Introduction
Spinal cord injury (SCI) refers to different degrees
of injury to the spinal cord due to a variety of external direct or
indirect traumas (1). SCI usually
leads to severe consequences, such as loss of sensation at the area
of injury, partial dysfunction of limbs (2). With the rapid development of modern
science, technology, industry and transportation, the incidence of
SCI has increased significantly; therefore, the epidemiological
investigations about SCI are also increasing. Most epidemiological
investigations demonstrate that the overall incidence rate of SCI
increases annually (3).
Additionally, previous age spectrum studies on SCI revealed that
SCI mostly occurs in young adults (especially due to traffic- and
factory-associated accidents) (2).
In addition, SCI has very high disability rate, which brings
serious economic and emotional burdens to individuals, families and
society (4).
SCI is divided into two stages: One is the injury to
specific regions of the spinal cord caused by the initial trauma;
the other is a secondary injury involving a series of biochemical,
molecular and cellular changes (5). SCI leads to a systemic inflammatory
response, and inflammatory cells invade other remote organs such as
the liver, lungs and kidneys, causing damage to these organs.
Inflammation and oxidative stress are two main factors of SCI, and
enhance the release of excitatory amino acids in the process of
cell apoptosis, to upregulate the generation of reactive oxygen
species and lipid peroxide, causing SCI-induced secondary injury
(6).
Protein kinase B (Akt) is a key kinase regulating
the proliferation, differentiation, apoptosis and death of cells.
Akt is activated by translocation to the inner surface of cell
membranes and subsequent phosphorylation (7). Phosphatidylinositol 3-kinase (PI3K)
is activated by the phosphorylation of the third hydroxyl group on
its inositol ring, which further phosphorylates inositol in the
cell membrane to translocate serine/threonine protein kinase
(8). Previous studies have
indicated that the activation of Akt protects nerves by inhibiting
the apoptosis of nervous cells, reducing the generation of oxygen
free radicals and suppressing the inflammatory reaction when SCI
occurs (7,9).
It is understood that the mitogen activated protein
kinase (MAPK) signaling pathway is an important intracellular
signal transduction pathway. The MAPK family has three subfamily
pathways, including extracellular regulated protein kinase
(ERK1/2), c-Jun N-terminal kinase (JNK) and P38 MAPK (10). When these MAPK pathways are
activated by a variety of factors such as lipopolysaccharide, they
will produce a large number of inflammatory mediators through
complex intracellular signal transduction, leading to inflammation
and promoting its development (10). Elevated phosphorylation is a sign
of activation of the ERK1/2, JNK and MAPK P38 signaling pathway
(11).
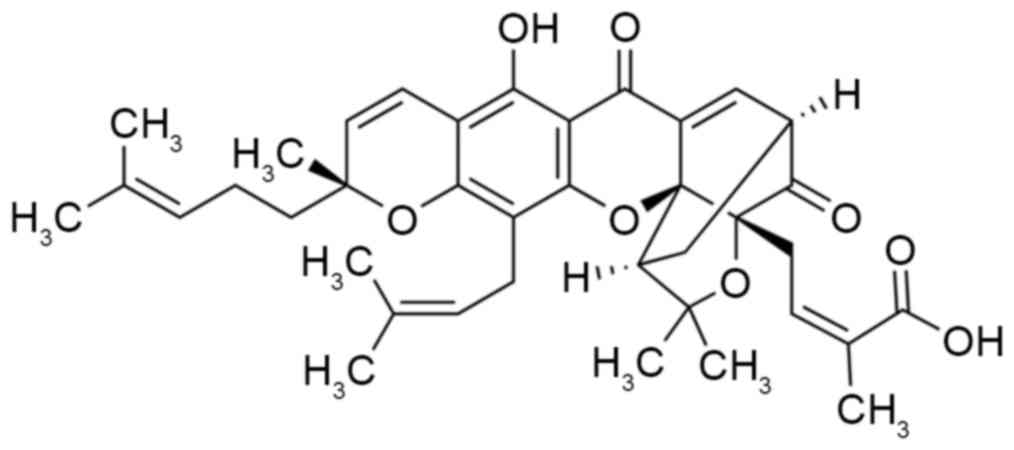
Gamboge is the dry resin secreted by Garcinia
hanburyi Hook.f. Gambogic acid, the main active constituent of
the resin produced by Garcinia hanburyi Hook.f, is a type of
natural small-molecule Xanthone drug (Fig. 1) (12). Gambogic acid has long been used as
anti-inflammatory, detoxification and insecticidal drug in
Southeast Asia (13). The present
study aimed to explore the protective effects of gambogic acid on
SCI, and its anti-inflammation mechanism in an SCI model in
vivo.
Materials and methods
Animals and in vivo treatment
Male Sprague-Dawley rats (weight, 200–220 g; n=24)
were purchased from the Animal Experiment Centre of Chongqing
Medical University (Chongqing, China) housed in a room controlled
for temperature (23±3°C) and relative humidity (40–60%), and had
free access to food and water. Animal care and study protocols were
carried out in accordance with the guidelines of the Institutional
Animal Care and Use Committee of the Second Affiliated Hospital of
Chongqing Medical University (Chongqing, China). Ethical approval
was received from the medical ethics committee of Hainan General
Hospital (Haikou, China). All rats were randomly assigned into
three groups (n=8/group): Sham-operated (sham), SCI model (SCI) and
gambogic acid (GA).
An SCI model was induced as previously described
(14). In the SCI model and GA
groups, rats were anesthetized with pentobarbital sodium (35 mg/kg,
intraperitoneally; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany),
and laminectomy was performed at the T9-T11 level in every rat,
which exposed the underlying cord. A weight-drop apparatus was used
to induce spinal cord contusion, at a height of 80 mm dropped onto
the exposed cord, representing moderate SCI. The skin and
musculature were sutured. In the sham group, rats underwent a sham
operation group without inducing SCI. In the GA group, rats
underwent SCI followed by 2 mg/kg/three days GA treatment for 10
weeks.
Behavioral testing and water content
of the spinal cord
All rats were assessed with the Basso, Beattie and
Bresnahan (BBB) scale test (15).
