Introduction
Osteoarthritis (OA) is the most common
musculoskeletal disease worldwide, which primarily affects the
knees, hands and feet, leading to a reduction in patient quality of
life. The incidence of symptomatic knee OA is highest amongst
individuals aged between 55 and 64 years, and the prevalence
increases with age. It has been reported that ~9.29% of the US
population is diagnosed with symptomatic knee OA by the age of 60
(1). The development of OA is
associated with various risk factors including genetic factors, an
epigenetic predisposition, aging, being female, injury, mechanical
stress, diet and lifestyle (2).
The natural process of aging is associated with OA and the key
chondrocyte senescence markers, including
senescent-associated-β-galactosidase, senescence-associated
heterochromatin foci, p53, p16, caveolin 1 and telomere length
(3). The progression of OA is
characterized by chondrocyte deterioration, degradation of the
extracellular matrix, subchondral bone remodeling, formation of
ectopic bone and synovitis. Under normal conditions, a balance
between matrix proteins and matrix-degrading enzymes is maintained
in chondrocytes (4). However, a
disruption in the normal resting state of chondrocytes may lead to
matrix remodeling, inappropriate hypertrophic maturation and
cartilage calcification. A previous study demonstrated that
interleukin (IL)-1 stimulates the synthesis and activity of matrix
metalloproteinases (MMPs), as well as other enzymes involved in
OA-induced cartilage destruction (5). Hypertrophic chondrocytes produce
catabolic proteins, including MMP13, Runt-related transcription
factor (Runx) 2 and type X collagen (Col X) (6). High levels of vascular endothelial
growth factor (VEGF) are also observed in patients with OA, which
implies that angiogenesis may contribute to the pathogenesis of OA
(7). Previous studies have
investigated novel drugs to repair cartilage lesions, such as
β-defensin-4, which is thought to be a protected marker in the
repair process of OA (8,9). In clinical practice, numerous drugs,
including nonsteroidal anti-inflammatory drugs, cyclooxygenase-2
(COX-2) inhibitors (10),
glucosamine, steroids and hyaluronan (11) have been used to delay OA in
patients. However, these drugs do not reverse the effects of OA.
Therefore, patients with OA must be treated with arthroplasty,
which is associated with a large economic burden. Therefore, it is
necessary to identify a novel agent for the treatment of OA.
Isoimperatorin [Iso; 4-(3-methyl-2-butenyl)-7H-furo
(3,2-g) benzopyran-7-one] is a linear furanocoumarin and one of the
main components of Prangos ferulacea (12). Iso is a potent medicinal herbal
compound that is also isolated from the roots of Angelicae
dahurica (13). A.
dahurica suppresses inducible nitric oxide synthase (iNOS) and
nitric oxide production (14). In
addition, as an extract from A. dahuricae, Iso has been used
as a vasodilatory, antiallergic and anticancer drug in clinical
practice (15–17). Notably, Iso also serves an active
anti-inflammatory role, and can inhibit COX-2 and prostaglandin E2
production in mouse models (18,19).
A previous study revealed that Iso can reduce the production of
reactive oxygen species via tumor necrosis factor-α-induced AKT,
extracellular signal-regulated kinase and protein kinase C
signaling in human cells (16,20).
However, whether Iso possesses chondroprotective properties remains
to be elucidated. The present study aimed to establish the effects
of Iso in delaying chondrocyte deterioration in OA in vivo
and in vitro, and to determine the potential underlying
mechanisms for novel treatments of OA. The results of the present
study demonstrated that Iso delayed the progression of OA in
vivo and in vitro. In addition, autophagy activation and
mammalian target or rapamycin complex 1 (mTORC1) inhibition were
responsible for the effects of Iso administration on OA.
Materials and methods
Animal model and treatments
C57/BL6 mice (age, 8 weeks; male; weight, 18–25 g)
were purchased from the Laboratory Animal Centre of the Southern
Medical University (Guangzhou, China). The mice had free access to
standard chow and water; the mice were maintained at an optimal
temperature (24–26°C) and at 70% humidity under a 12-h light/dark
cycle. The mice were anesthetized with 8 µl/g (anesthetic
volume/mice weight) of 1% Amobarbital sodium prior to surgery. The
OA model was established by selectively transecting the medial
meniscus (destabilization of the medial meniscus; DMM). Mice (n=15)
were randomly divided into the following three groups: Sham, OA and
OA + Iso (n=5/group). In the two surgical groups (OA and OA + Iso),
the right medial collateral ligaments were dissected, which was
followed by DMM to induce the OA model. In the sham-operated group,
only the skin of the right knee joint was resected. Following the
induction of OA, all mice in the OA + Iso group were treated with
Iso (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). Iso was
delivered intragastrically via oral administration (500 mg/g/day)
using syringe feeding. At 4 weeks post-surgery, mice from each
group were sacrificed and the right knee joints were collected.
Animal experiments were approved by the Animal Experimental Ethics
Committee of Southern Medical University (Guangdong, China).
Systematic assessment of OA
Histological staining and OA scoring
The right knee joints were isolated and all tissues
were fixed with 4% paraformaldehyde in 0.1 M phosphate-buffered
saline overnight at 4°C. They were then decalcified with 14.5% EDTA
(pH 7.4) at room temperature for ~20 days. Following
decalcification, the specimens were routinely embedded in paraffin,
cut into 5-µm sections along the sagittal plane and stained with
Safranin O/Fast Green and toluidine blue at room temperature for 15
min. Under an Olympus light microscope (magnification, ×200;
Olympus Corporation, Tokyo, Japan) three fields of tibial articular
cartilage were randomly selected and the number of chondrocytes was
calculated to obtain a mean value. OA was evaluated using the
Osteoarthritis Research Society International (OARSI)
semi-quantitative scoring system, in order to verify that cartilage
pathology varied in severity by location (21). In this system, the scores are
defined as follows: 0, normal cartilage; 0.5, loss of proteoglycan
with an intact surface; 1, superficial fibrillation without loss of
cartilage; 2, vertical clefts and loss of surface lamina; 3,
vertical clefts/erosion to the calcified layer lesion in 1–25% of
the quadrant width; 4, lesion reaches the calcified cartilage in
25–50% of the quadrant width; 5, lesion reaches the calcified
cartilage in 50–75% of the quadrant width; 6, lesion reaches the
calcified cartilage in >75% of the quadrant width. Three
different areas from the tibial plateau were randomly selected to
calculate and evaluate the OARSI scores. The average thickness of
the articular cartilage (TAC) in the tibial plateau was measured
using Image-Pro Plus version 6.0 software (Media Cybernetics, Inc.,
Rockville, MD, USA).
Immunohistochemistry
For immunohistochemical analysis, endogenous
peroxidase in deparaffinized sections (3–5 µm) was blocked via
incubation with 3% hydrogen peroxide at room temperature for 10
min. The sections were then incubated with rabbit anti-MMP13
(1:150; cat. no. A1606; ABclonal Biotech Co., Ltd., Woburn, MA,
USA), anti-Runx2 (1:75; cat. no. A2851; ABclonal Biotech Co.,
Ltd.), anti-Col X (1:75; cat. no. ab58632; Abcam, Cambridge, UK)
and anti-VEGF (1:100; cat. no. A5708; ABclonal Biotech Co., Ltd.)
at 4°C overnight. Subsequently, sections were incubated with
anti-rabbit secondary antibody (1:100; cat. no. RM3002; Beijing Ray
Antibody Biotech, Beijing, China) at 37°C for ~1 h.
