Introduction
Proto-oncogene Wnt-1 (Wnt)/β-catenin signaling is
associated with many developmental and physiological processes
during early stages of embryonic development (1–4). The
deregulation of Wnt signaling often leads to abnormalities,
including heart disease (5–8). In
the absence of the Wnt ligand, intracellular β-catenin is
constantly degraded by the adenomatous polyposis coli
(APC)/Axin/glycogensynthase kinase-3β (GSK-3β) complex. However,
once Wnt ligand binds to the membrane receptors Frizzled and low
density lipoprotein receptor related protein 5/6 (LRP5/6), it leads
to the activation of Dishevelled (Dvl), resulting in the
suppression of the APC/Axin/GSK-3β complex and activation of
nuclear β-catenin, and associated downstream genes, including Axin2
and myc proto-oncogene protein (c-Myc) (9–11).
Baicalin (5,6-dihydroxyflavone-7-O-D-glucuronic) is
an active ingredient extracted from dried roots of Scutellaria
baicalensis Georgi. Baicalin has been used for the clinical
treatment of certain acute and chronic inflammatory diseases.
Baicalin attenuates oxidative damage and apoptosis of myocardial
cells and protects against myocardial ischemia reperfusion injury
(12,13). Previous in vivo studies
suggested that cardioprotection mediated by baicalin was associated
with the modulation of the mitogen-activated protein kinase 1
pathway (14,15). Baicalin may decrease the activity
of the sympathetic nervous system by inhibiting the P2X3 receptor
in rat superior cervical ganglia to protect the myocardium from the
ischemic injury (16). Another
study demonstrated that baicalin can protect cardiomyocytes from
endoplasmic reticulum stress-induced apoptosis via the
DNA-damage-inducible transcript/CCAAT/enhancer-binding
protein/endothelia nitric oxide synthase/nitric oxide pathway
(17). However, the Wnt/β-catenin
signaling pathway-mediated molecular mechanism underlying the
cardioprotective effect of baicalin remains to be elucidated.
Since the concentration of reactive oxygen species
(ROS) is elevated in cardiac injury, including ischemic heart
disease (IHD), H2O2-treated cardiomyocyte
cell lines have commonly been used as an in vitro model of
cardiac injury to study the cardioprotective effects of drugs
against oxidative damage (18–23).
Therefore, in the present study, H2O2-treated
H9c2 cells were used to evaluate the effects of baicalin on cardiac
injury and on the activation of Wnt/β-catenin signal pathway.
Materials and methods
Cell culture, transfection and
treatment
Cells were cultured in Dulbecco's modified Eagle's
medium (DMEM; Wisent Inc., St Bruno, QC, Canada) supplemented with
10% fetal bovine serum (Biological Industries, Kibbutz Beit Haemek,
Israel), 100 U/ml penicillin and 100 mg/ml streptomycin (both,
purchased from Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) and cultured in a 37°C humidified incubator supplemented with
5% CO2. For the transfection assay, cells were plated at
1.0×105 cells/well and were transfected with small
interfering (si)RNA of β-catenin (Invitrogen; Thermo Fisher
Scientific, Inc.) or Stealth RNAi® negative control
duplexes (NC) (cat. no. 12935300, Invitrogen; Thermo Fisher
Scientific, Inc.) for 48 h using Lipofectamine 2000 transfection
reagent (Invitrogen; Thermo Fisher Scientific, Inc.). The following
β-catenin siRNA oligo sequences were used:
5′-UGUAGCAGGAGAUUAUGCAGCGUGG-3′ (forward) and
5′-CCACGCUGCAUAAUCUCCUGCUACA-3′ (reverse). H9c2 cells were
stimulated with 200 µM H2O2 for 2 h prior to
an MTT assay and with 100 µM H2O2 for 30 min
prior to the western blotting assay, to establish the myocardial
oxidative damage model. 293T cells were co-transfected with 1
ng/well V5-tagged Wnt3a (Wnt3a), 30 mM LiCl, 0.5 µM BIO or 40
ng/well β-cateninΔN (β-catenin truncation mutant with N-terminal
region deletion), as well as TOPFlash for 48 h before detecting RLU
(relative light units) values in each experimental group. Negative
control group was transfected with TOPFlash only and positive
control group was transfected with wnt3a or activator of
Wnt/β-catenin signaling pathway (LiCl, BIO or β-cateninΔN), as well
as TOPFlash but not treated with baicalin. Wnt3a was provided by X.
He and C. Niehrs (German Cancer Research Center) (24–27).
TOPFlash reporters were provided by RT. Moon (University of
Washington, School of Medicine) (28). LiCl, BIO and NaCl were purchased
from Sigma-Aldrich; Merck KGaA, Darmstadt, Germany. Baicalin was
purchased from Chengdu Must Bio-Technology Co., Ltd.; Chengdu,
Sichuan, China.
Reporter gene assay
A TOPFlash/Renilla reporter gene assay was
performed as previously described (24,29).
Cells were seeded in 96-well plates at 4×103 cells/well
and transfected with 40 ng TOPFlash plasmids for Wnt and β-catenin
and 10 ng Renilla luciferase control reporter vector. A
total of 48 h following transfection, cells were lysed with cell
lysis buffer (50 mM Tris-HCl pH 7.4, 150 mM NaCl, 1%
nadeoxycholale, 0.5 M EDTA, 1% Nonidet 40, 5 mM DTT, 10 µg
aprotinin, 10 µg leupeptin, 10 mM PMSF) and screened for luciferase
activity using the Dual-Luciferase Reporter Assay system according
to the manufacturer's protocol (Promega Corporation, Madison, WI,
USA).