A score of 0 indicated complete hind limb paralysis, and a score of
21 denoted completely normal locomotor function. After rats were
anesthetized with 10% chloral hydrate (3.5 mg/kg,
intraperitoneally), rats were sacrificed using decollation and
spinal cord tissue samples were weighed to obtain the wet weight
(g), then dried at 68°C for 48 h to obtain the dry weight (g).
Water content of spinal cord (%)=dry weight/wet weight ×100%.
Determination of inflammatory
cytokines, oxidative stress and caspase-3 activity
After rats were anesthetized with 10% chloral
hydrate (3.5 mg/kg, intraperitoneally), and peripheral blood was
acquired from the eye socket. Following sacrifice by decollation,
serum was acquired after centrifuging blood at 2,000 × g for 10 min
at 4°C. Inflammatory cytokines [tumor necrosis factor (TNF)-α (cat.
no. PT516; Beyotime Institute of Biotechnology, Haimen, China),
interleukin (IL)-6 (cat. no. PI328; Beyotime Institute of
Biotechnology), IL-12 (cat. no. H010; Beyotime Institute of
Biotechnology) and IL-1β (cat. no. PI303, Beyotime Institute of
Biotechnology)] and oxidative stress factors [malondialdehyde (MDA;
cat. no. S0131; Beyotime Institute of Biotechnology), superoxide
dismutase (SOD; cat. no. S0101; Beyotime Institute of
Biotechnology), glutathione (GSH; cat. no. S0052; Beyotime
Institute of Biotechnology) and glutathione peroxidase (GSH-PX;
cat. no. S0058; Beyotime Institute of Biotechnology)] were measured
using ELISA kits using fluorescence microplate reader (Model 680,
Bio-Rad Laboratories, Inc., Hercules, CA, USA) at a wavelength of
450 nm. Caspase-3 activity (cat. no. C1116; Beyotime Institute of
Biotechnology) was measured using an ELISA kit using a fluorescence
microplate reader at a wavelength of 405 nm.
Western blot analysis
Radioimmunoprecipitation assay lysis buffer
containing PMSF was added into spinal cord tissue samples for 30
min on ice, and the supernatant was harvested by centrifugation at
4°C at 8,000 × g for 10 min. The protein concentration was detected
using a bicinchoninic acid protein assay kit. Proteins (50 µg) were
separated by 6–10% SDS-PAGE and transferred to polyvinylidene
difluoride membranes. The membranes were blocked in TBS with Tween
20 containing 5% nonfat dry milk for 1 h at 37°C. They were then
incubated with the following primary antibodies: Anti-receptor
activator of nuclear factor-κB ligand (RANKL; cat. no. sc-9073;
1:500), anti-toll-like receptor 4 (TLR4; cat. no. sc-10741; 1:500),
anti-nuclear factor (NF)-κB (cat. no. sc-298, 1:500),
anti-phosphorylated (p)-NF-κB (cat. no. sc-136548; 1:500),
anti-p-p38 (cat. no. sc-7975-R, 1:500), anti-p38 (cat. no. sc-7149;
1:500), anti-PI3K (cat. no. sc-7174; 1:500), anti-Akt (cat. no.
sc-8312; 1:500) and anti-GADPH (cat. no. sc-25778; 1:500; all Santa
Cruz Biotechnology, Inc.) at 4°C overnight. After being washed
three times with TBST for 15 min, the membranes were incubated with
a horseradish peroxidase-conjugated anti-mouse IgG secondary
antibody (cat. no. sc-2030; 1:5,000; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA) for 1 h at 37°C and visualized by BeyoECL
Plus (cat. no. P0018A; Beyotime Institute of Biotechnology).
Statistical analysis
Data are presented as the mean ± standard error and
analyzed using SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA).
Comparisons of data between groups were performed by one-way
analysis of variance followed by Duncan's multiple range test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
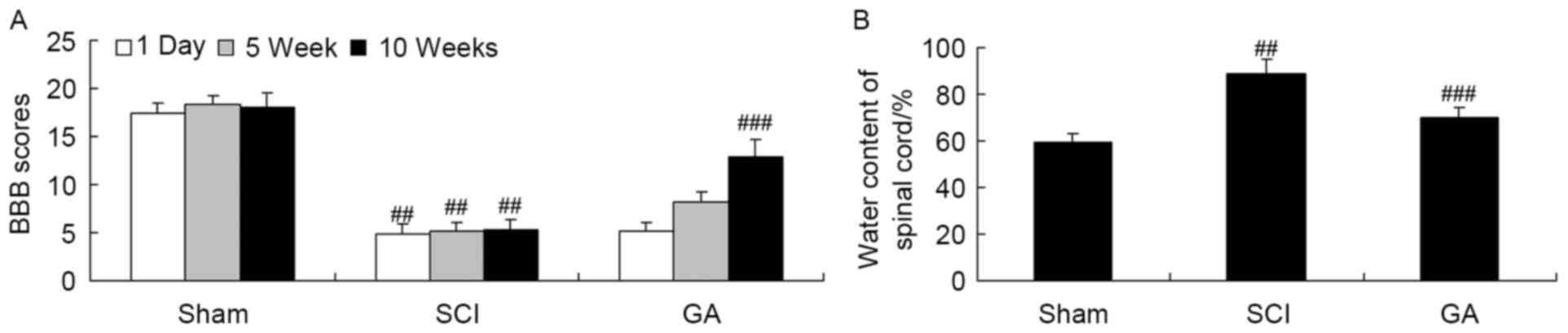
Gambogic acid effects on BBB scores
and the water content of the spinal cord in SCI rats
Firstly, SCI rats were used to investigate the
neuroprotective effects of gambogic acid. There was a significant
inhibition of BBB scores (Fig. 2A)
and increase of water content of the spinal cord (Fig. 2B) in the SCI model group, compared
with the sham group. Following treatment with gambogic acid for 10
weeks, BBB scores were significantly increased and water content of
spinal cord was significantly decreased in SCI rats by gambogic
acid treatment (Fig. 2).
