Introduction
Chronic rhinosinusitis (CRS) is clinically defined
as chronic inflammation of the nasal cavity and paranasal sinus
lasting over 12 weeks, including chronic rhinosinusitis with nasal
polyps (CRSwNP) and chronic rhinosinusitis without nasal polyps
(CRSsNP). Nasal mucosal inflammatory lesion has high heterogeneity
and is formed in a multi-step process caused by multiple factors
(1). Tissue remodeling is a
dynamic process of organic damage repair resulting from long-term
inflammatory stimulation (2).
Different types of CRS have different manifestations of mucous
tissue remodeling: CRSwNP mainly appears as hyperplasia of goblet
cells, extracellular matrix (ECM) proteinosis, and edema, while
CRSsNP primarily manifests as exuviation, collagenous fiber
precipitation, and submucous fibrosis (3,4).
Factors that influence the generation and degradation of ECM, such
as (transforming growth factor) TGF, oxidative stress, matrix
metalloproteinases (MMPs), tissue inhibitor of metalloproteinase,
and vascular endothelial growth factor (VEGF), can have effects on
tissue remodeling, although the mechanisms of network regulation by
these factors is still unclear (5).
Excessive generation of reactive oxygen species
(ROS) in cells or tissues results from noxious stimulation by
atmospheric pollution, smoke, drugs and trauma can lead to
disequilibrium of oxidant and antioxidant systems, resulting in
tissue damage, which in turn causes additional oxidative stress
(6). Based on previous studies,
oxidative stress is likely to play an important role in the
generation and development processes of nasal polyps (7–9).
Cekin et al discovered that the (malondialdehyde) MDA level
was increased in nasal polyps, while nitric oxide and (superoxide
dismutase) SOD levels were reduced; this findingindicates a
metabolic disorder involving free radicals (7). Bozkus et al found that
oxidative stress parameters in the blood and nasal polyps of
patients were higher than those of people without the disease, and
the level of oxidative stress was positively correlated with
patient age (8). Okur et al
reported that neutrophil granulocytes of patients with nasal polyp
could generate ROS, which is one means of releasing ROS by nasal
polyps (9).
Phosphoinositide 3-kinase (PI3K) is a bridge to link
extracellular stimulating factors and intracellular response
effects and possesses activities of serine/threonine kinase and
phosphatidylinositol kinase. Akt, the downstream effector protein
of PI3K, is a serine protease. Unphosphorylated Akt lacks
biological activity. Once it is phosphorylated, Akt (p-Akt) is able
to regulate cell growth, apoptosis, adhesion, migration,
infiltration, and metabolism through numerous pathways (10). phosphatase and tensin homolog gene
(PTEN) is the first discovered cancer suppressor gene with
bispecific phosphatase activity (11). PTEN is known to inhibit the
activity of PI3K, which then affects the phosphorylation of Akt
(12). The PI3K/PTEN/Akt signal
pathway has been demonstrated to play a role in a variety of tumors
including hematological neoplasms, liver cancer, intestinal cancer,
osteosarcoma, and lymphoma (13–16).
This pathway was recently found to be closely related to chronic
inflammatory diseases and autoimmune diseases such as asthma
(17).
However, it remains uncertain whether the
PI3K/PTEN/Akt pathway is associated with CRS. In this study, we
investigated the relationship between imbalanced expression of PTEN
and the components of the PI3K/Akt signaling pathway in rat nasal
epithelial cells. H2O2 was applied to
increase ROS levels to simulate oxidative stress in CRS.
Materials and methods
Cell grouping
Rat nasal epithelial cells were obtained from
Nanjing Cobioer Biotechnology Co., Ltd. (Jiangsu, China). Cells
were randomly divided into four groups: control,
H2O2, H2O2+PTEN and
H2O2+siPTEN group. In
H2O2+PTEN and
H2O2+siPTEN group, cells were respectively
infected by plasmids with PTEN gene and plasmids with silenced PTEN
gene. Recombinant plasmids were also purchased from Nanjing Cobioer
Biotechnology Co., Ltd. Cells in H2O2,
H2O2+PTEN and
H2O2+siPTEN group were treated by 50 µmol/l
of H2O2 (Shanghai Macklin Biochemical Co.,
Ltd., Shanghai, China) for 3 h. The appropriate concentration of
H2O2 was selected from 20, 50 and 80 µmol/l
by detecting cell viability through CCK-8 assay.
Cell transfection
Cells in logarithmic growth phase were seeded into a
6-well plate and cultured for 24 h. Recombinant plasmids expressing
either the PTEN gene or the silenced PTEN gene were transfected
into cells using Lipofectamine LTX according to the manufacturer's
instructions (Invitrogen; Thermo Fisher Scientific, Inc., Waltham,
MA, USA). Two micrograms of pIRES2-ZsGreen1-vector (Nanjing Vazyme
Biotechnology Co., Ltd., Jiangsu, China), 5 µl of Lipofectamine LTX
and 250 µl Opti-MEM (Shanghai Yeasen Biotechnology Co., Ltd.,
Shanghai, China) were well prepared, mixed, and then incubated at
room temperature for 25 min. Subsequently, 500 µl of the mixture
was added into each well of a 6-well plate with RPMI-1640 medium.
After 48 h culture, the transfected cells were harvested for the
indicated experiments. Western blot was used to detect the
expression of PTEN to assess the transfection efficiency.
CCK-8 assay
Cell viability in each group was detected through
using CCK-8 kit (Shanghai Yeasen Biotechnology Co., Ltd.). Cells
were grouped, and seeded into 96-well plates at amount of 100
µl/well, and then incubated at 37°C in 5% CO2 incubator
for 4 h. After added with 10 µl CCK reagent to each well, cells
were putted into 5% CO2 incubator at 37°C for 1–4 h.
Optical density (OD) value of each group was observed at 450 nm by
a spectrophotometer (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany).
Flow cytometry (FCM)
Cells in logarithmic phase were collected and seeded
into 6-well plates. Cells were digested by EDTA free trypsin,
stained with Annexin V-FITC and propidium iodide (all from Shanghai
Yeasen Biotechnology Co., Ltd.), and incubated in dark place for 15
min at room temperature afterwards. Cell cycle and apoptosis rate
of each group was detected by EPICS XL-MCL FCM (Beckman Coulter,
Inc., Brea, CA, USA) with excitation wavelength 488 nm and emission
wavelength 530 nm.