Cell isolation and protein
extraction
For total cellular protein extraction, cells were
lysed with a lysis buffer (50 mM Tris-HCl pH 7.4, 150 mM NaCl, 1%
nadeoxycholale, 0.5 M EDTA, 1% Nonidet 40, 5 mM DTT, 10 µg
aprotinin, 10 µg leupeptin, 10 mM PMSF) on ice for 15 min and
centrifuged at 4,000 × g for 15 min at 4°C, prior to the collection
of the supernatant. Nuclear extracts were prepared as follows:
Cells were resuspended in 400 µl hypotonic buffer (20 mM Tris-HCl
pH 7.4, 10 mM NaCl, 3 mM MgCl2; 10% Nonidet; 5 M DTT; 10
µg Aprotinin; 10 µg Leupeptin; 10 mM PMSF) and incubated on ice for
10 min, then centrifuged at 1,000 × g for 5 min at 4°C. Following
separation, the pellet was re-suspended in the lysis buffer on ice
for 20 min. Following centrifugation at 4,000 × g for 10 min at
4°C, the supernatant (nuclear extract) was collected.
Western blotting
Concentrations of proteins were quantified using the
bicinchoninic acid method. Subsequently, 30 µg proteins per lane
were subjected in 10% sodium dodecyl polyacrylamide gel (Sangon
Biotech Co., Ltd., Shanghai, China) using 1× SDS-PAGE running
buffer (cat. no. P0014; Beyotime Institute of Biotechnology,
Haimen, China), and then transferred onto a polyvinylidene fluoride
membrane (EMD Millipore, Billerica, MA, USA) using 1× western
transfer buffer (cat. no. P0021A; Beyotime Institute of
Biotechnology). Following blocking with 5% skimmed dry milk in TBS
(20 mM Tris-HCl pH 7.4, 150 mM NaCl) with 0.1% Tween 20 (TBST) for
1 h at room temperature, membranes were incubated with the
following polyclonal primary antibodies: Rabbit anti-Axin-2
(1:5,000; cat. no. ab32197; Abcam, Cambridge, UK), mouse anti-c-Myc
(1:5,000; cat. no. ab32, Abcam), rabbit anti-β-catenin (1:1,000;
cat. no. 8480; Cell Signaling Technology, Inc., Danvers, MA, USA),
rabbit anti-apoptosis regulator Bcl-2 (Bcl-2) (1:1,000; cat. no.
2870; Cell Signaling Technology, Inc.), rabbit anti-apoptosis
regulator BAX (Bax) (1:1,000; cat. no. 2772; Cell Signaling
Technology, Inc.), rabbit anti-cleaved caspase-3 (1:1,000; cat. no.
9664; Cell Signaling Technology, Inc.) and mouse anti-GAPDH
(1:1,000; cat. no. 60004-1-Ig; ProteinTech Group, Inc., Chicago,
IL, USA) at 4°C overnight. The membranes were subsequently washed
with TBST and incubated with horseradish peroxidase-conjugated goat
anti-rabbit IgG secondary antibody (1:25,000; cat. no. 31460;
Thermo Fisher Scientific, Inc.) or rabbit anti-mouse IgG secondary
antibody (1:25,000; cat. no. 27025; Thermo Fisher Scientific Inc.)
at room temperature for 1 h. Following washing by TBST, the
membranes were placed in enhanced chemifluorescence solution (EMD
Millipore) for 1 min. Immunoreactive bands were visualized by an
Image Quant LAS 500 Imager (GE Healthcare Life Sciences, Shanghai,
China), and the densitometry of bands were calculated by Quantity
One software (version 4.62; Bio-Rad laboratories Inc., Hercules,
CA, USA).
MTT assay
H9c2 cells were seeded in 96-well plates at a
density of 4×103 cells/well. MTT (0.5 mg/ml; Beyotime
Institute of Biotechnology) was added and cells were incubated for
4 h at 37°C prior to the addition of dimethyl sulfoxide. The
absorbance was measured at a wavelength of 490 nm according to the
manufacturer's protocol (SpectraMax® M2/M2e Multiskan;
Molecular Devices LLC, Sunnyvale, CA, USA). All experiments were
performed in quintuplicate.
Statistical analysis
All data are expressed as the mean ± standard
deviation. All statistical analyses were performed using SPSS
software (version 20.0; IBM Corp., Armonk, NY, USA). Statistical
significance was concluded by one-way analysis of variance,
followed by the Fisher post hoc test for multiple comparisons and
unpaired t-test for between two groups comparisons. All experiments
were replicated at least three times. P<0.05 was considered to
indicate a statistically significant difference.
