Introduction
Although highly active antiretroviral therapy
(HAART) has been proven to suppress human immunodeficiency virus
type 1 (HIV-1) replication to undetectable levels, interrupting
HAART allows the virus to rapidly increase in numbers when compared
with pretreatment levels (1,2).
Previous studies have demonstrated that latently infected cells are
major obstacles to treatment success, and these cells are not
eliminated by HAART (3–5). In addition, previous studies have
demonstrated that the estimated size of this latent reservoir is
105-106 cells/patient (6,7). It
has been proposed that, for the complete eradication of HIV-1,
patients would be required to receive HAART for >70 years
(6,7). However, this therapy is expensive and
associated with toxic effects. For these reasons, elimination of
the latent reservoir of HIV-1 is an important aim.
Although the mechanisms that establish and maintain
HIV-1 latency are not well defined, specific factors may contribute
to HIV-1 latency, including activator protein-1 (AP-1), nuclear
factor of activated T-cells (NFAT), nuclear factor-κB (NF-κB) and
specificity protein 1 (SP1) (8).
In addition, in latently infected cells, various studies have
demonstrated that the genomes of HIV-1 are present within genes
that are actively transcribed (9,10).
Therefore, transcriptional interference of the host gene may
contribute to viral latency (9–11).
In addition, histone deacetylation, DNA methylation, histone
methylation and additional specific epigenetic modifications may
silence HIV-1 transcription and expression (12).
To eradicate latent HIV-1 infection, the ‘shock and
kill’ strategy was proposed, which involves reactivation of latent
HIV-1 expression by various agents followed by the killing of
infectious cells by additional methods (13). Numerous potential agents that may
reactivate latent HIV-1 infection have been identified, and the
agents that have been investigated with regards to eradication of
latent HIV-1 are divided into the following seven groups: Histone
deacetylase inhibitors, including suberoylanilide hydroxamic
acid/vorinostat (14), valproic
acid (VPA) (15), suneroyl
bis-hydroxamic (16),
panobinostat, givinostat, belinostat (17) and M344 (18); cytokines and chemokines, including
tumor necrosis factor α (TNF-α) (19) and NF-κB (20); DNA methyltransferase inhibitors,
which primarily include decitabine (2′-deoxy-5-azacytidine) and its
analogs (21); histone
methyltransferase inhibitors, such as BIX01294 (22); protein kinase C activators such as
prostratin (23); positive
transcription elongation factor b activators, which include
hexamethylene bisacetamide (24);
and particular unclassified agents, such as disulfiram (25). However, due to limitations of
effectiveness and toxicity, the agents may not be suitable when
administered alone. Therefore, an increasing number of studies have
focused on identifying a cocktail of agents that reactivate latent
HIV-1 infections. Ideally, agents or agent cocktails used to
eradicate latent reservoirs should be highly efficient at
reactivating latent HIV-1, not induce global T-cell activation, and
must exhibit acceptable pharmacological and toxicological
properties. To the best of the author's knowledge, no agents that
effectively disrupt HIV-1 latency and exhibit low toxicities have
been identified thus far. Therefore, the identification of novel
agents, and particular agent combinations is required.
Quercetin is a flavonol that is present in various
plant-based foods, including onions, apples, citrus fruits,
berries, red grapes, red wine, broccoli, tea, flowers and bark
roots (26). It has been used as a
treatment for various conditions, including allergies, asthma,
bacterial infections, arthritis, gout, eye disorders, hypertension
and neurodegenerative disorders (26). Previous studies have indicated that
quercetin may potentially inhibit the HIV-1 integrase and reverse
transcriptase enzymes (27,28);
however, limited information regarding the role of quercetin in
combating latent HIV-1 infections is known. Therefore, the present
study aimed to investigate the ability of quercetin to reactivate
latent HIV-1. To achieve this, the ability of quercetin to induce
HIV-1 expression in latently infected cells, as well as the
potential underlying molecular mechanisms, were investigated. In
addition, the effect of quercetin in combination with additional
activators was investigated. The results demonstrated that
quercetin reactivated latent HIV-1 in an in vitro model of
HIV-1 latency potentially via the NF-κB signaling pathway, and
synergistically reactivated HIV-1 latency when combined with VPA or
prostratin. The results indicated that either of these combinations
may be useful as a potential anti-latency therapy.
Materials and methods
Cell culture and chemical
treatment
C11 cells, a type of latently infected Jurkat cell,
were generated in our laboratory (Shanghai, China) and have been
employed in previous studies (29–31).
Briefly, cells were transfected with a construct (donated by the
National Institutes of Health, Bethesda, MD, USA) that encodes
green fluorescent protein (GFP) as a marker for Tat-driven HIV-1
long terminal repeat (LTR) expression; lentiviral transfection was
performed as previously described (32). The C11 cells were cultured in
RPMI-1640 medium (Corning Incorporated, Corning, NY, USA)
supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), 100 U/ml penicillin and 100
µg/ml streptomycin (Invitrogen; Thermo Fisher Scientific, Inc.) at
37°C and 5% CO2. The HEK 293T human endothelial kidney
cell line was purchased from the American Type Culture Collection
(Manassas, VA, USA), and cells were cultured in Dulbecco's modified
Eagle's medium (Gibco; Thermo Fisher Scientific, Inc.) with 10%
FBS, 100 U/ml penicillin and 100 µg/ml of streptomycin at 37°C and
5% CO2. Quercetin was purchased from Sigma-Aldrich;
Merck KGaA (Darmstadt, Germany) and VPA was purchased from
InvivoGen (San Diego, CA, USA). Prostratin was purchased from LC
Laboratories (Woburn, MA, USA). Recombinant human TNF-α was
purchased from EMD Millipore (Billerica, MA, USA). Quercetin (100
mM), TNF-α (1 mg/ml), VPA (100 mM) and prostratin (10 mM) were
dissolved in anhydrous dimethylsulfoxide and stored at −20°C.
Visualization of GFP
As GFP was used as the marker of HIV-1 expression,
the expression of GFP was observed by fluorescence microscopy to
confirm reactivation. C11 cells (3×104) were treated at
37°C and 5% CO2 with quercetin (20 µM) or mock (0 µM),
C11 cells (6×104) were subsequently viewed using a Nikon
fluorescence microscope (Nikon Corporation, Tokyo, Japan). All
microscope samples were imaged across 10 random fields using a
Nikon E2 digital camera (Nikon Corporation), and images were
analyzed using EIS Element F 3.0 (Nikon Corporation).