ELISA
The levels of SOD and MDA were measured by applying
quantitative test kits (Shanghai Lanpai Biotechnology Co., Ltd.,
Shanghai, China). Cells were divided into control,
H2O2, H2O2+PTEN and
H2O2+siPTEN groups. Detection was conducted
according to the manufacturer's instructions. OD values were read
at 420 nm on a spectrophotometer (Sigma-Aldrich; Merck KGaA).
qPCR
Expression levels of PTEN, caspase-3, Bax and Bcl-2
mRNA were detected by means of qPCR. Cells were seeded into 6-well
plates at a density of 2×106 cells/well. Total RNA were
extracted with TRIzol (Thermo Fisher Scientific, Inc.) according to
the manufacture's instructions. Concentration of extracted RNA was
read through a UV spectrophotometer (Thermo Fisher Scientific,
Inc.). cDNA were synthesized by reverse transcription. Reduced
glyceraldehyde-phosphate dehydrogenase (GAPDH) was applied as the
internal control to monitor the efficiency of qPCR. All primers in
this study were designed by Sangon Biotech Co., Ltd. (Shanghai,
China). The specific primer sequences for each gene were listed as
the follows: 5′ACC AGG ACC AGA GGA AAC CT3′ and 5′TTT GTC AGG GTG
AGC ACA AGA3′ for PTEN (product: 126 bp); 5′ACC GCA CCC GGT TAC TAT
TC3′ and 5′CAA ATT CCG TGG CCA CCT TC3′ for caspase-3 (product: 148
bp); 5′TGG CGA TGA ACT GGA CAA CA3′ and 5′CAC GGA AGA AGA CCT CTC
GG3′ for Bax (product: 86 bp); 5′AGC ATG CGA CCT CTG TTT GA3′ and
5′TCA CTT GTG GCC CAG GTA TG3′ for Bcl-2 (product: 108 bp) and
5′GGC TCA TGA CCA CAG TCC AT3′ and 5′ACA TTG GGG GTA GGA ACA CG3′
for GAPDH (product: 202 bp). Each reaction was run in
triplicate.
Western blot
Cells were seeded in 6-well plates at a density of
2×106 cells/well, and grouped. Cells were harvested and
washed twice with PBS, protein lysed in ice-cold radio
immunoprecipitation assay buffer (Guangzhou Whiga Technology Co.,
Ltd., Guangdong, China) with freshly mixed 0.01% protease inhibitor
phenylmethanesulfonyl fluoride (Beijign O'BioLab Technology Co.,
Ltd., Beijing, China), then incubated for 30 min on ice. Cell lysis
was centrifuged at 10,000 × g for 5 min at 4°C, collected
supernatants containing 20–30 µg of protein were run on 10% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis gel and
electrophoretically transferred to a nitrocellulose membrane (Merck
KGaA). Protein expression level of PTEN, caspase-3, Bax, Bcl-2,
p-PI3K, PI3K, p-Akt and Akt were detected. GAPDH monoclonal
antibody was used to estimate protein loading. Blots were
visualized via an enhanced chemiluminescence (Thermo Fisher
Scientific, Inc.).
Statistical analysis
Data were expressed as mean ± SD. Differences among
groups were evaluated through variance analysis and Student's
t-test. Statistical significance was defined as P<0.05 or
P<0.01.
Results
Rat nasal epithelial cells were
successfully identified
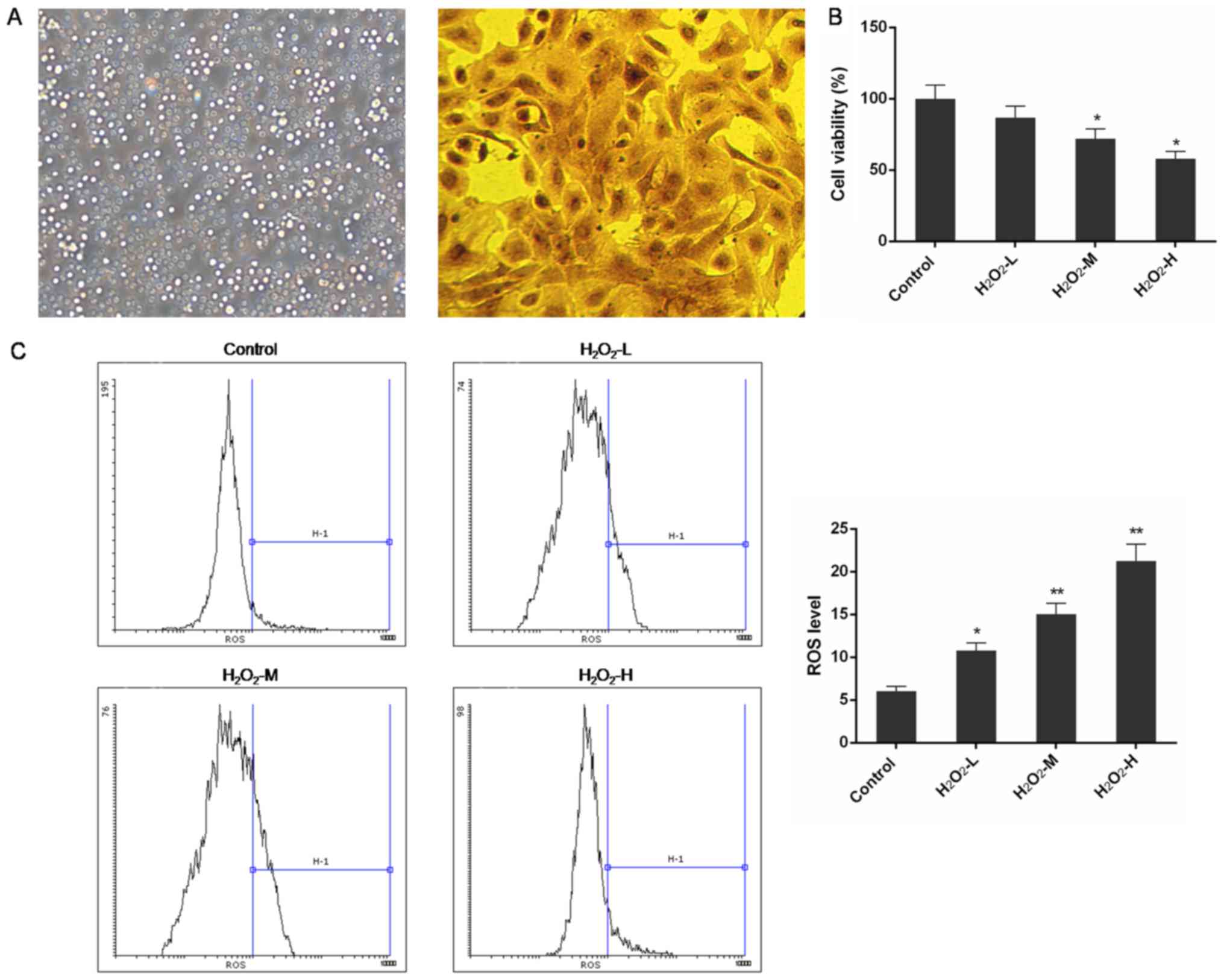
The in vitro observation under an inverted
microscope showed that after 24-h culture in serum medium, a
majority of rat nasal epithelial cells grew as single cells
adhering to the plate and were round or irregular in shape
(Fig. 1A). Cell nuclei formed oval
shapes, and the cell body was irregularly shaped. Cells were
positive for (diaminobenzidine) DAB, which was detected by
immunocytochemical staining with an anticytokeratin 19 antibody
(95% purity).
