Introduction
Glucocorticoid-induced osteoporosis (GIOP) is a
severe complication of prolonged systemic glucocorticoid use.
GIOP-mediated bone loss, and the associated risk of fracture, is a
relatively common disorder and the most prevalent type of secondary
osteoporosis (1). It is believed
that glucocorticoids may cause rapid bone loss, reduced bone
formation and increased bone resorption, in vitro and in
vivo (2,3). The therapeutic use of low doses of
oral glucocorticoids and mild endogenous hypercortisolism may also
be associated with bone loss (4).
However, patients treated with glucocorticoids are not often
evaluated and treated for this problem. Therefore, the exploration
of a novel and effective adjuvant therapy is required.
Icariin has been identified as a flavonoid isolated
from Herba Epimedii (Epimedium brevicornum), which is the
most commonly used Chinese herbal medicine in the treatment of
sexual dysfunction and osteoporosis (5,6). The
anti-osteoporotic role of icariin has been verified in
ovariectomized rats (7,8) and glucocorticoid-induced osteoporotic
rats (9). A 24-month randomized
double-blind placebo-controlled clinical trial indicated that an
Epimedium-derived phytoestrogen flavonoid preparation
containing 60 mg icariin, 15 mg daidzien and 3 mg genistein was
able to limit bone loss in late postmenopausal women (10). Notably, molecular mechanism studies
have suggested that icariin may induce osteoblast proliferation,
differentiation and mineralization via estrogen receptor-mediated
extracellular-signal related kinase and c-Jun N-terminal kinase
signal activation (11), and may
regulate the osteogenic differentiation of bone mesenchymal stem
cells in a mitogen activated protein kinase-dependent manner
(12). Furthermore, icariin, as an
inhibitor of cathepsin K, suppresses cartilage and bone degradation
in mice with collagen-induced arthritis (13). Previous study has indicated that
cathepsin K serves an important role in the pathogenesis of
rheumatoid arthritis (14).
Cathepsin K is expressed by osteoclasts during bone resorption, and
serves as the major collagenase responsible for organic bone matrix
degradation during the bone remodeling process (15). Excessive cathepsin K is a key
element in the pathogenesis of postmenopausal osteoporosis and
other skeletal disorders; therefore, cathepsin K may be a valuable
target in the development of novel therapeutic intervention
strategies (16). The expression
of cathepsin K and the effects of icariin in GIOP animal models
remains largely unknown.
MicroRNAs (miRNAs) are a class of small noncoding
(18–25 nucleotides), endogenous RNA molecules that regulate the
translation of messenger RNAs (mRNAs) by attaching to the target
3′-untranslated region (3′-UTR) of mRNAs (17,18).
The key roles of miRNAs involve regulation of cell proliferation,
differentiation and apoptosis of several cell types, including
osteoblasts and osteoclasts (19,20).
A growing number of studies have demonstrated pathogenic
alterations in various tissues that may be linked to abnormal miRNA
expression, including the regulation of bone development (21,22).
The present study hypothesized that a miRNA mechanism may be
involved in icariin-mediated inhibition of secondary osteoporosis
in mice, by targeting cathepsin K. This study aimed to improve the
understanding of the anti-osteoporotic action and underlying
molecular mechanism of icarrin, which may facilitate the treatment
of secondary osteoporotic patients.
Materials and methods
Animal treatment
All procedures were evaluated and approved by the
ethics committee of the Henan University of Traditional Chinese
Medicine (Henan, China). A total of 48 male C57BL/6J mice (age, 8
weeks; weight, 20±2 g) were obtained from Slac Laboratory Animal
(Shanghai, China; www.slaccas.com) and were allowed to acclimate to the
environment for 1 week. The mice were given free access to food and
tap water and were caged individually under controlled temperature
(23±2°C) and humidity (55±5%) with an artificial 12-h light/dark
cycle. The mice were randomly divided into four groups: Vehicle
group (n=12) were injected with normal saline; dexamethasone (DXM),
where mice were injected intramuscularly with 5 mg/kg body weight
DXM three times weekly, for 12 weeks (n=12); low dose icariin GIOP,
mice received oral icariin at a dose of 10 mg/kg/day for 12 weeks
(Icariin + L, n=12) combined with DXM; high dose icariin GIOP, mice
received oral icariin at a dose of 100 mg/kg/day, for 12 weeks
combined with DXM (Icariin + H, n=12).
Cell culture
Human embryonic kidney HEK293T cells [cat. no.
CRL-11268; American Type Culture Collection (ATCC), Manassas, VA,
USA] were maintained in Dulbecco's modified Eagle's medium (DMEM;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with
10% (v/v) fetal bovine serum (FBS; Sigma-Aldrich; Merck Millipore,
Darmstadt, Germany), 100 U/ml penicillin, and 100 mg/ml
streptomycin, in a 5% CO2 atmosphere at 37°C.