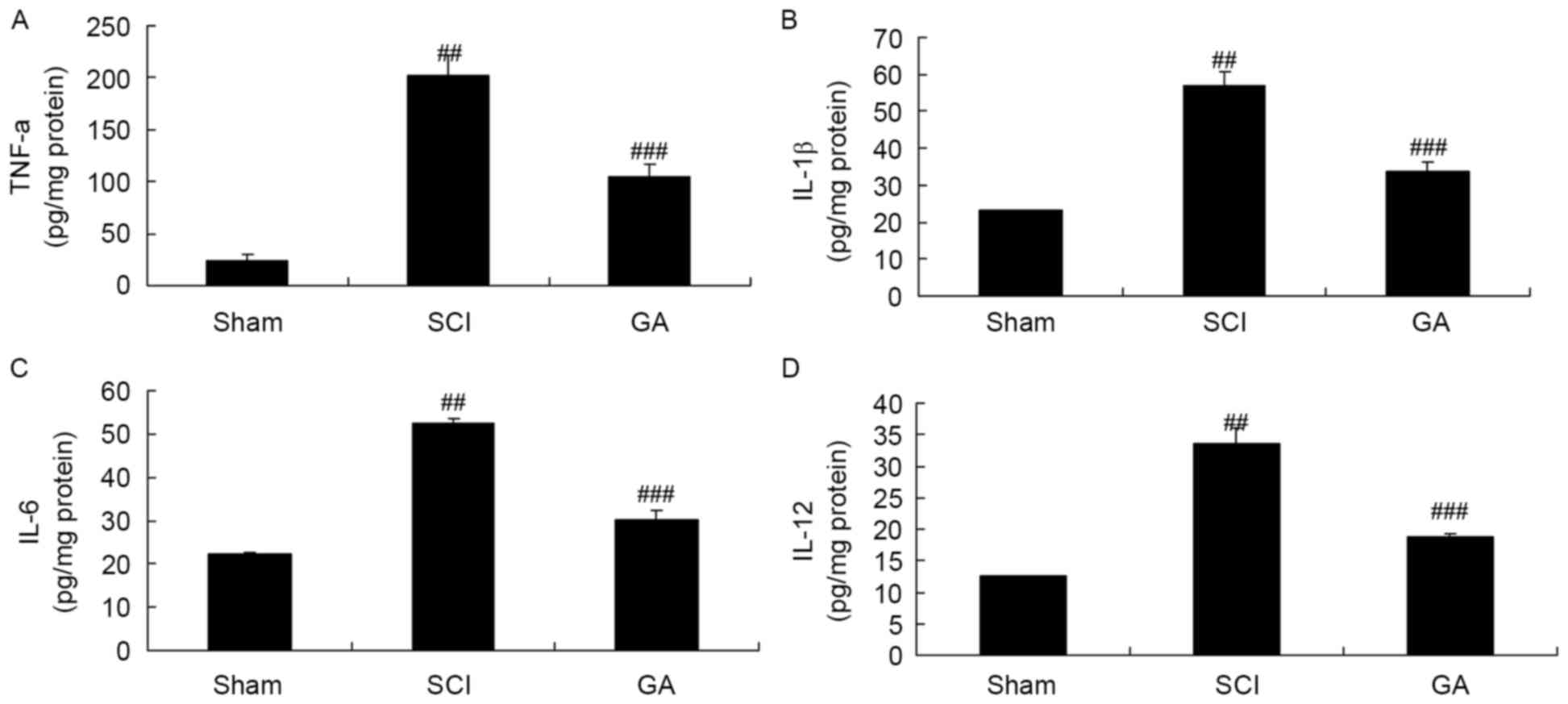
Gambogic acid effects on inflammatory
cytokines in SCI rats
In the SCI model group, TNF-α, IL-6, IL-12 and IL-1β
levels were significantly enhanced, compared with the sham group
(Fig. 3). Treatment with gambogic
acid significantly inhibited TNF-α, IL-6, IL-12 and IL-1β levels in
SCI rats, compared with the SCI model group (Fig. 3).
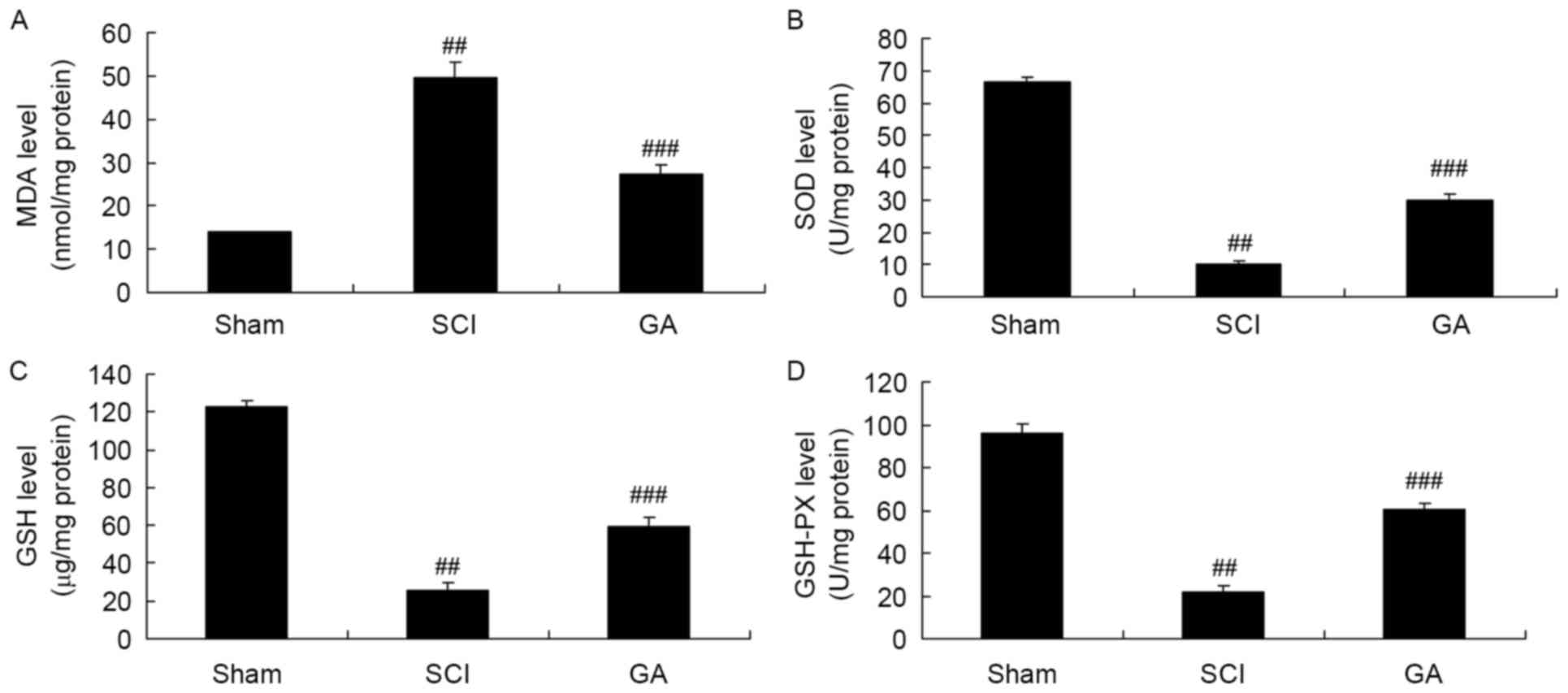
Gambogic acid effects on oxidative
stress in SCI rats
Next, there was a significant increase of MDA
activity and inhibition of SOD, GSH and GSH-PX activities in the
SCI model group, compared with sham group (Fig. 4). Treatment with gambogic acid
significantly reduced MDA activity and increased SOD, GSH and
GSH-PX activity inhibition in the SCI rats, compared with the SCI
model (Fig. 4).