Immunohistochemistry was performed to detect MMP13, Runx2, Col X
and VEGF using a diaminobenzidine tetrahydrochloride kit (cat. no.
ZLI-9017; OriGene Technologies, Inc., Beijing, China) for 1 min
followed by counterstaining with hematoxylin for 15 sec at room
temperature. The percentage of positive cells in three random
fields among the total area was calculated to obtain a mean value
using an Olympus light microscope (light, magnification, ×200;
Olympus Corporation).
Isolation and culture of murine primary
chondrocytes
Chondrocytes were isolated from the knees of
4-day-old C57/BL6 male mice (Laboratory Animal Centre of the
Southern Medical University) via digestion at 37°C with 0.25%
trypsin-EDTA for 30 min and then 0.25% collagenase II for 6 h. The
cells were suspended following centrifugation at 1,000 × g for 5
min at room temperature in Dulbecco's modified Eagle's medium-F12
(cat. no. 11320-033; Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with 5% fetal bovine serum (Thermo
Fisher Scientific, Inc.), 100 U/ml penicillin and 100 µg/ml
streptomycin (Life Technologies; Thermo Fisher Scientific, Inc.),
and then cultured at 37°C in an atmosphere containing 5%
CO2 and 95% humidity.
Cell viability assay
The cytotoxicity of Iso on chondrocytes was assessed
using a Cell Counting kit-8 (CCK8) assay (cat. no. KGA317; Nanjing
KeyGen Biotech Co., Ltd., Nanjing, China). The cells were seeded
onto 96-well culture plates at 1×105 cells/well and
allowed to adhere overnight at 37°C. Following incubation, cells
were treated with 1–100 mM Iso at 37°C for 24 h, the culture medium
was then removed and 10 µg/100 µl CCK8 was added to each well for
incubation at 37°C for 4 h. The optical density was measured at 450
nm using a microplate reader.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
The primary chondrocytes were seeded onto 96-well
culture plates at 1×105 cells/well and allowed to adhere
overnight at 37°C. Following 48 h, the cells were serum starved
overnight then treated with 1–50 µM Iso for 1 h, prior to
stimulation with 10 ng/ml IL-1 (cat. no. 211-11B; Peprotech, Inc.,
Rocky Hill, NJ, USA) at room temperature for 24 h. Following 24 h,
cells were collected for RT-qPCR and western blotting. RNA was
extracted from monolayer cultures of chondrocytes using TRIzol
reagent (Thermo Fisher Scientific, Inc.). Total RNA was isolated
with methylene trichloride and isopropanol, then reversed
transcribed into cDNA using the PrimeScript™ RT Reagent kit (cat.
no. RR037A; Takara Bio, Inc., Otsu, Japan), according to the
manufacturer's protocol. RT-qPCR was performed in triplicate using
the ChamQ™ SYBR qPCR Master Mix (cat. no. Q311; Vazyme, Piscataway,
NJ, USA). The cycle parameters of RT were as follows: 10 min at
30°C, 50 min for 42°C, and 95°C at 5 min. In addition, the
following qPCR thermocycling conditions were used: 1 cycle at 95°C
for 10 min, followed by 40 cycles of 15 sec at 95°C, 1 min at 60°C
and 15 sec at 72°C. A melting curve was generated to verify
gene-specific amplification. The primers were designed using
Primer3-Blast online software (https://blast.ncbi.nlm.nih.gov/Blast.cgi). The primer
pairs used for the present study are presented in Table I. Relative gene expression levels
were calculated using the 2−ΔΔCq method (22). Target mRNA levels were normalized
to the reference gene GADPH (Table
I).
 | Table I.Primer sequences for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primer sequences for reverse
transcription-quantitative polymerase chain reaction.
| Gene ID | Gene | Forward
(5′-3′) | Reverse
(5′-3′) |
|---|
| NM_008607.2 | MMP13 |
CTTCTTCTTGTTGAGCTGGACTC |
CTGTGGAGGTCACTGTAGACT |
| NM_001145920.2 | Runx2 |
AAATTAACGCCAGTCGGAGC |
CCACTTCTCGGTCTGACGAC |
| NM_009925.4 | Col X |
TTCTGCTGCTAATGTTCTTGACC |
GGGATGAAGTATTGTGTCTTGGG |
| NM_001025250.3 | VEGF |
CTGCCGTCCGATTGAGACC |
CCCCTCCTTGTACCACTGTC |
| NM_001289726.1 | GAPDH |
AGGTCGGTGTGAACGGATTTG |
TGTAGACCATGTAGTTGAGGTCA |
Cartilage protein extraction and western blot
analysis
Western blot analyses were conducted on the protein
of lysates from cultured chondrocytes, which were treated as
described prior to RT-qPCR. The cells were treated with 1 M
Tris-HCl (pH 6.8) buffer containing 10% SDS (Sigma-Aldrich; Merck
KGaA) and 20% glycerol for 10 min at 97°C. The cell lysates were
centrifuged at 1,000 × g for 5 min at room temperature and the
supernatants were stored at −20°C. For electrophoresis, the
concentration of the supernatants was measured using a
bicinchoninic acid assay; 100 µg supernatants were separated by 10%
SDS-PAGE. The proteins were transferred onto nitrocellulose
membranes, blocked with 5% non-fat milk containing 0.02% Tween at
room temperature for ~1 h, and incubated overnight at 4°C with
anti-β-actin (cat. no. RM2001; 1:2,000; Beijing Ray Antibody
Biotech), anti-Beclin1 (cat. no. 3495; 1:2,000; Cell Signaling
Technology, Inc., Danvers, MA, USA), anti-p62 (cat. no. 5114;
1:2,000; Cell Signaling Technology, Inc.), anti-S6 kinase (S6K;
cat. no. 9202; 1:3,000; Cell Signaling Technology, Inc.),
anti-phosphorylated (p)-S6K (cat. no. 9204; 1:3,000; Cell Signaling
Technology, Inc.), anti-eukaryotic translation initiation factor
4E/binding protein 1 (4E/BP1; cat. no. 9452; 1:3,000; Cell
Signaling Technology, Inc.) and anti-P-4E/BP1 (cat. no. 2855;
1:3,000; Cell Signaling Technology, Inc.). The blots were then
probed with horseradish peroxidase-conjugated secondary antibodies
(cat. no. RM3002; 1:5,000; Beijing Ray Antibody Biotech) at room
temperature for 1 h. The antigen-antibody complexes were visualized
with an Enhanced Chemiluminescence Plus detection kit (PerkinElmer,
Inc., Waltham, MA, USA).
Statistical analysis
Data are presented as the mean ± standard deviation.