H2O2 treatment
decreased cell viability and increased ROS level
The CCK-8 assay indicated that the viability of rat
nasal epithelial cells was significantly reduced by
H2O2 treatment. H2O2
affected cell proliferation in a concentration-dependent manner and
the differences were significant in the
H2O2-M (50 µM) and
H2O2-H (80 µM) groups when compared with
normal control cells (P<0.05) (Fig.
1B). Additionally, the ROS levels in
H2O2-treated groups were markedly enhanced
and the effect was in a concentration-depended manner (P<0.05 or
P<0.01) (Fig. 1C).
The expression of PTEN was upregulated
in cells transfected with plasmids containing the PTEN gene and
inhibited in cells transfected with siRNA against the PTEN
gene
The expression of PTEN in each experimental
condition was quantified by qPCR and western blot analysis. In NC
and mock-transfected groups, which were transfected with empty
plasmids, the mRNA and protein levels of PTEN were similar to the
control group. In the PTEN-transfected group, PTEN mRNA and protein
were both found to be overexpressed compared with the control group
(P<0.01). In contrast, in cells transfected with siRNA against
the PTEN gene, the expression of both PTEN mRNA and protein was
substantially inhibited (P<0.01) (Fig. 2A and B).
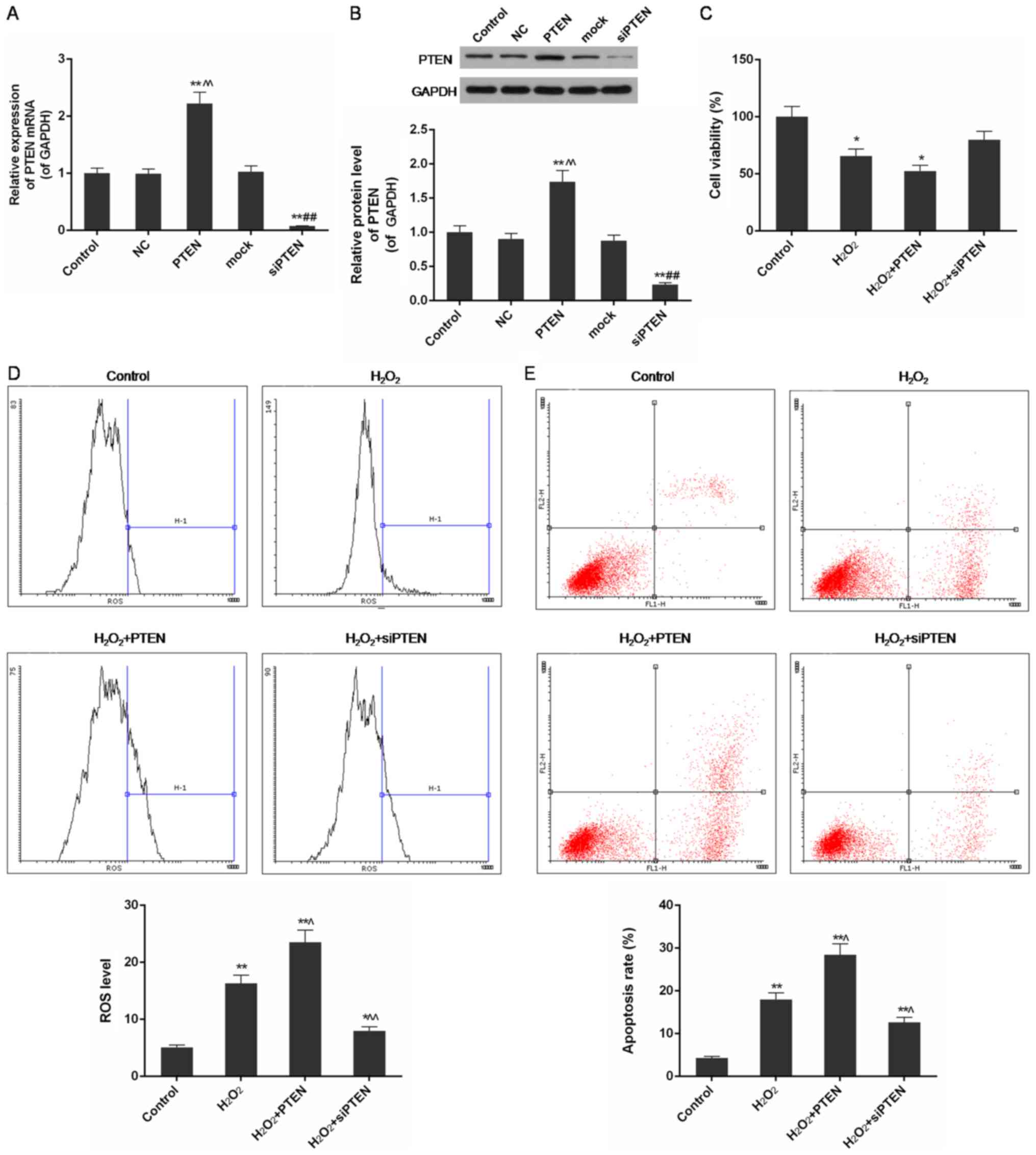 | Figure 2.Expression levels of PTEN with
infected plasmids, and cell viability, ROS level and apoptosis rate
in control, H2O2,
H2O2+PTEN and
H2O2+siPTEN group. (A) The expression of PTEN
mRNA was significantly upregulated in cells infected by plasmids
with PTEN gene and was obviously downregulated in cells infected by
plasmids with silenced PTEN gene. (B) The protein level of PTEN was
significantly increased in cells infected by plasmids with PTEN
gene and was obviously reduced in cells infected by plasmids with
silenced PTEN gene. (C) PTEN protein aggravated the reduction of
cell viability in H2O2 induced injury. (D)
PTEN further increased ROS level in H2O2
injured cells while inhibition of PTEN expression decreased it. (E)
PTEN further promoted apoptosis of H2O2
injured cells when PTEN gene silencing inhibited it. Data were
presented as mean ± SD, n=3, *P<0.05 and **P<0.01 vs. control
group, ^P<0.05 and ^^P<0.01 vs.
H2O2 (50 µmol/l) group. PTEN, phosphatase and
tensin homolog; ROS, reactive oxygen species; si, small
interfering; NC, negative control. |
PTEN levels correlated with reduced
cell viability and increased ROS level and apoptosis rate in
H2O2-injured cells
Using the CCK-8 assay, we found that cell viability
and proliferation were decreased by H2O2
treatment and further inhibited in PTEN overexpressing cells, but
the injury was improved in the PTEN silenced group (P<0.05)
(Fig. 2C). Through FCM analysis,
ROS levels in the H2O2-treated group were
found to be increased compared with control cells (P<0.01). The
level of ROS was further increased in the
H2O2+PTEN group, but was inhibited in the
H2O2+siPTEN group compared with the group
treated with only H2O2 (P<0.05 or
P<0.01) (Fig. 2D).
