection of Authenticated Cell Cultures (Salisbury, UK; cat. no.
86012804). The cells were cultured in Gibco® RPMI 1640
(Thermo Fisher Scientific, Inc., Waltham, MA, USA), supplemented
with 2 mM L-glutamine, 1% (v/v) antibiotics (penicillin, 100 U/ml
and streptomycin, 100 µg/ml) and 10% (v/v) fetal calf serum (Thermo
Fisher Scientific, Inc.) at 37°C in a 5% CO2
environment. For the flux experiments, 12-well dishes (for 24 h
exposure time) and 24-well dishes (for 48 h exposure time) were
used (both Sarstedt AG & Co., Nümbrecht, Germany). The
experiments were begun with an initial cell number of
9.0×104 cells/well in the 12-well plates and
7.0×104 cells/well in the 24-well plates. Following 24 h
of pre-culture, the culture media of the cells were exchanged for
culture media with different fibers. Stock solutions with 5 mg of
the respective fibers suspended in 1 ml PBS (cat. no. BIO-37108;
Bioline Reagents, Ltd., London, UK) were diluted with fresh culture
medium to a concentration of 1 µg/cm2, and 25
µg/cm2 for glass fibers and MWCN or 5 µg/cm2
for the two types of asbestos. Control cells were mock-treated with
PBS buffer. Following 24 h culture for the first culture approach
(low concentration), and 48 h for the second approach (high
concentration), the culture supernatants were collected,
centrifuged at 460 × g at room temperature for 5 min in order to
remove fibers from the medium and immediately frozen at −80°C. The
cells in the corresponding wells were trypsinized, diluted 1:2 with
trypan blue and counted using a Neubauer chamber. The frozen
culture supernatants were heated for 15 min at 95°C and
subsequently centrifuged at 8,000 × g at room temperature for 10
min (31). Glucose, lactate,
pyruvate, glutamine, glutamate and serine were determined using a
bench top random clinical analyzer, as described by Unterluggauer
et al (32). The conversion
rates of the metabolites in nmol/(hx105 cells) were
calculated as the difference between medium samples from dishes
with cells and medium samples incubated in parallel dishes without
cells.
Statistical analysis
Results are presented as the mean ± standard error
of the mean for each condition, compared with the mean of the