Flow cytometry
C11 cells (6×104) were washed with
phosphate-buffered saline (PBS) and incubated with the following
treatments: Quercetin (20 µM) or mock treatment for 72 h; quercetin
(20 µM) or mock treatment for 0, 24, 48, 72 and 96 h; quercetin (5,
10, 20 and 40 µM) or mock treatment for 72 h; quercetin (20 µM),
mock (0 µM) treatment, VPA (2 mM), prostratin (200 nM), quercetin
(20 µM) + VPA (2 mM) and quercetin (20 µM) + prostratin (200 nM)
for 72 h. Cells were cultured in RPMI-1640 medium supplemented with
10% FBS, 100 U/ml penicillin and 100 µg/ml streptomycin, at 37°C
and 5% CO2. Cells were washed with 1 ml PBS for 5 min
and resuspended in 300 µl PBS. GFP expression was measured using a
FACScan flow cytometer (BD Biosciences, Franklin lakes, NJ, USA),
and FACS data were analyzed with FlowJo software version 10.0
(FlowJo LLC, Inc., Ashland, OR, USA). GFP-associated fluorescence
was differentiated from background fluorescence by the gating of
live cells (10,000 events in total) and by two-parameter analysis.
For all analyses, three independent experiments were performed, and
samples were analyzed in triplicate.
Cytotoxicity assay
Cell proliferation and viability were measured using
a Cell Counting Kit-8 assay (CCK-8; Dojindo Molecular Technologies,
Inc., Rockville, MD, USA) (30,33).
C11 cells were seeded in 96-well plates (~4×104 cells
per well) before they were treated with 0, 5, 10, 20, 40, 80 and
160 µM quercetin for 48 h at 37°C and 5% CO2. This was
followed by the addition of 10 µl CCK-8 solution to each well of
the plate. Following incubation for 4 h at 37°C, the absorbance was
read at 450 nm using a microplate reader. For each sample, the half
maximal inhibitory concentration (IC50) was measured in
triplicate and at least three independent assays were
performed.
Transient transfection and luciferase
assays
HEK 293T cells were plated at 1×105
cells/well in 24-well plates at 24 h prior to transfection, and
were transfected using Lipofectamine® 2000 according to
the manufacturer's protocol (Invitrogen; Thermo Fisher Scientific,
Inc.). The HIV-1 LTR-luc (1.0 µg; donated by Dr Warner C. Greene,
Duke University Medical Center, Durham, NC, USA) (34), HIV-1 LTR lacking two κВ enhancers
[1.0 µg; HIV-1-LTR (ΔκВ)-luc], HIV-1 LTR lacking an AP-1 enhancer
[1.0 µg; HIV-1-LTR (ΔAP-1)-luc], HIV-1 LTR lacking an SP1 enhancer
[1.0 µg; HIV-1-LTR (ΔSP1)-luc; all donated by Dr Andrew D. Badley
(Division of Infectious Diseases, Mayo Clinic, Rochester, MN, USA)
(35) or pRL-SV40 (0.1 µg; Promega
Corp., Madison, WI, USA) vector constructs were formulated into
liposomes and transfected into HEK 293T cells. At 24 h
post-transfection, the cells were mock (0 µM)-treated, or treated
with quercetin (20 µM) or TNF-α (10 ng/ml) at 37°C and 5%
CO2. At 48 h post-treatment, cells were lysed with
passive buffer (Yeasen Co., Ltd., Shanghai, China) and the
luciferase activity was measured using a
Dual-Luciferase® Reporter assay kit (Promega Corp.) and
normalized by Renilla luciferase activity, according to the
manufacturer's instructions.
Cell nuclear protein extraction and
electrophoretic mobility shift assay (EMSA)
Nuclear extracts from C11 cells following treatment
with different agents were obtained as previously described
(30,36). Briefly, C11 cells
(3×104) were treated with quercetin (10, 20 and 40 µM)
for 3 h or TNF-α (10 ng/ml) for 30 min, before they were washed
twice with PBS and resuspended in 100 µl ice-cold buffer A [10 mM
HEPES-NaOH, pH 7.9; 10 mM KCl; 1.5 mM MgCl2; 0.5 mM
dithiothreitol (DTT); and 0.2 mM phenylmethane sulfonyl fluoride
(PMSF)] and 0.6% nonidet P-40 for 15 min followed by centrifugation
at 15,000 × g for 2 min at 4°C. The supernatant contained
cytoplasmic protein, and was discarded. The precipitated nuclear
pellet was washed once with buffer A and resuspended in 60 µl
ice-cold buffer B (20 mM HEPES-NaOH, pH 7.9; 420 mM NaCl; 1.5 mM
MgCl2; 0.2 mM EDTA; 0.5 mM DTT; 0.2 mM PMSF; 25% glycerol). The
mixture was incubated on ice for 30 min with intermittent mixing
followed by centrifugation at 15,000 × g for 15 min at 4°C. The
supernatant, containing nuclear proteins, was collected and the
protein concentration was measured with a bicinchoninic acid kit
(Beyotime Institute of Biotechnology, Haimen, China). Proteins were
stored at −80°C for EMSA analysis.
The EMSA for NF-κВ was performed using the
LightShift™ Chemiluminescent EMSA kit (Pierce; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol.
Briefly, 10 µM biotin-labeled double-stranded NF-κВ
oligonucleotides (5′-AGTTGAGGGGACTTTCCCAGG-3′ and
3′-TCAACTCCCCTGAAAGGGTCC-5′) (Shanghai Ruidi Biological Technology
Co., Ltd., Shanghai, China) were incubated with 20 µg nuclear
protein extracts at room temperature for 20 min. The samples were
subsequently subjected to 5% non-denaturing polyacrylamide gel
electrophoresis in Tris/borate/EDTA buffer and transferred to a
nylon membrane. After attaching to the membrane by UV-crosslinking,
the DNA-protein complexes were detected by LightShift™
chemiluminescence and analyzed by autoradiography. Cold competition
was performed in the presence of 100-fold excess non-labeled
consensus oligonucleotides for 10 min prior to the addition of
labeled oligonucleotides.
Statistical analysis
Data are representative of three independent
experiments. Values were presented as the mean ± standard
deviation. One-way analysis of variance and Tukey's post hoc test
were performed using SPSS version 19.0 (IBM SPSS, Armonk, NY, USA).
P<0.05 was considered to indicate a significant difference.