Results
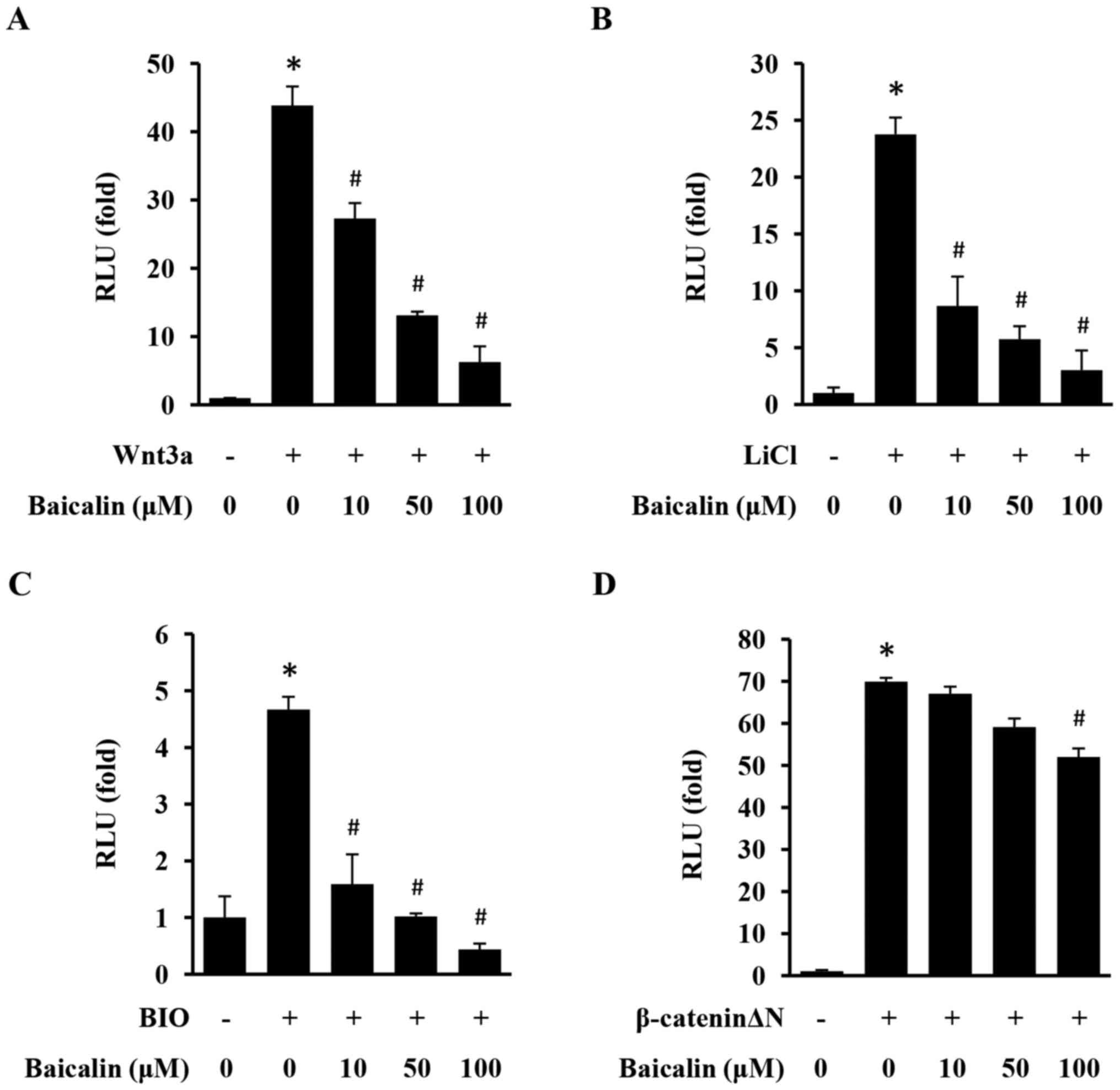
Baicalin inhibits the activation of
the Wnt/β-catenin pathway in 293 cells
The TOPFlash/Renilla reporter gene assay was
used to evaluate the effect of baicalin on the activation of the
Wnt/β-catenin pathway in 293 cells. As presented in Fig. 1, Wnt/β-catenin signaling was
significantly activated upon stimulation with Wnt3a, LiCl, BIO and
β-cateninΔN. However, baicalin induced a specific and significant
inhibitory effect on the activation of TOPFlash with in a
dose-dependent manner, demonstrating that baicalin efficiently
inhibits the Wnt/β-catenin signaling pathway.