results from control samples. All statistical analyses were
performed using the statistical software package SSPS 17.0 (SPSS
Inc., Chicago, IL, USA). A comprehensive one-way analysis of
variance with repeated measures combined with a Student Newman
Keuls post hoc test was performed. P<0.05 was considered to
indicate a statistically significant difference.
Results and Discussion
Characterization of the fibrous dusts
by electron microscopy
UICC crocidolite South African, a ferrous rod-like
fiber with the chemical formula Na2
(Fe32+Fe23+[(OH)2Si8O22]),
is composed of 130×106 WHO fibers/mg with a length of
>5 µm, a diameter of <3 µm, and a length:diameter ratio
>3:1. Crocidolite is a rigid and rod-like fiber with a
characteristic iron content (13).
UICC chrysotile ‘A’ Rhodesian (chemical structural formula,
Mg6 [(OH)8Si4O10], with
an approximately equal Mg/Si distribution) is composed of
800×106 WHO fibers/mg. These fibers are of a curly,
pliable structure (13). The WHO
fraction of the glass fibers is composed of 0.26×106
fibers/mg. EDX analysis revealed the following chemical
composition: 70.0% SiO2; 14.3% CaO; 9.7%
Na2O; 2.5% MgO; 2.3% K2O; and 1.2%
Al2O3. Glass fibers are characteristic of an
amorphous material. A diffraction pattern was not detectable by
transmission electron microscopy (Helmig et al,
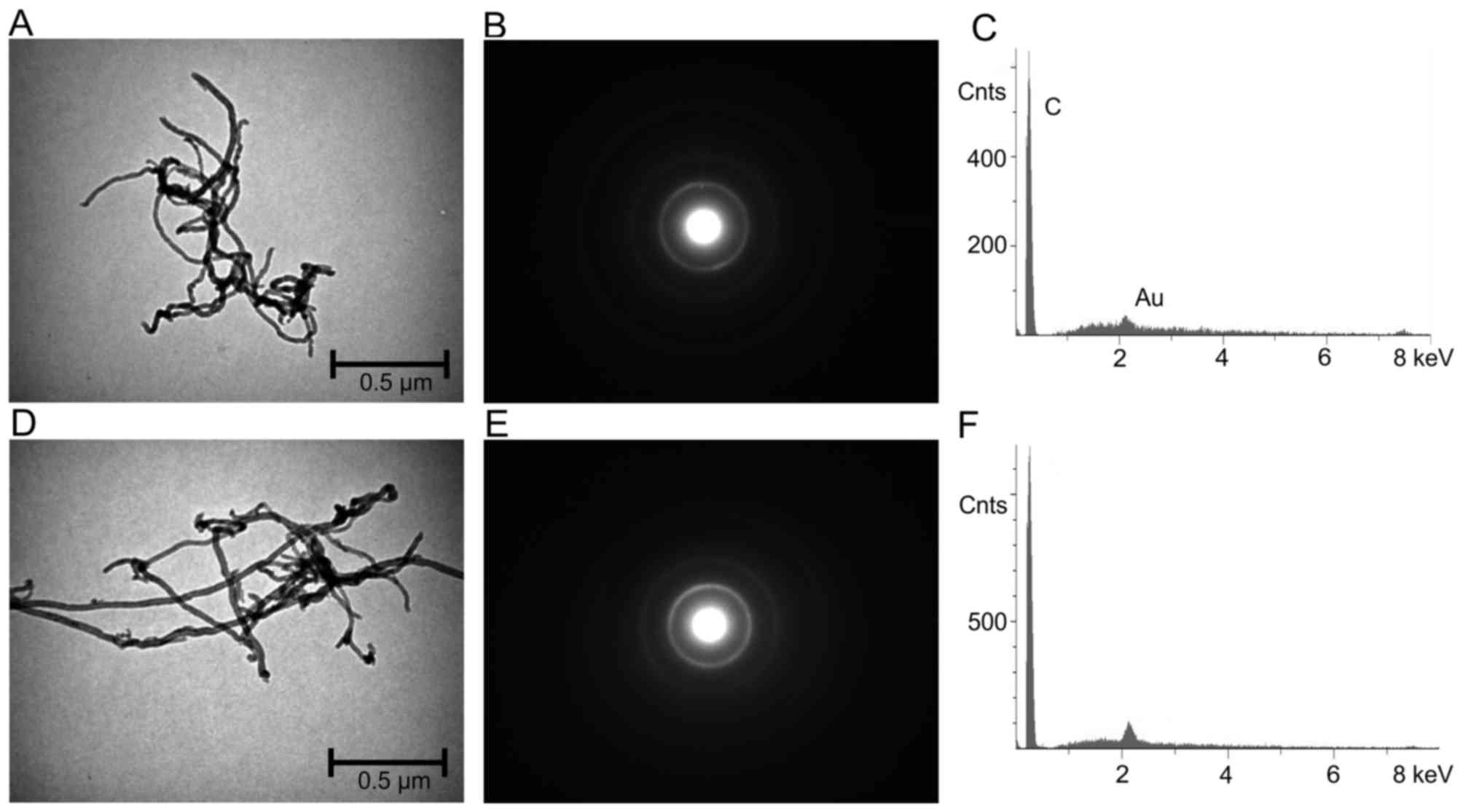
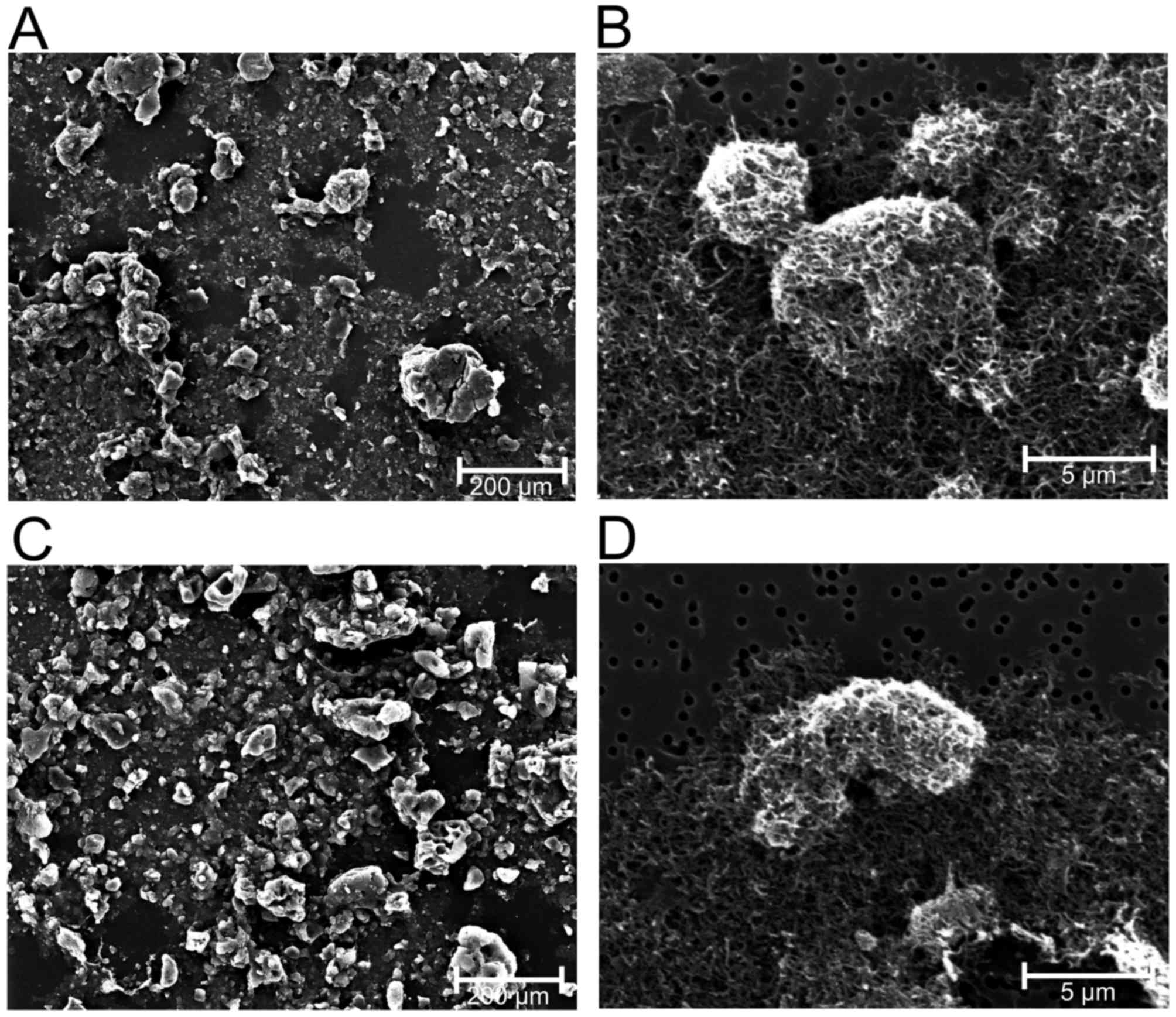
unpublished). In contrast with chrysotile, crocidolite and glass
fibers, the nano-sized 1–2 µm MWCN (CP-0012-SG) and 5–15 µm MWCN
(CP-0009-SG) agglomerate to larger units with diameters of ~5 µm in
a suspension (Figs. 2 and 3).
Impact of chrysotile, crocidolite,
glass fibers, MWCN 5–15 µm and MWCN 1–2 µm on the proliferation
rate of A549 cells
The aim of the present study was to investigate the
impact of two dosages of the selected fibrous dusts on glycolysis,
glutaminolysis and serine metabolism in cell culture: A low
concentration with little or no impact on the cell proliferation
rate of A549 cells, and a high concentration with a more severe
impact on cell proliferation. The dosages were not selected to
define the no-observed-adverse-effect-level or the
lowest-observed-adverse-effect-level of the corresponding fibers.