Results
Quercetin reactivates latent HIV-1
replication
In order to assess the induction of HIV-1 expression
in latently infected cells by quercetin, C11 cells that were
established in our lab were employed. These cells are Jurkat T
cells latently infected with a single provirus integrated into
intron 3 of the RNA binding protein with serine rich domain 1 gene,
combined with a GFP gene and under the control of the HIV-1 LTR,
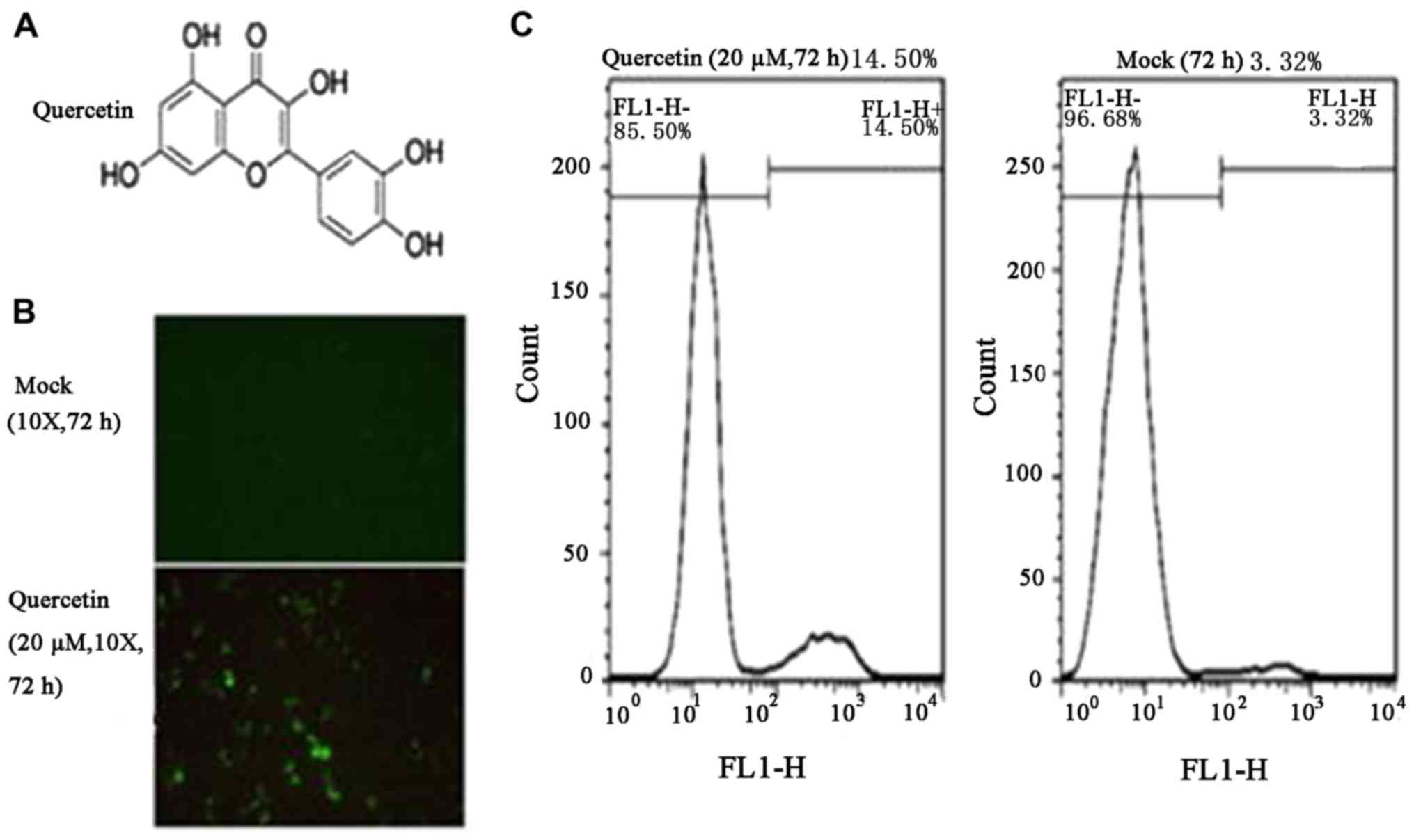
and was used as a marker of HIV-1 LTR expression (31). The structure of quercetin is
presented in Fig. 1A. Quercetin
(20 µM) was used to treat C11 cells for 72 h, and fluorescence
microscopy analysis indicated ~10% C11 cells were positive for
HIV-1 LTR expression (Fig. 1B).
Subsequently, as GFP was used as a marker of HIV-1 expression, flow
cytometry was performed to detect the percentage of GFP-positive
cells (Fig. 1C). The results
indicated that the HIV-1 transcriptional activity increased to
14.50% following treatment with quercetin (20 µM) for 72 h,
compared with 3.12% in mock-treated cells. To analyze the kinetics
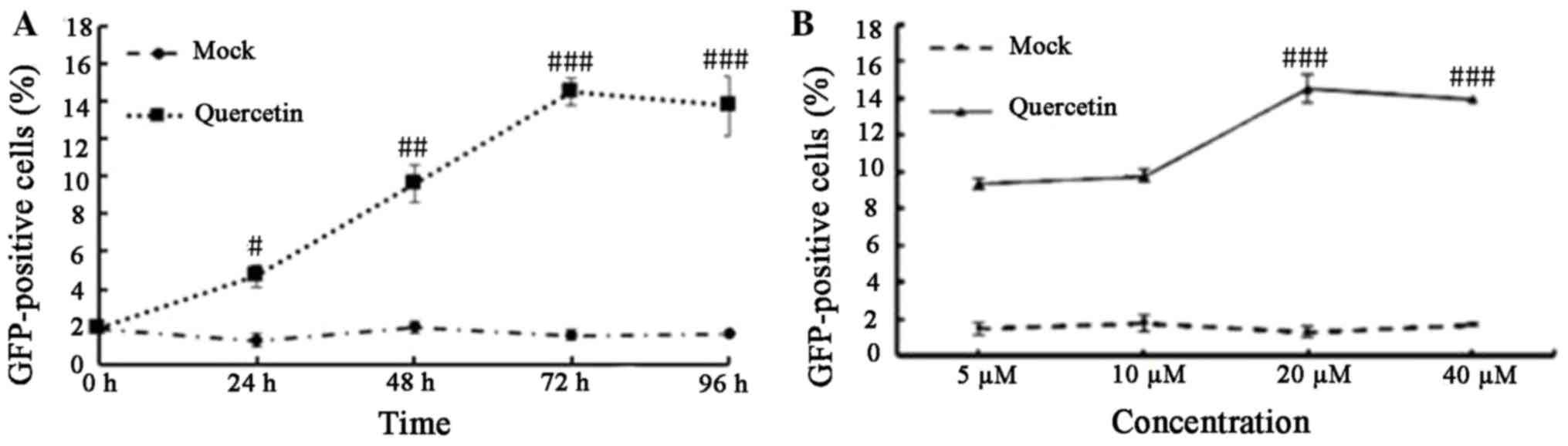
of HIV-1 LTR expression induced by quercetin, a kinetics experiment
was performed where quercetin (20 µM) or mock-treated C11 cells
were cultured for 1-4 days. At each time point, flow cytometry
analysis was performed to determine the proportion of
GFP-expressing cells. Following treatment with quercetin, the
percentage of GFP-expressing cells increased for the first 3 days
and then plateaued at day 4, whereas no increase in GFP-positive
cells was observed in the mock-treated group over the same time
period (Fig. 2A). These results
indicated that quercetin may affect HIV-1 expression in a
time-dependent manner. To determine the effect of increasing
concentrations of quercetin on HIV-1 production, cells were treated
with 5, 10, 20 and 40 µM quercetin for 72 h. The percentage of
GFP-expressing cells was increased by between 3- and 6-fold
compared with the mock-treated cells (Fig. 2B). The results demonstrated that
quercetin induced HIV-1 LTR reactivation in a
concentration-dependent manner.