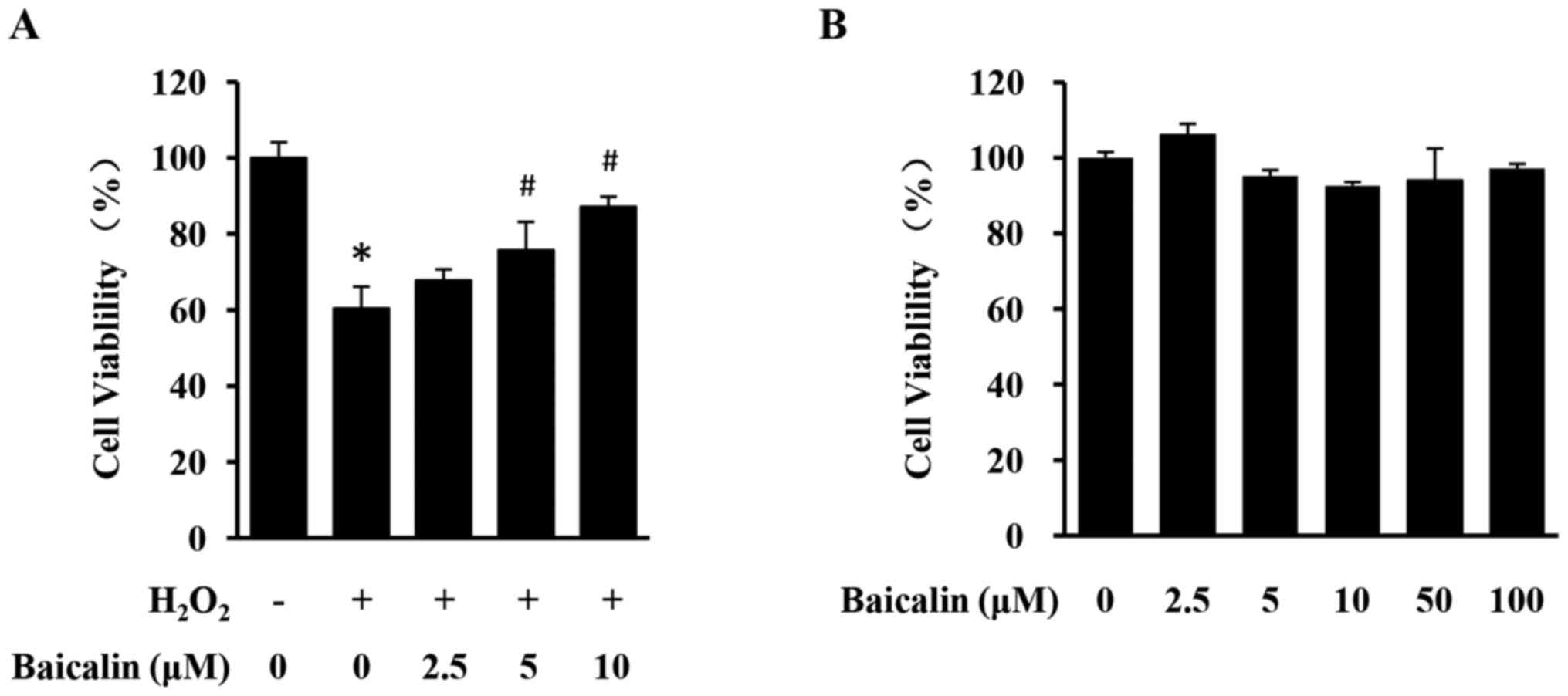
Baicalin protects against H2O2-induced
loss of H9c2 cell viability
To evaluate the cardioprotective effects of baicalin
against H2O2-induced cardiotoxicity,
H2O2-stimulated H9c2 cells were treated with
different concentrations of baicalin and cell viability was
assessed using an MTT assay. Stimulation with
H2O2 led to a significant decrease in H9c2
cell viability, which was significantly reversed by the treatment
with baicalin in a dose-dependent manner (Fig. 2A). Treatment with baicalin alone
exhibited no effect on the viability of H9c2 cells even at
increased concentrations (Fig.
2B). Therefore, baicalin can protect against oxidative damage
of cardiomyocytes in vitro, without causing apparent
cytotoxicity.
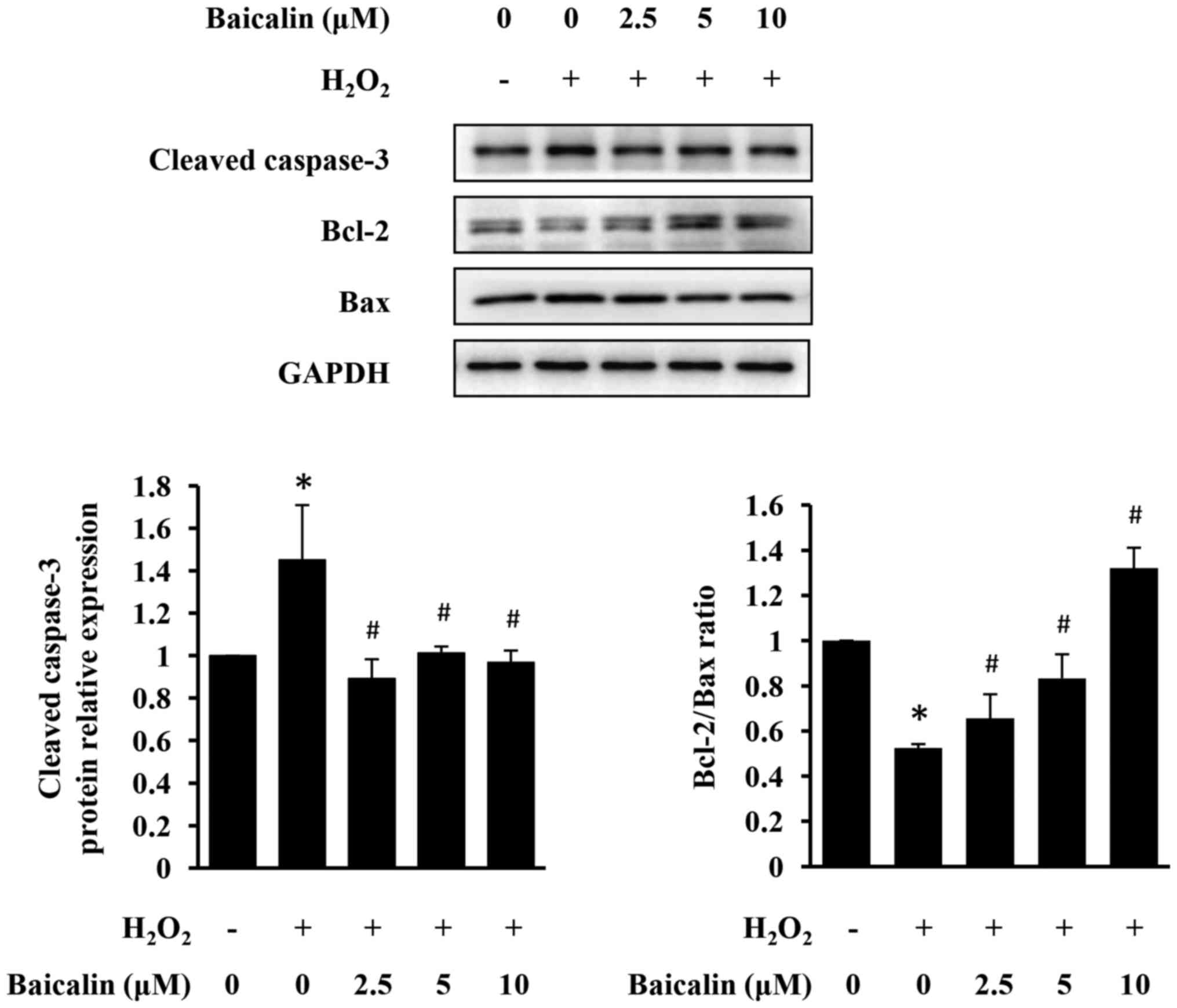
Baicalin prevents H2O2-induced
apoptosis of H9c2 cells
The effect of baicalin on
H2O2-induced apoptosis of H9c2 cells was
examined using western blot analysis. As presented in Fig. 3, H2O2
