Introduction
Acute liver failure (ALF) is a type of severe liver
disease which frequently occurs in patients with no medical history
of liver disease, characterized by sudden apoptosis of liver cells
over a short period, rapid deterioration of liver function and
complications including hepatic encephalopathy and coagulation
disorders (1). Due to high
incidence, rapid development, severe illness, poor prognosis and
high mortality rate, acute liver failure seriously endangers the
life of patients and has become a challenge to clinicians (2). ALF exhibits a complex pathogenesis,
and is a complicated pathophysiological process involving numerous
factors. Endotoxemia and special liver toxic substances are
important factors that lead to the incidence and development of
liver failure (1). It is believed
that lipopolysaccharide (LPS)/D-galactosamine (D-GalN) may lead to
the injury of liver cells in mice by various mechanisms including
liver cell apoptosis, generation of free radicals and lipid
peroxidation (3). Research
regarding the role of hepatocyte apoptosis and oxidative stress in
the pathogenesis of ALF is of primary concern (4). It has previously been demonstrated
that hepatic tissues of patients with ALF generate increased
reactive oxygen species (ROS), resulting in an oxidation-reduction
imbalance and thereby oxidative stress injury, which results in the
apoptosis of liver cells by a mitochondrial pathway. Therefore,
antioxidants and anti-apoptotic therapeutics are necessary in the
treatment of ALF (5). Excluding
liver transplantation, there is no effective therapeutic strategy
in the treatment of ALF (6).
Therefore, early therapeutic intervention in ALF has high research
value and clinical significance.
A previous study suggested that transcription factor
nuclear factor erythroid 2-related factor (Nrf) 2 is a key factor
in the regulation of numerous antioxidants, which may maintain the
balance of oxidation and reduction, inhibit apoptosis and protect
against inflammation (7). The
expression of Nrf2 is decreased under normal circumstances, very
few stable Nrf2 units translocate into the nucleus and bind to
antioxidant response elements (ARE) (8). However, the balance is disturbed
under oxidative stress; Nrf2 escapes the Keap1-mediated ubiquitin
degradation pathway and rapidly translocates into the nucleus,
binds to ARE to initiate the transcription of phase II detoxifying
enzymes and various antioxidant enzyme genes downstream of ARE,
thus resisting external harmful stimuli. Target genes in Nrf2
include heme oxygenase (HO-1), superoxide dismutase (SOD),
dependent coenzyme/II oxidoreductase-1 and glutamylcysteine
synthase. Deficiency or activation disorders of Nrf2 may increase
the sensitivity of stimuli, leading to cell dysfunction and
apoptosis, prolonged inflammatory repair and other pathological
alterations (7,9).
Intestinal endotoxemia is important in liver
failure. The pathophysiological process of endotoxin-induced liver
injury involves a variety of signaling molecules and signal
transduction pathways, of which the LPS/Toll like receptor (TLR)4
signal transduction pathway exhibits a predominant role (10). TLR4/nuclear factor (NF)-κB are
classical inflammatory signals, and high expression of TLR4/NF-κB
is associated with liver injury. It has been indicated that
therapeutic intervention of the expression of TLR4 and NF-κB may
improve liver function to a certain extent.
Oxymatrine (Fig.
1), additionally termed matrine, is an alkaloid with a
tetracyclicquinolizidine structure extracted from Sophora
alopecuroides L. (11), which
belongs to the Ningxia herbal medicine Sophora. With
anti-inflammatory, hepatoprotective and anti-neoplastic functions,
Oxymatrine is effective in the treatment of cardiovascular disease
(12). As a drug with various
pharmacological effects, Oxymatrine has been applied to the
treatment of hepatitis B and liver fibrosis, in addition to
preventing chronic kidney disease development into renal
interstitial fibrosis in patients, with low rates of adverse
reaction (11,13). Therefore, the specific purpose of
the present study was to investigate the protective effects of
oxymatrine against LPS/D-GalN-induced acute liver failure and
potential mechanisms in the mouse model.