H2O2 treatment significantly increased
apoptosis, and the rate was further increased in the
H2O2-PTEN group but was decreased in the
H2O2-siPTEN group (P<0.05 or P<0.01)
(Fig. 2E).
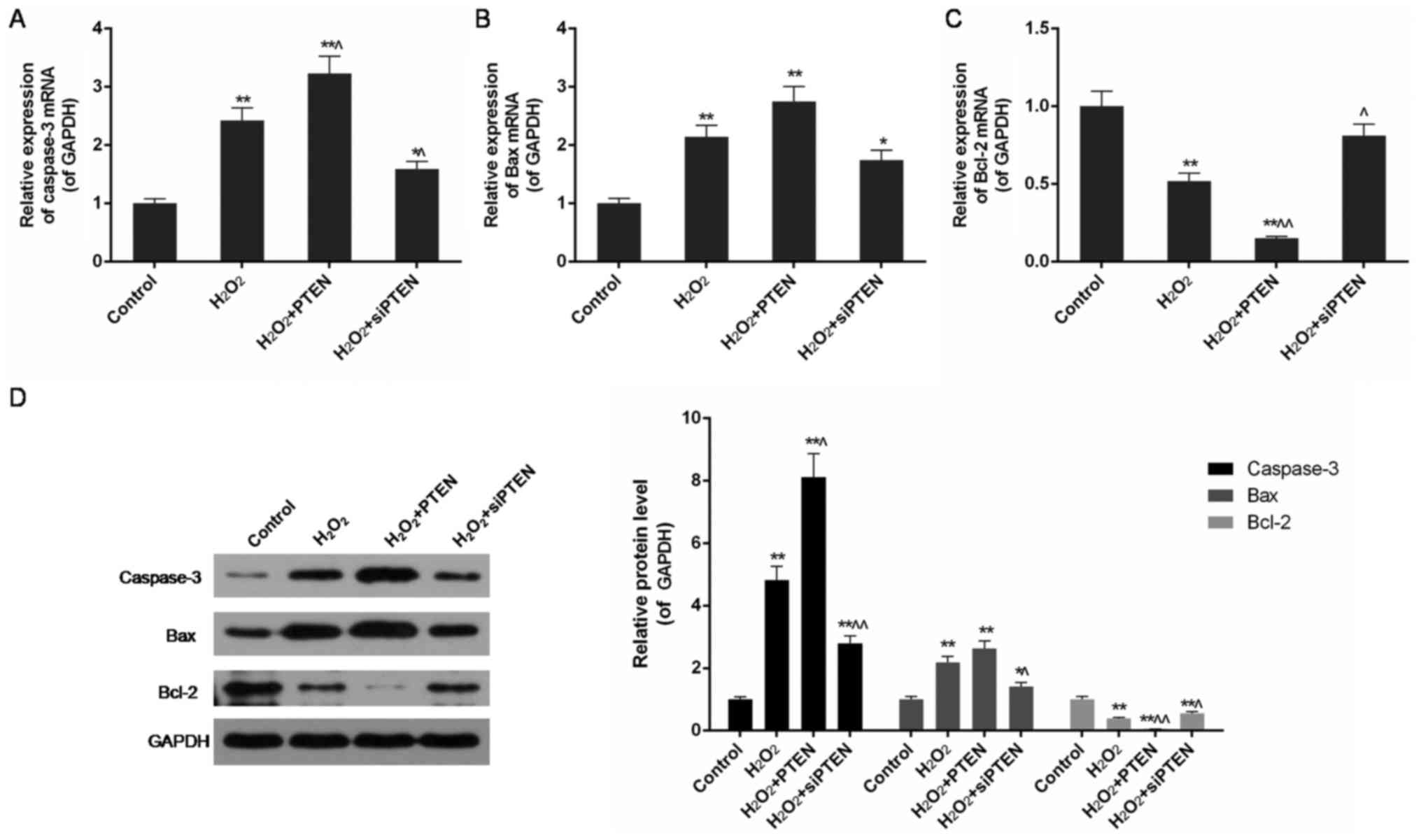
PTEN upregulated the expression of
caspase-3 and Bax in H2O2-injured cells
In the H2O2-treated group, in
agreement with the increased apoptosis rate described above, high
expression of caspase-3 and Bax was also detected by qPCR and
western blot analysis (P<0.01). In PTEN overexpressing cells,
the expression of caspase-3 and Bax was further upregulated, while
that of Bcl-2 was significantly reduced compared with the
H2O2-treated group (P<0.05 or P<0.01).
In contrast, disrupting the expression of the PTEN gene
downregulated the expression of caspase-3 and Bax but upregulated
Bax following H2O2-induced injury (P<0.05
or P<0.01) (Fig. 3).
PTEN decreased SOD and catalase (CAT)
levels and increased MDA content in
H2O2-injured cells
Using an ELISA-based assay, a remarkable decrease of
SOD and CAT levels and a significant increase of MDA were observed
in H2O2-treated cells, compared with the
control group (P<0.01). Compared with the
H2O2-treated group, overexpression of PTEN in
the H2O2-PTEN treated group significantly
reduced the level of SOD and CAT but increased MDA, whereas
inhibition of PTEN expression in the
H2O2-siPTEN group increased SOD and CAT
levels and reduced MDA in H2O2-injured cells
(P<0.05 or P<0.01) (Fig.
4A-C).