One-way analysis of variance followed by a post hoc Tukey test was
used to determine statistical differences. All experiments were
repeated at least three times, and representative experiments are
shown. Data analyses were performed using SPSS version 19.0
software (IBM Corp., Armonk, NY, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
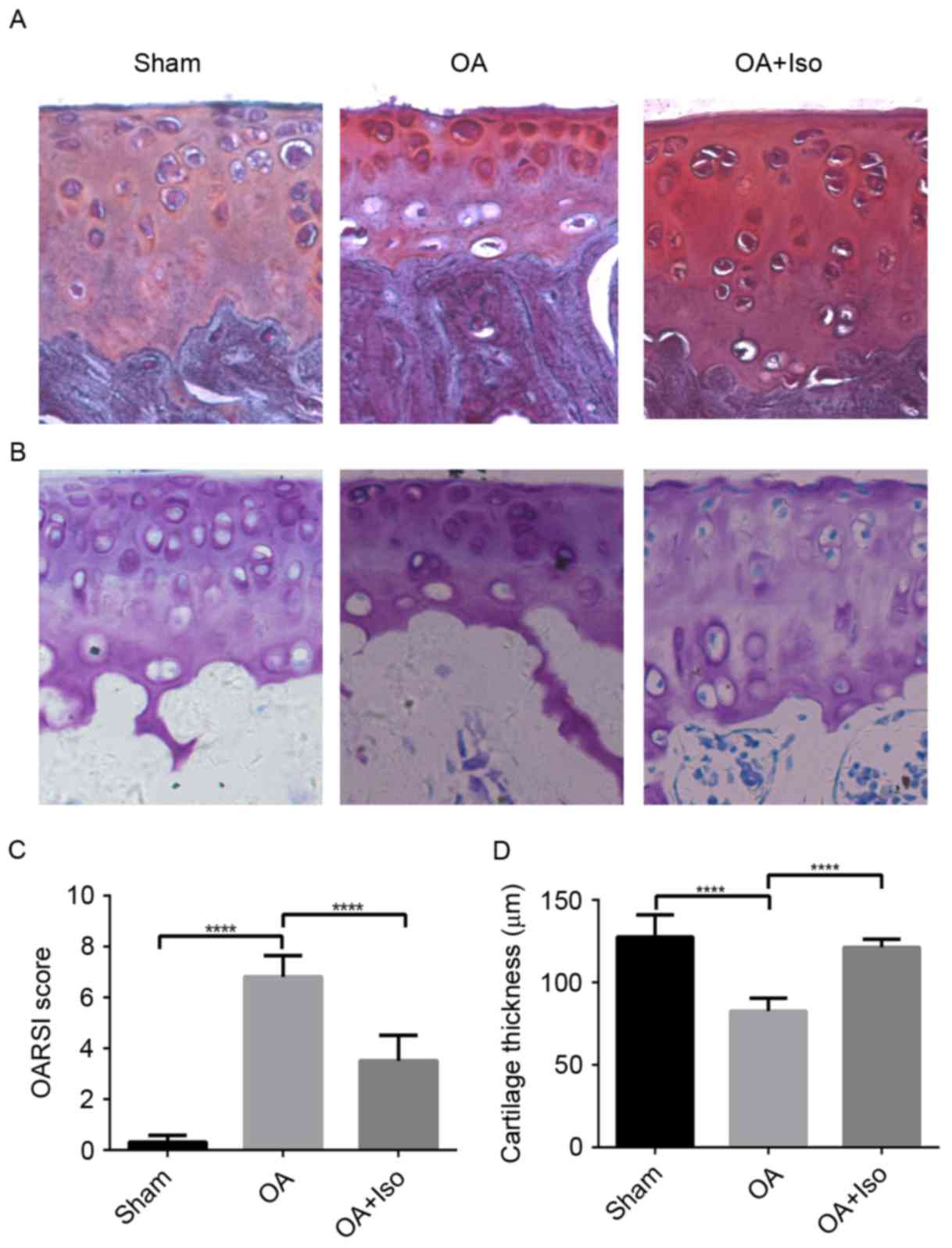
Iso treatment delays the progression
of experimental OA
To confirm the effects of Iso on delaying OA
progression, Safranin O/Fast Green staining was performed and the
OARSI scoring system was used to evaluate the severity of OA. The
results revealed that there were more fibrillations on the surface
of the articular cartilage in the OA group than in the OA + Iso
group. In addition, in the OA group, less chondrocytes were
observed than in the OA + Iso group (Fig. 1A). TAC was assessed by toluidine
blue staining (Fig. 1B). TAC was
reduced in the OA group when compared with the OA + Iso group. The
histological OARSI score in the tibial plateau was increased in the
OA group when compared with the Sham group; however, it was
significantly decreased in the OA + Iso group compared with in the
OA group (Sham, 0.30±0.27; OA, 6.80±0.84; OA + Iso, 3.50±1.0;
P<0.05; Fig. 1C; Table II). These results indicated that
Iso treatment ameliorated degradation of articular cartilage, as
verified in Fig. 1D. Cartilage
thickness was significantly decreased in the OA group compared with
in the Sham group; however, thickness was significantly increased
in the OA + Iso group compared with in the OA group (Sham,
127.62±13.36; OA, 82.53±7.90; OA + Iso, 121.16±4.97; P<0.05;
Fig. 1D; Table II).
 | Table II.OARSI scoring and TAC of the tibial
plateau in different groups. |
Table II.
OARSI scoring and TAC of the tibial
plateau in different groups.
| Group | OARSI score | TAC (µm) |
|---|
| Sham |
0.30±0.27a |
127.62±13.36a |
| OA | 6.80±0.84 | 82.53±7.90 |
| OA + Iso |
3.50±1.00a |
121.16±4.97a |
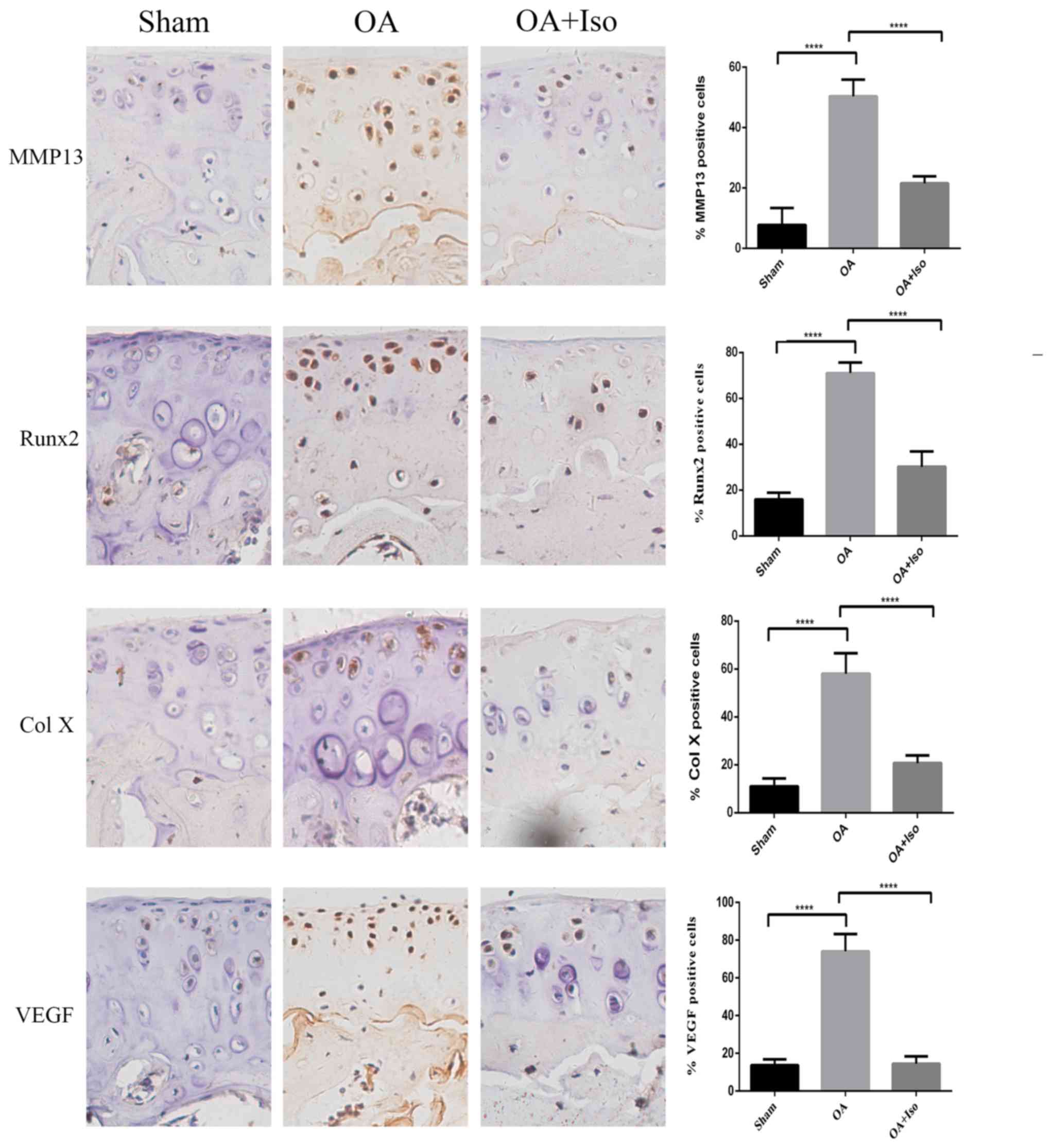
Iso reduces the expression of MMP13,
Runx2, Col X and VEGF in experimental OA
To investigate the mechanism underlying the
protective effects of Iso on cartilage, chondrocyte hypertrophy and
angiogenesis were evaluated. The expression levels of MMP13, Runx2,
Col X (all hypertrophic chondrocyte markers) and VEGF (an
angiogenesis marker) were detected by immunohistochemistry