Materials and methods
Animals and experimental protocol
Male, C57BL/6 mice (weight, 20–22 g; age, 6 weeks
old) were obtained from the Center of Experimental Animals of
Chongqing University (Chongqing, China) and were housed under
standard conditions (temperature, 22±2°C; humidity, 55±5%, 12-h
light/dark cycle) with free access to food and water. All animal
experiments carried out in the present study were approved by the
Care and Use of Laboratory Animals of Fuling Center Hospital of
Chongqing (Chongqing, China). Mice were randomly assigned to
normal, ALF model or Oxymatrine groups. In the ALF model and
Oxymatrine groups, mice were administrated 4 mg/kg LPS and 600
mg/kg D-GalN (intraperitoneal injection) for 24 h. Then, mice of
the Oxymatrine group were treated with 120 mg/kg/day oxymatrine for
4 weeks. Mice in the normal and ALF model groups were treated with
normal saline.
Blood clinical analyses
Following treatment with Oxymatrine, blood samples
were collected and centrifuged at 2,000 × g for 10 min at 4°C. The
serum was then collected and used to measure aspartate transaminase
(AST) and alanine aminotransferase (ALT) levels using ELISA kits
(C010-2 and C009-2 respectively; Nanjing Jiancheng Bioengineering
Institute, Nanjing, China).
Analysis of oxidative stress and
inflammation
Following treatment with Oxymatrine, the tissue
samples were homogenized in lysis buffer (Beyotime Institute of
Biotechnology, Jiangsu, China) and used to analyze superoxide
dismutase (SOD), glutathione peroxidase (GSH-Px), malondialdehyde
(MDA), tumor necrosis factor (TNF)-α and myeloperoxidase (MPO)
activities using ELISA kits (A001-1-1, A005, A003-1, H052 and A044
respectively; Nanjing, Jiancheng Bioengineering Institute).
Western blot analysis
Following treatment with Oxymatrine, the tissues
samples were homogenized in radioimmunoprecipitation assay buffer
(Beyotime Institute of Biotechnology), and the protein
concentrations were determined using a BCA protein assay reagent
(Beyotime Institute of Biotechnology). A total of 50–60 µg proteins
were separated on 10% SDS-PAGE gel and transferred onto
polyvinylidene membranes (Thermo Fisher Scientific, Inc., Waltham,
MA, USA). Following blocking with 5% skim milk for 1 h at 37°C, the
membranes were incubated with primary antibodies against Nrf2
(1:500; cat. no. sc-81342;), HO-1 (1:500; cat. no. sc-136256) TLR4
(1:300; cat. no. sc-293072), myeloid differentiation primary
response 88 (MyD88; sc-11356; 1:300), NF-κB (1:300; cat. no.
sc-136970) and GAPDH (1:300; cat. no. sc-59540) all obtained from
Santa Cruz Biotechnology, Inc. (Dallas, TX, USA) at 4°C overnight.
Following washing with TBST 3 times, the membranes were probed with
a horseradish peroxidase-conjugated secondary antibody (1:5,000;
Cell Signaling Technology, Inc., Danvers, MA USA) at room
temperature for 2 h. Protein was developed with the ECL Plus
Western Blotting Detection system (GE Healthcare Life Sciences,
Little Chalfont, UK) and analyzed using Image_Lab version 3.0
(Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
Data are presented as the mean ± standard deviation
using SPSS version 19.0 (IBM Corp., Armonk, NY, USA). Comparison
among groups was determined by one-way analysis of variance
followed by the Tukey post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Oxymatrine increases survival rate in
ALF mice
In LPS/D-GalN -induced ALF mice, survival rate was
40% which was a decreased value compared with normal control group.
Treatment with Oxymatrine significantly increased survival rate of
LPS/D-GalN-induced ALF mice, compared with the LPS/D-GalN-induced
ALF model group (Fig. 2).
Oxymatrine decreases plasma AST and
ALT serum levels in ALF mice
Conversely, AST and ALT serum levels of ALF model
mice were increased compared with normal control group. Treatment
with Oxymatrine significantly reduced AST and ALT serum levels in
ALF mice, compared with ALF model group (Fig. 3).