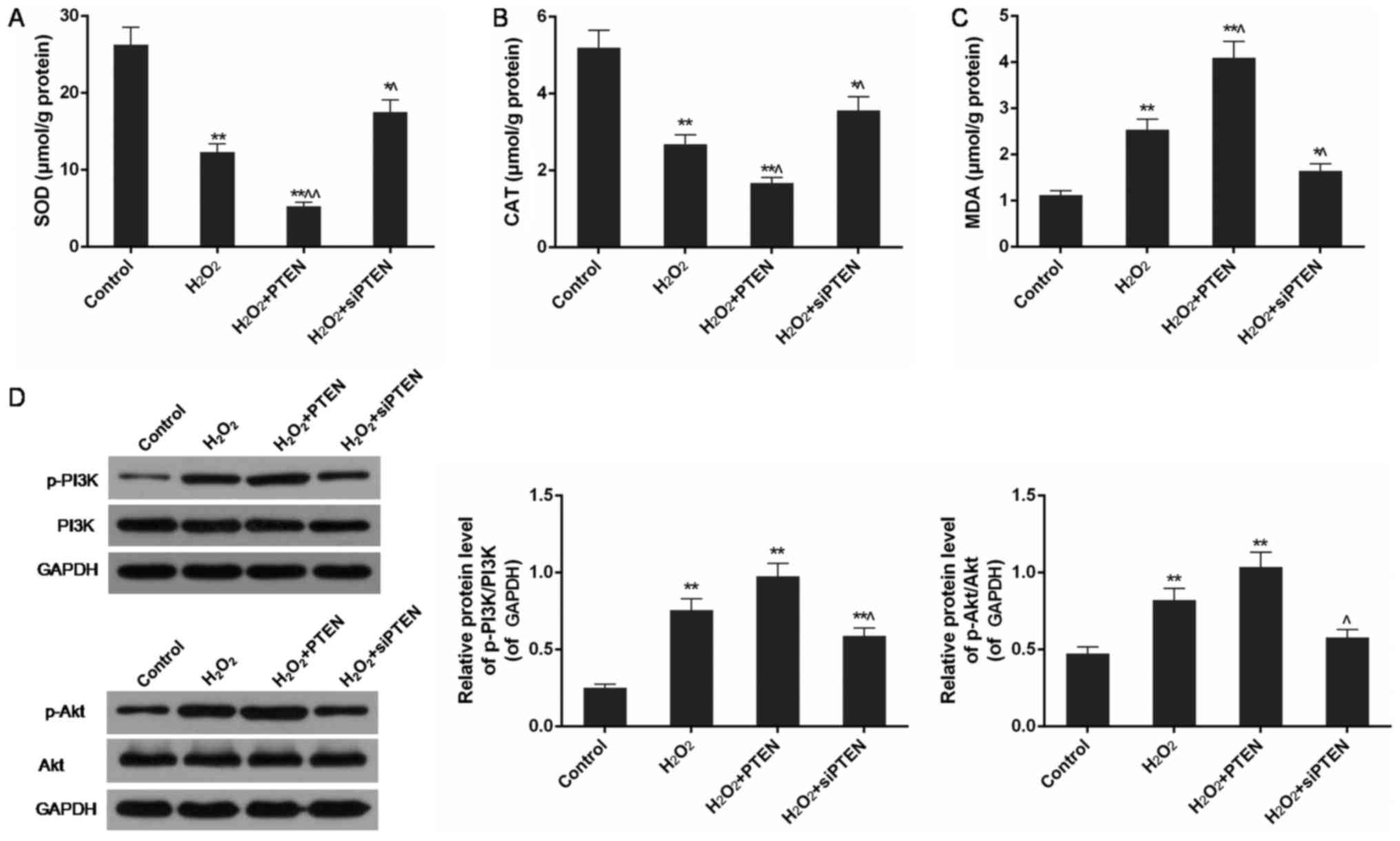 | Figure 4.The contents of SOD, CAT and MDA and
the expression level of PI3K/Akt pathway in control,
H2O2, H2O2+PTEN and
H2O2+siPTEN group. (A) The level of SOD was
further decreased in H2O2+PTEN group but
increased in H2O2+siPTEN group based on
H2O2 group. (B) The level of CAT was further
decreased in H2O2+PTEN group but increased in
H2O2+siPTEN group based on
H2O2 group. (C) The level of MDA was further
increased in H2O2+PTEN group but decreased in
H2O2+siPTEN group based on
H2O2 group. (D) The phosphorylation of PI3K
and Akt was further promoted in H2O2+PTEN
group but inhibited in H2O2+siPTEN group
based on H2O2 group. Data were presented as
mean ± SD, n=3, *P<0.05 and **P<0.01 vs. control group,
^P<0.05 and ^^P<0.01 vs.
H2O2 (50 µmol/l) group. PTEN, phosphatase and
tensin homolog; si, small interfering; SOD, superoxide dismutase;
CAT, catalase; MDA, malondialdehyde; p, phosphorylated; PI3K,
phosphoinositide 3-kinase; Akt, protein kinase B. |
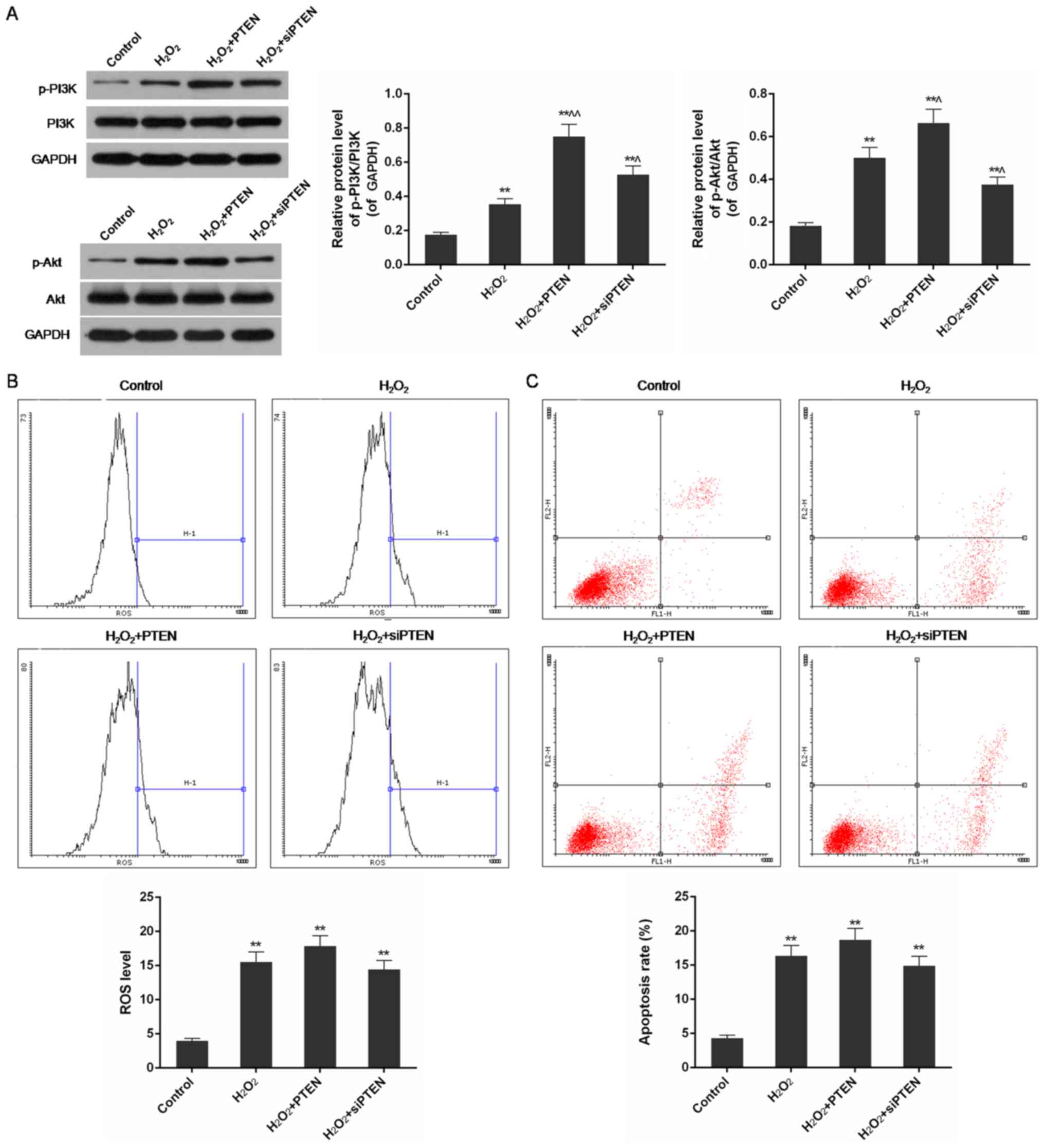
PTEN promoted phosphorylation of PI3K
and Akt in H2O2-injured cells
Western blot analysis showed that the levels of
phosphorylated PI3K and Akt were significantly increased in
H2O2-injured cells in comparison with control
cells (P<0.01). Overexpression of PTEN in the
H2O2-PTEN group slightly promoted the
increase of p-PI3K/PI3K and p-Akt/Akt ratios. In contrast,
silencing of the PTEN gene inhibited the activation of PI3K
and Akt in H2O2-injured cells compared with
the H2O2-treatedgroup (P<0.05) (Fig. 4D).