(Fig. 2). The percentage of
MMP13-positive cells in the articular cartilage was significantly
higher in the OA group compared with in the Sham group; however, it
was lower in the OA + Iso group compared with in the OA group
(Sham, 7.76±5.66%; OA, 50.40±5.46%; OA + Iso, 21.60±2.25%;
P<0.05; Fig. 2). A similar
result was obtained for the percentage of Runx2-positive cells
(Sham, 15.88±2.92%; OA, 71.19±4.71%; OA + Iso, 30.15±6.66%;
P<0.05; Fig. 2) and Col
X-positive cells (Sham, 10.94±3.34%; OA, 61.65±6.47%; OA + Iso,
20.72±3.19%; P<0.05; Fig. 2),
as the percentage in the articular cartilage was significantly
higher in the OA group compared with in the Sham group; however, it
was significantly lower in the OA + Iso group compared with in the
OA group. These results indicated that Iso reduced hypertrophic
alterations in articular cartilage in the experimental OA model. In
addition, the percentage of VEGF-positive cells in the articular
cartilage was significantly higher in the OA group compared with in
the Sham group; however, it was significantly lower in the OA + Iso
group compared with in the OA group (Sham, 13.70±3.08%; OA,
74.82±9.18%; OA + Iso, 14.43±3.98%; P<0.05; Fig. 2). These results suggested that Iso
treatment may delay the degradation of articular cartilage, in
part, through the inhibition of angiogenesis.
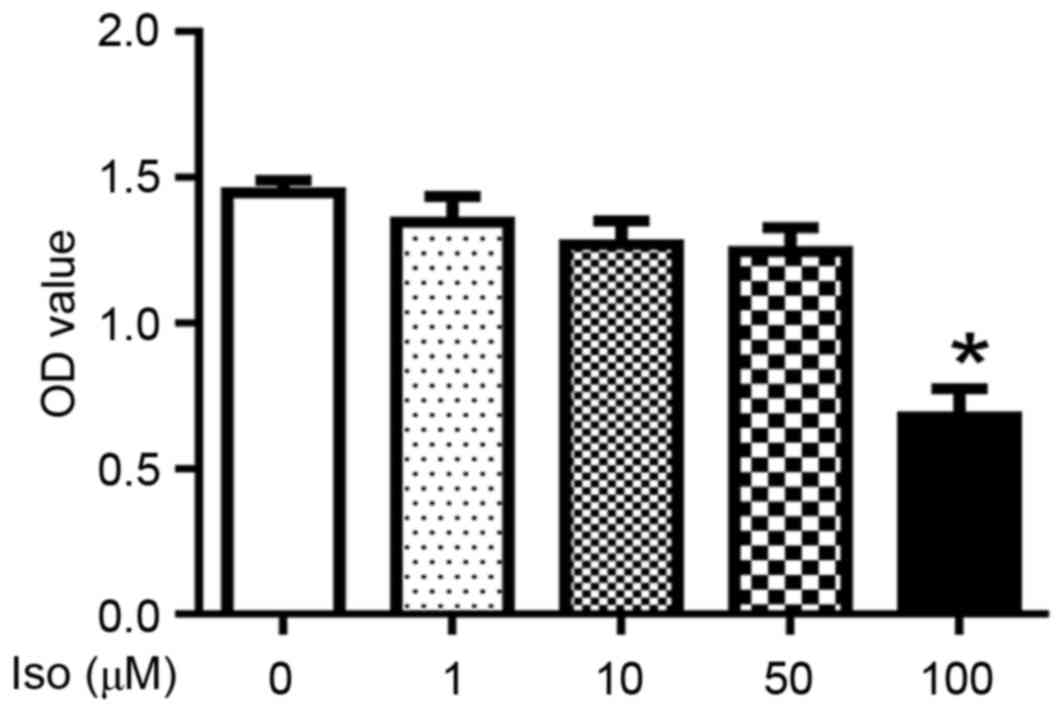
Effects of Iso on the viability of
primary chondrocytes
The potential cytotoxicity of Iso on mouse primary
chondrocytes was evaluated using CCK8 assay. Iso, at concentrations
between 1 and 50 µM, exhibited no toxicity in chondrocytes;
however, 100 µM Iso significantly reduced the viability of
chondrocytes (P<0.05; Fig. 3).
Therefore, 1, 10 and 50 µM Iso were used in the subsequent
experiments.
Iso treatment reduces MMP13, Runx2,
Col X and VEGF expression in primary chondrocytes
The effects of various concentrations of Iso on
murine primary chondrocytes treated with 10 ng/ml IL-1 were
determined. IL-1 significantly upregulated the expression of MMP13
(Fig. 4A), Runx2 (Fig. 4B), Col X (Fig. 4C) and VEGF (Fig. 4D). However, Iso inhibited the
effects of IL-1, decreasing the expression of MMP13, Runx2, Col X
(chondrocyte hypertrophy) and VEGF (angiogenesis). These results
demonstrated that Iso may inhibit chondrocyte hypertrophy and
angiogenesis in vivo and in vitro.