upregulated the expression of cleaved caspase-3 and increased the
pro-apoptotic Bax/Bcl-2 ratio in H9c2 cells, indicating
pro-apoptotic property of H2O2. However,
baicalin significantly inhibited H2O2-induced
cardiomyocyte apoptosis, in a dose-dependent manner (Fig. 3).
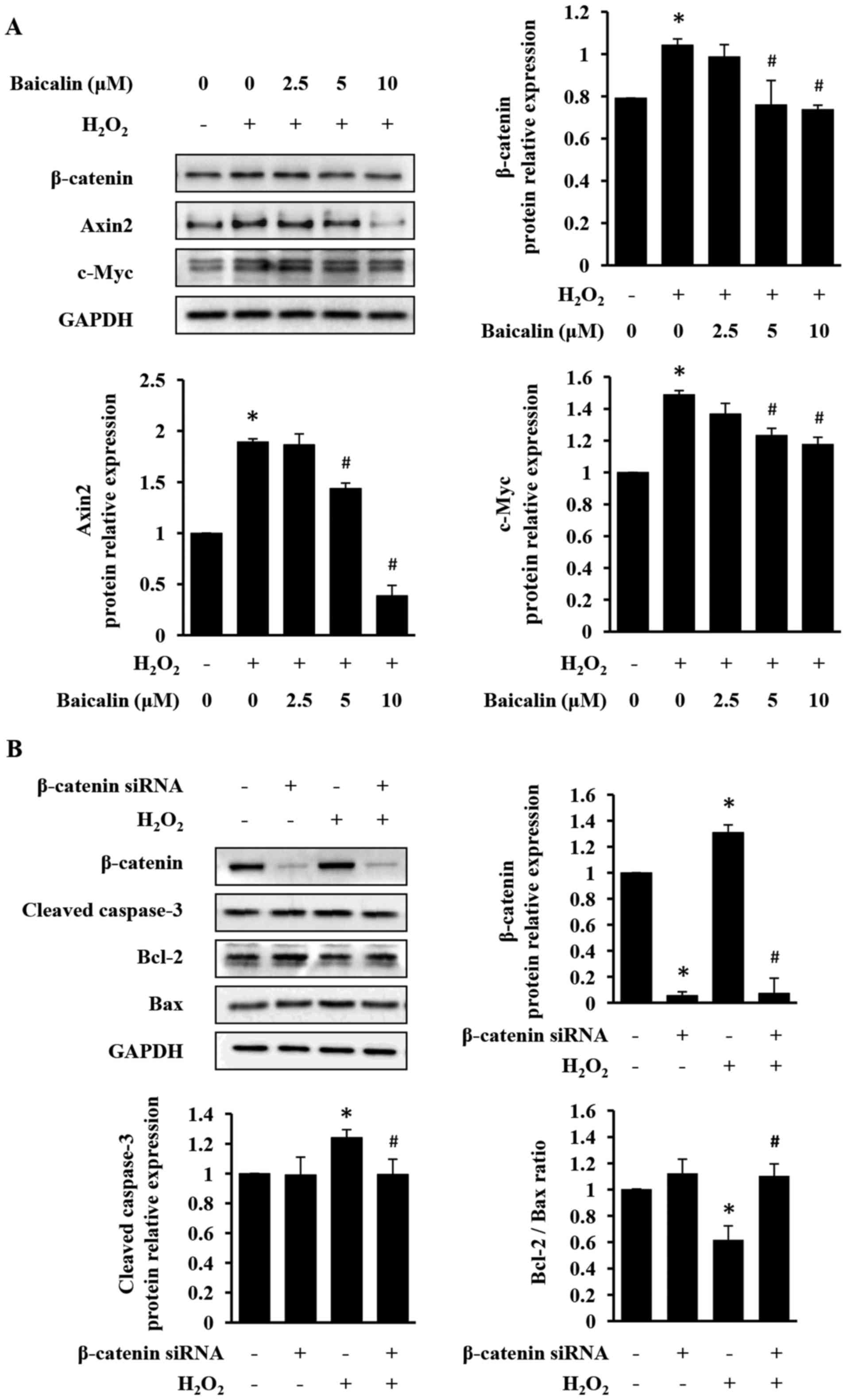
Baicalin inhibits apoptosis by
suppressing β-catenin expression in H9c2 cells
To investigate the mechanism underlying the
anti-apoptotic and cardioprotective activities of baicalin, its
effect on the expression levels of β-catenin and its downstream
targets axin-2 and c-Myc in H9c2 cells was determined. As presented
in Fig. 4A, baicalin treatment
significantly suppressed H2O2-induced
expression of β-catenin, axin-2 and c-Myc. Additionally,
siRNA-mediated downregulation of β-catenin expression prevented
apoptosis in H2O2-treated H9c2 cells
(Fig. 4B). LiCl, a GSK-3β
inhibitor, commonly used to stabilize β-catenin, was used to
further verify above-observed results (30–32).