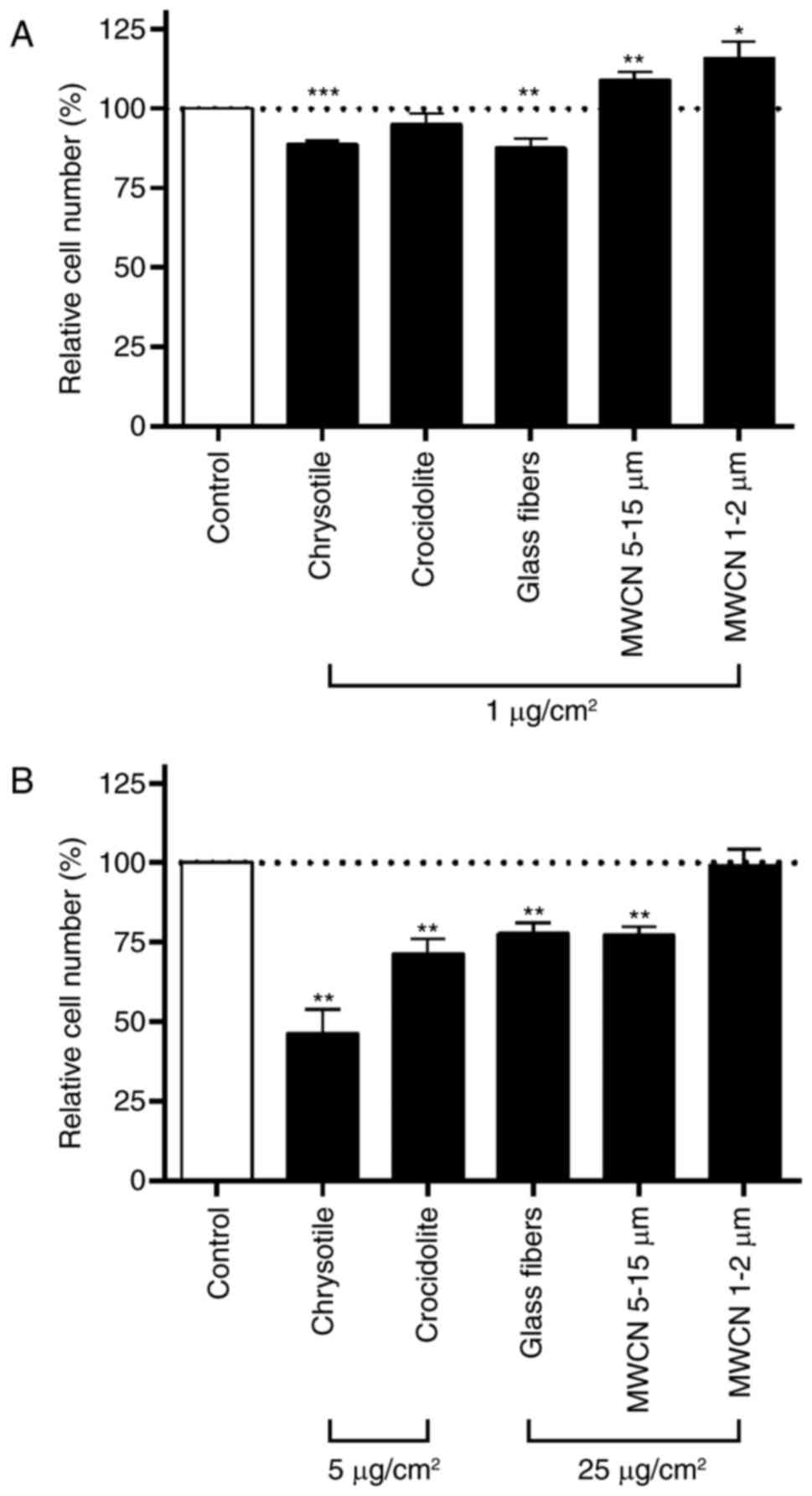
For all fibers tested, the low dosage was 1 µg/cm2 with
an incubation time of 24 h (Fig.
4A). The high concentration was 25 µg/cm2 for glass
fibers, MWCN 5–15 µm and MWCN 1–2 µm (Fig. 4B). Concentrations >25
µg/cm2 led to an impenetrable dust carpet over the cell
monolayer and were therefore not used in the present study.
Chrysotile and crocidolite asbestos had a significantly increased
inhibitory effect on cell proliferation in A549 cells compared with
glass fibers or MWCN. For the two asbestos fibers, a dosage of 25
µg/cm2 for 48 h was cytotoxic. In order to achieve
comparable inhibitory effects on the cell proliferation rate among
the different fibers tested at the high dosage, chrysotile and
crocidolite were applied at a concentration of 5 µg/cm2
for 48 h. In the experiments for all dusts tested, neither the low
dosage of 1 µg/cm2 nor the high dosage (5
µg/cm2 and 25 µg/cm2, respectively) had an
impact on cell viability, which corresponded to a number of other
studies published in the literature (33–35).
