Introduction
With high incidence and mortality rates, lung cancer
is one of the most common malignancies worldwide. The incidence of
lung cancer in China currently increasing, and presents a serious
threat to human health (1). Among
all lung cancers, ~80–85% are non-small cell lung cancers (NSCLC).
The available treatments for patients with NSCLC are
unsatisfactory; and patients demonstrate an overall 5-year survival
rate of <15% (2). With poor
rates of early diagnosis and high rates of disease recurrence and
metastasis, the curative effect of surgical treatment for NSCLC
remains unsatisfactory. Therefore, the development of drugs for the
effective treatment of NSCLC is very important.
Following years of investigation and practice,
important progress has been made in the treatment of lung cancer
with specific drugs (3). However,
the efficacy of current existing therapeutic drugs is not
satisfactory (4). In recent years,
the epidermal growth factor receptor-tyrosine kinase inhibitor
(EGFR-TKI) appears to offer a novel and effective therapeutic
option for the treatment of lung cancer. A previous study indicated
that EGFR-TKI prolongs the progression-free survival of patients
with NSCLC that are sensitive to the EGFR-TKI (5). However, treatment with EGFR-TKI will
inevitably result in drug resistance (6–10).
A previous study demonstrated that the B-cell
lymphoma 2 (Bcl-2) expression was significantly increased in
patients with NSCLC harboring EGFR mutations that were resistant to
EGFR-TKI treatment (11). The
anti-apoptotic gene, Bcl-2, is a key downstream effector involved
in EGFR-TKI drug resistance mechanism (11). TW37 is a gossypol derivative and is
an effective small molecule inhibitor of Bcl-2. It prevents Bcl-2
activation and inhibits multiple Bcl-2 family members. Recent
studies indicate that TW37 may inhibit the growth of various cancer
cells by inducing S-phase cell cycle arrest via regulating the
expression of several important cell cycle-associated genes
(12,13).
In a pre-experiment performed by the authors of the
present study (data unpublished), TW37 was demonstrated to induce
apoptosis of the NSCLC cell line, H1975/EGFR-TKI, which has
developed secondary resistance to EGFR-TKI. The present study was
designed to investigate the effect of TW37 on H1975/EGFR-TKI cell
growth and explore the underlying mechanisms.
Materials and methods
Reagents
RPMI-1640 medium, fetal bovine serum (FBS),
penicillin, streptomycin and polyvinylidene fluoride (PVDF)
membranes were purchased from Sigma Aldrich; Merck Millipore
(Darmstadt, Germany). Primary antibodies against Bcl-2 (cat no.
15071), AKT serine/threonine kinase 1 (AKT) (cat no. 4685),
phosphorylated (p)-AKT (cat no. 4060) and GAPDH (cat no. 5174),
together with the corresponding secondary antibodies, anti-rabbit
IgG, horse radish peroxidase (HRP)-linked antibody (cat no. 7074)
and anti-mouse IgG HRP-linked antibody (cat no. 7076), were
purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA).
The MTT reagent and enhanced chemiluminescence (ECL) Plus reagent
were purchased from Gibco; Thermo Fisher Scientific, Inc. (Waltham,
MA, USA). A PIP3 Mass ELISA kit (cat no. K-2500) was purchased from
Echelon Biosciences (Salt Lake City, UT, USA). The Annexin
V-fluorescein isothiocyanate (FITC) apoptosis detection kit was
purchased from Vazyme (Piscataway, NJ, USA). The TW37 and
hematoxylin stain were obtained from Selleck Chemicals (Shanghai,
China). The Transwell chamber was purchased from Jinan PengBo
Biotechnology Co., Ltd. (Jinan, China).
Cell culture
The H1975/EGFR-TKI cell line, which harbors
mutations in the EGFR gene at exons 21 (L858R) and 20 (T790M), was
obtained from the American Type Culture Collection (Manassas, VA,
USA), and cultured in RPMI-1640 supplemented with 10% FBS, 100 U/ml
penicillin and 100 µg/ml streptomycin. Cells were incubated at 37°C
in a humidified atmosphere of 5% CO2, and were passaged
when they reached 90% confluence.