Serum and urine chemistries
Mice were placed in metabolic cages for 24 h to
collect and recorded the volume of urine at week 12. Blood were
collected after the animals were sacrificed at week 12 by
intraperitoneal injection of sodium pentobarbital (2%; 200 mg/kg;
cat. no. P3761; Sigma-Aldrich; Merck Millipore). The concentrations
of calcium (Ca, absorbance was measured at 490 nm) and creatinine
(Cr, absorbance was measured at 630 nm) in serum and urine were
measured by standard colorimetric methods, using a microplate
reader (BioTek Instruments, Winooski, VT, USA). The urinary Ca
level was corrected against the urinary Cr concentration. Serum
levels of alkaline phosphatase (ALP, cat. no. P0321; Beyotime
Institute of Biotechnology, Haimen, China), tartrate resistant acid
phosphatase (TRAP, cat. no. P0332; Beyotime Institute of
Biotechnology), osteocalcin (OCN, cat. no. 60–1305; Immutopics,
Inc., San Clemente, CA, USA) and deoxypyridinoline (DPD, cat. no.
Ek-M20919; EK-Bioscience, Biotechnology Co., Ltd., Shanghai, China)
were detected using mouse bioactive ELISA assays and measured using
a SpectraMax M5 ELISA plate reader (Molecular Devices, LLC,
Sunnyvale, CA, USA).
Osteoclast formation in RAW264.7
cells
RAW264.7 cells (cat. no. TIB 71; ATCC) were cultured
in α-MEM + 10% FBS (Sigma-Aldrich; Merck Millipore) and were plated
in 6-well dishes at 1×105 cells/well and were grown for
5 days with RANKL (20 ng/ml, cat. no. 462-TR; R&D Systems,
Minneapolis, MN, USA) induction of osteoclastogenesis, as
previously described (23).
Caspase-3 activity assay
Osteoclast (5×104) lysates were prepared
in 2 ml of lysis buffer, containing 25 mM HEPES, pH 7.5, 5 mM EDTA
and 1 mM EGTA (all from Sigma-Aldrich; Merck Millipore), 5 mM
MgCl2 and 10 mM sucrose (both from Thermo Fisher
Scientific, Inc.), 5 mM dithiothreitol (DTT), 1%
3-[-(3-chloramidopropyl) dimethylammonio]-1-propanesulfonic acid
(CHAPS), protease inhibitor cocktail (10 µl/ml), and 1 mM PMSF (all
from Sigma-Aldrich; Merck Millipore). After 30 min incubation at
0°C, cell lysates were centrifuged at 12,000 × g for 15 min at 4°C,
and the supernatants were collected. The activity of caspase-3 was
determined using the caspase-3 activity assay kit (cat. no. C1116;
Beyotime Institute of Biotechnology), according to the
manufacturer's instructions. Release of p-nitroaniline was measured
at a wavelength of 405 nm using a SpectraMax M5 ELISA plate reader,
according to the manufacturer's instructions.
Quantification of apoptosis by flow
cytometry
Quantitative assessment of apoptotic osteoclasts
(5×104) was performed by terminal deoxynucleotidyl
transferase-mediated deoxyuridine triphosphate nick end labeling
(TUNEL) method, which examines DNA-strand breaks during apoptosis,
using an ApoAlert™ DNA Fragmentation Assay kit (BD Biosciences,
Franklin Lakes, NJ, USA). Cells were trypsinized, fixed with 4%
paraformaldehyde and permeabilized with 0.1% Triton X-100 in 0.1%
sodium citrate. The cells were washed with PBS three times and
subsequently incubated with the reaction mixture for 60 min at
37°C. Cells were immediately analyzed using a FACScan flow
cytometer equipped with the CellQuest version 5.1 (BD
Biosciences).
Bone histomorphology
The tibias were collected after the animals were
sacrificed and were decalcified in 0.5 M EDTA (pH=8.0) and embedded
in paraffin by standard histological procedures. Sections (5 µm)
were cut and stained with hematoxylin & eosin and safranin O,
and were visualized under a Leica DM 2500 microscope (Leica
Microsystems GmbH, Wetzlar, Germany). The trabecular bone
microarchitecture of the proximal metaphysis of the tibia was
measured using a SkyScan 1076 microtomography scanner (Bruker
Corporation, Ettlingen, Germany), with a slice thickness of 22 µm.
Bone morphometric parameters, including volumetric bone mineral
density (BMD), bone volume over total volume (BV/TV), trabecula
number (Tb.N), trabecula thickness (Tb.Th) and trabecula separation
(Tb.Sp) were measured, as previously described (24). Femurs were placed in a 3-point
bending configuration on an Instron Microtester 5848 system
(Instron, Norwood, MA USA), to measure maximum load and stiffness,
as previously described (25).
Transfection of miRNA-186 mimics and
inhibitor
The 6-carboxyfluorescein-conjugated
2′-OMe-oligonucleotides were chemically synthesized and purified by
high-performance liquid chromatography (GenePharma Co., Ltd.,
Shanghai, China). The 2′-OMe-miRNA (miR)-186 mimic was composed of
RNA duplexes with the following sequence:
5′-CAAAGAAUUCUCCUUUUGGGCU-3′. The 2′-OMe-miR-1271 inhibitor
sequence was: 5′-AGCCCAAAAGGAGAAUUCUUUG-3′, and the 2′-Ome-scramble
sequence was: 5′-CUUGGUCAAGUCGCGAUCCGUA-3′. Osteoclasts
(1×105) were transfected using Lipofectamine
2000™ (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), at a final concentration of 100 nM. The culture
medium was changed 24 h post transfection. Cells were harvested for
analysis after 48 h.
Luciferase reporter gene activity
assay
The 3′-UTR of the cathepsin K gene containing the
predicated target site for miR-186 was synthesized by GenePharma.