PTEN promoted activation of PI3K and
Akt even when the PI3K/Akt pathway was inhibited by perifosine in
H2O2-injured cells
In H2O2-injured cells with
inhibition of PI3K/Akt pathway, the levels of p-PI3K/PI3K and
P-Akt/Akt were observed to be downregulated compared with
uninhibited H2O2-injured cells, but were
still higher than those in non-treated cells (Figs. 4D and 5A). Inhibition of the PI3K/Akt pathway
had a slight influence on the activation of PI3K and Akt. In
comparison with the H2O2-treated group,
phosphorylation of PI3K and Akt was promoted in the
H2O2-PTEN group but was inhibited in the
H2O2-siPTEN treated group (P<0.05)
(Fig. 5A).
 | Figure 5.Expression of PI3K/Akt pathway, ROS
level and apoptosis rate in control, H2O2,
H2O2+PTEN and
H2O2+siPTEN group with perifosine treatment.
(A) PTEN promoted the activation of PI3K and Akt even when PI3K/Akt
pathway was inhibited in H2O2 injured cells.
(B) ROS level was reduced in H2O2-PTEN group
but were increased in H2O2-siPTEN group when
PI3K/Akt pathway was inhibited. (C) Apoptosis rate was reduced in
H2O2-PTEN group but were increased in
H2O2-siPTEN group when PI3K/Akt pathway was
inhibited. Data were presented as mean ± SD, n=3, *P<0.05 and
**P<0.01 vs. control group, ^P<0.05 and
^^P<0.01 vs. H2O2 (50 µmol/l)
group. PTEN, phosphatase and tensin homolog; si, small interfering;
p, phosphorylated; PI3K, phosphoinositide 3-kinase; Akt, protein
kinase B; ROS, reactive oxygen species. |
ROS levels and apoptosis rates were
reduced in the H2O2-PTEN group but were
increased in the H2O2-siPTEN group when the
PI3K/Akt pathway was inhibited
With inhibition of PI3K/Akt pathway by perifosine,
ROS levels and apoptosis rates in H2O2 group
increased compared with control and uninhibited
H2O2-treated groups. In comparison with
uninhibited cells under the same condition of PTEN expression, ROS
level and apoptosis rate were decreased in
H2O2-PTEN group but increased in
H2O2-siPTEN group (Figs. 2D and E and 5B and C). There was no significant
difference in ROS levels and apoptosis rates among
H2O2, H2O2-PTEN and
H2O2-siPTEN groups (Fig. 5C and D).
Discussion
It has been demonstrated that the PTEN/PI3K/Akt
signaling pathway, which is activated by extracellular stimuli such
as viruses and inflammatory factors through G protein-coupled
receptors or TLR/IL-1RS signaling, plays a key role in regulating
biological functions of cells (18,19).
After activation of PI3K, a secondary messenger, PIP3, is formed on
cell membranes to act on inactivated Akt protein and
phosphoinositide-dependent kinase-1 (PDK1) to promote
phosphorylation and activation of Akt. p-Akt regulates a variety of
biological activities such as cell proliferation, apoptosis,
differentiation, apoptosis, migration, glucose transport and
release of inflammatory cytokines through activating downstream
target proteins. As it is still difficult to directly detect the
expression of PI3K, the level of PI3K is generally inferred through
measuring the level of p-Akt by western blot because it is the only
protein known to be affected by PI3K (20,21).
PTEN can dephosphorylate PI(3,4,5)P3 to
PI(4,5)P2 and PI(3,4)P2 to
PI(4)P to switch off the pathway
of promoting phosphorylation of Akt by PI3K, resulting in decreased
p-Akt levels and negative regulation of the PI3K signaling pathway.
Several lines of evidence support an antagonistic relationship
between PTEN and Akt (22–27). It was previously reported that
membrane-associated guanylate kinases MAGI2 and MAGI3, which act
upstream protein of PTEN, were able to enhance the activity of PTEN
and inhibit the activity of Akt (23). Additionally, PTEN could inhibit
upstream of PI3K to inhibit activation of PI3K (24). Goo et al discovered that
high levels of p-Akt in rats inhibited the PTEN gene (25). Hua et al found that p-Akt
was highly expressed in prostate cancer cells when the PTEN
gene was altered or inactivated (26). Bai et al demonstrated a
reciprocal relationship between levels of PI3K/Akt and PTEN in
gastric (27).
Recently, an increasing number of studies have
indicated that the PI3K/PTEN/Akt signaling pathway not only is
closely related to tumorigenesis, but plays an important part in
chronic inflammatory diseases and autoimmune disease such as
rheumatic arthritis, systemic lupus erythematosus, bronchial asthma
and chronic obstructive pneumonia, which may result from the
regulatory functions of this pathway on lymphocytes and
inflammatory cellular factors (28–31).
CRS is an upper respiratory inflammatory disease with a
complicated, multi-causal pathogenesis. The surface of the nasal
cavity and paranasal sinus are a pseudostratified ciliated columnar
epithelium, which is composed of four types of cells,
includingciliated columnar epithelium, basal cells and goblet
cells. Goblet cells can generate glycoproteins, which have an
important role in maintaining viscidity and resilience of mucus.
Mucus possesses cleaning functions to clear intranasal
microparticle residues and potential accessory substancesfor
inflammation, and regular and oriented swing of cilium can remove
mucus from the nasopharynx and throat (32,33).
A previous study verified that weak expression of PTEN as well as
strong expression of PI3K could both inhibit apoptosis of ciliated
cells in mucosa of the upper respiratory tract and induce
metaplasia of these cells to goblet cells, resulting in increasing
secretion of mucus in the mucosal epithelium and decreasing the
function of expelling mucus (34,35).
The study applied rat nasal epithelial cells injured by
H2O2 to stimulate oxidative stress in CRS
patients. According to the features of the PI3K/PTEN/Akt signaling
pathway in regulation of cellular functions, we hypothesized that
the expression of PTEN in nasal epithelial cells under oxidative
stress is higher than in basal conditions, but the level of p-Akt
is additionally increased, and therefore, PTEN and p-Akt should be
in an antagonistic relationship with each other in the nasal
epithelial cells of CRS patients. However, although we observed
pro-apoptotic effects of PTEN on nasal epithelial cells, the
assumption of a relationship between PTEN and p-Akt was not
validated in this study.