Oxymatrine exhibits varied effects on
SOD, GSH-Px and MDA activities in ALF mice
It was observed that there was a significant
decrease in SOD and GSH-Px, and an increase in MDA activities in
the ALF model group, compared with normal control group.
Pre-treatment with Oxymatrine significantly increased SOD and
GSH-Px activities and decreased MDA activities in ALF mice,
compared with the ALF model group (Fig. 4).
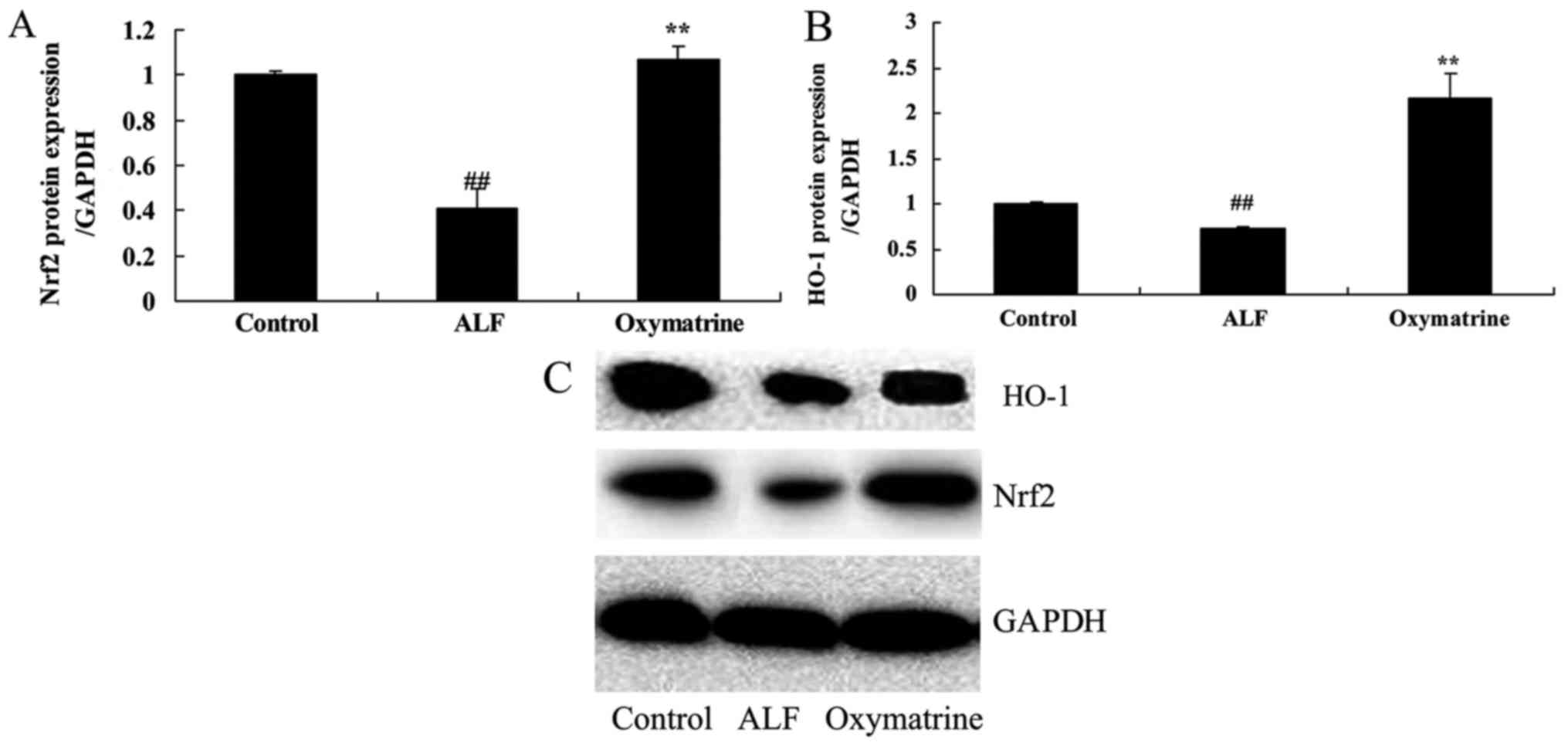
Oxymatrine activates Nrf2 and HO-1
protein expression in ALF mice
To explore the potential effects of Oxymatrine on
ALF mice, Nrf2 and HO-1 protein expression was measured using
western blot analysis. The results of the western blot analysis
demonstrated that Nrf2 and HO-1 protein expression of ALF mice was
downregulated in ALF mice, compared with normal control group.