The fragment was inserted into the multiple cloning site in the
pMIR-REPORT luciferase miRNA expression reporter vector (Ambion;
Thermo Fisher Scientific, Inc.). HEK293T cells (5×104;
cat. no. CRL-11268; ATCC) were co-transfected with luciferase
reporters containing cathepsin K 3′-UTR and miR-186 mimics using
Lipofectamine 2000. The cell lysates were harvested following 48 h
transfection, and the luciferase activity was measured using a dual
luciferase reporter assay kit (cat. no. RG027; Beyotime Institute
of Biotechnology) according to manufacturer's instructions.
Reverse transcription-quantitative PCR
(RT-qPCR.)
The tibias were crushed in liquid nitrogen and RNA
extraction was performed using TRIzol® according to the
manufacturer's instructions (Invitrogen; Thermo Fisher Scientific,
Inc.). Synthesis of cDNA was performed by reverse transcription of
2 µg total RNA, using Moloney murine leukemia virus reverse
transcriptase (Invitrogen; Thermo Fisher Scientific, Inc.), with
oligo (dT)15 primers (Fermentas; Thermo Fisher
Scientific, Inc., Pittsburgh, PA, USA), according to the
manufacturer's instructions. The first strand cDNAs served as the
template for the qPCR and were performed using an ABI 7300
thermocycler (Applied Biosytems; Thermo Fisher Scientific, Inc.).
GAPDH served as an internal control to determine the relative
expression of the target genes. The reaction conditions were set
according to the kit protocol. The level of miR-186 was quantified
using a mirVana qRT-PCR miRNA Detection kit (Ambion; Thermo Fisher
Scientific, Inc.) and SYBR-Green (cat. no. D7260; Beyotime
Institute of Biotechnology). Following the cycling reactions were
used: 95°C for 10 min, followed by 40 cycles of 95°C for 15 sec,
58°C for 30 sec, and 72°C for 30 sec. U6 served as the internal
control. The quantitation cycle (Cq) method (26) was used to determine relative
expression levels. The PCR primers (Sangon Biotech, Co., Ltd.,
Shanghai, China) used in the present study were as follows:
forward, 5′-CCAGGGCGTACGGAGGCCATT-3′ and reverse,
5′-GACCAAATTACGGCGTAGCCTC-3′ for ALP; forward,
5′-AGCATAAGGGTCCAAGTCCAA-3′ and reverse, 5′-TACCAAAAGCGGCGTAGTTA-3′
for TRAP; forward, 5′-AGGCGGAGGTCGATGCCCCG-3′ and reverse,
5′-CACGATGATGTCACCCTCGATGT-3′ for cathepsin K, forward,
5′-ACCGTGAGTTGTCCGTAGCATC-3′ and reverse,
5′-CGTAAGGGTCCGATACATCTC-3′ for osteoprotegerin (OPG); forward,
5′-CGTAGGTAGCCGGGTAGGATC-3′ and reverse, 5′-GCGTGGGAGCCTGATGCTCA-3′
for receptor activator of nuclear factor-κB ligand (RANKL);
forward, 5′-GGCAGGTGCTTCAGAACTGG-3′ and reverse,
5′-GTGGTGGCAGGTAGGTATGG-3′ for Runt-related transcription factor 2
(Runx2); forward, 5′-TGAGCTGGAACGTCACGTGC-3′ and reverse,
5′-AAGAGGAGGCCAGCCAGACA-3′ for osterix; forward,
5′-CTCAGGGTTTCAGTGGTT-3′ and reverse, 5′-TTTCCACGAGCACCCATC-3′ for
collagen type 1, α1 chain (Col1α1); and forward,
5′-ACAGGGGAGGTGATAGCATT-3′ and reverse,
5′-GACCAAAAGCCTTCATACATCTC-3′ for GAPDH.
Western blot analysis
The tibias were homogenized and extracted in NP-40
buffer, followed by 5–10 min boiling and centrifugation (12,000 × g
for 15 min at 4°C) to obtain the supernatant. Samples containing 50
µg protein were separated by 10% SDS-PAGE and transferred onto
nitrocellulose membranes (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). The membranes were incubated at room temperature for 4 h with
primary cathepsin K antibodies (1:500; R&D Systems). The blots
were washed 3 times with TBS with Tween-20 (TBST), and incubated
with donkey anti-goat immunoglobulin G (IgG; cat. no. OARA01784)
and donkey anti-mouse IgG secondary antibodies (cat. no. OARA01848)
at room temperature for 2 h, conjugated to IRDye 800CW Infrared Dye
(LI-COR Biosciences, Lincoln, NE, USA), diluted 1:10,000. The
membranes were washed 3 times with TBST. Blots were visualized
using the Odyssey Infrared Imaging System (LI-COR Biosciences) and
normalized to β-actin expression, to correct for unequal loading,
using a mouse monoclonal anti-β-actin antibody (cat. no. AP0060;
Bioworld Technology, Inc., St. Louis Park, MN, USA).
Statistical analysis
Data are presented as the mean ± standard deviation
for each group. All statistical analyses were performed using PRISM
version 4.0 (GraphPad Software, Inc., La Jolla, CA, USA).
Inter-group differences were analyzed by one-way analysis of
variance, followed by a post hoc Tukey's honest significant
difference test for multiple comparisons. P<0.05 was considered
to indicate a statistically significant difference.