Quercetin synergistically reactivates
latent HIV-1 production
As quercetin is effective and less toxic than
prostratin (37), the present
study assessed whether quercetin synergistically reactivates HIV-1
in C11 cells when combined with VPA or prostratin. C11 cells were
treated with quercetin alone (20 µM), VPA alone (2 mM), prostratin
alone (200 nM), quercetin (20 µM) + VPA (2 mM, quercetin (20 µM) +
prostratin (200 nM) or received mock treatment for 72 h. A
synergistic interaction between two activators indicates that
combined treatment results in a level of activation that is higher
than the sum of the activation induced by each activator when
applied individually (38). As
demonstrated in Fig. 3, the
percentage of GFP-positive cells was 14.6% in the quercetin alone
(20 µM), 11.4% in VPA alone (2 mM), 22.6% in prostratin alone (200
nM), 44.4% in quercetin + VPA, 53.8% in quercetin + prostratin and
1.8% in the mock-treated groups. These results indicate that
quercetin, in combination with VPA or prostratin, resulted in
synergistic reactivation of latent HIV-1 production in C11
cells.
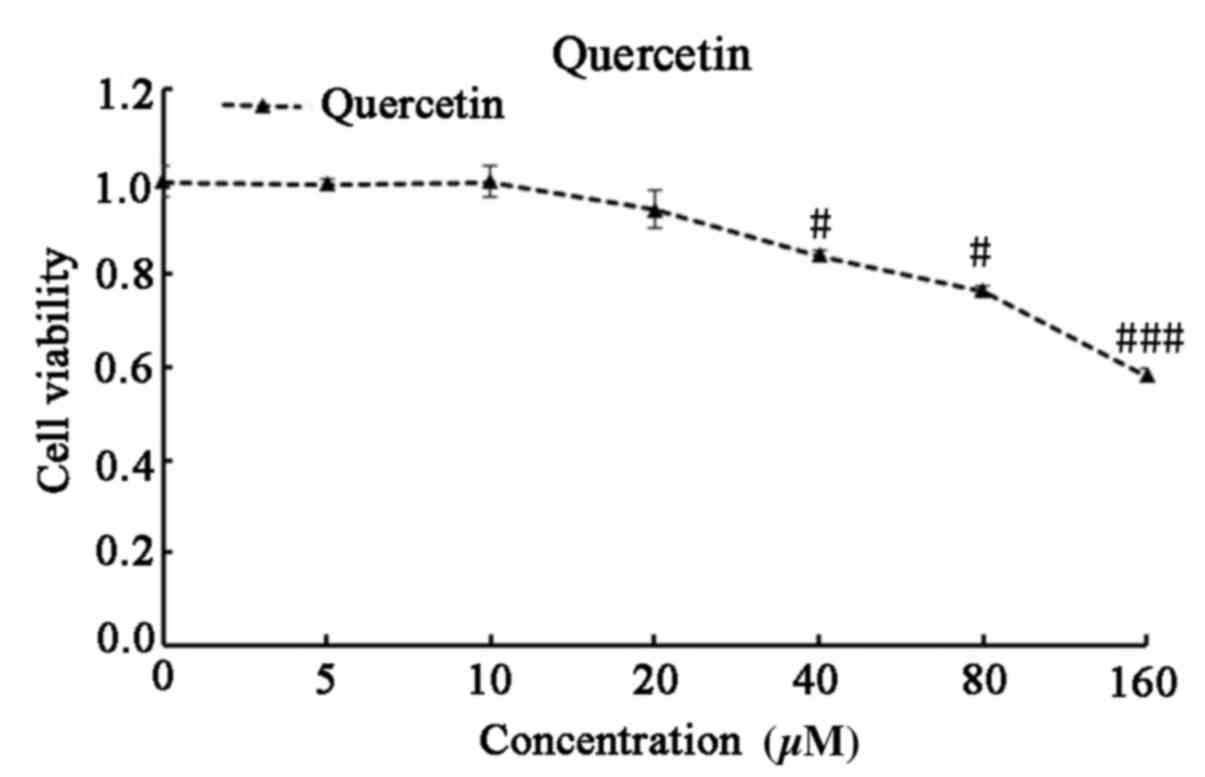
Quercetin exhibits no apparent
toxicity in vitro
The toxicity of quercetin was investigated to
determine whether it may be ideal therapeutic agent for
reactivation of latent HIV-1. C11 cells were treated with 0, 5, 10,
20, 40, 80 and 160 µM quercetin for 72 h, and cell viability was
subsequently analyzed using CCK-8 assay. At its active
concentration (20 µM), quercetin exhibited no significant toxicity
in C11 cells (Fig. 4).
Quercetin activates the HIV-1 LTR
through induction of NF-κB
The present study subsequently investigated the
signaling pathway through which quercetin may mediate activation of
the HIV-1 LTR. Binding sites for a number of inducible
transcription factors, including NF-κB, AP-1 and SP1, are located
within the HIV-1 LTR (39). To
determine the role of particular transcription factors in the
activation of the HIV LTR by quercetin, the present study employed
HEK 293T cells that were transfected with luciferase reporter
plasmids containing the wild-type HIV-1 LTR, LTR lacking two κB
enhancers, LTR lacking AP-1 enhancers or LTR lacking SP1 enhancers.