Oxymatrine significantly increased Nrf2 and HO-1 protein expression
in ALF mice, compared with ALF model group (Fig. 5).
Oxymatrine decreases plasma TNF-α and
MPO levels in ALF mice
The results demonstrated that TNF-α and MPO
levels of the ALF model group were upregulated, compared with
normal control group. Treatment with Oxymatrine significantly
decreased TNF-α and MPO levels in ALF mice, compared with
ALF model group (Fig. 6).
Oxymatrine suppresses TLR4, MyD88 and
NF-κB protein expression in ALF mice
The present study next sought to investigate the
role of TLR4, MyD88 and NF-κB. Protein expression was measured
using Western blot analysis. As presented in Fig. 7, TLR4, MyD88 and NF-κB protein
expression in ALF mice was increased, compared with normal control
group. Oxymatrine significantly suppressed TLR4, MyD88 and NF-κB
protein expression in ALF mice, compared with ALF model group
(Fig. 7).
Discussion
The incidence of liver disease is high in China due
to hepatitis B and C viral infections. According to the report of
the World Health Organization, ~2 billion people are infected with
hepatitis B worldwide and 350 million of these cases are chronic
infections (3). In addition, ~1
million fatal cases of liver cirrhosis, liver failure and
hepatocellular carcinoma are induced by hepatitis B every year
(14). Hepatic cirrhosis accounts
for 10–20% of all liver diseases based on pathological features and
progression of disease (15).
Liver failure may be divided into four categories, including acute
liver failure, sub-acute liver failure, acute-on-chronic liver
failure and chronic liver failure, each of which account for
~0.25–5% of advanced liver diseases, however the mortality rate may
be up to 60–80% (2,15). Chronic or acute/subacute liver
failure frequently occurs in China, accounting for >90% of all
liver diseases (16). The present
study firstly observed that Oxymatrine effectively increased the
survival rate and reduced AST and ALT activities in LPS/
D-GalN-induced ALF mice. Li et al (17) reported that the effects of
oxymatrine prevents against arsenic trioxide-induced liver
injury.
The body has developed a set of complex systems in
response to the damage that may result from oxidative stress, one
of which is ARE (18). ARE is a
cis-acting element located upstream of phase II detoxifying enzymes
and antioxidant protein/enzyme genes (19). It has previously been demonstrated
that Nrf2 is the activator of ARE and the key transcription factor
which regulates the oxidative stress response, the sensor for toxic
substances and exogenous oxidative stress, and is closely
associated with incidence and development of inflammation,
respiratory system disease, malignant tumors, precancerous lesions
and cardiovascular diseases (8).
Under normal circumstances, Nrf2 is in an inactive state via
binding to cytoplasmic specific receptor Keap-1 in the form of a
heterologous dipolymer (19). It
is activated by oxidative stress, uncouples from Keap1 and
translocates into the nucleus to bind to ARE. The activated ARE
then mediates target gene transcription, to increase the resistance
of cells to oxidative stress. SOD, HO-1 and various other Nrf2
target genes are expressed in the liver. HO-1, additionally termed
heat shock protein 32, is an endogenous antioxidant enzyme which is
of current research interest (20). It has previously been demonstrated
that HO-1 and metabolic products of heme may protect against
oxidative stress, and the targeted activation of HO-1 may prevent
injury to the liver (9,21). The results of the present study
demonstrated that Oxymatrine significantly increased SOD and GSH-Px
activities and decreased MDA activities in ALF mice through
activating Nrf2/HO-1 expression. Jiang et al (22) demonstrated that Oxymatrine
ameliorates renal ischemia-reperfusion injury via the Nrf2/HO-1
pathway.