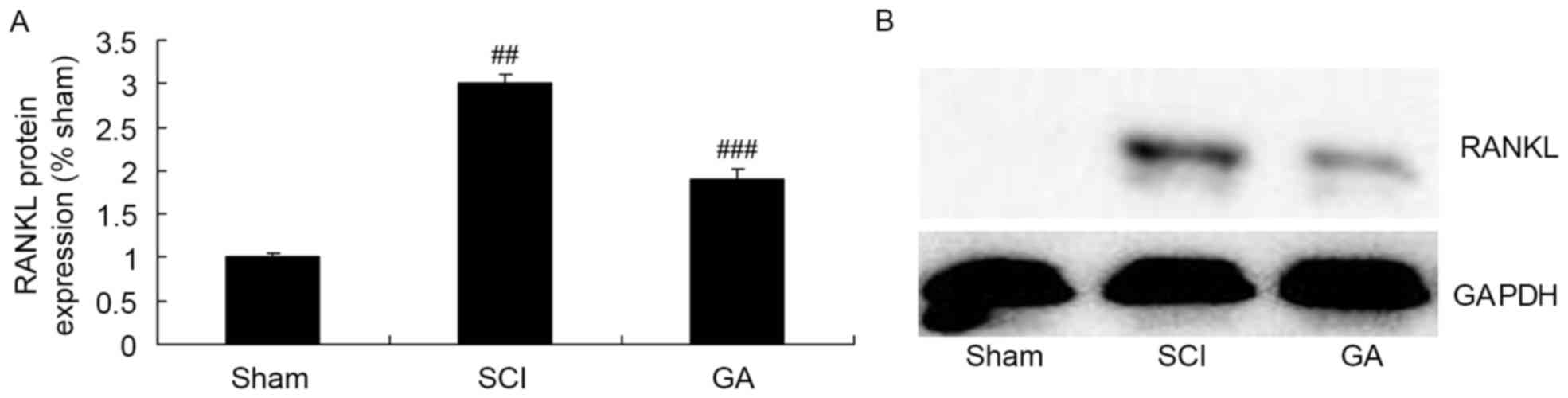
Gambogic acid effects on RANKL protein
expression in SCI rats
Western blotting was used to determine RANKL protein
expression in SCI rats. In the SCI model group, there was a
significant increase of RANKL protein expression, compared with the
sham group (Fig. 5). As presented
in Fig. 5, treatment with gambogic
acid significantly suppressed increase of RANKL protein expression
in SCI rats, compared with the SCI model group.
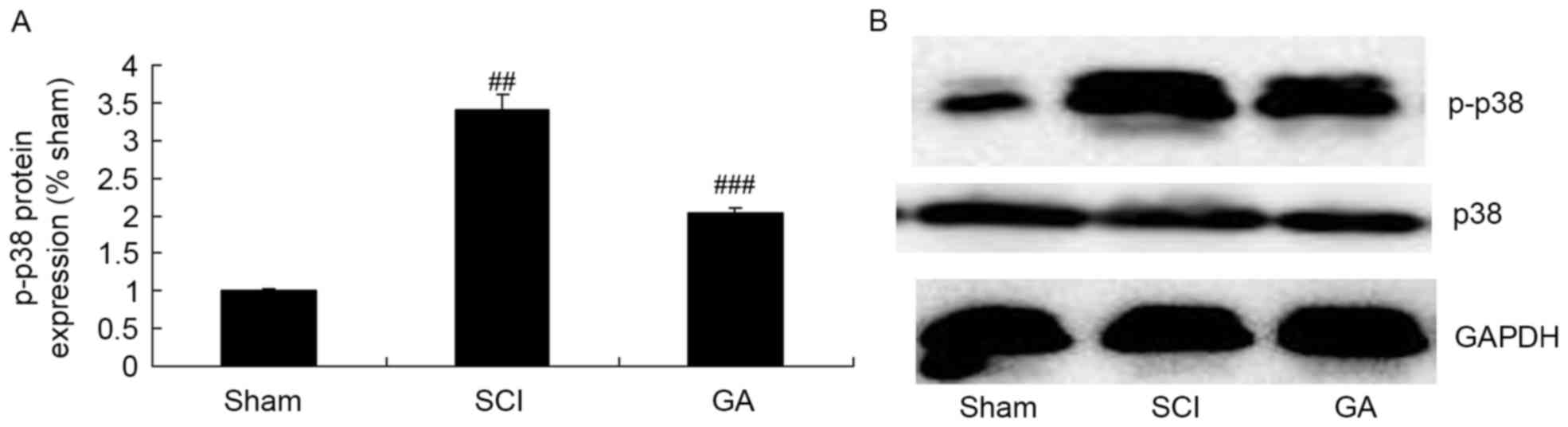
Gambogic acid effects on p-p38 protein
expression in SCI rats
The SCI model group exhibited a significant increase
of p-p38 protein expression in SCI model rats, compared with the
sham group (Fig. 6). Treatment
with gambogic acid significantly suppressed p-p38 protein
expression in SCI rats, compared with the SCI model group (Fig. 6).