TNF-α was selected as a positive control. Compared with mock
controls, TNF-α induced ~3.65-fold upregulation of the
HIV-1-LTR-luc reporter, ~2.98-fold upregulation of the HIV-1-LTR
(ΔAP-1)-luc and ~3.11-fold upregulation of HIV-1-LTR (ΔSP1) -luc
reporter; however, TNF-α failed to activate the HIV-1-LTR (ΔκB)-luc
reporter (Fig. 5A). Similarly,
quercetin induced ~1.56-fold upregulation of the HIV-1-LTR-luc
reporter, ~1.53-fold upregulation of the HIV-1-LTR (ΔAP-1)-luc and
~1.58-fold upregulation of the HIV-1-LTR (ΔSP1)-luc reporters,
whereas it failed to activate the HIV-1-LTR (ΔκB)-luc reporter.
These results indicated that NF-κB transcription factors may serve
an important role in quercetin-mediated activation of latent HIV-1
LTR expression. In order to further confirm the involvement of the
NF-κB signaling pathway in quercetin-mediated activation of latent
HIV-1 LTR expression, an EMSA was performed to assess whether
quercetin treatment was a sufficient stimulus for NF-κB nuclear
translocation and DNA binding. Nuclear extracts from C11 cells
treated with quercetin or TNF-α were incubated with biotin-labeled
NF-κB enhancer oligonucleotides. The results demonstrated that
quercetin increased the translocation of NF-κB to the nucleus in a
concentration-dependent manner (Fig.
5B). These results provided further evidence to suggest that
quercetin-mediated regulation of HIV-1 gene expression may occur
via the NF-κB signaling pathway.
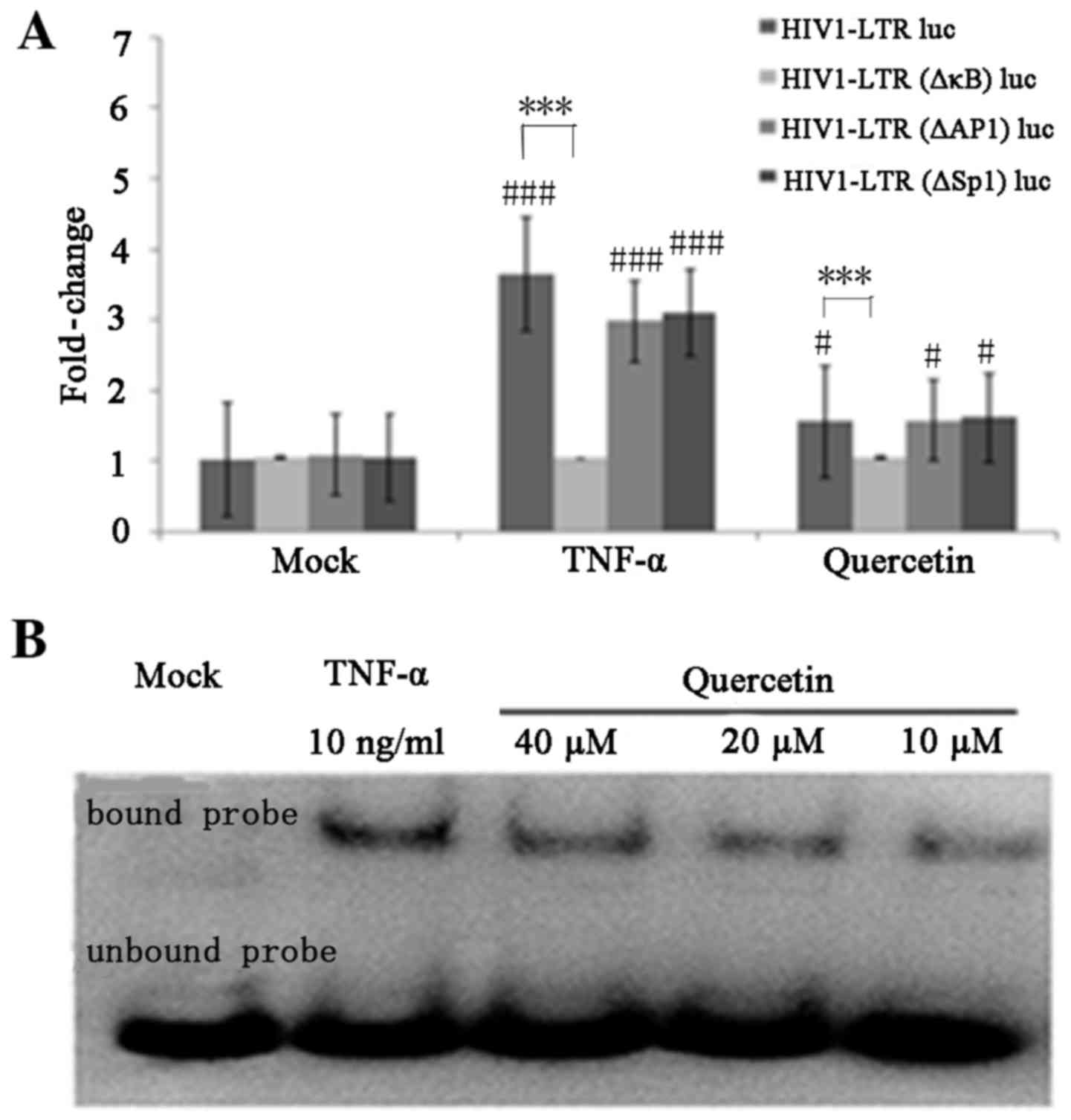 | Figure 5.Quercetin activates the HIV-1 LTR via
induction of NF-κB. (A) HEK 293T cells were transfected with
HIV1-LTR luc, HIV1-LTR (ΔκB) luc, HIV1-LTR (ΔAP-1) luc and HIV1-LTR
(ΔSP1) luc. At 24 h post-transfection, the cells were mock-treated
or treated with quercetin (20 µM) or TNF-α (10 ng/ml). Luc activity
was measured after 48 h of treatment. Data are presented as the
mean ± standard deviation. (B) Quercetin stimulates nuclear NF-κB
DNA binding. C11 cells were treated with quercetin (10, 20 and 40
µM) for 3 h or with TNF-α (10 ng/ml) for 30 min. Nuclear extracts
were isolated and subject to an electrophoretic mobility shift
assay with biotin-labeled NF-κB enhancer DNA probes.