Results
Effects of icariin on physiological
and biochemical properties
Administration of DXM significantly downregulated
the serum Ca level, compared with the control group, and icariin
treatment did not recover these levels in GIOP mice (Fig. 1A). In contrast, DXM exposure
markedly increased the level of urine Ca; however, high dose
icariin-treatment reversed this upregulation in GIOP mice (Fig. 1B). ELISA analysis of bone formation
and resorption markers indicated that DXM induced high bone
turnover in mice, as evidenced by a significant increase in serum
ALP (Fig. 1C), TRAP (Fig. 1D) OCN (Fig. 1E) and urinary DPD/Cr (Fig. 1F) levels. However, mice in the high
dose icariin + H group demonstrated decreased serum ALP, TRAP and
OCN and urinary DPD/Cr.
 | Figure 1.Effects of icariin on physiological
and biochemical parameters. (A) Serum and (B) urine Ca were
measured by standard colorimetric methods. Serum levels of (C) ALP,
(D) TRAP, (E) OCN and (F) urine levels of DPD were detected by
ELISA. Data are expressed as mean ± standard deviation
(n=12/group). *P<0.05, **P<0.01 and ***P<0.001 vs. DXM
group. Ca, calcium; Cr, creatinine; ALP, alkaline phosphatase;
TRAP, tartrate resistant acid phosphatase; OCN, osteocalcin; DPD,
deoxypyridinoline; DXM, dexamethasone; L, low dose; H, high dose;
U, units. |
Effects of icariin on bone
microarchitecture and biomechanical parameters
BMD decreased by 27.73 and 42.59% at weeks 6 and 12,
respectively, following administration of DXM (P<0.001), whereas
administration with high-dose icariin (100 mg/kg) significantly
restored the DXM-induced reduction in BMD in GIOP mice (Fig. 2A). A gross decrease in bone mass
was observed following 12 weeks of DXM administration, which was
inhibited when icariin was co-administered (Fig. 2B). Furthermore, structural
parameters of the trabecular bone network in the tibial proximal
metaphysis were measured. The results demonstrated that DXM-treated
mice demonstrated significant decreases in trabecular BV/TV, Tb.N
and Tb.Th, and increases in Tb.Sp, compared with the control group
(Fig. 2C). Notably, icariin
treatment in GIOP mice reversed the DXM-induced bone
microarchitectural deteriorations. Bone biomechanical parameters,
including maximum load and stiffness, decreased by 61.24 and 45.83%
respectively, following DXM treatment. Mice in the icarrin + H
group demonstrated significantly restoration of the DXM-induced
biomechanical alterations in the tibial proximal metaphysis
(Fig. 2D).
 | Figure 2.Effects of icariin on bone
microarchitecture and biomechanical parameters. (A) Volumetric BMD
was measured by micro-CT. (B) Hematoxylin and eosin staining of the
trabecular bone zone below the growth plate of the tibial proximal
metaphysis (magnification, ×50). (C) BV/TV, Tb.N, Tb.Th and Tb.Sp
were assessed by micro-CT. (D) Maximum load and stiffness were
assessed by micro-force testing. Data are expressed as mean ±
standard deviation (n=12/group). *P<0.05, **P<0.01 and
***P<0.001 vs. DXM group. BMD, bone mineral density; BV/TV, bone
volume over total volume; Tb.N, trabecula number; Tb.Th, trabecula
thickness; Tb.Sp, trabecula separation; DXM, dexamethasone; L, low
dose; H, high dose; N, Newtons; CT, computed tomography. |
Effects of icariin on the mRNA
expression of key regulators in bone metabolism
ALP and TRAP mRNA were significantly increased in
the DXM group, compared with the control group (Fig. 3A and B); however, these were
reduced by high-dose icariin co-administration. Furthermore,
expression of the bone formation-associated genes Runx2, osterix
and Col1α1 were decreased with DXM administration compared with the
vehicle group, and this was prevented by high dose icariin
treatment in DXM-induced GIOP mice (Fig. 3C). To further investigate the
molecular mechanism underlying DXM-induced inhibition of bone
formation and promotion of bone resorption, RT-qPCR was used to
assess the osteoclastogenesis-associated genes RANKL and OPG, which
are produced by the osteoblast. The results indicated that DXM
significantly elevated the expression of RANKL, and significantly
reduced the RANKL/OPG ratio. However, these alterations in RANKL
expression and the RANKL/OPG ratio were reversed by icariin
co-administration (Fig. 3D).
 | Figure 3.Effects of icariin on the mRNA
expression of key regulators in bone metabolism. The mRNA
expression of (A) ALP and (B) TRAP were measured by reverse
transcription-quantitative polymerase chain reaction. The mRNA
expression of (C) Runx2, Osterix and Col1a1, and (D) OPG and RANKL
were measured by RT-qPCR. Data are expressed as mean ± standard
deviation (n=/group). *P<0.05, **P<0.01 and ***P<0.001 vs.
DXM group. ALP, alkaline phosphatase; TRAP, tartrate resistant acid
phosphatase; Runx2, runt related transcription factor 2; Col1α1,
collage type 1 α 1 chain; OPG, osteoprotegerin; RANKL, receptor
activator of nuclear factor κB ligand; DXM, dexamethasone; L, low
dose; H, high dose. |
Effects of icariin on cathepsin K and
miR-186 expression
Cathepsin K mRNA and protein expression levels were
significantly upregulated in DXM-treated mice, compared with the
control group (Fig. 4A and B).