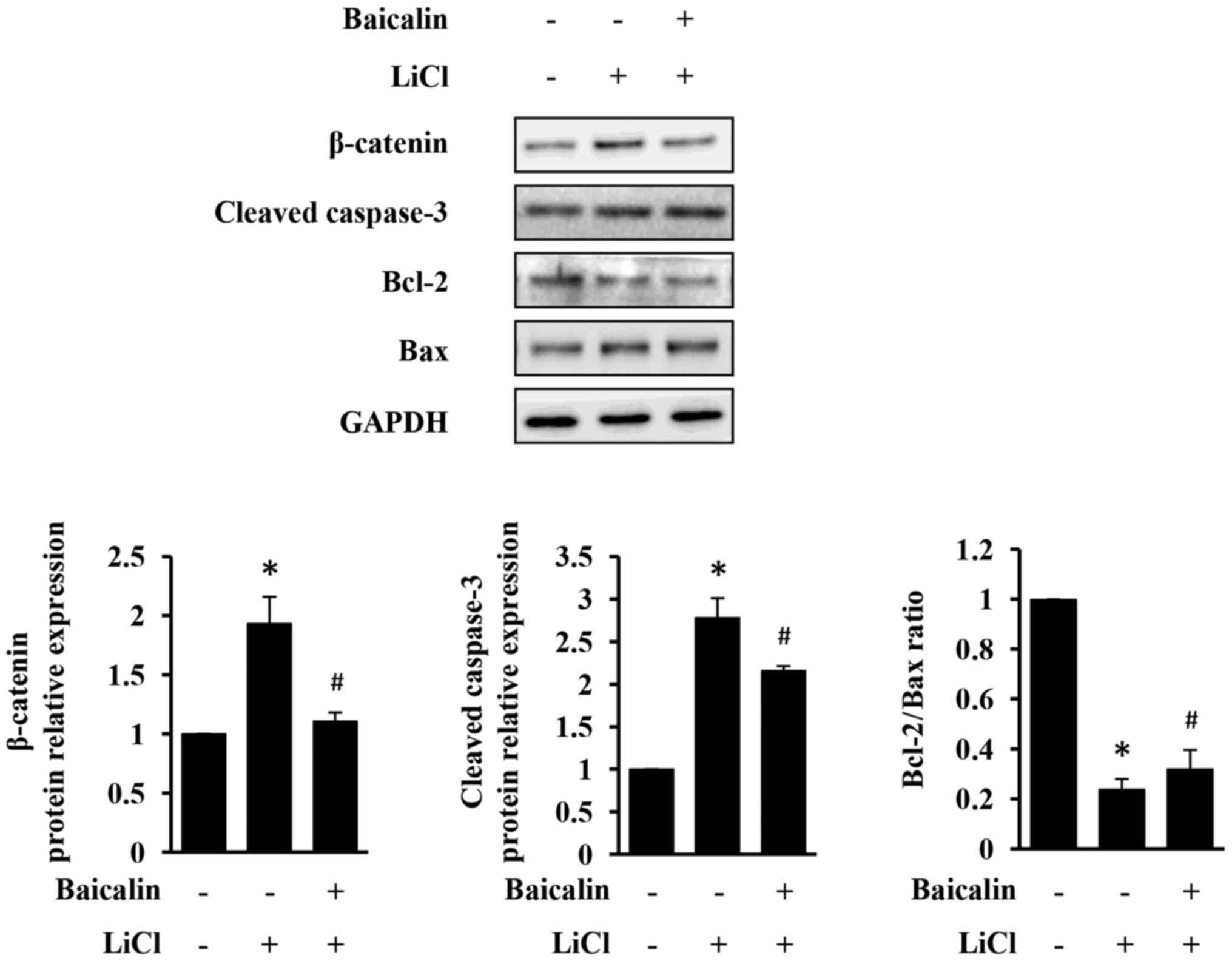
As presented in Fig. 5, LiCl
increased β-catenin expression, which in turn induced apoptosis of
H9c2 cells. However, LiCl-induced injury in H9c2 cells was
significantly neutralized by the treatment with baicalin (Fig. 5). Taken together, the results of
the present study indicate that baicalin exerts an anti-apoptotic
activity, likely through the suppression of the Wnt/β-catenin
pathway.