At the low dosage (1 µg/cm2 for 24 h) only chrysotile
and glass fibers led to a significant inhibition (12%) of cell
proliferation compared with mock-treated controls (Fig. 4A). In the case of chrysotile, the
inhibition of cell proliferation rose to 54% at the high
concentration (5 µg/cm2 for 48 h; Fig. 4B). Crocidolite asbestos exerted no
impact at the low dosage and decreased cell proliferation by 29% at
the high dosage (5 µg/cm2 for 48 h). Similar growth
inhibitory effects of chrysotile and crocidolite have been
described in bronchial epithelial BEAS 2B cells (36) and human embryonic lung HEL-299
cells (37). The growth inhibitory
effect of glass fibers increased from 12% inhibition at the low
dosage to 22% inhibition at the high dosage (5 µg/cm2
for 48 h). MWCN at 1–2- and 5–15-µm lengths induced a significant
increase in cell proliferation (MWCN 1–2 µm, 16% and MWCN 5–15 µm,
9%) at the low dosage (1 µg/cm2 for 24 h). At the high
dosage (25 µg/cm2 for 48 h) only the 5–15 µm MWCN led to
an inhibition of cell proliferation of 23%, whereas the shorter 1–2
µm MWCN had no impact on cell proliferation compared with the
mock-treated control cells. By quantifying the DNA content, Tabet
et al (38) estimated a
reduction in the cell number of 15–20% when A549 cells were
incubated for 24, 48 and 72 h with 20 µg/cm2 MWCN at a
length of 100 µm (Graphistrength C10; ARKEMA, Colombes, France);
whereas, under the same conditions in mesothelial MeT5A cells, no
significant effect of MWCN was observed. In the same study, 48 h
incubation with 20 µg/cm2 crocidolite significantly
increased the DNA content in A549 cells, whereas an MTT assay
indicated a significant downregulation of cell viability.
Impact of chrysotile, crocidolite,
glass fibers, MWCN 5–15 µm and MWCN 1–2 µm on glycolysis,
glutaminolysis and serine metabolism
The impact of the fibrous dusts on glycolysis,
glutaminolysis and serine metabolism was investigated by direct
measurement of the conversion rates of glucose, pyruvate, lactate,
glutamine, glutamate and serine in the culture supernatants of
mock-treated and fibrous dust-treated A549 cells. In differentiated
tissues, in the presence of oxygen, glucose is completely degraded
to CO2 and water via cytosolic glycolysis, the
mitochondrial citric acid cycle and endoxidation in order to
regenerate energy (Fig. 1). In low
oxygen conditions, pyruvate is reduced to lactate within the
cytosol. By contrast, in proliferating cells, including A549 cells,
glycolytic pyruvate is released as lactate even in the presence of
oxygen (Fig. 1). In addition to
energy regeneration in proliferating cells, glycolytic
intermediates serve as precursors for the synthesis of important
cellular building blocks, including nucleic acids, amino acids and
phospholipids, which are essential for cells with a high
proliferation rate (Fig. 1)
(29,30). For energy regeneration,
proliferating cells utilize a novel efficient source of energy,
which is the degradation of the amino acid glutamine (27,29,39).
Thereby extracellular glutamine is desaminated to glutamate within
the mitochondria. A certain amount of the glutamate is directly
released from the cells and may be identified as glutamate
production in the culture supernatants of the cells (Fig. 1). A further quantity of the
glutamate is converted to α-ketoglutarate and is infiltrated into
the citric acid cycle for energy regeneration. In addition,
glutamate provides the amino group for serine synthesis from
glycerate 3-phosphate (P) and is a component of glutathione, an
important metabolite for the detoxification of reactive oxygen
species (ROS). A certain amount of the malate within the citric
acid cycle is decarboxylated to pyruvate and released as lactate;
thus, glutaminolysis summarizes the degradation of the amino acid
glutamine to malate, CO2, pyruvate, lactate and citric
acid. Additionally, glutamine is a precursor of purine and
pyrimidine synthesis (Fig. 1).
Pyruvate is either consumed by the cells when the metabolite is
added into the medium, or is released from the cells in the absence
of extracellular pyruvate. Serine is a precursor for phospholipid,
glycine and cysteine synthesis and donates one-carbon units to
folate, all of which are necessary for cellular building block
synthesis (40). When the rate of
serine synthesis exceeds the amount of serine necessary for the
synthesis of cellular components, serine is released from the
cells. However, when the cells are unable to synthesize serine in
sufficient amounts, serine is consumed from the medium (40,41).
At the low dosage tested in the present study (1
µg/cm2 dust for 24 h) none of the fibrous dusts tested
led to a significant alteration in the conversion rates of glucose,
lactate, pyruvate, glutamine, glutamate and serine (Fig. 5). At high dosages, the most marked
effect on the metabolic conversion rates was observed with
chrysotile (Fig. 5 A-E). Glucose
and glutamine consumption, in addition to pyruvate, lactate and
glutamate production, significantly increased within 48 h
incubation of A549 cells with 5 µg chrysotile/cm2. The
other fibrous dusts did also increase the conversion rates of these
metabolites. However, the extent of the effects decreased in the
following order: Chrysotile (5 µg/cm2)>crocidolite (5
µg/cm2)>glass fibers (25 µg/cm2)>MWCN
5–15 µm (25 µg/cm2)>MWCN 1–2 µm (25
µg/cm2).