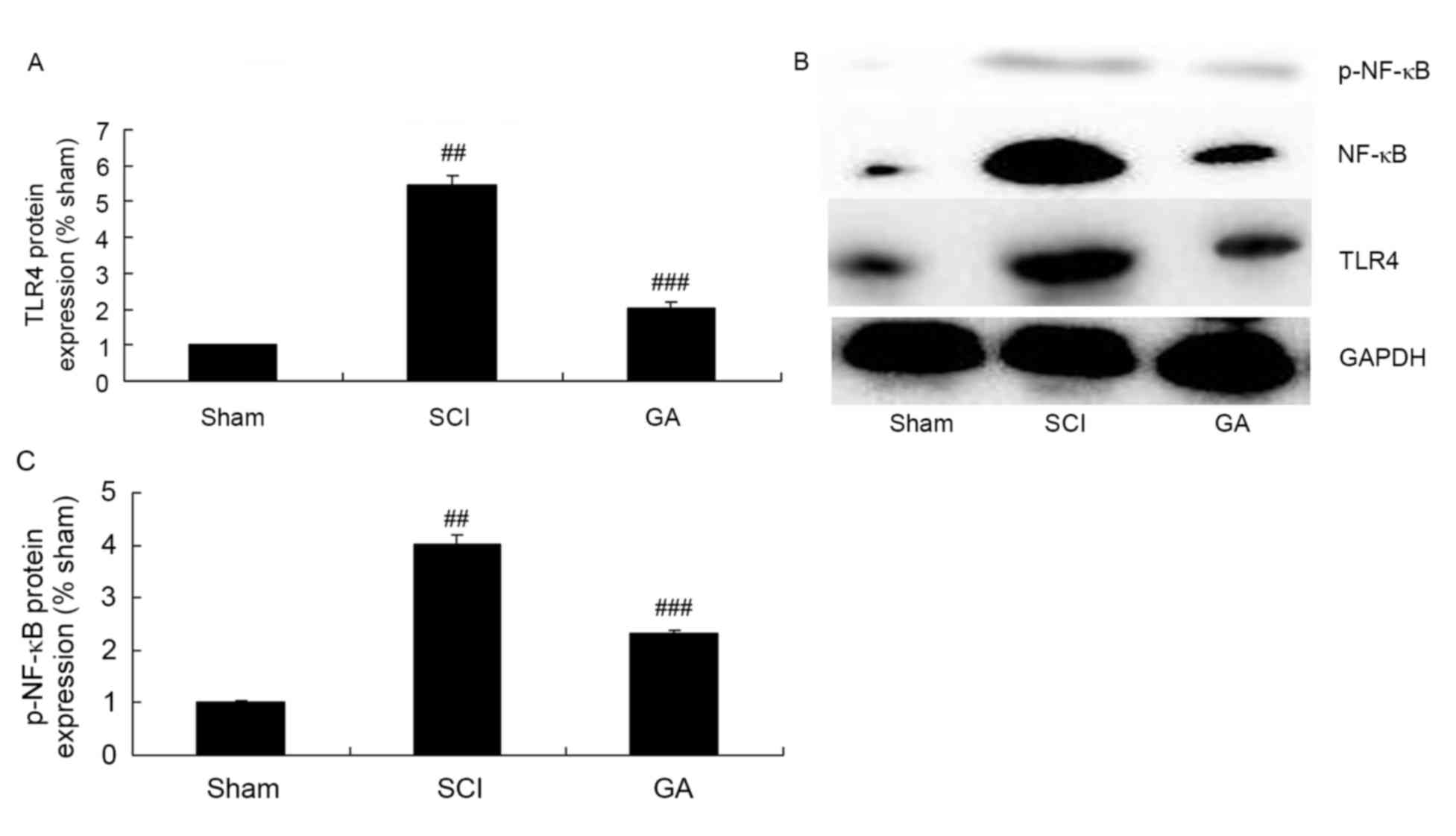
Gambogic acid effects on TLR4/NF-κB
protein expression in SCI rats
To explore the anti-inflammation mechanism of
gambogic acid in SCI, TLR4/NF-κB protein expression were measured
using western blot analysis. The results of western blot analysis
demonstrated a significant increase of TLR4 and p-NF-κB protein
expression in the SCI model group, compared with the sham group
(Fig. 7). However, gambogic acid
significantly suppressed TLR4 and p-NF-κB protein expression in SCI
rats, compared with the SCI model group (Fig. 7).
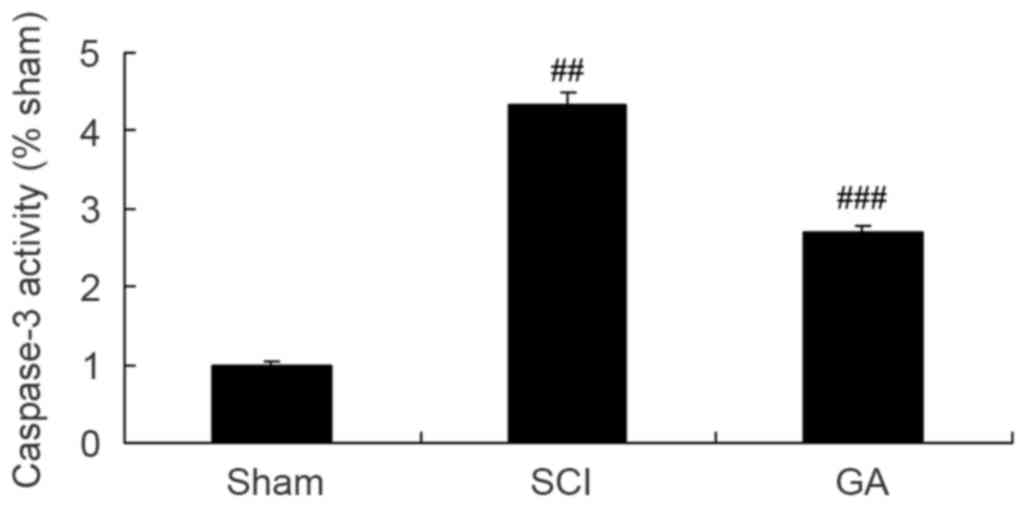
Gambogic acid effects on caspase-3
activity in SCI rats
As presented in Fig.