However, these were reversed when icariin was co-administered in
GIOP mice. Furthermore, the tibial levels of miR-186 were
suppressed in GIOP mice compared with the control group; however,
these levels were increased in icariin-supplemented GIOP mice
(Fig. 4C). Notably, Spearman's
rank correlation analysis indicated that the expression levels of
cathepsin K protein and miR-186 were inversely correlated in DXM
and icariin + H group mice (Fig.
4D). To further investigate the effect of cathepsin K on
cartilaginous degeneration, safranin O staining was performed to
observe the upper epiphyseal cartilage in the proximal tibias. The
thickness of cartilage adjacent to the epiphyseal plate was reduced
in the proximal tibia of the DXM mice, suggesting that DXM induces
a delayed formation of new cartilage on the upper epiphyseal plate.
However, the thickness of newly formed cartilage of the GIOP mice
was improved by high concentrations of icariin (Fig. 4E).
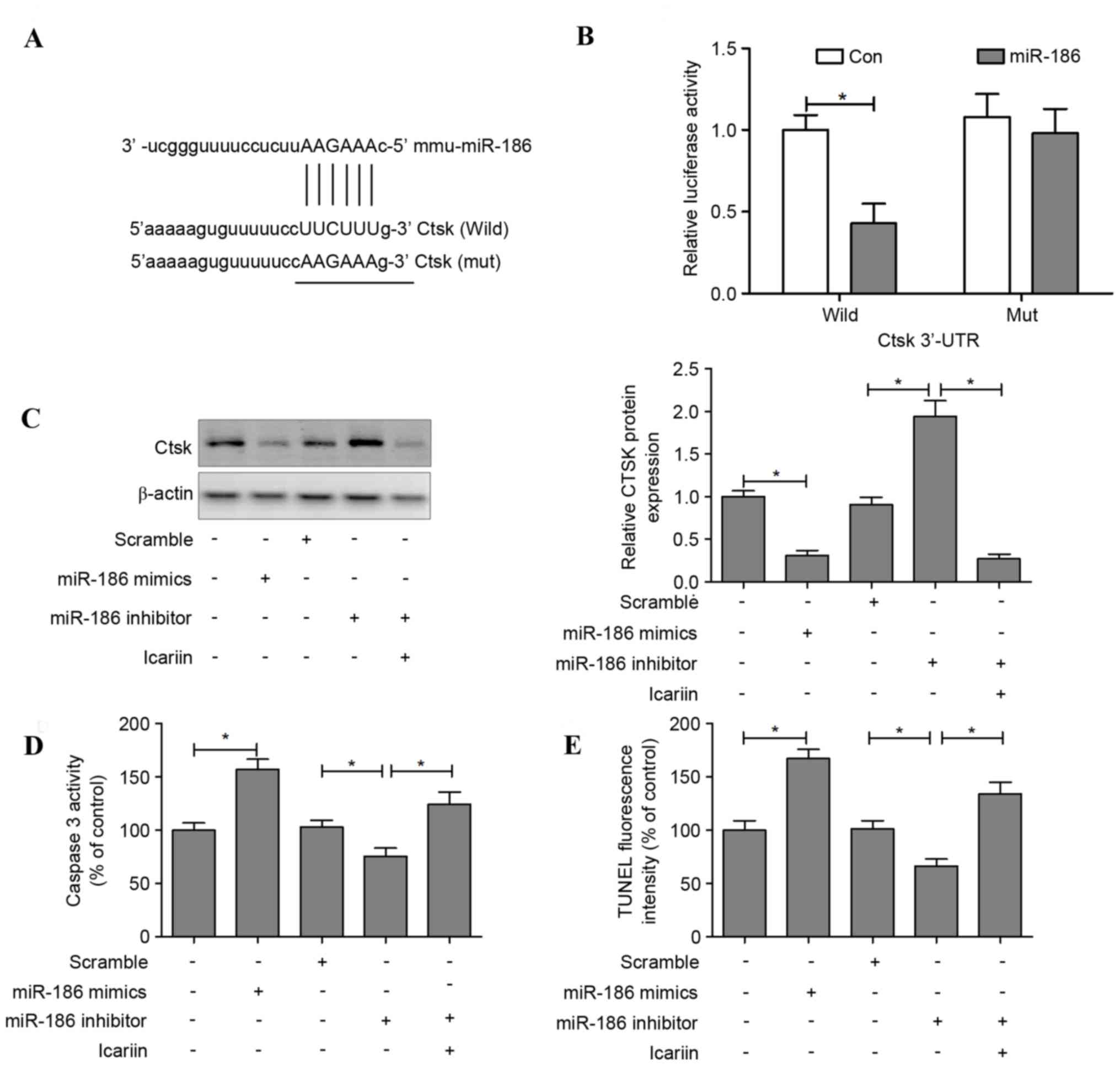
Cathepsin K is a direct target of
miR-186 in osteoclasts
The target prediction tools TargetScan, PicTar and
microRNA.org were used to identify potential target
genes of miR-186; the results indicated that miR-186 may regulate
cathepsin K (Fig. 5A). To
investigate whether miR-186 may directly regulate cathepsin K
expression, a luciferase reporter construct containing the 3′-UTR
of cathepsin K was used. The wild-type and mutant cathepsin K
luciferase expression vectors were transfected alongside an miR-186
mimic into HEK293T, and the level of luciferase enzyme activity was
measured. Overexpression of miR-186 suppressed the luciferase
activity of the reporter gene; however, a mutation in the miRNA
binding site abolished the luciferase activity repression,
confirming that the cathepsin K 3′-UTR contains a seed site for
miR-186 (Fig. 5B).