#P<0.05 and ###P<0.001 vs. mock treated
cells; ***P<0.001 vs. HIV1-LTR (ΔκB) luc-infected cells. HIV-1,
human immunodeficiency virus type 1; NF-κB, nuclear factor-κB; LTR,
long terminal repeat; luc, luciferase; AP-1, activator protein-1;
SP1, specificity protein 1; TNF-α, tumor necrosis factor-α. |
Discussion
Although recent studies investigating the clinical
consequences of latent HIV-1 infection have made progress, the
persistence of the latent reservoir of integrated HIV-1 proviruses
in resting CD4+ T cells is a major obstacle for viral
eradication (40). Therefore the
development of an effective treatment for HIV-1 infection remains a
challenge (41). Reactivation of
the latent provirus in patients receiving HAART is a promising
strategy for the depletion of the latent viral reservoir (42). In order to achieve this aim,
Katlama et al (43)
proposed a three-tiered strategy to reactivate the latent cells and
eliminate viral reservoirs. The first and most important step is to
reactivate the expression of the latent HIV-1 provirus. Therefore,
various studies have focused on agents that target different
mechanisms of HIV-1 latency, including VPA, TNF-α and prostratin
(44). However, the toxicity and
ineffectiveness of these agents in clinical trials and the
necessity for prolonged treatment limit the applications of these
agents (45). It is thought that
treatment with a combination of agents may be less toxic and more
effective (46). Therefore, the
identification of improved treatment combinations that demonstrate
increased specificity is required.
Quercetin, is a flavonol that is used in the
treatment of allergies, asthma, bacterial infections, arthritis,
gout, eye disorders, hypertension and neurodegenerative disorders
(27). Quercetin has demonstrated
the ability to treat HIV-1 as a drug in HAART (47); however, there is limited
information regarding the effect of quercetin on HIV-1 latency.
Therefore, the present study used a simple in vitro model of
latent HIV-1 infection in order to investigate whether quercetin
reactivates latently infected C11 cells. A plasmid vector encoding
GFP under the control of the HIV-1 LTR was transfected into C11
cells, and used as a marker of HIV-1 expression. The expression of
the HIV-1 LTR was detected by fluorescence microscopy and flow
cytometry (30). The results
demonstrated that quercetin effectively reactivated HIV-1 latency
and exhibited low toxicity in C11 cells at concentrations ≤20 µM.
In addition, the results demonstrated that latent HIV-1 replication
was activated by quercetin in a time- and concentration-dependent
manner.
Compared with prostrotin and additional activators,
the effect of quercetin alone on HIV-1 reactivation is weak;
however, quercetin is less toxic to cells (37,46).
Therefore, the current study investigated whether synergistic
activation of HIV-1 occurred when quercetin was combined with VPA
or prostratin in C11 cells. VPA and prostratin were selected as
they demonstrate a potent effect on the reactivation of infection
in latently infected cell lines and ex vivo primary cells
(48,49). The results of the present study
demonstrated that co-treatment with quercetin plus VPA or quercetin
plus prostratin induced HIV-1 expression in a higher percentage of
C11 cells when compared with each activator alone. These results
indicated that quercetin combined with VPA or prostratin may lead
to synergistic reactivation of HIV-1 production at a lower
concentration in C11 cells, therefore, lower concentrations of
these agents may be used to reactivate latent HIV-1 cells. These
results are consistent with previous studies demonstrating that
co-treatment with an NK-κB inducer and histone deacetylase
inhibitor synergistically increased the proportion of J-Lat cells
displaying GFP fluorescence when compared with treatment with each
compound in isolation (36,50),
and one study reported that prostratin synergizes with other
activators to promote activation of latent HIV via NF-κB (51).
Viral and cellular transcription factors with
binding sites in the HIV-1 LTR serve an important role in the
expression of latent HIV-1. The HIV-1 LTR contains several
DNA-binding sites for various cellular transcription factors,
including NFAT, AP-1, SP1, NF-κB, lymphoid enhancer binding factor
1, COUP transcription factor 2, ETS proto-oncogene 1 and upstream
stimulatory factor (52,53). Of these, NF-κB serves an important
role in the reactivation pathway of latent HIV-1. A previous study
reported that quercetin inhibited inflammation associated with
NF-κB in specific cell lines (54). Therefore, the present study
investigated whether the NF-κB signaling pathway may be involved in
the quercetin-mediated activation of the latent HIV-1 LTR in C11
cells. The results indicated that quercetin effectively reactivated
the wild-type HIV-1 LTR-luc, the HIV-LTR (ΔAP-1)-luc and the
HIV-LTR (ΔSP1)-luc reporters, whereas, it failed to activate the
LTR reporter lacking the κB enhancers. In addition, the results
indicated that nuclear translocation of NF-κB was induced by
quercetin in a concentration-dependent manner as determined by EMSA
analysis. Together, the results of the current study indicate that
quercetin may activate HIV-1 gene expression via the NF-κB
signaling pathway.
In conclusion, the HIV-1 viral reservoir is a major
obstacle to the eradication of the provirus in patients receiving
HAART. A current therapeutic strategy, termed ‘shock-and-kill’, has
been proposed as a promising solution to eradicate HIV-1 reservoirs
in the presence of HAART. For this therapy to be successful, the
primary and most important aim is to identify a method of inducing
latent HIV-1 gene expression. Due to the limitations of
ineffectiveness and toxicity, the agents that have previously been
investigated have failed in the clinic (44). Therefore, the identification of
agents that exhibit lower toxicity is required. The current study
demonstrated that quercetin is a potent activator of HIV-1 latency
that lacks obvious cytotoxicity and may function via the NF-κB
signaling pathway. Notably, the results indicated that quercetin
synergizes with VPA or prostratin in the induction of HIV-1
transcription. In addition, a previous study demonstrated that the
majority of agents that have been identified, and that do not cause
global T cell activation, are ineffective in the clinic (44). Therefore, it is important to
identify agents that may reactivate latent HIV-1 without global T
cell activation, The results of the current study suggest that
quercetin may meet these criteria. However, this study employed an
in vitro model of HIV-1 latency, therefore further
investigation into the effects of quercetin in a wider population
of latent HIV-1 infected cells from HAART-treated patients will be
required to explore this agent as a potential drug candidate. In
addition, further investigation as to whether quercetin may lead to
global T cell activation should be performed.
Acknowledgements
The present study was supported by the Shanghai
Scientific Research Plan Project (grant no. 14401902000) and the
National Natural Science Foundation of China (grant no.
31501032).
References
|
1
|
Davey RT Jr, Bhat N, Yoder C, Chun TW,
Metcalf JA, Dewar R, Natarajan V, Lempicki RA, Adelsberger JW,
Miller KD, et al: HIV-1 and T cell dynamics after interruption of
highly active antiretroviral therapy (HAART) in patients with a
history of sustained viral suppression. Proc Natl Acad Sci USA.
96:pp. 15109–15114. 1999; View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dahabieh MS, Battivelli E and Verdin E:
Understanding HIV latency: The road to an HIV cure. Annu Rev Med.