8, a significant increase of caspase-3 activity was observed in
the SCI model group, compared with the sham group. After treatment
with gambogic acid for 10 weeks, caspase-3 activity was
significantly inhibited in SCI rats, compared with the SCI model
group (Fig. 8).
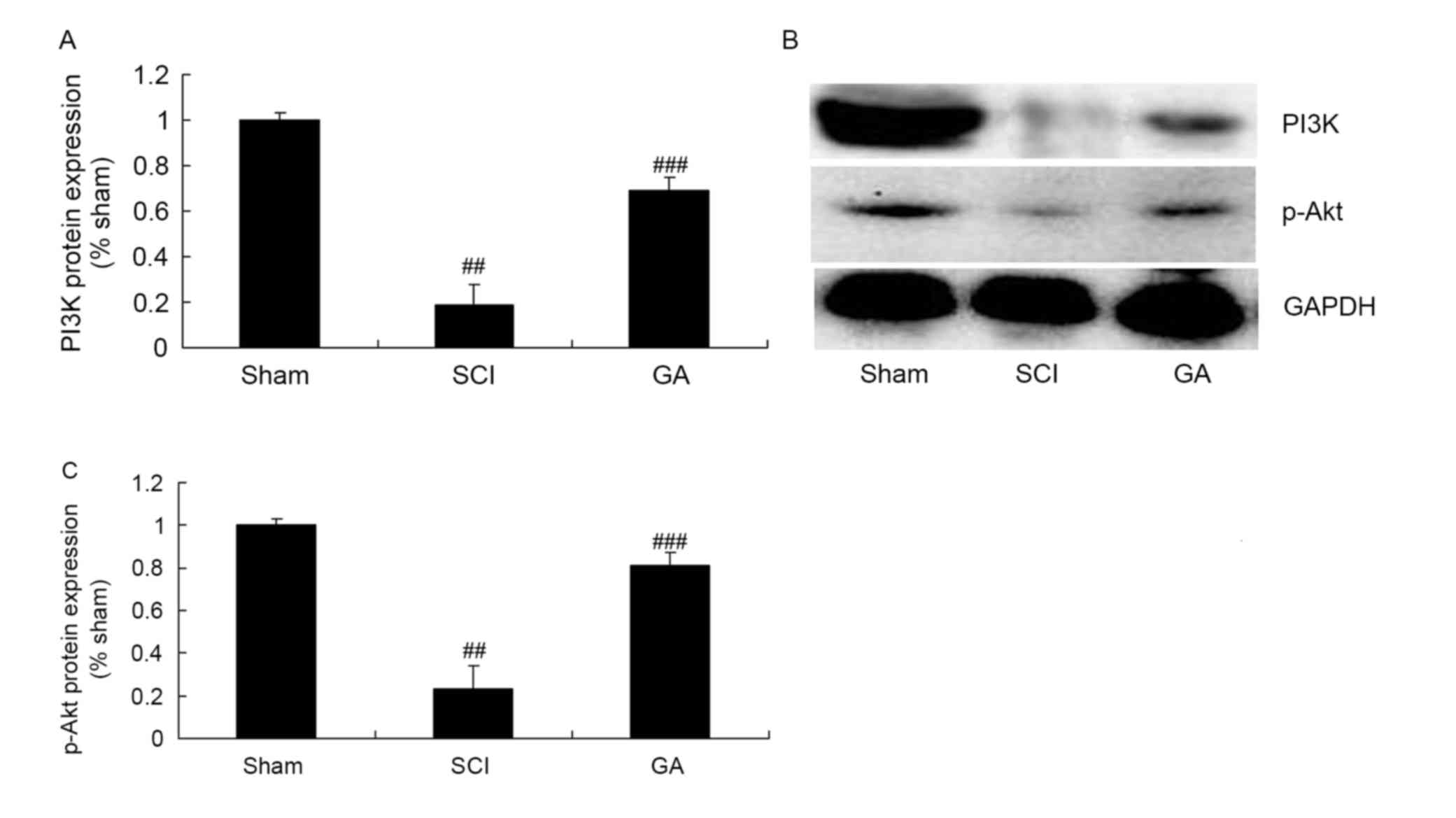
Gambogic acid effects on PI3K/Akt
protein expression in SCI rats
To investigate the anti-apoptosis mechanism of
gambogic acid in SCI, PI3K/Akt protein expression was measured
using western blot analysis. As presented in Fig. 9, PI3K and p-Akt protein expression
levels were significantly inhibited in the SCI model group,
compared with the sham group. Gambogic acid significantly induced
PI3K and p-Akt protein expression levels in SCI rats, compared with
the SCI model group (Fig. 9).
Discussion
SCI, a central nervous system disorder featuring the
loss of the sensation, movement and reflexes, and dysfunction of
sphincter under the area of injury, results from vertebral fracture
and compression to the spinal cord due to mechanical injury
(2). Common causes of SCI include
traffic accidents, sports injuries, bullet injury, falls and
natural disasters (16). At
present, SCI is characterized by high incidence, high disability
rate and a huge economic burden (17). The treatment of SCI is a hotspot
and difficult problem in clinical research (17). The results of the present study
demonstrated that gambogic acid increased BBB scores and decreased
the water content of the spinal cord in SCI rats.
Normally, the spinal cord forms an immune privileged
region through the blood-brain-barrier, preventing invasion by
antibodies and immune cells. However, when the spinal cord is
injured, the blood-brain-barrier will be destroyed, thereby leading
to an intensive local immune inflammatory reaction (18). ILs, TNF-α inflammatory factors and
chemical factors will be released to assist to repair the body.
However, excessive activation of the inflammatory response also
damages normal cells (19).
Therefore, it is important to study interventions in the
inflammatory response, as appropriately controlling the
inflammatory response can prevent further damage to the body,
providing novel ideas for the treatment of SCI (20). The present study confirmed that
gambogic acid significantly reduced the levels of inflammatory
cytokines (TNF-α, IL-6, IL-12 and IL-1β), oxidative stress factors
(MDA, SOD, GSH and GSH-PX) in SCI rats. Cascão et al
(21) reported that potent
anti-inflammatory effects of gambogic acid suppressed
antigen-induced arthritis.