TLR4 is one of the natural immune recognition
receptors which is primarily used to recognize LPS and mediate
transmembrane signal transduction. LPS activates Kupffer cells (KC)
via a TLR-mediated signal transduction pathway, with a mechanism as
follows: LPS and TLR4 interact with each other to transfer LPS into
TLR4/lymphocyte antigen 96 (MD2) by LPS binding protein (LBP) and
cluster of differentiation 14, and then LPS binds to TLR4/MD2 to
induce the accumulation of TLR4, leading to the activation of
intracellular MyD88 dependent pathway, and thereby promoting the
expression and activation of NF-κB in cells, so as to stimulate
cells to produce a variety of inflammatory mediators including TNF,
IL-1, IL-6, nitric oxide, leukotrienes and thus resulting in the
inflammatory reaction (23–25).
This aids in the body's removal of endotoxins, however an excessive
inflammatory reaction results in liver cell apoptosis and necrosis
(26). It was demonstrated that
Oxymatrine significantly suppressed TNF-α and MPO activities
via the TLR4/MyD88/NF-κB-dependent inflammatory signaling pathways.
Fan et al (27) reported
that Oxymatrine protects rat brains against focal ischemia and
downregulates TLR4, TLR2, MyD88 and NF-κB.
In conclusion, oxymatrine effectively attenuated
LPS/D-GalN-induced ALF through oxidative damage, by activation of
Nrf2/HO-1 and suppression of TLR4-dependent inflammatory signaling
pathways. Identification of the mechanism underlying these effects
of Oxymatrine may aid in the development of a novel therapeutic
strategy and subsequent clinical application in the treatment of
ALF in the future.
Acknowledgements
The present study was partially supported by Health
and Family Planning Commission of Chongqing municipal, China (grant
no. 2016ZDXM038).
References
|
1
|
Yonekawa C, Nakae H, Tajimi K and Asanuma
Y: Effectiveness of combining plasma exchange and continuous
hemodiafiltration in patients with postoperative liver failure.
Artif Organs. 29:324–328. 2005. View Article : Google Scholar
|
|
2
|
Nalos M, Leverve X, Huang S, Weisbrodt L,
Parkin R, Seppelt I, Ting I and Mclean A: Half-molar sodium lactate
infusion improves cardiac performance in acute heart failure: A
pilot randomised controlled clinical trial. Crit Care. 18:R482014.
View Article : Google Scholar :
|
|
3
|
Lee WM, Hynan LS, Rossaro L, Fontana RJ,
Stravitz RT, Larson AM, Davern TJ II, Murray NG, McCashland T,
Reisch JS, et al: Intravenous N-acetylcysteine improves
transplant-free survival in early stage non-acetaminophen acute
liver failure. Gastroenterology. 137(856–864): 864.e12009.
|
|
4
|
Moniaux N, Song H, Darnaud M, Garbin K,
Gigou M, Mitchell C, Samuel D, Jamot L, Amouyal P, Amouyal G, et
al: Human
hepatocarcinoma-intestine-pancreas/pancreatitis-associated protein
cures fas-induced acute liver failure in mice by attenuating
free-radical damage in injured livers. Hepatology. 53:618–627.
2011. View Article : Google Scholar
|
|
5
|
Shi M, Zhang Z, Xu R, Lin H, Fu J, Zou Z,
Zhang A, Shi J, Chen L, Lv S, et al: Human mesenchymal stem cell
transfusion is safe and improves liver function in acute-on-chronic
liver failure patients. Stem Cells Transl Med. 1:725–731. 2012.