66:407–421. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lohse N, Hansen AB, Pedersen G, Kronborg
G, Gerstoft J, Sørensen HT, Vaeth M and Obel N: Survival of persons
with and without HIV infection in Denmark, 1995–2005. Ann Intern
Med. 146:87–95. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Finzi D, Hermankova M, Pierson T, Carruth
LM, Buck C, Chaisson RE, Quinn TC, Chadwick K, Margolick J,
Brookmeyer R, et al: Identification of a reservoir for HIV-1 in
patients on highly active antiretroviral therapy. Science.
278:1295–3100. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wong JK, Hezareh M, Günthard HF, Havlir
DV, Ignacio CC, Spina CA and Richman DD: Recovery of
replication-competent HIV despite prolonged suppression of plasma
viremia. Science. 278:1291–1295. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Strain MC, Little SJ, Daar ES, Havlir DV,
Gunthard HF, Lam RY, Daly OA, Nguyen J, Ignacio CC, Spina CA, et
al: Effect of treatment, during primary infection, on establishment
and clearance of cellular reservoirs of HIV-1. J Infect Dis.
191:1410–1418. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Siliciano JD, Kajdas J, Finzi D, Quinn TC,
Chadwick K, Margolick JB, Kovacs C, Gange SJ and Siliciano RF:
Long-term follow-up studies confirm the stability of the latent
reservoir for HIV-1 in resting CD4+ T cells. Nat Med. 9:727–728.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mehla R, Bivalkar-Mehla S, Zhang R, Handy
I, Albrecht H, Giri S, Nagarkatti P, Nagarkatti M and Chauhan A:
Bryostatin modulates latent HIV-1 infection via PKC and AMPK
signaling but inhibits acute infection in a receptor independent
manner. PLoS One. 5:e111602010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Han Y, Lin YB, An W, Xu J, Yang HC,
O'Connell K, Dordai D, Boeke JD, Siliciano JD and Siliciano RF:
Orientation-dependent regulation of integrated HIV-1 expression by
host gene transcriptional readthrough. Cell Host Microbe.
4:134–146. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shan L, Yang HC, Rabi SA, Bravo HC, Shroff
NS, Irizarry RA, Zhang H, Margolick JB, Siliciano JD and Siliciano
RF: Influence of host gene transcription level and orientation on
HIV-1 latency in a primary-cell model. J Virol. 85:5384–5393. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lenasi T, Contreras X and Peterlin BM:
Transcriptional interference antagonizes proviral gene expression
to promote HIV latency. Cell Host Microbe. 4:123–133. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mbonye U and Karn J: Control of HIV
latency by epigenetic and non-epigenetic mechanisms. Curr HIV Res.
9:554–567. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Margolis DM, Garcia JV, Hazuda DJ and
Haynes BF: Latency reversal and viral clearance to cure HIV-1.
Science. 353:aaf65172016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Del Prete GQ, Shoemaker R, Oswald K, Lara
A, Trubey CM, Fast R, Schneider DK, Kiser R, Coalter V, Wiles A, et
al: Effect of suberoylanilide hydroxamic Acid (SAHA) administration
on the residual virus pool in a model of combination antiretroviral
therapy-mediated suppression in SIVmac239-infected indian rhesus
macaques. Antimicrob Agents Chemother. 58:6790–6806. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mazzoccoli G, Longhitano C and Vinciguerra
M: Cardio-hepatic metabolic derangements and valproic acid. Curr
Clin Pharmacol. 9:165–170. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huber K, Doyon G, Plaks J, Fyne E, Mellors
JW and Sluis-Cremer N: Inhibitors of histone deacetylases:
Correlation between isoform specificity and reactivation of HIV
type 1 (HIV-1) from latently infected cells. J Biol Chem.
286:22211–22218. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rasmussen TA, Schmeltz Søgaard O,
Brinkmann C, Wightman F, Lewin SR, Melchjorsen J, Dinarello C,
Østergaard L and Tolstrup M: Comparison of HDAC inhibitors in
clinical development: Effect on HIV production in latently infected
cells and T-cell activation. Hum Vaccin Immunother. 9:993–1001.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hao Y, Zhang Y, Zhou X, Qu X, Wang P, Liu
S, Lu D and Zhu H: Selective histonedeacetylase inhibitor M344
intervenes in HIV-1 latency through increasing histone acetylation
and activation of NF-kappaB. PLoS One. 7:e488322012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Folks TM, Clouse KA, Justement J, Rabson
A, Duh E, Kehrl JH and Fauci AS: Tumor necrosis factor alpha
induces expression of human immunodeficiency virus in a chronically
infected T-cell clone. Proc Natl Acad Sci USA. 86:pp. 2365–2368.
1989; View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang P, Qu X, Wang X, Liu L, Zhu X, Zeng H
and Zhu H: As2O3 synergistically reactivate latent HIV-1 by
induction of NF-κB. Antiviral Res. 100:688–697. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fernandez G and Zeichner SL: Cell
line-dependent variability in HIV activation employing DNMT
inhibitors. Virol J. 7:2662010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Imai K, Togami H and Okamoto T:
Involvement of histone H3 lysine 9 (H3K9) methyltransferase G9a in
the maintenance of HIV-1 latency and its reactivation by BIX01294.
J Biol Chem. 285:16538–16545. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Williams SA, Chen LF, Kwon H, Fenard D,
Bisgrove D, Verdin E and Greene WC: Prostratin antagonizes HIV
latency by activating NF-kappaB. J Biol Chem. 279:42008–42017.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vlach J and Pitha PM: Hexamethylene
bisacetamide activates the human immunodeficiency virus type 1
provirus by an NF-kappa B-independent mechanism. J Gen Virol.
74:2401–2408. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xing S, Bullen CK, Shroff NS, Shan L, Yang
HC, Manucci JL, Bhat S, Zhang H, Margolick JB, Quinn TC, et al:
Disulfiram reactivates latent HIV-1 in a Bcl-2-transduced primary
CD4+ T cell model without inducing global T cell activation. J
Virol. 85:6060–6064. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Larson AJ, Symons JD and Jalili T:
Therapeutic potential of quercetin to decrease blood pressure:
Review of efficacy and mechanisms. Adv Nutr. 3:39–46. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fesen MR, Kohn KW, Leteurtre F and Pommier
Y: Inhibitors of human immunodeficiency virus integrase. Proc Natl
Acad Sci USA. 90:pp. 2399–2403. 1993; View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tanaka R, Tsujii H, Yamada T, Kajimoto T,
Amano F, Hasegawa J, Hamashima Y, Node M, Katoh K and Takebe Y:
Novel 3alpha-methoxyserrat-14-en-21beta-ol (PJ-1) and
3beta-methoxyserrat-14-en-21beta-ol (PJ-2)-curcumin, kojic acid,
quercetin, and baicalein conjugates as HIV agents. Bioorg Med Chem.