In the condition of H2O2
treatment, we observed an increase in the level of ROS in rat nasal
epithelial cells, particularly when PTEN was upregulated, along
with an increase in the apoptosis rate. Inhibition of PTEN
expression by gene silencing could mitigate
H2O2 induced injury on rat nasal epithelial
cells. Through detecting the expression of apoptosis related genes,
we found that high levels of PTEN protein further upregulated the
expression of pro-apoptotic genes caspase-3 and Bax and
downregulated that of the anti-apoptotic gene Bcl-2 to promote
apoptosis. Meanwhile, H2O2 treatment reduced
SOD and CAT levels, especially in PTEN overexpressing cells while
additionally increasing MDA levels compared with control cells. SOD
and CAT are essential natural scavengers of superoxide radicals and
hydrogen peroxide to prevent organisms from undergoing oxidative
stress injury (36). MDA, the
final product of lipid oxidation, is a common indicator of lipid
peroxidation of the cellular membrane (37). The changes in these oxidative
parameters in this study imply that H2O2
successfully induced oxidative stress in nasal epithelial cells and
that high PTEN expression further worsens the induced injury.
Under oxidative stress conditions, activation of the
PI3K/Akt signaling pathway was found to be promoted in comparison
with control cells. In the H2O2-PTEN group,
overexpression of PTEN further upregulated phosphorylation of PI3K
and Akt. In contrast, in the H2O2-siPTEN
group, inhibition of PTEN expression decreased activation of
PI3K/Akt pathway. These results indicate thatthe PTEN and PI3K/Akt
pathways are positively correlated in
H2O2-injured nasal epithelial cells, which is
controversial with previous reports and our hypothesis that PTEN
has antagonistic effect on activation of PI3K. Through inhibiting
the PI3K/Akt pathway by perifosine, it was observed that the levels
of p-PI3K/PI3K and p-Akt/Akt were downregulated in the
H2O2-treated group but were still high in the
PTEN overexpressing group. However, under these conditions, ROS
levels and apoptosis rates in H2O2-injured
nasal epithelial cells were slightly reduced, and the differences
among H2O2, H2O2-PTEN
and H2O2-siPTEN groups were not significant.
Several experiments support the ideathat apart from inhibiting
phosphorylation of Akt and activation of PI3K, PTEN also plays a
role in regulating pathways related to cell growth, proliferation
and metabolism, including the JAK/STAT, FAK, ERK1/2, and RhoA-ROCK
pathways (38,39). It remains possible that there might
be unknown regulatory protein(s) acting in the PI3K/PTEN/Akt
signaling pathway to regulate the expression of PTEN and that the
PTEN and PI3K/Akt pathwaysmay be two distinct signaling pathways
with cross-interaction.
A significant body of evidence suggests an
antagonistic relationship between PTEN and p-Akt. However, our
study found that the expression of both p-Akt and PTEN in
H2O2-injured nasal epithelial cells was
higher than in control cells and that PTEN and p-Akt levels were
positively correlated. Unknown regulatory protein(s) possibly exist
in the PI3K/PTEN/Akt pathway or it may be that the PTEN and
PI3K/Akt pathways are two distinct signaling pathways that
cross-interact with each other. Further studies are required to
explore the internal mechanisms of these molecules in this
context.
Acknowledgements
Funded by Zhejiang Provincial Natural Science
Foundation of China (LY17H130002) and Wenzhou Public Welfare
Science and Technology Project (Y20150270).
References
|
1
|
Fokkens WJ, Lund VJ, Mullol J, Bachert C,
Alobid I, Baroody F, Cohen N, Cervin A, Douglas R, Gevaert P, et
al: EPOS 2012: European position paper on rhinosinusitis and nasal
polyps 2012. A summary for otorhinolaryngologists. Rhinology.
50:1–12. 2012.PubMed/NCBI
|
|
2
|
Al-Muhsen S, Johnson JR and Hamid Q:
Remodeling in asthma. J Allergy Clin Immunol. 128:451–464. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dennis SK, Lam K and Luong A: A review of
classification schemes for chronic rhinosinusitis with nasal
polyposis endotypes. Laryngoscope Investig Otolaryngol. 1:130–134.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kato A: Immunopathology of chronic
rhinosinusitis. Allergol Int. 64:121–130. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang Y, Zhang N, Lan F, Van Crombruggen K,
Fang L, Hu G, Hong S and Bachert C: Transforming growth factor-beta
1 pathways in inflammatory airway diseases. Allergy. 69:699–707.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Srivastava S, Singh D, Patel S and Singh
MR: Role of enzymatic free radical scavengers in management of
oxidative stress and autoimmune disorders. Int J Biol Macromol.
101:502–517. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cekin E, Ipcioglu OM, Erkul BE, Kapucu B,
Ozcan O, Cincik H and Gungor A: The association of oxidative stress
and nasal polyposis. J Int Med Res. 37:325–330. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bozkus F, San I, Ulas T, Iynen I, Yesilova
Y, Guler Y and Aksoy N: Evaluation of total oxidative stress
parameters in patients with nasal polyps. Acta Otorhinolaryngol
Ital. 33:248–253. 2013.PubMed/NCBI
|
|
9
|
Okur E, Inanc F, Yildirim I, Kilinc M and
Kilic MA: Malondialdehyde level and adenosine deaminase activity in
nasal polyps. Otolaryngol Head Neck Surg. 134:37–40. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tanaka Y, Hosoyama T, Mikamo A, Kurazumi
H, Nishimoto A, Ueno K, Shirasawa B and Hamano K: Hypoxic
preconditioning of human cardiosphere-derived cell sheets enhances
cellular functions via activation of the PI3K/Akt/mTOR/HIF-1α
pathway. Am J Transl Res. 9:664–673. 2017.PubMed/NCBI
|
|
11
|
Li J, Yen C, Liaw D, Podsypanina K, Bose
S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R, et al:
PTEN, a putative protein tyrosine phosphatase gene mutated in human
brain, breast, and prostate cancer. Science. 275:1943–1947. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Getahun A, Wemlinger SM, Rudra P, Santiago
ML, van Dyk LF and Cambier JC: Impaired B cell function during
viral infections due to PTEN-mediated inhibition of the PI3K
pathway. J Exp Med. 214:931–941. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tian H, Ge C, Zhao F, Zhu M, Zhang L, Huo
Q, Li H, Chen T, Xie H, Cui Y, et al: Downregulation of AZGP1 by
Ikaros and histone deacetylase promotes tumor progression through
the PTEN/Akt and CD44s pathways in hepatocellular carcinoma.