The NF-κB signaling pathway serves an important role
in the incidence and development of SCI, and has many downstream
targets. IκB can be phosphorylated by a variety of inflammatory
factors, and the subunit p65 is activated and then enters into the
nucleus (22). The activation of
subunit p65 enhances the transcription and expression of IL-1B and
TNF-α, which can activate the NF-κB signaling pathway again. The
MAPK signaling pathway is widely distributed in mammals. NF-κB, one
of the targets of the MAPK signaling pathway, is involved in
inflammatory responses, as well as the proliferation,
differentiation and apoptosis of cells (23). The MAPK pathway is highly
conserved, involving three types of kinases, which can be triggered
by a variety of factors such as growth factors, cytokines, hormones
and proteins (24). Through the
cascade reaction, the MAPK pathway alters the secretion of certain
cytokines to initiate their biological functions, so as to
influence the development and prognosis of inflammatory responses
(24). The activation of the MAPK
signaling pathway may increase production of cytokines such as
TNF-α, IL-6, IL-12 and IL-1β, leading to inflammation and immune
responses (25). In the present
study, it was demonstrated that gambogic acid significantly
suppressed TLR4 and NF-κB protein expression and induced the p-p38
MAPK signaling pathway in SCI rats.
The mechanism of the differentiation of human bone
marrow stromal cells (hBMSCs) into neurons and glial cells in
vitro induced by RANKL needs further investigation, and it may
be due to the following mechanisms: i) RANKL binds to and activates
RANK; ii) RANK interacts with TNF receptor-associated factors to
activate NF-κB; iii) NF-κB enters into the nucleus from the
cytoplasm rapidly, to bind to the κB site of target genes and
induce the transcription of corresponding target genes; iv) the
expression of transcription factors involved in the differentiation
of neural cells are up-regulated when selective genes are switched
on or off, to pass signals required in the differentiation of
neural cells and thus inducing the differentiation of hBMSCs into
neural cells (26,27). In the present study, gambogic acid
significantly suppressed the increase of RANKL protein expression
in SCI rats. Pandey et al (13) demonstrated that gambogic acid
inhibits multiple myeloma mediated osteoclastogenesis through the
NF-κB and RANKL signaling pathways.
Akt is the central point of the cellular signal
transduction pathway, and is usually stimulated by cytokines and
growth factors to pass signals, thereby inducing changes under
stress (28). Akt serves an
important role in the metabolism, survival, proliferation,
differentiation and other key biological functions of cells
(9). In addition, Akt serves as an
important central control factor in regulating the survival of
neurons in the central nervous system, and its signal transduction
participates in the survival, development, proliferation,
differentiation, axonal growth and synaptic plasticity of neurons
(29). In this study, it was
demonstrated that gambogic acid significantly inhibited caspase-3
activity and induced PI3K and p-Akt protein expression levels in
SCI rats, which demonstrated that the PI3K/Akt signaling pathway
may be involved in the anti-apoptosis mechanism of gambogic acid in
SCI. Ma et al (12)
demonstrated that Gambogic acid inhibits osteoclast formation via
RANKL, p38 and Akt.
In conclusion, the results of the present study
revealed that gambogic acid inhibits SCI and SCI-induced
inflammation, oxidative stress and apoptosis through the TLR4/NF-κB
protein/p38 and Akt signaling pathways. Thus, gambogic acid may be
a promising approach to treat SCI in the future.
References
|
1
|
Raithatha R, Carrico C, Powell ES,
Westgate PM, Ii Chelette KC, Lee K, Dunsmore L, Salles S and Sawaki
L: Non-invasive brain stimulation and robot-assisted gait training
after incomplete spinal cord injury: A randomized pilot study.
Neuro Rehabilitation. 38:15–25. 2016.
|
|
2
|
Ness LL and Field-Fote EC: Effect of
whole-body vibration on quadriceps spasticity in individuals with
spastic hypertonia due to spinal cord injury. Restor Neurol
Neurosci. 27:621–631. 2009.
|
|
3
|
Biglari B, vd Linden PH, Simon A, Aytac S,
Gerner HJ and Moghaddam A: Use of Medihoney as a non-surgical
therapy for chronic pressure ulcers in patients with spinal cord
injury. Spinal Cord. 50:165–169. 2012. View Article : Google Scholar
|
|
4
|
Nussbaum EL, Flett H, Hitzig SL,
McGillivray C, Leber D, Morris H and Jing F: Ultraviolet-C
irradiation in the management of pressure ulcers in people with
spinal cord injury: A randomized, placebo-controlled trial. Arch
Phys Med Rehabil. 94:650–659. 2013. View Article : Google Scholar
|
|
5
|
Cooney SJ, Zhao Y and Byrnes KR:
Characterization of the expression and inflammatory activity of
NADPH oxidase after spinal cord injury. Free Radic Res. 48:929–939.
2014. View Article : Google Scholar :
|
|
6
|
Lu T, Zhang C, Chai M and An Y:
Isoquercetin ameliorates tunicamycin-induced apoptosis in rat
dorsal root ganglion neurons via suppressing ROS-dependent
endoplasmic reticulum stress. Biomed Pharmacother. 80:343–351.
2016. View Article : Google Scholar
|
|
7
|
Jung SY, Kim DY, Yune TY, Shin DH, Baek SB
and Kim CJ: Treadmill exercise reduces spinal cord injury-induced
apoptosis by activating the PI3K/Akt pathway in rats. Exp Ther Med.
7:587–593. 2014. View Article : Google Scholar
|
|
8
|
Zhang P and Ma X: Effect of rutin on
spinal cord injury through inhibition of the expression of MIP-2
and activation of MMP-9, and downregulation of Akt phosphorylation.
Mol Med Rep. 12:7554–7560. 2015. View Article : Google Scholar
|
|
9
|
Kim JH, Kim SH, Cho SR, Lee JY, Kim JH,
Baek A and Jung HS: The modulation of neurotrophin and epigenetic
regulators: Implication for astrocyte proliferation and neuronal
cell apoptosis after spinal cord injury. Ann Rehabil Med.
40:559–567. 2016. View Article : Google Scholar :
|
|
10
|
Cao J, Wang JS, Ren XH and Zang WD: Spinal
sample showing p-JNK and P38 associated with the pain signaling
transduction of glial cell in neuropathic pain. Spinal Cord.