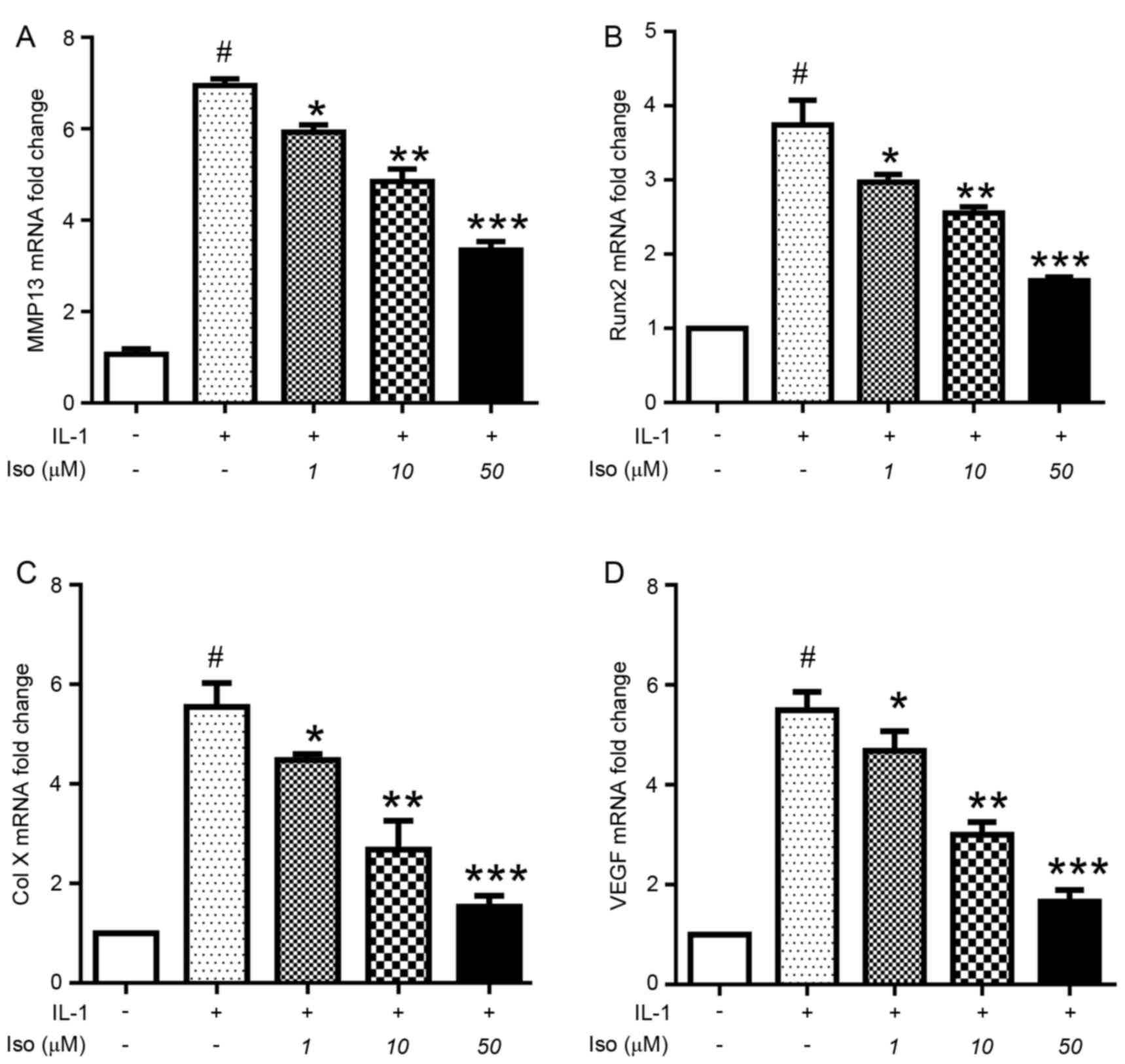 | Figure 4.Iso treatment induces a reduction in
the mRNA expression levels of MMP13, Runx2, Col X and VEGF in
murine primary chondrocytes. Chondrocytes were pretreated with
various concentrations of Iso (0, 1, 10 or 50 µM) for 1 h, followed
by stimulation with IL-1 (10 ng/ml) for 24 h. The chondrocytes were
collected for gene expression analysis by reverse
transcription-quantitative polymerase chain reaction. Iso inhibited
the mRNA expression levels of (A) MMP13, (B) Runx2, (C) Col X and
(D) VEGF in a dose-dependent manner. Data are presented as the mean
± standard deviation of three experiments. #P<0.05 vs
negative control; *P<0.05; **P<0.01; ***P<0.005 vs. IL-1
treatment only. Col X, type X collagen; IL, interleukin; Iso,
isoimperatorin; MMP, matrix metalloproteinase; Runx2, Runt-related
transcription factor; VEGF, vascular endothelial growth factor. |
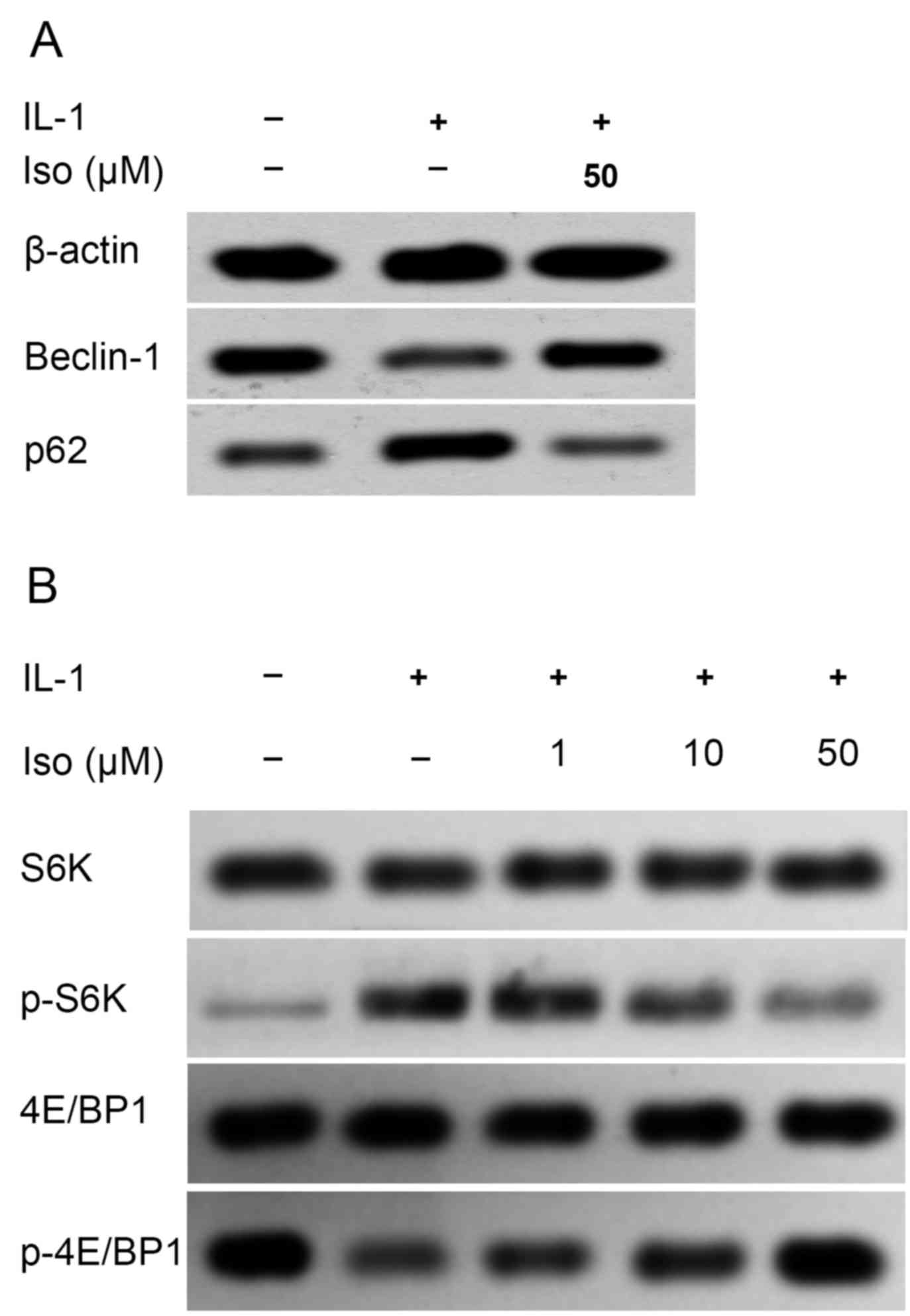
Iso activates autophagy signaling by
enhancing expression of Beclin 1 and inhibiting that of p62
Recent evidence suggests that autophagy may be
involved in the progression of OA (23); therefore, the present study
examined whether Iso attenuated OA by modulating autophagy
signaling. Autophagy was inhibited following IL-1 stimulation, as
indicated by the reduced expression of Beclin 1 and increased
expression of p62. However, Iso treatment inhibited the effects of
IL-1 by enhancing the expression of Beclin 1 and reducing that of
p62, indicating that Iso may attenuate OA by activating autophagy
(Fig. 5A).