Transfection with an miR-186 mimic decreased the
levels of cathepsin K in osteoclasts, whereas transfection with
miR-186 inhibitor increased these levels. However, cathepsin K
protein expression was reduced when icariin was administered
alongside the miR-186 inhibitor (Fig.
5C). In addition, apoptotic features induced by transfection
with miR-186 mimic or inhibitor were assessed. The results
demonstrated that caspase-3 activity was significantly increased in
the miR-186 mimic group compared with the control group. In
contrast, osteoclasts transfected with an miR-186 inhibitor
suppressed caspase-3 activity, and this downregulation of caspase-3
was reversed when icariin was co-administered with the miR-186
inhibitor (Fig. 5D). TUNEL
staining indicated that transfection with the miR-186 mimic induced
cell apoptosis, whereas the miR-186 inhibitor suppressed cell
apoptosis, and icariin treatment reversed the suppression of cell
apoptosis in miR-186 inhibitor-treated osteoclasts (Fig. 5E).
Discussion
The present study revealed that cathepsin K is
upregulated in GIOP mice, and this is characterized by a reduction
in bone volume and strength, accompanied with a high bone turnover,
as evidenced by a significant increase in bone formation and
resorption markers. Furthermore, when the expression of cathepsin K
was blocked by icariin, the bone abnormalities were normalized.
Notably, the protective effects of icariin against bone
deteriorations in GIOP mice involved activation of miR-186, which
serves to suppress cathepsin K.
It has previously been suggested that glucocorticoid
treatment may influence bone remodeling and calcium homeostasis
(27). Recent studies have
indicated that DXM-exposure exhibits the typical features of GIOP
in rabbits or mice, evidenced by the alterations in the basic bone
microarchitecture and biomechanical parameters (2,28). A
study investigating the pharmacological mechanisms of icariin
revealed multiple targets in bone metabolism, and cathepsin K was
identified as the critical node in the associated signaling
networks (13). Growing evidence
has suggested that cathepsin K inhibition may specifically block
bone resorption without interfering with other pathways (16,29,30).
In a randomized trial, the cathepsin K inhibitor odanacatib
demonstrated an ability to decrease bone resorption, maintain bone
formation and increase areal and volumetric BMD and increase
estimated bone strength, in the hip and spine (31). Icariin has been identified as an
inhibitor of cathepsin K and may suppress cartilage and bone
degradation in mice with collagen-induced arthritis (13). In vivo, icariin has
demonstrated significant anti-osteoporosis effects in
ovariectomized rats and mice, glucocorticoid-induced rats and OPG
knockout mice (7,8). However, in vitro studies have
indicated that icariin may reduce TRAP activity and increase
osteogenic differentiation, calcium deposition and mineralized
nodule formation in induced bone marrow stromal and RAW264.7 cells
(32,33). The present study revealed that high
concentrations of icariin inhibits DXM-induced high bone turnover
and cathepsin K upregulation in GIOP mice.
Several miRNAs have been associated with
glucocorticoid-induced osteogenic differentiation and bone
deterioration (34). Specifically,
miR-29a ameliorates glucocorticoid-induced suppression of
osteoblast differentiation by regulating β-catenin acetylation
(35), and miR-29a overexpression
may represent an alternative strategy for alleviating
glucocorticoid-induced bone deterioration (36). Furthermore, miR-216a reverses DXM
suppression of osteogenesis, promotes osteoblast differentiation
and enhances bone formation by regulating the c-Casitas B-lineage
lymphoma-mediated phosphoinositide 3-kinase/protein kinase B
pathway (37). The results of the
present study suggested that icariin prevents DXM-induced bone loss
by inhibiting activated cathepsin K and increasing miR-186 levels.
Notably, the expression levels of cathepsin K protein and miR-186
were inversely correlated in DXM and icariin + H group mice, and
bioinformatics analysis suggested that miR-186 may regulate
cathepsin K via binding to a seed region in the 3′-UTR of cathepsin
K. These results suggested that miR-186 and cathepsin K serve a
role in icariin-mediated protection against DXM-induced bone
deterioration.
In conclusion, the present study demonstrated that
icariin may significantly ameliorate bone deterioration in GIOP
mice, and the underlying mechanism may be mediated, at least
partially, via activation of miR-186 and subsequent suppression of
cathepsin K. These results may facilitate understanding of the
underlying molecular mechanisms in DXM-induced osteoporosis, and
may provide evidence to support the use of icariin as a therapeutic
approach in the management of glucocorticoid-induced bone loss, and
the disequilibrium of calcium homeostasis.