53:92–97. 2015. View Article : Google Scholar
|
|
11
|
Malon JT and Cao L: Calcitonin
gene-related peptide contributes to peripheral nerve injury-induced
mechanical hypersensitivity through CCL5 and p38 pathways. J
Neuroimmunol. 297:68–75. 2016. View Article : Google Scholar :
|
|
12
|
Ma J, Ma Y, Liu X, Chen S, Liu C, Qin A
and Fan S: Gambogic acid inhibits osteoclast formation and
ovariectomy-induced osteoporosis by suppressing the JNK, p38 and
Akt signalling pathways. Biochem J. 469:399–408. 2015. View Article : Google Scholar
|
|
13
|
Pandey MK, Karelia D and Amin SG: Gambogic
acid and its role in chronic diseases. Adv Exp Med Biol.
928:375–395. 2016. View Article : Google Scholar
|
|
14
|
Wu Y, Streijger F, Wang Y, Lin G, Christie
S, Mac-Thiong JM, Parent S, Bailey CS, Paquette S, Boyd MC, et al:
Parallel metabolomic profiling of cerebrospinal fluid and serum for
identifying biomarkers of injury severity after acute human spinal
cord injury. Sci Rep. 6:387182016. View Article : Google Scholar :
|
|
15
|
Zhang H, Wang L, Wen S, Xiang Q, Xiang X,
Xu C, Wan Y, Wang J, Li B, Wan Y, et al: Magnetic resonance imaging
tracking and assessing repair function of the bone marrow
mesenchymal stem cells transplantation in a rat model of spinal
cord injury. Oncotarget. 8:58985–58999. 2017.
|
|
16
|
Giuliano F, Sanchez-Ramos A, Löchner-Ernst
D, Del Popolo G, Cruz N, Leriche A, Lombardi G, Reichert S, Dahl P,
Elion-Mboussa A and Casariego J: Efficacy and safety of tadalafil
in men with erectile dysfunction following spinal cord injury. Arch
Neurol. 64:1584–1592. 2007. View Article : Google Scholar
|
|
17
|
Derakhshanrad N, Saberi H, Yekaninejad MS,
Eskandari G, Mardani A, Rahdari F and Meybodi KT: Safety of
granulocyte colony-stimulating factor (G-CSF) administration for
postrehabilitated motor complete spinal cord injury patients: An
open-label, phase I study. Cell Transplant. 22 Suppl 1:S139–S146.
2013. View Article : Google Scholar
|
|
18
|
Segal JL, Gonzales E, Yousefi S,
Jamshidipour L and Brunnemann SR: Circulating levels of IL-2R,
ICAM-1, and IL-6 in spinal cord injuries. Arch Phys Med Rehabil.
78:44–47. 1997. View Article : Google Scholar
|
|
19
|
Badner A, Vawda R, Laliberte A, Hong J,
Mikhail M, Jose A, Dragas R and Fehlings M: Early intravenous
delivery of human brain stromal cells modulates systemic
inflammation and leads to vasoprotection in traumatic spinal cord
injury. Stem Cells Transl Med. 5:991–1003. 2016. View Article : Google Scholar :
|
|
20
|
Khayrullina G, Bermudez S and Byrnes KR:
Inhibition of NOX2 reduces locomotor impairment, inflammation, and
oxidative stress after spinal cord injury. J Neuroinflammation.
12:1722015. View Article : Google Scholar :
|
|
21
|
Cascão R, Vidal B, Raquel H, Neves-Costa
A, Figueiredo N, Gupta V, Fonseca JE and Moita LF: Potent
anti-inflammatory and antiproliferative effects of gambogic acid in
a rat model of antigen-induced arthritis. Mediators Inflamm.
2014:1953272014. View Article : Google Scholar :
|
|
22
|
Yuan B, Liu D and Liu X: Spinal cord
stimulation exerts analgesia effects in chronic constriction injury
rats via suppression of the TLR4/NF-κB pathway. Neurosci Lett.
581:63–68. 2014. View Article : Google Scholar
|
|
23
|
Pratheeshkumar P, Son YO, Wang X, Divya
SP, Joseph B, Hitron JA, Wang L, Kim D, Yin Y, Roy RV, et al:
Cyanidin-3-glucoside inhibits UVB-induced oxidative damage and
inflammation by regulating MAP kinase and NF-κB signaling pathways
in SKH-1 hairless mice skin. Toxicol Appl Pharmacol. 280:127–137.
2014. View Article : Google Scholar :
|
|
24
|
Luo Y, Fu C, Wang Z, Zhang Z, Wang H and
Liu Y: Asiaticoside attenuates the effects of spinal cord injury
through antioxidant and anti-inflammatory effects, and inhibition
of the p38-MAPK mechanism. Mol Med Rep. 12:8294–8300. 2015.
View Article : Google Scholar
|
|
25
|
Horvath RJ, Landry RP, Romero-Sandoval EA
and DeLeo JA: Morphine tolerance attenuates the resolution of
postoperative pain and enhances spinal microglial p38 and
extracellular receptor kinase phosphorylation. Neuroscience.
169:843–854. 2010. View Article : Google Scholar :
|
|
26
|
Liu HJ, Yan H, Yan J, Li H, Chen L, Han LR
and Yang XF: Substance P promotes the proliferation, but inhibits
differentiation and mineralization of osteoblasts from rats with
spinal cord injury via RANKL/OPG system. PLoS One. 11:e01650632016.
View Article : Google Scholar :
|
|
27
|
Maïmoun L, Couret I, Mariano-Goulart D,
Dupuy AM, Micallef JP, Peruchon E, Ohanna F, Cristol JP, Rossi M
and Leroux JL: Changes in osteoprotegerin/RANKL system, bone
mineral density, and bone biochemicals markers in patients with
recent spinal cord injury. Calcif Tissue Int. 76:404–411. 2005.
View Article : Google Scholar
|
|
28
|
Zhang P, Zhang L, Zhu L, Chen F, Zhou S,
Tian T, Zhang Y, Jiang X, Li X, Zhang C, et al: The change tendency
of PI3K/Akt pathway after spinal cord injury. Am J Transl Res.
7:2223–2232. 2015.
|
|
29
|
Stover J and Nagatomi J: Cyclic pressure
stimulates DNA synthesis through the PI3K/Akt signaling pathway in
rat bladder smooth muscle cells. Ann Biomed Eng. 35:1585–1594.
2007. View Article : Google Scholar
|