View Article : Google Scholar :
|
|
6
|
Larsen FS, Schmidt LE, Bernsmeier C,
Rasmussen A, Isoniemi H, Patel VC, Triantafyllou E, Bernal W,
Auzinger G, Shawcross D, et al: High-volume plasma exchange in
patients with acute liver failure: An open randomised controlled
trial. J Hepatol. 64:69–78. 2016. View Article : Google Scholar
|
|
7
|
Huang YJ, Chen P, Lee CY, Yang SY, Lin MT,
Lee HS and Wu YM: Protection against acetaminophen-induced acute
liver failure by omentum adipose tissue derived stem cells through
the mediation of Nrf2 and cytochrome P450 expression. J Biomed Sci.
23:52016. View Article : Google Scholar :
|
|
8
|
Mobasher MA, González-Rodriguez A,
Santamaría B, Ramos S, Martín MÁ, Goya L, Rada P, Letzig L, James
LP, Cuadrado A, et al: Protein tyrosine phosphatase 1B modulates
GSK3β/Nrf2 and IGFIR signaling pathways in acetaminophen-induced
hepatotoxicity. Cell Death Dis. 4:e6262013. View Article : Google Scholar :
|
|
9
|
Wang CF, Wang ZY and Li JY: Dual
protective role of HO-1 in transplanted liver grafts: A review of
experimental and clinical studies. World J Gastroenterol.
17:3101–3108. 2011.
|
|
10
|
Xu CL, Hao YH, Lu YP, Tang ZS, Yang XC, Wu
J, Zheng X, Wang BJ, Liu J and Yang DL: Upregulation of toll-like
receptor 4 on T cells in PBMCs is associated with disease
aggravation of HBV-related acute-on-chronic liver failure. J
Huazhong Univ Sci Technolog Med Sci. 35:910–915. 2015. View Article : Google Scholar
|
|
11
|
Zhao HW, Zhang ZF, Chai X, Li GQ, Cui HR,
Wang HB, Meng YK, Liu HM, Wang JB, Li RS, et al: Oxymatrine
attenuates CCl4-induced hepatic fibrosis via modulation of
TLR4-dependent inflammatory and TGF-β1 signaling pathways. Int
Immunopharmacol. 36:249–255. 2016. View Article : Google Scholar
|
|
12
|
Zhang W, Zhang J, Liu YK, Liu J, Wang X,
Xu Q, Wang Y, Xu X and Dai G: Cardioprotective effects of
oxymatrine on isoproterenol-induced heart failure via regulation of
DDAH/ADMA metabolism pathway in rats. Eur J Pharmacol. 745:29–35.
2014. View Article : Google Scholar
|
|
13
|
Fu L, Xu Y, Tu L, Huang H, Zhang Y, Chen
Y, Tao L and Shen X: Oxymatrine inhibits aldosterone-induced rat
cardiac fibroblast proliferation and differentiation by attenuating
smad-2,-3 and −4 expression: An in vitro study. BMC Complement
Altern Med. 16:2412016. View Article : Google Scholar :
|
|
14
|
Qin G, Shao JG, Wang B, Shen Y, Zheng J,
Liu XJ, Zhang YY, Liu YM, Qin Y and Wang LJ: Artificial liver
support system improves short- and long-term outcomes of patients
with HBV-associated acute-on-chronic liver failure: A single-center
experience. Medicine (Baltimore). 93:e3382014. View Article : Google Scholar :
|
|
15
|
Sterneck M, Settmacher U, Ganten T,
Sarrazin C, Speidel N, Broering D, Heyne N, Paulus E, Mertens M and
Fischer L: Improvement in gastrointestinal and health-related
quality of life outcomes after conversion from mycophenolate
mofetil to enteric-coated mycophenolate sodium in liver transplant
recipients. Transplant Proc. 46:234–240. 2014. View Article : Google Scholar
|
|
16
|
Kribben A, Gerken G, Haag S,
Herget-Rosenthal S, Treichel U, Betz C, Sarrazin C, Hoste E, Van
Vlierberghe H, Escorsell A, et al: Effects of fractionated plasma
separation and adsorption on survival in patients with
acute-on-chronic liver failure. Gastroenterology. 142:782–789.e3.