Discussion
The present study demonstrated that baicalin
attenuated H2O2-induced H9c2 cell injury
through inhibition of the Wnt/β-catenin signaling pathway. It was
demonstrated that β-catenin serves a crucial role in enhancing
H2O2-induced injury of H9c2 cells, which is
attenuated by pretreatment with baicalin. Previous studies have
demonstrated that ROS can modulate the Wnt/β-catenin signaling
pathway via regulation of the redox-dependent interaction between
Dvl and nucleoredoxin (NRX). NRX normally interacts with Dvl and
inhibits activation of the Wnt/β-catenin pathway. However, under
oxidative stress, NRX dissociates from Dvl and enables Dvl to
activate Wnt/β-catenin signaling (33). An association between Dvl and NRX
supports the results of the present study, and suggests that ROS
can promote the activation of Wnt/β-catenin signaling, suggesting
that baicalin can potentially regulate the expression of
Dvl/NRX.
The present study indicates that the suppression of
Wnt/β-catenin signaling pathway can be a potential strategy for the
treatment of IHD. It has been previously demonstrated that
insulin-like growth factor binding protein 4 is a robust inhibitor
of Wnt/β-catenin signaling, which can effectively protect the heart
from the ischemic injury (8,34),
which further supports the results of the present study. Baicalin
is commonly used in the clinical treatment of various diseases.
Whether baicalin can be applied for the treatment of IHD remains to
be elucidated.
In conclusion, the present study is, to the best of
the authors' knowledge, the first one to demonstrate the role of
baicalin in the modulation of Wnt/β-catenin signaling pathway.
Baicalin suppresses the activation of Wnt/β-catenin signaling
pathway via regulation of β-catenin, leading to the protection
against H2O2-induced apoptosis of H9c2 cells.
The present study provides novel insights and theoretical basis for
the potential use of baicalin in the treatment of IHD.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81302884) and the
Developmental Fund of Chen Keji Integrative Medicine (grants no.
CKJ 2016005, 2016003 and 2016002).
Glossary
Abbreviations
Abbreviations:
|
APC
|
adenomatous polyposis coli
|
|
GSK-3β
|
glycogensynthase kinase-3β
|
|
LRP5/6
|
low-density lipoprotein
receptor-related protein 5/6
|
|
Dvl
|
Dishevelled
|
|
NRX
|
nucleoredoxin
|
|
ROS
|
reactive oxygen species
|
|
IHD
|
ischemic heart disease
|
References
|
1
|
Dohn TE and Waxman JS: Distinct phases of
Wnt/β-catenin signaling direct cardiomyocyte formation in
zebrafish. Dev Biol. 361:364–376. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gessert S and Kühl M: The multiple phases
and faces of wnt signaling during cardiac differentiation and
development. Circ Res. 107:186–199. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gibb N, Lavery DL and Hoppler S: Sfrp1
promotes cardiomyocyte differentiation in Xenopus via
negative-feedback regulation of Wnt signalling. Development.
140:1537–1549. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yamashita JK, Takano M, Hiraoka-Kanie M,
Shimazu C, Peishi Y, Yanagi K, Nakano A, Inoue E, Kita F and
Nishikawa S: Prospective identification of cardiac progenitors by a
novel single cell-based cardiomyocyte induction. FASEB J.
19:1534–1536. 2005.PubMed/NCBI
|
|
5
|
Barandon L, Couffinhal T, Ezan J, Dufourcq
P, Costet P, Alzieu P, Leroux L, Moreau C, Dare D and Duplàa C:
Reduction of infarct size and prevention of cardiac rupture in
transgenic mice overexpressing FrzA. Circulation. 108:2282–2289.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mirotsou M, Zhang Z, Deb A, Zhang L,
Gnecchi M, Noiseux N, Mu H, Pachori A and Dzau V: Secreted frizzled
related protein 2 (Sfrp2) is the key Akt-mesenchymal stem
cell-released paracrine factor mediating myocardial survival and
repair. Proc Natl Acad Sci USA. 104:pp. 1643–1648. 2007; View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang Z, Deb A, Zhang Z, Pachori A, He W,
Guo J, Pratt R and Dzau VJ: Secreted frizzled related protein 2
protects cells from apoptosis by blocking the effect of canonical
Wnt3a. J Mol Cell Cardiol. 46:370–377. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhu W, Shiojima I, Ito Y, Li Z, Ikeda H,
Yoshida M, Naito AT, Nishi J, Ueno H, Umezawa A, et al: IGFBP-4 is
an inhibitor of canonical Wnt signalling required for
cardiogenesis. Nature. 454:345–349. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Funato Y and Miki H: Redox regulation of
Wnt signalling via nucleoredoxin. Free Radic Res. 44:379–388. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kafka A, Bašić-Kinda S and Pećina-Šlaus N:
The cellular story of dishevelleds. Croat Med J. 55:459–467. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
MacDonald BT, Tamai K and He X:
Wnt/beta-catenin signaling: Components, mechanisms, and diseases.
Dev Cell. 17:9–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kong F, Luan Y, Zhang ZH, Cheng GH, Qi TG
and Sun C: Baicalin protects the myocardium from
reperfusion-induced damage in isolated rat hearts via the
antioxidant and paracrine effect. Exp Ther Med. 7:254–259. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu M, Chen X, Gu Y, Peng T, Yang D, Chang
RC, So KF, Liu K and Shen J: Baicalin can scavenge peroxynitrite
and ameliorate endogenous peroxynitrite-mediated neurotoxicity in
cerebral ischemia-reperfusion injury. J Ethnopharmacol.