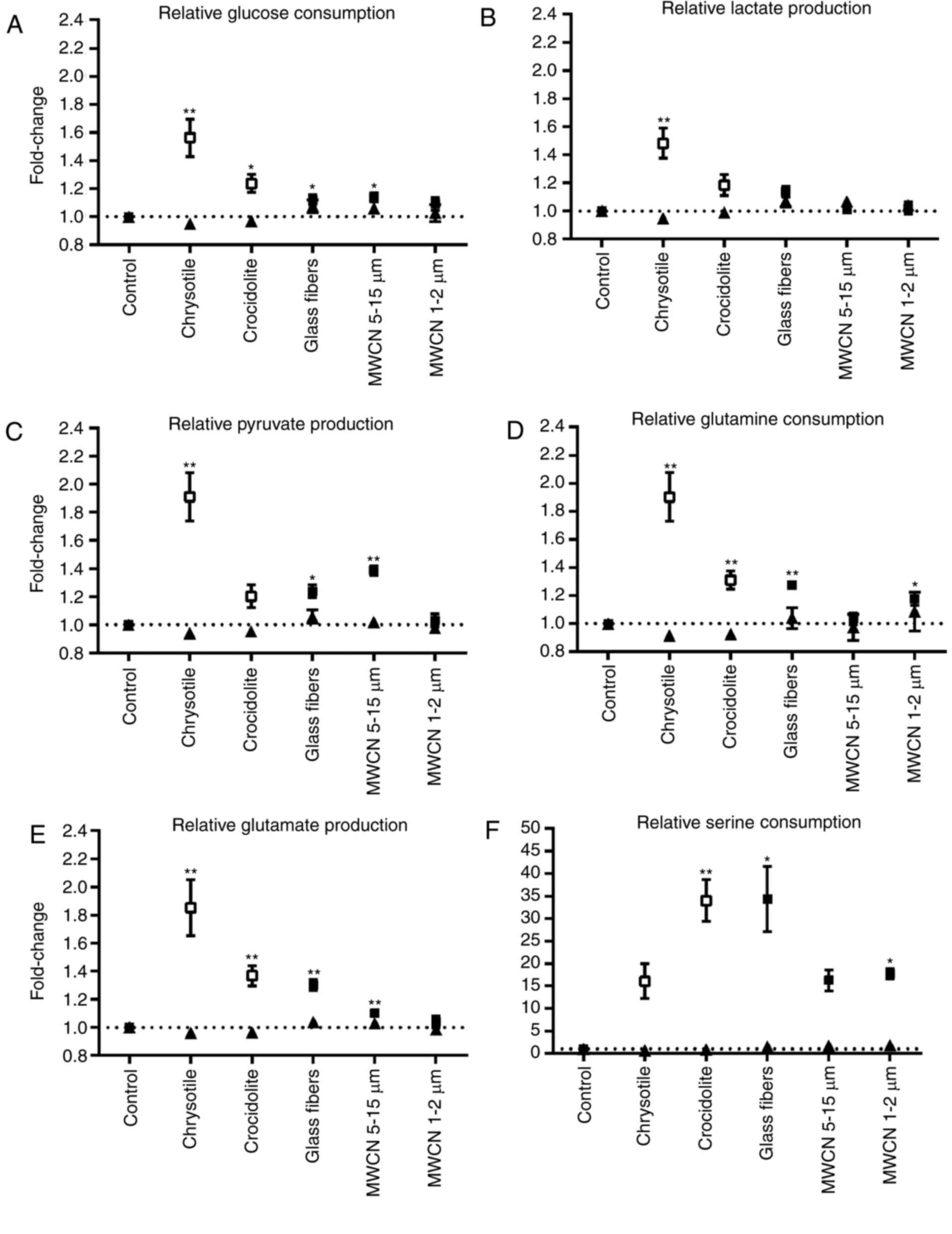 | Figure 5.Relative metabolic conversion rates
in dust-treated A549 cells compared with mock-treated controls. The
mean value of mock-treated controls was set at 1 and (A) glucose
consumption rates, (B) lactate production rates, (C) pyruvate
production rates, (D) glutamine consumption rates, (E) glutamate
production rates and (F) serine consumption rates of dust-treated
cells were calculated compared with the control value. Data are
presented as the mean ± standard error of the mean. ▲, dust
concentration=1 µg/cm2; control, n=14; all dust-treated
cells, n=6. □, concentration of chrysotile and crocidolite=5
µg/cm2; ■, concentration of all other dusts=25
µg/cm2; control n=6; all dust-treated cells, n=4.
*P<0.05; **P<0.01 vs. control. |
At first glance the increase in glucose and
glutamine consumption observed for the fibrous dusts tested in the
present study points to an increase in the metabolic activity
within the cells. However, the simultaneous increase in pyruvate,
lactate and glutamate release indicates that the amount of glucose
and glutamine channeled into the debranching synthetic processes
for cellular components was decreased in the presence of fibrous
dusts at non-toxic concentrations. This assumption is additionally
supported by the increase in serine uptake observed for certain of
the fibrous dusts tested (Fig.
5F). The amino acid serine is synthesized from the glycolytic
intermediate glycerate 3-P and the glutaminolytic intermediate
glutamate (Fig. 1). Serine
metabolism is regulated, inter alia, by the transcription
factors cellular tumor antigen p53 (p53) and Myc proto-oncogene
protein (Myc) (40). p53
suppresses the expression of phosphoglycerate dehydrogenase
(PHGDH), the first enzyme in serine synthesis. Myc has been
demonstrated to enhance the expression of PHGDH, in addition to
phosphoserine aminotransferase 1 and phosphoserine phosphatase, the
two following enzymes within serine synthesis. Concurrently with
hypoxia-inducible factor 1-α (HIF1α), Myc activates the expression
of serine hydroxymethyltransferase 2, which catalyzes the
reversible reaction of serine and tetrahydrofolate to glycine and
5,10-methylene tetrahydrofolate. According to results published by
Matsuoka et al (22),
treatment with chrysotile and crocidolite led to a dose- and
time-dependent increase in Ser15 phosphorylation and the
stabilization of p53 in A549 cells. A p53-regulated downregulation
of serine synthesis may be an explanation for the increased serine
uptake observed in the chrysotile- and crocidolite-treated A549
cells in the present study (Fig.
5F). A crocidolite-associated stabilization of p53 in A549
cells was additionally described by Johnson and Jaramillo (23). In the same study, JM 100 glass
microfiber did not induce an increase in p53 (23); whereas, in the present study, in
glass fiber-treated A549 cells the increase in serine uptake was
among the highest compared with the other fibers tested. In
addition, continuous exposure of primary human lung small airway
epithelial cells immortalized with human telomerase reverse
transcriptase for 6 months to 0.02 µg/cm2 dispersed
carbon nanotubes (single-walled and MWCN) and crocidolite induced
an overexpression of cMyc, while p53 was underexpressed (42). In addition to numerous other
cellular targets, Myc is an important transcriptional upregulator
of glycolytic enzymes and glutaminolytic glutaminase (43), which may serve a regulatory role in
the fiber-dependent increase in glucose and glutamine consumption
observed in the present study. However, the increase in glutamate
production in the present study was an indication that a high
amount of glutamine consumed in the fibrous dust-treated cells was
not converted in glutaminolysis (Fig.
5D and E). Further investigations into the level of gene
expression regulation are required to clarify the underlying
mechanism of the fiber-dependent increase in glucose, glutamine and
serine uptake observed in A549 cells in the present study.