2012. View Article : Google Scholar
|
|
17
|
Li L, Liu Q, Fan L, Xiao W, Zhao L, Wang
Y, Ye W, Lan F, Jia B, Feng H, et al: Protective effects of
oxymatrine against arsenic trioxide-induced liver injury.
Oncotarget. 8:12792–12799. 2017.
|
|
18
|
Crespo I, Miguel BS, Laliena A, Alvarez M,
Culebras JM, González-Gallego J and Tuñón MJ: Melatonin prevents
the decreased activity of antioxidant enzymes and activates nuclear
erythroid 2-related factor 2 signaling in an animal model of
fulminant hepatic failure of viral origin. J Pineal Res.
49:193–200. 2010.
|
|
19
|
Xu W, Hellerbrand C, Köhler UA, Bugnon P,
Kan YW, Werner S and Beyer TA: The Nrf2 transcription factor
protects from toxin-induced liver injury and fibrosis. Lab Invest.
88:1068–1078. 2008. View Article : Google Scholar
|
|
20
|
Fang Fang, Li H, Qin T, Li M and Ma S:
Thymol improves high-fat diet-induced cognitive deficits in mice
via ameliorating brain insulin resistance and upregulating
NRF2/HO-1 pathway. Metab Brain Dis. 32:385–393. 2017. View Article : Google Scholar
|
|
21
|
Chen X, Gong X, Jiang R, Wang B, Kuang G,
Li K and Wan J: Resolvin D1 attenuates CCl4-induced acute liver
injury involving up-regulation of HO-1 in mice. Immunopharmacol
Immunotoxicol. 38:61–67. 2016. View Article : Google Scholar
|
|
22
|
Jiang G, Liu X, Wang M, Chen H, Chen Z and
Qiu T: Oxymatrine ameliorates renal ischemia-reperfusion injury
from oxidative stress through Nrf2/HO-1 pathway. Acta Cir Bras.
30:422–429. 2015. View Article : Google Scholar
|
|
23
|
Luo JX, Zhang Y, Hu XY, Chen G, Liu XY,
Nie HM, Liu JL and Wen DC: Aqueous extract from Aconitum
carmichaelii Debeaux reduces liver injury in rats via
regulation of HMGB1/TLR4/NF-KappaB/caspase-3 and PCNA signaling
pathways. J Ethnopharmacol. 183:187–192. 2016. View Article : Google Scholar
|
|
24
|
Liu H, Zhang W, Dong S, Song L, Zhao S, Wu
C, Wang X, Liu F, Xie J, Wang J and Wang Y: Protective effects of
sea buckthorn polysaccharide extracts against LPS/d-GalN-induced
acute liver failure in mice via suppressing TLR4-NF-κB signaling. J
Ethnopharmacol. 176:69–78. 2015. View Article : Google Scholar
|
|
25
|
Liang Q, Zhang J, Tang W, Geng Q, Xu X and
Jiang W: Triptolide attenuates acute small-for-size liver graft
injury in rats by inhibition of Toll-like receptor 4. Transplant
Proc. 46:3303–3308. 2014. View Article : Google Scholar
|
|
26
|
Shah N, de Oca Montes M, Jover-Cobos M,
Tanamoto K, Muroi M, Sugiyama K, Davies NA, Mookerjee RP, Dhar DK
and Jalan R: Role of toll-like receptor 4 in mediating multiorgan
dysfunction in mice with acetaminophen induced acute liver failure.
Liver Transpl. 19:751–761. 2013. View
Article : Google Scholar
|
|
27
|
Fan H, Li L, Zhang X, Liu Y, Yang C, Yang
Y and Yin J: Oxymatrine downregulates TLR4, TLR2, MyD88, and
NF-kappaB and protects rat brains against focal ischemia. Mediators
Inflamm. 2009:7047062009. View Article : Google Scholar
|