References
|
1
|
Spreafico A, Frediani B, Francucci CM,
Capperucci C, Chellini F and Galeazzi M: Role of apoptosis in
osteoporosis induced by glucocorticoids. J Endocrinol Invest. 31
Suppl 7:S22–S27. 2008.
|
|
2
|
Yongtao Z, Kunzheng W, Jingjing Z, Hu S,
Jianqiang K, Ruiyu L and Chunsheng W: Glucocorticoids activate the
local renin-angiotensin system in bone: Possible mechanism for
glucocorticoid-induced osteoporosis. Endocrine. 47:598–608. 2014.
View Article : Google Scholar
|
|
3
|
Hofbauer LC, Gori F, Riggs BL, Lacey DL,
Dunstan CR, Spelsberg TC and Khosla S: Stimulation of
osteoprotegerin ligand and inhibition of osteoprotegerin production
by glucocorticoids in human osteoblastic lineage cells: Potential
paracrine mechanisms of glucocorticoid-induced osteoporosis.
Endocrinology. 140:4382–4389. 1999. View Article : Google Scholar
|
|
4
|
Shaker JL and Lukert BP: Osteoporosis
associated with excess glucocorticoids. Endocrinol Metab Clin North
Am. 34:341–356. 2005. View Article : Google Scholar
|
|
5
|
Li C, Li Q, Mei Q and Lu T:
Pharmacological effects and pharmacokinetic properties of icariin,
the major bioactive component in Herba Epimedii. Life Sci.
126:57–68. 2015. View Article : Google Scholar
|
|
6
|
Zhang X, Liu T, Huang Y, Wismeijer D and
Liu Y: Icariin: Does it have an osteoinductive potential for bone
tissue engineering? Phytother Res. 28:498–509. 2014. View Article : Google Scholar
|
|
7
|
Yang L, Yu Z, Qu H and Li M: Comparative
effects of hispidulin, genistein, and icariin with estrogen on bone
tissue in ovariectomized rats. Cell Biochem Biophys. 70:485–490.
2014. View Article : Google Scholar
|
|
8
|
Liu M, Zhong C, He RX and Chen LF: Icariin
associated with exercise therapy is an effective treatment for
postmenopausal osteoporosis. Chin Med J (Engl). 125:1784–1789.
2012.
|
|
9
|
Feng R, Feng L, Yuan Z, Wang D, Wang F,
Tan B, Han S, Li T, Li D and Han Y: Icariin protects against
glucocorticoid-induced osteoporosis in vitro and prevents
glucocorticoid-induced osteocyte apoptosis in vivo. Cell Biochem
Biophys. 67:189–197. 2013. View Article : Google Scholar
|
|
10
|
Zhang G, Qin L and Shi Y:
Epimedium-derived phytoestrogen flavonoids exert beneficial effect
on preventing bone loss in late postmenopausal women: A 24-month
randomized, double-blind and placebo-controlled trial. J Bone Miner
Res. 22:1072–1079. 2007. View Article : Google Scholar
|
|
11
|
Song L, Zhao J, Zhang X, Li H and Zhou Y:
Icariin induces osteoblast proliferation, differentiation and
mineralization through estrogen receptor-mediated ERK and JNK
signal activation. Eur J Pharmacol. 714:15–22. 2013. View Article : Google Scholar
|
|
12
|
Wu Y, Xia L, Zhou Y, Xu Y and Jiang X:
Icariin induces osteogenic differentiation of bone mesenchymal stem
cells in a MAPK-dependent manner. Cell Prolif. 48:375–384. 2015.
View Article : Google Scholar
|
|
13
|
Sun P, Liu Y, Deng X, Yu C, Dai N, Yuan X,
Chen L, Yu S, Si W, Wang X, et al: An inhibitor of cathepsin K,
icariin suppresses cartilage and bone degradation in mice of
collagen-induced arthritis. Phytomedicine. 20:975–979. 2013.
View Article : Google Scholar
|
|
14
|
Skoumal M, Haberhauer G, Kolarz G, Hawa G,
Woloszczuk W, Klingler A, Varga F and Klaushofer K: The imbalance
between osteoprotegerin and cathepsin K in the serum of patients
with longstanding rheumatoid arthritis. Rheumatol Int. 28:637–641.
2008. View Article : Google Scholar
|
|
15
|
Lewiecki EM: Odanacatib, a cathepsin K
inhibitor for the treatment of osteoporosis and other skeletal
disorders associated with excessive bone remodeling. IDrugs.
12:799–809. 2009.
|
|
16
|
Bone HG, McClung MR, Roux C, Recker RR,
Eisman JA, Verbruggen N, Hustad CM, DaSilva C, Santora AC and Ince
BA: Odanacatib, a cathepsin-K inhibitor for osteoporosis: A
two-year study in postmenopausal women with low bone density. J
Bone Miner Res. 25:937–947. 2010.
|
|
17
|
Dole NS and Delany AM: MicroRNA variants
as genetic determinants of bone mass. Bone. 84:57–68. 2016.
View Article : Google Scholar
|
|
18
|
Kagiya T: MicroRNAs and osteolytic bone
metastasis: The roles of microRNAs in tumor-induced osteoclast
differentiation. J Clin Med. 4:1741–1752. 2015. View Article : Google Scholar :
|
|
19
|
Xia Z, Chen C, Chen P, Xie H and Luo X:
MicroRNAs and their roles in osteoclast differentiation. Front Med.