The increase in glucose, glutamine and serine
consumption observed in the fibrous dust-treated A549 cells may be
interpreted as a metabolic mechanism to compensate for the
inhibition of cellular building block synthesis. However, the
inhibition of cell proliferation suggests an interruption of the
metabolic regulation between the provision of cellular components
and energy regeneration. Few studies have addressed the effect of
fibrous dusts on the metabolism of cells. In accordance with the
results of the present study, Riganti et al (44) described a dose-dependent inhibition
of the oxidative pentose-P pathway in A549 cells due to the direct
interaction of crocidolite with glucose 6-P dehydrogenase (G6PDH).
The oxidative pentose-P pathway provides ribose 5-P for nucleic
acid synthesis in addition to reduced nicotinamide adenine
dinucleotide phosphate (NADPH) + H+ for glutathione
recycling, for example (Fig. 1).
G6PDH is responsible for NADPH + H+ regeneration, which
is necessary for the recycling of glutathione, the principal
antioxidant cellular pathway (Fig.
1). An inhibition of G6PDH and glutathione recycling is a
conceivable explanation for the increase in ROS production
described in asbestos- (37,45,46),
MWCN- (46) and glass fiber-
(45) treated cells. In the
present study, the cells were incubated with 5 µg
crocidolite/cm2 for 48 h. Notably, in the study of
Golladay et al (47), 24 h
incubation of A549 cells with 3 µg/cm2 crocidolite led
to a release of 75% of the intracellular glutathione into the
medium, which was not induced by nonspecific membrane damage. High
ROS concentrations have been demonstrated to stabilize the alpha
subunit of HIF1 (48), which is an
important transcriptional upregulator of glycolytic enzymes.
ROS-induced HIF1α stabilization and HIF1-induced upregulation of
important glycolytic enzymes may be an explanation for the observed
increase in glycolytic conversion rates in the dust-treated A549
cells (Fig. 5A-C). Notably, in
pulmonary alveolar macrophages treated with 25 µg/cm2
chrysotile fibers for 18 h, a decrease in ATP levels of 20–30% has
been described (49) which
indicates that, in addition to the synthesis of cellular building
blocks, energy regeneration is impaired by treatment with
chrysotile.
Classification of observed
effects
For the concentrations tested in the present study,
and with respect to the inhibitory effect on cell proliferation
(Fig. 4) as well as the extent of
the metabolic alterations (Fig.
5), the results revealed the following ranking among the fibers
tested: Chrysotile>crocidolite>glass fibers>MWCN 5–15
µm>MWCN 1–2 µm. For the asbestos fibers and MMVF this ranking
correlated best with the number of fibers (chrysotile, 800 million
fibers/mg>crocidolite, 130 million fibers/mg>glass fibers,
0.26 million fibers/mg). In these fibers, the effects on cell
proliferation and cellular metabolism decreased with a decreasing
number of fibers. It appeared that the results observed for MWCN
5–15 µm and MWCN 1–2 µm were not consistent with this trend.
Nano-sized fibers (MWCN) are characterized by greater numbers of
fibers per unit of mass compared with chrysotile, crocidolite and
glass fibers. However, the effects of MWCN 5–15 µm and 1–2 µm on
cell proliferation and cellular metabolism were the lowest in the
present study (Figs. 4 and
5). Therefore, the behavior of the
MWCN in fluids was examined using SEM, which demonstrated the
strong tendency to form agglomerates of MWCN 5–15 µm and MWCN 1–2
µm (Fig. 3). Compared with the
longer MWCN 5–15 µm, MWCN 1–2 µm agglomerate to larger units.
Consequently, the functionally-relevant number of fibers is
markedly decreased by agglomeration. This explains why the observed
effects of MWCN on cell proliferation and metabolism were the
lowest by mass in the ranking produced in the present study.
In conclusion, the determination of glycolytic,
glutaminolytic and serine conversion rates in the culture
supernatants of cell cultures is a rapid and effective method of
obtaining an impression of the impact of chemicals on the metabolic
signature and physiological status of cells. For the fibrous dusts
tested in the present study, the metabolic signature in A549 cells
revealed a ranking which correlated best with the relevant number
of fibers. Based on these results, in future experiments, a
comparable concentration-dependent analysis of the intracellular
concentrations of the transcription factors HIF1, c-Myc and p53,
the protein content and enzymatic activity of important enzymes in
glycolysis, glutaminolysis and serine metabolism, the metabolic
intermediates of the corresponding pathways and the energy charge
may help to localize the molecular targets of the fibrous dusts
tested and to explain the underlying molecular mechanism of the
ranking between the different dusts disclosed in the present study
(50).
References
|
1
|
Thermal Insulation Manufactures
Association, Nomenclature Committee, . Manmade vitreous fibers.
Nomenclature, chemistry and physical properties. The Committe;
Stanford, CT: 1991
|
|
2
|
IARC Working Group on the Evaluation of
Carcinogenic Risks to Humans, . Man-made vitreous fibres. IARC
Monogr Eval Carcinog Risks Hum. 81:1–381. 2002.PubMed/NCBI
|
|
3
|
IARC Working Group on the Evaluation of
Carcinogenic Risks to Humans, . Arsenic, metals, fibres, and dusts.
IARC Monogr Eval Carcinog Risks Hum. 100:11–465. 2012.PubMed/NCBI
|
|
4
|
World Health Organization, . Determination
of airborne fibre number concentrations. A recommended method, by
phase-contrast optical microscopy, membrane filter method. World
Health Organization; Geneva: 1997
|
|
5
|
Schinwald A, Chernova T and Donaldson K:
Use of silver nanowires to determine thresholds for fibre
length-dependent pulmonary inflammation and inhibition of
macrophage migration in vitro. Part Fibre Toxicol. 9:472012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schinwald A and Donaldson K: Use of
back-scatter electron signals to visualise cell/nanowires
interactions in vitro and in vivo; frustrated phagocytosis of long
fibres in macrophages and compartmentalisation in mesothelial cells
in vivo. Part Fibre Toxicol. 9:342012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rittinghausen S, Hackbarth A, Creutzenberg
O, Ernst H, Heinrich U, Leonhardt A and Schaudien D: The
carcinogenic effect of various multi-walled carbon nanotubes
(MWCNTs) after intraperitoneal injection in rats. Part Fibre
Toxicol. 11:592014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Poland CA, Duffin R, Kinloch I, Maynard A,
Wallace WA, Seaton A, Stone V, Brown S, Macnee W and Donaldson K:
Carbon nanotubes introduced into the abdominal cavity of mice show
asbestos-like pathogenicity in a pilot study. Nat Nanotechnol.