Disease attenuation following Iso
treatment may be partly attributable to inhibition of the mTORC1
signaling pathway
The mTORC1 signaling pathway is a well-known key
regulator of inflammation and angiogenesis in OA (24,25).
To investigate the potential mechanism through which Iso exerts its
anti-OA effect, the phosphorylation status of its two direct
downstream targets in the mTORC1 signaling pathway, S6K and 4E-BP1
(26), were determined by western
blot analysis. Iso decreased the phosphorylation of S6K and
increased 4E/BP1 phosphorylation in a dose-dependent manner, which
suggested that the mTORC1 pathway was inactivated by Iso (Fig. 5B).
Discussion
Iso is a traditional Chinese herb with
anti-inflammatory properties that is widely used in China. In the
present study, the anti-OA properties of Iso were evaluated in
vivo and in vitro. Iso was revealed to prevent articular
cartilage degeneration by preventing chondrocyte hypertrophy and
aberrant angiogenesis via MMP13, Runx2, Col X and VEGF expression,
and activating autophagy by downregulating the mTORC1 signaling
pathway in a dose-dependent manner. These findings were consistent
in both of the models investigated: The DMM-induced OA mouse model
and in IL-1-treated murine chondrocytes.
OA is a chronic degenerative process that leads to
chondrocyte hypertrophy and cartilage damage (27). In OA, the rupture of cartilage
homeostasis causes a subset of factors to promote cartilage damage,
which leads to matrix remodeling, cartilage calcification and
hypertrophy-like changes, and aggravates the progression of OA
(6,21,22,28).
Articular cartilage refers to permanent cartilage that does not
undergo terminal differentiation under normal conditions. However,
when stimulated, chondrocyte differentiation leads to hypertrophy
and apoptosis (28). MMP13, Runx2
and Col X are produced by hypertrophic chondrocytes and are
important standard markers for chondrocyte hypertrophy (16,18,21).
Runx2 is a major transcription factor involved in chondrocyte
differentiation and is overexpressed in OA cartilage, elevating the
production of Col X and MMP13 (28–30).
Angiogenesis is also thought to affect the function and homeostasis
of cartilage in the pathogenesis of OA (31). VEGF is a secreted mitogen
associated with angiogenesis that has been reported to regulate the
vascular invasion of the hypertrophic cartilage in OA; one isoform
of which, VEGF-A, promotes subsequent cartilage vascularization
(32,33). A meta-analysis revealed that VEGF
is upregulated in patients with OA (7) and that it contributes to the severity
of knee OA (34,35). Intravenous and intra-articular
administration of the anti-VEGF antibody bevacizumab facilitates
articular cartilage repair and attenuates the severity of OA
(36,37). Therefore, it is hypothesized that
Iso may ameliorate OA by reducing MMP13, Runx2, Col X and VEGF
production.
In the present study, the mechanism underlying the
action of Iso on primary chondrocytes was also evaluated. Numerous
signaling pathways are involved in cartilage damage in OA. Among
these, the present study focused on the autophagy-associated mTORC1
signaling pathway due to its vital function in the progression of
OA. Autophagy is an essential cellular homeostatic process for
cartilage survival that protects articular cartilage. Previous
studies have demonstrated that the genetic ablation of autophagy
protein 5 in chondrocytes accelerates age-associated OA (34,38).
Autophagy is decreased in OA; however, the administration of
rapamycin, a specific inhibitor of mTOR signaling, reverses this
effect (39,40). Autophagy is involved in numerous
pathological processes, including anti-inflammatory,
anti-hypertrophic and anti-angiogenesis activities. It is also
involved in chondrocyte hypertrophy during cartilage degradation
and counteracts degenerative alterations, including cartilage
hypertrophy and the terminal differentiation of chondrocytes
(41,42). Peroxisome proliferator-activated
receptor (PPAR)γ deficiency leads to increased cartilage
degradation and the aberrant production of inflammatory (iNOS and
COX-2) and hypertrophic (MMP13) markers associated with upregulated
mTOR signaling and autophagy inactivation. This results in
increased inflammatory and hypertrophic activity in the articular
cartilage and OA (24). mTOR is
associated with angiogenesis, and the administration of rapamycin
decreases VEGF production (25)
and disrupts angiogenesis (43).
mTOR interacts with numerous aspects of different cellular
processes, including survival, growth and metabolism (40–42,44,45).
An increasing body of evidence has indicated that mTOR signaling
regulates the modulation of autophagy. mTOR suppression can
activate autophagy, leading to a reduction in the severity of OA
(46,47). Intra-articular administration of
rapamycin also reduces the severity of articular cartilage
degradation by reducing mTOR signaling (48,49).
Enhancement of the synthesis of endogenous n-3 polyunsaturated
fatty acids (PUFAs) from n-6 PUFAs attenuates OA through the
inhibition of mTORC1 and the promotion of autophagy in cartilage
chondrocytes (50). In addition,
cartilage-specific deletion of mTOR upregulates autophagy and
delays OA (51). Conversely,
suppression of regulated in development and DNA damage response 1,
an endogenous inhibitor of mTOR, leads to mTOR activation and
defective autophagy in OA (52).
PPARγ deficiency also results in aberrant mTOR signaling, which
induces a metabolic balance in cartilage homeostasis (24,53).
It may be hypothesized that activation of autophagy is beneficial
to articular cartilage, leading to mTOR suppression and the
attenuation of OA (54,55). These findings indicated that Iso
may activate autophagy by downregulating the mTORC1 signaling
pathway. However, the exact mechanism associated with Iso and the
mTORC1 autophagy pathway remains unclear, and it has yet to be
established whether autophagy and mTORC1 are direct targets of Iso.
Further studies are required to elucidate the underlying mechanism
by which Iso regulates the mTORC1 autophagic signaling pathway.
The present study also investigated the in
vivo effects of Iso on articular cartilage in a DMM model over
a period of 4 weeks. The results revealed that Iso ameliorated the
OA-associated effects, and that 4 weeks was sufficient to induce OA
using DMM.
The present study had a few limitations. A more
in-depth study would provide more information regarding the effects
of Iso on cartilage deterioration in DMM. In future studies, the
course of DMM-induced OA should be evaluated for 8 weeks. In
addition, further insight into the effects of Iso on other aspects
of OA is required, including subchondral bone, synovium and
meniscus. The incidence of OA increases with age and the treatment
options for OA are limited. In the late stages of OA, patients
require arthroplasty, which has a large economic burden. Therefore,
more attention should be given to developing novel therapies that
delay the progression of OA, including exploiting novel drugs from
medicinal herbs.