150:116–124. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu X, Gu J, Fan Y, Shi H and Jiang M:
Baicalin attenuates acute myocardial infarction of rats via
mediating the mitogen-activated protein kinase pathway. Biol Pharm
Bull. 36:988–994. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun SJ, Wu XP, Song HL and Li GQ: Baicalin
ameliorates isoproterenol-induced acute myocardial infarction
through iNOS, inflammation, oxidative stress and P38MAPK pathway in
rat. Int J Clin Exp Med. 8:22063–22072. 2015.PubMed/NCBI
|
|
16
|
Zhang J, Liu S, Xu B, Li G, Li G, Huang A,
Wu B, Peng L, Song M, Xie Q, et al: Study of baicalin on
sympathoexcitation induced by myocardial ischemia via P2X3 receptor
in superior cervical ganglia. Auton Neurosci. 189:8–15. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shen M, Wang L, Yang G, Gao L, Wang B, Guo
X, Zeng C, Xu Y, Shen L, Cheng K, et al: Baicalin protects the
cardiomyocytes from ER stress-induced apoptosis: Inhibition of CHOP
through induction of endothelial nitric oxide synthase. PLoS One.
9:e883892014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Berg K, Jynge P, Bjerve K, Skarra S, Basu
S and Wiseth R: Oxidative stress and inflammatory response during
and following coronary interventions for acute myocardial
infarction. Free Radic Res. 39:629–636. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sun Y: Myocardial repair/remodelling
following infarction: Roles of local factors. Cardiovasc Res.
81:482–490. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Diestel A, Drescher C, Miera O, Berger F
and Schmitt KR: Hypothermia protects H9c2 cardiomyocytes from H2O2
induced apoptosis. Cryobiology. 62:53–61. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Eguchi M, Liu Y, Shin EJ and Sweeney G:
Leptin protects H9c2 rat cardiomyocytes from H2O2-induced
apoptosis. FEBS J. 275:3136–3144. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Law CH, Li JM, Chou HC, Chen YH and Chan
HL: Hyaluronic acid-dependent protection in H9C2 cardiomyocytes: A
cell model of heart ischemia-reperfusion injury and treatment.
Toxicology. 303:54–71. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Park ES, Kang JC, Jang YC, Park JS, Jang
SY, Kim DE, Kim B and Shin HS: Cardioprotective effects of
rhamnetin in H9c2 cardiomyoblast cells under H2 O2 -induced
apoptosis. J Ethnopharmacol. 153:552–560. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen J, Yan H, Ren DN, Yin Y, Li Z, He Q,
Wo D, Ho MS, Chen Y, Liu Z, et al: LRP6 dimerization through its
LDLR domain is required for robust canonical Wnt pathway
activation. Cell Signal. 26:1068–1074. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mao B, Wu W, Davidson G, Marhold J, Li M,
Mechler BM, Delius H, Hoppe D, Stannek P, Walter C, et al: Kremen
proteins are Dickkopf receptors that regulate Wnt/beta-catenin
signalling. Nature. 417:664–667. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mao B, Wu W, Li Y, Hoppe D, Stannek P,
Glinka A and Niehrs C: LDL-receptor-related protein 6 is a receptor
for Dickkopf proteins. Nature. 411:321–325. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tamai K, Semenov M, Kato Y, Spokony R, Liu
C, Katsuyama Y, Hess F, Saint-Jeannet JP and He X:
LDL-receptor-related proteins in Wnt signal transduction. Nature.
407:530–535. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Veeman MT, Slusarski DC, Kaykas A, Louie
SH and Moon RT: Zebrafish prickle, a modulator of noncanonical
Wnt/Fz signaling, regulates gastrulation movements. Curr Biol.
13:680–685. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ren DN, Chen J, Li Z, Yan H, Yin Y, Wo D,
Zhang J, Ao L, Chen B, Ito TK, et al: LRP5/6 directly bind to
Frizzled and prevent Frizzled-regulated tumour metastasis. Nat
Commun. 6:69062015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Caraci F, Gili E, Calafiore M, Failla M,
La Rosa C, Crimi N, Sortino MA, Nicoletti F, Copani A and Vancheri
C: TGF-beta1 targets the GSK-3beta/beta-catenin pathway via ERK
activation in the transition of human lung fibroblasts into
myofibroblasts. Pharmacol Res. 57:274–282. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang Y, Lam JB, Lam KS, Liu J, Lam MC, Hoo
RL, Wu D, Cooper GJ and Xu A: Adiponectin modulates the glycogen
synthase kinase-3beta/beta-catenin signaling pathway and attenuates
mammary tumorigenesis of MDA-MB-231 cells in nude mice. Cancer Res.
66:11462–11470. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang Y, Qiu WJ, Liu DX, Neo SY, He X and
Lin SC: Differential molecular assemblies underlie the dual
function of Axin in modulating the WNT and JNK pathways. J Biol
Chem. 276:32152–32159. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Funato Y, Michiue T, Asashima M and Miki
H: The thioredoxin-related redox-regulating protein nucleoredoxin
inhibits Wnt-beta-catenin signalling through dishevelled. Nat Cell
Biol. 8:501–508. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wo D, Peng J, Ren DN, Qiu L, Chen J, Zhu
Y, Yan Y, Yan H, Wu J, Ma E, et al: Opposing roles of Wnt
inhibitors IGFBP-4 and Dkk1 in cardiac ischemia by differential
targeting of LRP5/6 and β-catenin. Circulation. 134:1991–2007.
2016. View Article : Google Scholar : PubMed/NCBI
|