3:423–428. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
van Berlo D, Wilhelmi V, Boots AW,
Hullmann M, Kuhlbusch TA, Bast A, Schins RP and Albrecht C:
Apoptotic, inflammatory, and fibrogenic effects of two different
types of multi-walled carbon nanotubes in mouse lung. Arch Toxicol.
88:1725–1737. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
DFG, . Fibrous Dust [MAK Value
Documentation]. Wiley-VCH Verlag GmbH & Co; KGaA, Weinheim: pp.
142–338. 1997
|
|
11
|
Nymark P, Lindholm PM, Korpela MV, Lahti
L, Ruosaari S, Kaski S, Hollmén J, Anttila S, Kinnula VL and
Knuutila S: Gene expression profiles in asbestos-exposed epithelial
and mesothelial lung cell lines. BMC Genomics. 8:622007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hevel JM, Olson-Buelow LC, Ganesan B,
Stevens JR, Hardman JP and Aust AE: Novel functional view of the
crocidolite asbestos-treated A549 human lung epithelial
transcriptome reveals an intricate network of pathways with
opposing functions. BMC Genomics. 9:3762008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Helmig S, Dopp E, Wenzel S, Walter D and
Schneider J: Induction of altered mRNA expression profiles caused
by fibrous and granular dust. Mol Med Rep. 9:217–228.
2014.PubMed/NCBI
|
|
14
|
Armand L, Biola-Clier M, Bobyk L,
Collin-Faure V, Diemer H, Strub JM, Cianferani S, Van Dorsselaer A,
Herlin-Boime N, Rabilloud T and Carriere M: Molecular responses of
alveolar epithelial A549 cells to chronic exposure to titanium
dioxide nanoparticles: A proteomic view. J Proteomics. 134:163–173.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lieber M, Smith B, Szakal A, Nelson-Rees W
and Todaro G: A continuous tumor-cell line from a human lung
carcinoma with properties of type II alveolar epithelial cells. Int
J Cancer. 17:62–70. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Witherden IR, Bon EJ Vanden, Goldstraw P,
Ratcliffe C, Pastorino U and Tetley TD: Primary human alveolar type
II epithelial cell chemokine release: Effects of cigarette smoke
and neutrophil elastase. Am J Respir Cell Mol Biol. 30:500–509.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Foster KA, Oster CG, Mayer MM, Avery ML
and Audus KL: Characterization of the A549 cell line as a type II
pulmonary epithelial cell model for drug metabolism. Exp Cell Res.
243:359–366. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Giard DJ, Aaronson SA, Todaro GJ, Arnstein
P, Kersey JH, Dosik H and Parks WP: In vitro cultivation of human
tumors: Establishment of cell lines derived from a series of solid
tumors. J Natl Cancer Inst. 51:1417–1423. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wottrich R, Diabaté S and Krug HF:
Biological effects of ultrafine model particles in human
macrophages and epithelial cells in mono- and co-culture. Int J Hyg
Environ Health. 207:353–361. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Castell JV, Donato MT and Gómez-Lechón MJ:
Metabolism and bioactivation of toxicants in the lung. The in vitro
cellular approach. Exp Toxicol Pathol. 57 Suppl 1:S189–S204. 2005.
View Article : Google Scholar
|
|
21
|
Nagatomo H, Morimoto Y, Ogami A, Hirohashi
M, Oyabu T, Kuroda K, Higashi T and Tanaka I: Change of heme
oxygenase-1 expression in lung injury induced by chrysotile
asbestos in vivo and in vitro. Inhal Toxicol. 19:317–323. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Matsuoka M, Igisu H and Morimoto Y:
Phosphorylation of p53 protein in A549 human pulmonary epithelial
cells exposed to asbestos fibers. Environ Health Perspect.
111:509–512. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Johnson NF and Jaramillo RJ: p53, Cip1,
and Gadd153 expression following treatment of A549 cells with
natural and man-made vitreous fibers. Environ Health Perspect. 105
Suppl 5:S1143–S1145. 1997. View
Article : Google Scholar
|
|
24
|
Signorelli S, Jennings P, Leonard MO and
Pfaller W: Differential effects of hypoxic stress in alveolar
epithelial cells and microvascular endothelial cells. Cell Physiol
Biochem. 25:135–144. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jing XG, Chen TF, Huang C, Wang H, An L,
Cheng Z and Zhang GJ: MiR-15a expression analysis in non-small cell
lung cancer A549 cells under local hypoxia microenvironment. Eur
Rev Med Pharmacol Sci. 21:2069–2074. 2017.PubMed/NCBI
|
|
26
|
ATCC®.org.: ‘A549 cell line:
CCl-185 product description, documentation p53 hotspot mutation
data’. https://www.lgcstandards-atcc.org/~/media/2B3C84F951E24E668C78EB70809C7613.ashx
|
|
27
|
DeBerardinis RJ, Mancuso A, Daikhin E,
Nissim I, Yudkoff M, Wehrli S and Thompson CB: Beyond aerobic
glycolysis: Transformed cells can engage in glutamine metabolism
that exceeds the requirement for protein and nucleotide synthesis.