In conclusion, the results of the present study
demonstrated that Iso ameliorated articular cartilage degradation
in a murine DMM model. A reduction in MMP13, Runx2, Col X and VEGF
expression was observed in cartilage from Iso-treated mice
following DMM surgery. In addition, autophagy was activated and
mTORC1 was inhibited in murine primary chondrocytes. These
observations indicated that Iso may ameliorate the pathological
alterations induced by OA by delaying chondrocyte deterioration,
activating autophagy and inhibiting mTORC1, which suggests that Iso
may be an effective therapeutic approach for attenuating articular
cartilage degradation and thus, treating OA.
Acknowledgements
The present study was partially supported by the
Research Fund for the Doctoral Program of Higher Education of China
(grant no. 20124433110021).
Glossary
Abbreviations
Abbreviations:
|
OA
|
osteoarthritis
|
|
DMM
|
destabilization of the medial
meniscus
|
|
MMP
|
matrix metalloproteinase
|
|
Runx2
|
Runt-related transcription factor
2
|
|
Col X
|
type X collagen
|
|
VEGF
|
vascular endothelial growth factor
|
|
Iso
|
isoimperatorin
|
|
COX-2
|
cyclooxygenase-2
|
|
TAC
|
thickness of articular cartilage
|
|
OARSI
|
Osteoarthritis Research Society
International
|
|
mTORC1
|
mammalian target of rapamycin complex
1
|
References
|
1
|
Losina E, Weinstein AM, Reichmann WM,
Burbine SA, Solomon DH, Daigle ME, Rome BN, Chen SP, Hunter DJ,
Suter LG, et al: Lifetime risk and age at diagnosis of symptomatic
knee osteoarthritis in the US. Arthritis Care Res (Hoboken).
65:703–711. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Musumeci G, Aiello F, Szychlinska M, Di
Rosa M, Castrogiovanni P and Mobasheri A: Osteoarthritis in the
XXIst century: Risk factors and behaviours that influence disease
onset and progression. Int J Mol Sci. 16:6093–6112. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Musumeci G, Szychlinska MA and Mobasheri
A: Age-related degeneration of articular cartilage in the
pathogenesis of osteoarthritis: Molecular markers of senescent
chondrocytes. Histol Histopathol. 30:1–12. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Aigner T, Sachse A, Gebhard PM and Roach
HI: Osteoarthritis: Pathobiology-targets and ways for therapeutic
intervention. Adv Drug Deliv Rev. 58:128–149. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jacques C, Gosset M, Berenbaum F and Gabay
C: The role of IL-1 and IL-1Ra in joint inflammation and cartilage
degradation. Vitam Horm. 74:371–403. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Loeser RF, Goldring SR, Scanzello CR and
Goldring MB: Osteoarthritis: A disease of the joint as an organ.
Arthritis Rheum. 64:1697–1707. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yuan Q, Sun L, Li JJ and An CH: Elevated
VEGF levels contribute to the pathogenesis of osteoarthritis. BMC
Musculoskelet Disord. 15:4372014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Musumeci G, Carnazza ML and Loreto C,
Leonardi R and Loreto C: β-Defensin-4 (HBD-4) is expressed in
chondrocytes derived from normal and osteoarthritic cartilage
encapsulated in PEGDA scaffold. Acta Histochem. 114:805–812. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Musumeci G, Carnazza ML, Leonardi R and
Loreto C: Expression of β-defensin-4 in ‘an in vivo and ex vivo
model’ of human osteoarthritic knee meniscus. Knee Surg Sports
Traumatol Arthrosc. 20:216–222. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Philp AM, Davis ET and Jones SW:
Developing anti-inflammatory therapeutics for patients with
osteoarthritis. Rheumatology (Oxford). 56:869–881. 2017.PubMed/NCBI
|
|
11
|
Goldring MB and Goldring SR:
Osteoarthritis. J Cell Physiol. 213:626–634. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang S, Chen Q and He L: Development and
validation of a gas chromatography-mass spectrometry method for the
determination of isoimperatorin in rat plasma and tissue:
Application to the pharmacokinetic and tissue distribution study. J
Chromatogr B Analyt Technol Biomed Life Sci. 852:473–478. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wei Y and Ito Y: Preparative isolation of
imperatorin, oxypeucedanin and isoimperatorin from traditional
Chinese herb ‘bai zhi’ Angelica dahurica (Fisch. ex Hoffm) Benth.
et Hook using multidimensional high-speed counter-current
chromatography. J Chromatogr A. 1115:112–117. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kang OH, Chae HS, Oh YC, Choi JG, Lee YS,
Jang HJ, Kim JH, Kim YC, Sohn DH, Park H and Kwon DY:
Anti-nociceptive and anti-inflammatory effects of Angelicae
dahuricae radix through inhibition of the expression of inducible
nitric oxide synthase and NO production. Am J Chin Med. 36:913–928.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zheng YM, Shen JZ, Wang Y, Lu AX and Ho
WS: Anti-oxidant and anti-cancer activities of Angelica dahurica
extract via induction of apoptosis in colon cancer cells.
Phytomedicine. 23:1267–1274. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nie H, Meng LZ, Zhou JY, Fan XF, Luo- Y
and Zhang GW: Imperatorin is responsible for the vasodilatation
activity of Angelica Dahurica var. Formosana regulated by nitric
oxide in an endothelium-dependent manner. Chin J Integr Med.
15:442–447. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen YF, Tsai HY and Wu TS:
Anti-inflammatory and analgesic activities from roots of Angelica
pubescens. Planta Med. 61:2–8. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Moon TC, Jin M, Son JK and Chang HW: The
effects of isoimperatorin isolated from Angelicae dahuricae on
cyclooxygenase-2 and 5-lipoxygenase in mouse bone marrow-derived
mast cells. Arch Pharm Res. 31:210–215. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ban HS, Lim SS, Suzuki K, Jung SH, Lee S,
Lee YS, Shin KH and Ohuchi K: Inhibitory effects of furanocoumarins
isolated from the roots of Angelica dahurica on prostaglandin E2
production. Planta Med. 69:408–412. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Moon L, Ha YM, Jang HJ, Kim HS, Jun MS,
Kim YM, Lee YS, Lee DH, Son KH, Kim HJ, et al: Isoimperatorin,
cimiside E and 23-O-acetylshengmanol-3-xyloside from Cimicifugae
rhizome inhibit TNF-α-induced VCAM-1 expression in human
endothelial cells: Involvement of PPAR-γ upregulation and PI3K,
ERK1/2 and, PKC signal pathways. J Ethnopharmacol. 133:336–344.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Glasson SS, Chambers MG, Van Den Berg WB
and Little CB: The OARSI histopathology initiative -
recommendations for histological assessments of osteoarthritis in
the mouse. Osteoarthritis Cartilage. 18 Suppl 3:S17–S23. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lotz M and Caramés B: Autophagy: A new
therapeutic target in cartilage injury and osteoarthritis. J Am
Acad Orthop Surg. 20:261–262. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vasheghani F, Zhang Y, Li YH, Blati M,
Fahmi H, Lussier B, Roughley P, Lagares D, Endisha H, Saffar B, et
al: PPARγ deficiency results in severe, accelerated osteoarthritis
associated with aberrant mTOR signalling in the articular
cartilage. Ann Rheum Dis. 74:569–578. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Guba M, von Breitenbuch P, Steinbauer M,
Koehl G, Flegel S, Hornung M, Bruns CJ, Zuelke C, Farkas S,
Anthuber M, et al: Rapamycin inhibits primary and metastatic tumor
growth by antiangiogenesis: Involvement of vascular endothelial
growth factor. Nat Med. 8:128–135. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Laplante M and Sabatini DM: Regulation of
mTORC1 and its impact on gene expression at a glance. J Cell Sci.