17:5238–5246. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ying H, Zhang Y, Zhou X, Qu X, Wang P, Liu
S, Lu D and Zhu H: Selective histonedeacetylase inhibitor M344
intervenes in HIV-1 latency through increasing histone acetylation
and activation of NF-kappaB. PLoS One. 7:e488322012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang P, Qu X, Wang X, Liu L, Zhu X, Zeng H
and Zhu H: As2O3 synergistically reactivate latent HIV-1 by
induction of NF-κB. Antiviral Res. 100:688–697. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ding D, Qu X, Li L, Zhou X, Liu S, Lin S,
Wang P, Liu S, Kong C, Wang X, et al: Involvement of histone
methyltransferase GLP in HIV-1 latency through catalysis of H3K9
dimethylation. Virology. 440:182–189. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kutner RH, Zhang XY and Reiser J:
Production, concentration and titration of pseudotyped HIV-1-based
lentiviral vectors. Nat Protoc. 4:495–505. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lin S, Zhang Y, Ying H and Zhu H: HIV-1
reactivation induced by apicidin involves histone modification in
latently infected cells. Curr HIV Res. 9:202–208. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang FX, Xu Y, Sullivan J, Souder E,
Argyris EG, Acheampong EA, Fisher J, Sierra M, Thomson MM, Najera
R, et al: IL-7 is a potent and proviral strain-specific inducer of
latent HIV-1 cellular reservoirs of infected individuals on virally
suppressive HAART. J Clin Invest. 115:128–137. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bren GD, Whitman J, Cummins N, Shepard B,
Rizza SA, Trushin SA and Badley AD: Infected cell killing by HIV-1
protease promotes NF-kappaB dependent HIV-1 replication. PLoS One.
3:e21122008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lilling G, Elena N, Sidi Y and
Bakhanashvili M: p53-associated 3′→5′ exonuclease activity in
nuclear and cytoplasmic compartments of cells. Oncogene.
22:233–245. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kulkosky J, Culnan DM, Roman J, Dornadula
G, Schnell M, Boyd MR and Pomerantz RJ: Prostratin: Activation of
latent HIV-1 expression suggests a potential inductive adjuvant
therapy for HAART. Blood. 98:3006–3015. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Reuse S, Calao M, Kabeya K, Guiguen A,
Gatot JS, Quivy V, Vanhulle C, Lamine A, Vaira D, Demonte D, et al:
Synergistic activation of HIV-1 expression by deacetylase
inhibitors and prostratin: Implications for treatment of latent
infection. PLoS One. 4:e60932009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Victoriano AF and Okamoto T:
Transcriptional control of HIV replication by multiple modulators
and their implication for a novel antiviral therapy. AIDS Res Hum
Retroviruses. 28:125–138. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Persaud D, Zhou Y, Siliciano JM and
Siliciano RF: Latency in human immunodeficiency virus Type 1
infection: No easy answers. J Virol. 77:1659–1665. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shan L and Siliciano RF: From reactivation
of latent HIV-1 to elimination of the latent reservoir: The
presence of multiple barriers to viral eradication. Bioessays.
35:544–552. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shishido T, Wolschendorf F, Duverger A,
Wagner F, Kappes J, Jones J and Kutsch O: Selected drugs with
reported secondary cell-differentiating capacity prime latent HIV-1
infection for reactivation. J Virol. 86:9055–9069. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Katlama C, Deeks SG, Autran B,
Martinez-Picado J, van Lunzen J, Rouzioux C, Miller M, Vella S,
Schmitz JE, Ahlers J, et al: Barriers to a cure for HIV: New ways
to target and eradicate HIV-1 reservoirs. Lancet. 381:2109–2117.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Rasmussen TA and Lewin SR: Shocking HIV
out of hiding: Where are we with clinical trials of latency
reversing agents? Curr Opin HIV AIDS. 11:394–401. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Xing S and Siliciano RF: Targeting HIV
latency: Pharmacologic strategies toward eradication. Drug Discov
Today. 18:541–551. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Darcis G, Van DB and Van LC: HIV latency:
should we shock or lock? Trends Immunol. 38217–22. (8)2017.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Nair MPN, Saiyed ZM, Gandhi NH and
Ramchand CN: The Flavonoid, Quercetin, Inhibits HIV-1 Infection in
normal peripheral blood mononuclear cells. Am J Infect Dis.
5:135–141. 2009. View Article : Google Scholar
|
|
48
|
Moog C, Kuntz-Simon G, Caussin-Schwemling
C and Obert G: Sodium valproate, an anticonvulsant drug, stimulates
human immunodeficiency virus type 1 replication independently of
glutathione levels. J Gen Virol. 77:1993–1999. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hezareh M: Prostratin as a new therapeutic
agent targeting HIV viral reservoirs. Drug News Perspect.
18:496–500. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Márquez N, Calzado MA, Sánchez-Duffhues G,
Pérez M, Minassi A, Pagani A, Appendino G, Diaz L, Muñoz-Fernández
MA and Muñoz E: Differential effects of phorbol-13-monoesters on
human immunodeficiency virus reactivation. Biochem Pharmacol.
75:1370–1380. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chan JK, Bhattacharyya D, Lassen KG,
Ruelas D and Greene WC: Calcium/calcineurin synergizes with
prostratin to promote NF-κB dependent activation of latent HIV.
PLoS One. 8:e777492013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Suñé C and García-Blanco MA: Sp1
transcription factor is required for in vitro basal and
Tat-activated transcription from the human immunodeficiency virus
type 1 long terminal repeat. J Virol. 69:6572–6576. 1995.PubMed/NCBI
|
|
53
|
Perkins ND, Edwards NL, Duckett CS,
Agranoff AB, Schmid RM and Nabel GJ: A cooperative interaction
between NF-kappaB and Sp1 is required for HIV-1 enhancer
activation. EMBO J. 12:3551–3558. 1993.PubMed/NCBI
|
|
54
|
Indra MR, Karyono S, Ratnawati R and Malik
SG: Quercetin suppresses inflammation by reducing ERK1/2
phosphorylation and NF kappaB activation in Leptin-induced human
umbilical vein endothelial cells (HUVECs). BMC Res Notes.
6:2752013. View Article : Google Scholar : PubMed/NCBI
|