5:414–419. 2011. View Article : Google Scholar
|
|
20
|
Arfat Y, Xiao WZ, Ahmad M, Zhao F, Li DJ,
Sun YL, Hu L, Zhihao C, Zhang G, Iftikhar S, et al: Role of
microRNAs in osteoblasts differentiation and bone disorders. Curr
Med Chem. 22:748–758. 2015. View Article : Google Scholar
|
|
21
|
Gamez B, Rodriguez-Carballo E and Ventura
F: MicroRNAs and post-transcriptional regulation of skeletal
development. J Mol Endocrinol. 52:R179–R197. 2014. View Article : Google Scholar
|
|
22
|
Taipaleenmäki H, Hokland Bjerre L, Chen L,
Kauppinen S and Kassem M: Mechanisms in endocrinology: micro-RNAs:
Targets for enhancing osteoblast differentiation and bone
formation. Eur J Endocrinol. 166:359–371. 2012. View Article : Google Scholar
|
|
23
|
Cuetara BL, Crotti TN, O'Donoghue AJ and
McHugh KP: Cloning and characterization of osteoclast precursors
from the RAW264.7 cell line. In Vitro Cell Dev Biol Anim.
42:182–188. 2006. View Article : Google Scholar :
|
|
24
|
Parfitt AM, Drezner MK, Glorieux FH, Kanis
JA, Malluche H, Meunier PJ, Ott SM and Recker RR: Bone
histomorphometry: Standardization of nomenclature, symbols and
units. Report of the ASBMR Histomorphometry Nomenclature Committee.
J Bone Miner Res. 2:595–610. 1987. View Article : Google Scholar
|
|
25
|
Turner CH and Burr DB: Basic biomechanical
measurements of bone: A tutorial. Bone. 14:595–608. 1993.
View Article : Google Scholar
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
27
|
Jensen PR, Andersen TL, Hauge EM,
Bollerslev J and Delaisse JM: A joined role of canopy and reversal
cells in bone remodeling-lessons from glucocorticoid-induced
osteoporosis. Bone. 73:16–23. 2014. View Article : Google Scholar
|
|
28
|
Tamura Y, Kawao N, Yano M, Okada K,
Okumoto K, Chiba Y, Matsuo O and Kaji H: Role of plasminogen
activator inhibitor-1 in glucocorticoid-induced diabetes and
osteopenia in mice. Diabetes. 64:2194–2206. 2015. View Article : Google Scholar
|
|
29
|
Panwar P, Soe K, Guido RV, Bueno RV,
Delaisse JM and Bromme D: A novel approach to inhibit bone
resorption: Exosite inhibitors against cathepsin K. Br J Pharmacol.
173:396–410. 2016. View Article : Google Scholar
|
|
30
|
Helali AM, Iti FM and Mohamed IN:
Cathepsin K inhibitors: A novel target but promising approach in
the treatment of osteoporosis. Curr Drug Targets. 14:1591–1600.
2013. View Article : Google Scholar
|
|
31
|
Brixen K, Chapurlat R, Cheung AM, Keaveny
TM, Fuerst T, Engelke K, Recker R, Dardzinski B, Verbruggen N,
Ather S, et al: Bone density, turnover, and estimated strength in
postmenopausal women treated with odanacatib: A randomized trial. J
Clin Endocrinol Metab. 98:571–580. 2013. View Article : Google Scholar
|
|
32
|
Fan JJ, Cao LG, Wu T, Wang DX, Jin D,
Jiang S, Zhang ZY, Bi L and Pei GX: The dose-effect of icariin on
the proliferation and osteogenic differentiation of human bone
mesenchymal stem cells. Molecules. 16:10123–10133. 2011. View Article : Google Scholar
|
|
33
|
Cui J, Zhu M, Zhu S, Wang G, Xu Y and Geng
D: Inhibitory effect of icariin on Ti-induced inflammatory
osteoclastogenesis. J Surg Res. 192:447–453. 2014. View Article : Google Scholar
|
|
34
|
Li T, Li H, Li T, Fan J, Zhao RC and Weng
X: MicroRNA expression profile of dexamethasone-induced human bone
marrow-derived mesenchymal stem cells during osteogenic
differentiation. J Cell Biochem. 115:1683–1691. 2014. View Article : Google Scholar
|
|
35
|
Ko JY, Chuang PC, Chen MW, Ke HC, Wu SL,
Chang YH, Chen YS and Wang FS: MicroRNA-29a ameliorates
glucocorticoid-induced suppression of osteoblast differentiation by
regulating β-catenin acetylation. Bone. 57:468–475. 2013.
View Article : Google Scholar
|
|
36
|
Wang FS, Chuang PC, Lin CL, Chen MW, Ke
HJ, Chang YH, Chen YS, Wu SL and Ko JY: MicroRNA-29a protects
against glucocorticoid-induced bone loss and fragility in rats by
orchestrating bone acquisition and resorption. Arthritis Rheum.
65:1530–1540. 2013. View Article : Google Scholar
|
|
37
|
Li H, Li T, Fan J, Li T, Fan L, Wang S,
Weng X, Han Q and Zhao RC: miR-216a rescues dexamethasone
suppression of osteogenesis, promotes osteoblast differentiation
and enhances bone formation, by regulating c-Cbl-mediated PI3K/AKT
pathway. Cell Death Differ. 22:1935–1945. 2015. View Article : Google Scholar :
|