Proc Natl Acad Sci USA. 104:pp. 19345–19350. 2007; View Article : Google Scholar :
|
|
28
|
Eigenbrodt E, Kallinowski F, Ott M,
Mazurek S and Vaupel P: Pyruvate kinase and the interaction of
amino acid and carbohydrate metabolism in solid tumors. Anticancer
Res. 18:3267–3274. 1998.
|
|
29
|
Mazurek S, Boschek CB, Hugo F and
Eigenbrodt E: Pyruvate kinase type M2 and its role in tumor growth
and spreading. Semin Cancer Biol. 15:300–308. 2005. View Article : Google Scholar
|
|
30
|
Christofk HR, Heiden MG Vander, Harris MH,
Ramanathan A, Gerszten RE, Wei R, Fleming MD, Schreiber SL and
Cantley LC: The M2 splice isoform of pyruvate kinase is important
for cancer metabolism and tumour growth. Nature. 452:230–233. 2008.
View Article : Google Scholar
|
|
31
|
Mazurek S, Michel A and Eigenbrodt E:
Effect of extracellular AMP on cell proliferation and metabolism of
breast cancer cell lines with high and low glycolytic rates. J Biol
Chem. 272:4941–4952. 1997. View Article : Google Scholar
|
|
32
|
Unterluggauer H, Mazurek S, Lener B,
Hütter E, Eigenbrodt E, Zwerschke W and Jansen-Dürr P: Premature
senescence of human endothelial cells induced by inhibition of
glutaminase. Biogerontology. 9:247–259. 2008. View Article : Google Scholar
|
|
33
|
Li P, Liu T, Kamp DW, Lin Z, Wang Y, Li D,
Yang L, He H and Liu G: The c-Jun N-terminal kinase signaling
pathway mediates chrysotile asbestos-induced alveolar epithelial
cell apoptosis. Mol Med Rep. 11:3626–3634. 2015. View Article : Google Scholar
|
|
34
|
Leyva FJ and Roberts K: Crocidolite
induces prostaglandin I(2) release mediated by vitronectin receptor
and cyclooxygenase-2 in lung cells. Lung. 188:133–141. 2010.
View Article : Google Scholar :
|
|
35
|
Srivastava RK, Lohani M, Pant AB and
Rahman Q: Cyto-genotoxicity of amphibole asbestos fibers in
cultured human lung epithelial cell line: Role of surface iron.
Toxicol Ind Health. 26:575–582. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nymark P, Jensen KA, Suhonen S, Kembouche
Y, Vippola M, Kleinjans J, Catalán J, Norppa H, van Delft J and
Briedé JJ: Free radical scavenging and formation by multi-walled
carbon nanotubes in cell free conditions and in human bronchial
epithelial cells. Part Fibre Toxicol. 11:42014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ueki A: Biological effects of asbestos
fibers on human cells in vitro-especially on lymphocytes and
neutrophils. Ind Health. 39:84–93. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tabet L, Bussy C, Amara N, Setyan A,
Grodet A, Rossi MJ, Pairon JC, Boczkowski J and Lanone S: Adverse
effects of industrial multiwalled carbon nanotubes on human
pulmonary cells. J Toxicol Environ Health A. 72:60–73. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lobo C, Ruiz-Bellido MA, Aledo JC, Márquez
J, Núnez de Castro I and Alonso FJ: Inhibition of glutaminase
expression by antisense mRNA decreases growth and tumourigenicity
of tumour cells. Biochem J. 348:257–261. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yang M and Vousden KH: Serine and
one-carbon metabolism in cancer. Nat Rev Cancer. 16:650–662. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mazurek S, Zwerschke W, Jansen-Dürr P and
Eigenbrodt E: Effects of the human papilloma virus HPV-16 E7
oncoprotein on glycolysis and glutaminolysis: Role of pyruvate
kinase type M2 and the glycolytic-enzyme complex. Biochem J.
356:247–256. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang L, Stueckle TA, Mishra A, Derk R,
Meighan T, Castranova V and Rojanasakul Y: Neoplastic-like
transformation effect of single-walled and multi-walled carbon
nanotubes compared to asbestos on human lung small airway
epithelial cells. Nanotoxicology. 8:485–507. 2014.doi:
10.3109/17435390.2013.801089. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Dang CV: Rethinking the Warburg effect
with Myc micromanaging glutamine metabolism. Cancer Res.
70:859–862. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Riganti C, Aldieri E, Bergandi L, Fenoglio
I, Costamagna C, Fubini B, Bosia A and Ghigo D: Crocidolite
asbestos inhibits pentose phosphate oxidative pathway and glucose
6-phosphate dehydrogenase activity in human lung epithelial cells.
Free Radic Biol Med. 32:938–949. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cardinali G, Kovacs D, Maresca V, Flori E,
Del l'Anna ML, Campopiano A, Casciardi S, Spagnoli G, Torrisi MR
and Picardo M: Differential in vitro cellular response induced by
exposure to synthetic vitreous fibers (SVFs) and asbestos
crocidolite fibers. Exp Mol Pathol. 81:31–41. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Garza KM, Soto KF and Murr LE:
Cytotoxicity and reactive oxygen species generation from aggregated
carbon and carbonaceous nanoparticulate materials. Int J
Nanomedicine. 3:83–94. 2008.PubMed/NCBI
|
|
47
|
Golladay SA, Park SH and Aust AE: Efflux
of reduced glutathione after exposure of human lung epithelial
cells to crocidolite asbestos. Environ Health Perspect. 105 Suppl
5:S1273–S1277. 1997. View Article : Google Scholar
|
|
48
|
Finkel T: Signal transduction by
mitochondrial oxidants. J Biol Chem. 287:4434–4440. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Nadeau D and Lane DA: The cytotoxicity of
chrysotile asbestos fibers to pulmonary alveolar macrophages. I.
Effects of inhibitors of ADP-ribosyl transferase. Cell Biol
Toxicol. 4:13–30. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Pink M, Verma N, Rettenmeier AW and
Schmitz-Spanke S: Integrated proteomic and metabolomic analysis to
assess the effects of pure and benzo[a]pyrene-loaded carbon black
particles on energy metabolism and motility in the human
endothelial cell line EA.hy926. Arch Toxicol. 88:913–934. 2014.
View Article : Google Scholar : PubMed/NCBI
|