Carcinogenesis. 38:207–217. 2017.PubMed/NCBI
|
|
14
|
Yu M, Mu Y, Qi Y, Qin S, Qiu Y, Cui R and
Zhong M: Odontogenic ameloblast-associated protein (ODAM) inhibits
human colorectal cancer growth by promoting PTEN elevation and
inactivating PI3K/AKT signaling. Biomed Pharmacother. 84:601–607.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xiao J, Yu W, Hu K, Li M, Chen J and Li Z:
miR-92a promotes tumor growth of osteosarcoma by targeting PTEN/AKT
signaling pathway. Oncol Rep. 37:2513–2521. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang X, Chen Y, Zhao P, Zang L, Zhang Z
and Wang X: MicroRNA-19a functions as an oncogene by regulating
PTEN/AKT/pAKT pathway in myeloma. Leuk Lymphoma. 58:932–940. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yadav UC, Naura AS, Aguilera-Aguirre L,
Boldogh I, Boulares HA, Calhoun WJ, Ramana KV and Srivastava SK:
Aldose reductase inhibition prevents allergic airway remodeling
through PI3K/AKT/GSK3β pathway in mice. PLoS One. 8:e574422013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guillermet-Guibert J, Bjorklof K, Salpekar
A, Gonella C, Ramadani F, Bilancio A, Meek S, Smith AJ, Okkenhaug K
and Vanhaesebroeck B: The p110beta isoform of phosphoinositide
3-kinase signals downstream of G protein-coupled receptors and is
functionally redundant with p110gamma. Proc Natl Acad Sci USA.
105:8292–8297. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schmid MC, Avraamides CJ, Dippold HC,
Franco I, Foubert P, Ellies LG, Acevedo LM, Manglicmot JR, Song X,
Wrasidlo W, et al: Receptor tyrosine kinases and TLR/IL1Rs
unexpectedly activate myeloid cell PI3kγ, a single convergent point
promoting tumor inflammation and progression. Cancer Cell.
19:715–727. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Padala RR, Karnawat R, Viswanathan SB,
Thakkar AV and Das AB: Cancerous perturbations within the ERK,
PI3K/Akt, and Wnt/β-catenin signaling network constitutively
activate inter-pathway positive feedback loops. Mol Biosyst.
13:830–840. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xuan W, Feng X, Qian C, Peng L, Shi Y, Xu
L, Wang F and Tan W: Osteoclast differentiation gene expression
profiling reveals chemokine CCL4 mediates RANKL-induced osteoclast
migration and invasion via PI3K pathway. Cell Biochem Funct.
35:171–177. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Stocker H, Andjelkovic M, Oldham S,
Laffargue M, Wymann MP, Hemmings BA and Hafen E: Living with lethal
PIP3 levels: Viability of flies lacking PTEN restored by a PH
domain mutation in Akt/PKB. Science. 295:2088–2091. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu X, Hepner K, Castelino-Prabhu S, Do D,
Kaye MB, Yuan XJ, Wood J, Ross C, Sawyers CL and Whang YE: Evidence
for regulation of the PTEN tumor suppressor by a membrane-localized
multi-PDZ domain containing scaffold protein MAGI-2. Proc Natl Acad
Sci USA. 97:4233–4238. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schneider E, Keppler R, Prawitt D,
Steinwender C, Roos FC, Thüroff JW, Lausch E and Brenner W:
Migration of renal tumor cells depends on dephosphorylation of Shc
by PTEN. Int J Oncol. 38:823–831. 2011.PubMed/NCBI
|
|
25
|
Goo CK, Lim HY, Ho QS, Too HP, Clement MV
and Wong KP: PTEN/Akt signaling controls mitochondrial respiratory
capacity through 4E-BP1. PLoS One. 7:e458062012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hua S, Yao M, Vignarajan S, Witting P,
Hejazi L, Gong Z, Teng Y, Niknami M, Assinder S, Richardson D and
Dong Q: Cytosolic phospholipase A2α sustains pAKT, pERK and AR
levels in PTEN-null/mutated prostate cancer cells. Biochim Biophys
Acta. 1831:1146–1157. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bai ZG, Ye YJ, Shen DH, Lu YY, Zhang ZT
and Wang S: PTEN expression and suppression of proliferation are
associated with Cdx2 overexpression in gastric cancer cells. Int J
Oncol. 42:1682–1691. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Banham-Hall E, Clatworthy MR and Okkenhaug
K: The therapeutic potential for PI3K inhibitors in autoimmune
rheumatic diseases. Open Rheumatol J. 6:245–258. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tamura N: Recent findings on
phosphoinositide-3 kinase in rheumatic diseases. Nihon Rinsho
Meneki Gakkai Kaishi. 35:8–13. 2012.(In Japanese). View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jang HY, Kwon OK, Oh SR, Lee HK, Ahn KS
and Chin YW: Mangosteen xanthones mitigate ovalbumin-induced airway
inflammation in a mouse model of asthma. Food Chem Toxicol.
50:4042–4050. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Juss JK, Hayhoe RP, Owen CE, Bruce I,
Walmsley SR, Cowburn AS, Kulkarni S, Boyle KB, Stephens L, Hawkins
PT, et al: Functional redundancy of class I phosphoinositide
3-kinase (PI3K) isoforms in signaling growth factor-mediated human
neutrophil survival. PLoS One. 7:e459332012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dong J, Shang Y, Inthavong K, Tu J, Chen
R, Bai R, Wang D and Chen C: From the cover: Comparative numerical
modeling of inhaled nanoparticle deposition in human and rat nasal
cavities. Toxicol Sci. 152:284–296. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Biswas K, Chang A, Hoggard M, Radcliff FJ,
Jiang Y, Taylor MW, Darveau R and Douglas RG: Toll-like receptor
activation by sino-nasal mucus in chronic rhinosinusitis.
Rhinology. 55:59–69. 2017.PubMed/NCBI
|
|
34
|
Langlois MJ, Roy SA, Auclair BA, Jones C,
Boudreau F, Carrier JC, Rivard N and Perreault N: Epithelial
phosphatase and tensin homolog regulates intestinal architecture
and secretory cell commitment and acts as a modifier gene in
neoplasia. FASEB J. 23:1835–1844. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tyner JW, Kim EY, Ide K, Pelletier MR,
Roswit WT, Morton JD, Battaile JT, Patel AC, Patterson GA, Castro
M, et al: Blocking airway mucous cell metaplasia by inhibiting EGFR
antiapoptosis and IL-13 transdifferentiation signals. J Clin
Invest. 116:309–321. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
El-Shitany NA and Eid B: Proanthocyanidin
protects against cisplatin-induced oxidative liver damage through
inhibition of inflammation and NF-κβ/TLR-4 pathway. Environ
Toxicol. 32:1952–1963. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dede H, Takmaz O, Ozbasli E, Dede S and
Gungor M: Higher level of oxidative stress markers in small for
gestational age newborns delivered by cesarean section at term.
Fetal Pediatr Pathol. 36:232–239. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chetram MA and Hinton CV: PTEN regulation
of ERK1/2 signaling in cancer. J Recept Signal Transduct Res.
32:190–195. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yang S and Kim HM: The RhoA-ROCK-PTEN
pathway as a molecular switch for anchorage dependent cell
behavior. Biomaterials. 33:2902–2915. 2012. View Article : Google Scholar : PubMed/NCBI
|