126:1713–1719. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Berenbaum F, Griffin TM and Liu-Bryan R:
Review: Metabolic regulation of inflammation in osteoarthritis.
Arthritis Rheumatol. 69:9–21. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
van der Kraan PM and van den Berg WB:
Chondrocyte hypertrophy and osteoarthritis: Role in initiation and
progression of cartilage degeneration? Osteoarthritis Cartilage.
20:223–232. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Higashikawa A, Saito T, Ikeda T, Kamekura
S, Kawamura N, Kan A, Oshima Y, Ohba S, Ogata N, Takeshita K, et
al: Identification of the core element responsive to runt-related
transcription factor 2 in the promoter of human type X collagen
gene. Arthritis Rheum. 60:166–178. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kamekura S, Kawasaki Y, Hoshi K, Shimoaka
T, Chikuda H, Maruyama Z, Komori T, Sato S, Takeda S, Karsenty G,
et al: Contribution of runt-related transcription factor 2 to the
pathogenesis of osteoarthritis in mice after induction of knee
joint instability. Arthritis Rheum. 54:2462–2470. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yi JW, Lee WS, Kim SB, Heo YM and Chae DS:
Effect of zoledronate on the expression of vascular endothelial
growth factor-A by articular chondrocytes and synovial cells: An in
vitro study. J Bone Metab. 21:249–255. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Neve A, Cantatore FP, Corrado A, Gaudio A,
Ruggieri S and Ribatti D: In vitro and in vivo angiogenic activity
of osteoarthritic and osteoporotic osteoblasts is modulated by VEGF
and vitamin D3 treatment. Regul Pept. 184:81–84. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Takimoto A, Nishizaki Y, Hiraki Y and
Shukunami C: Differential actions of VEGF-A isoforms on
perichondrial angiogenesis during endochondral bone formation. Dev
Biol. 332:196–211. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Saetan N, Honsawek S, Tanavalee A,
Yuktanandana P, Meknavin S, Ngarmukos S, Tanpowpong T and Parkpian
V: Relationship of plasma and synovial fluid vascular endothelial
growth factor with radiographic severity in primary knee
osteoarthritis. Int Orthop. 38:1099–1104. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim HR, Lee JH, Kim KW, Kim BM and Lee SH:
The relationship between synovial fluid VEGF and serum leptin with
ultrasonographic findings in knee osteoarthritis. Int J Rheum Dis.
19:233–240. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nagai T, Sato M, Kobayashi M, Yokoyama M,
Tani Y and Mochida J: Bevacizumab, an anti-vascular endothelial
growth factor antibody, inhibits osteoarthritis. Arthritis Res
Ther. 16:4272014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nagai T, Sato M, Kutsuna T, Kokubo M,
Ebihara G, Ohta N and Mochida J: Intravenous administration of
anti-vascular endothelial growth factor humanized monoclonal
antibody bevacizumab improves articular cartilage repair. Arthritis
Res Ther. 12:R1782010. View
Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bouderlique T, Vuppalapati KK, Newton PT,
Li L, Barenius B and Chagin AS: Targeted deletion of Atg5 in
chondrocytes promotes age-related osteoarthritis. Ann Rheum Dis.
75:627–631. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li YS, Zhang FJ, Zeng C, Luo W, Xiao WF,
Gao SG and Lei GH: Autophagy in osteoarthritis. Joint Bone Spine.
83:143–148. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Caramés B, Taniguchi N, Otsuki S, Blanco
FJ and Lotz M: Autophagy is a protective mechanism in normal
cartilage, and its aging-related loss is linked with cell death and
osteoarthritis. Arthritis Rheum. 62:791–801. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Borzì RM, Guidotti S, Minguzzi M, Facchini
A, Platano D, Trisolino G, Filardo G, Cetrullo S, D'Adamo S,
Stefanelli C, et al: Polyamine delivery as a tool to modulate stem
cell differentiation in skeletal tissue engineering. Amino Acids.
46:717–728. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yang RT, Zhang C, Liu Y, Zhou HH and Li
ZB: Autophagy prior to chondrocyte cell death during the
degeneration of Meckel's cartilage. Anat Rec (Hoboken).
295:734–741. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Alvarez-García O, García-López E, Loredo
V, Gil-Peña H, Rodríguez-Suárez J, Ordóñez FA, Carbajo-Pérez E and
Santos F: Rapamycin induces growth retardation by disrupting
angiogenesis in the growth plate. Kidney Int. 78:561–568. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Laplante M and Sabatini DM: Regulation of
mTORC1and its impact on gene expression at a glance. J Cell Sci.
126:1713–1719. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Howell JJ, Ricoult SJ, Ben-Sahra I and
Manning BD: A growing role for mTOR in promoting anabolic
metabolism. Biochem Soc Trans. 41:906–912. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Caramés B, Hasegawa A, Taniguchi N, Miyaki
S, Blanco FJ and Lotz M: Autophagy activation by rapamycin reduces
severity of experimental osteoarthritis. Ann Rheum Dis. 71:575–581.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Bohensky J, Leshinsky S, Srinivas V and
Shapiro IM: Chondrocyte autophagy is stimulated by HIF-1 dependent
AMPK activation and mTOR suppression. Pediatr Nephrol. 25:633–642.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Matsuzaki T, Matsushita T, Tabata Y, Saito
T, Matsumoto T, Nagai K, Kuroda R and Kurosaka M: Intra-articular
administration of gelatin hydrogels incorporating
rapamycin-micelles reduces the development of experimental
osteoarthritis in a murine model. Biomaterials. 35:9904–9911. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Takayama K, Kawakami Y, Kobayashi M, Greco
N, Cummins JH, Matsushita T, Kuroda R, Kurosaka M, Fu FH and Huard
J: Local intra-articular injection of rapamycin delays articular
cartilage degeneration in a murine model of osteoarthritis.
Arthritis Res Ther. 16:4822014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Huang MJ, Wang L, Jin DD, Zhang ZM, Chen
TY, Jia CH, Wang Y, Zhen XC, Huang B, Yan B, et al: Enhancement of
the synthesis of n-3 PUFAs infat-1 transgenic mice inhibits mTORC1
signalling and delays surgically induced osteoarthritis in
comparison with wild-type mice. Ann Rheum Dis. 73:1719–1727. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhang Y, Vasheghani F, Li YH, Blati M,
Simeone K, Fahmi H, Lussier B, Roughley P, Lagares D, Pelletier JP,
et al: Cartilage-specific deletion of mTOR upregulates autophagy
and protects mice from osteoarthritis. Ann Rheum Dis. 74:1432–1440.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Alvarez-Garcia O, Olmer M, Akagi R,
Akasaki Y, Fisch KM, Shen T, Su AI and Lotz MK: Suppression of
REDD1 in osteoarthritis cartilage, a novel mechanism for
dysregulated mTOR signaling and defective autophagy. Osteoarthritis
Cartilage. 24:1639–1647. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Dell'Accio F and Sherwood J: PPARγ/mTOR
signalling: Striking the right balance in cartilage homeostasis.
Ann Rheum Dis. 74:477–479. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Chagin AS: Effectors of mTOR-autophagy
pathway: Targeting cancer, affecting the skeleton. Curr Opin
Pharmacol. 28:1–7. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Shi J, Zhang C, Yi Z and Lan C: Explore
the variation of MMP3, JNK, p38 MAPKs, and autophagy at the early
stage of osteoarthritis. IUBMB Life. 68:293–302. 2016. View Article : Google Scholar : PubMed/NCBI
|