Cell proliferation assay
An MTT assay was performed to determine the
proliferation of H1975/EGFR-TKI cells. H1975/EGFR-TKI cells growing
in log-phase were trypsinized using 0.25% trypsin, and seeded in
96-well plates at a density of 5×103 cells/well. Cells
were then treated with 250, 500 and 750 nmol/l TW37 for 24 h. MTT
reagent was subsequently added to the culture medium at a final
concentration of 0.5 mg/ml, and cells were incubated for additional
4 h. Dimethyl sulfoxide (Sigma-Aldrich) was added to dissolve the
formazan product. After incubating for 10 min at 37°C, the optical
density at 490 nm was measured using a spectrophotometer.
Experiments were repeated in triplicate and the results are
presented as the mean ± standard deviation.
Apoptosis analysis
Flow cytometry analysis was performed to determine
alterations in the level of apoptosis following treatment of
H1975/EGFR-TKI cells with TW37. H1975/EGFR-TKI cells were treated
with 250, 500 and 750 nmol/l TW37 for 24 h. Cells were then washed
with cold phosphate-buffered saline (PBS) and fixed in 70% ethanol
at 4°C for 30 min, prior to exposing the cells to 20 µg/ml RNase I
and 50 µg/ml PI for 30 min at 37°C, before they were labeled with
Annexin V-FITC, according to the manufacturer's instructions
(Vazyme). The level of apoptosis was assessed using a flow
cytometer (BD Biosciences, Franklin Lakes, NJ, USA) was applied for
cell analysis. Experiments were repeated three times.
Migration assay
To measure cell migration ability, an in
vitro scratch assay was performed. H1975/EGFR-TKI cells were
seeded in 6-well plates at a density of 5×105
cells/well. Cells were treated with 0, 250, 500 and 750 nmol/l TW37
and incubated in a humidified atmosphere at 37°C with 5%
CO2 for ~24 h, until the cells were 80% confluent. A
scratch-wound was generated using a 10-µl pipette tip, and cells
were washed three times with PBS before they were incubated in RPMI
1640 medium for 24 h. Images were captured under an inverted light
microscope (Olympus Corporation, Tokyo, Japan). The migration
distance of cells was measured using ImageJ software (version 1.47;
National Institutes of Health, Bethesda, USA). Experiments were
repeated at least in triplicate.
Transwell invasion assay
A transwell assay was performed to measure the
invasion ability of H1975/EGFR-TKI cells treated with TW37. Cells
were seeded into the upper transwell chamber together with a
Matrigel-coated membrane matrix, and were treated with 0, 250, 500
and 750 nmol/l TW37. RPMI-1640 medium containing 10% FBS was added
to the lower chamber as a chemoattractant. The cells were incubated
for 24 h. At the end of the experiment, the non-invading cells on
the upper surface were removed, and the cells on the lower surface
were fixed with 95% ethanol for 20 min and stained with 0.1%
hematoxylin for 30 min, at room temperature. Stained cells were
observed under an inverted light microscope (Olympus Corporation,
Tokyo, Japan). A total of three independent experiments were
performed.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The mRNA expression levels of Bcl-2 and the GAPDH
housekeeping gene in H1975/EGFR-TKI cells were measured by RT-qPCR
analysis. Total RNA was extracted from the cells using TRIzol
reagent (Takara Biotechnology Co., Ltd., Dalian, China) according
to the manufacturer's instructions. A total of 1 µg RNA was then
reverse transcribed into cDNA using a Revert Aid RT Reverse
Transcription kit (Invitrogen; Thermo Fisher Scientific, Inc.)
according to manufacturer's instructions. cDNA was subsequently
amplified by qPCR using the following thermal cycling parameters:
Initial activation at 95°C for 10 min, followed by 40 cycles of
denaturation at 95°C for 10 sec, annealing at 60°C for 60 sec and
extension at 72°C for 15 sec. The primers for Bcl-2 and GAPDH were
as follows: Bcl-2, forward, 5′-ATGTGTGTGGAGAGCGTCAA-3′, and
reverse, 5′-ACAGTTCCACAAAGGCATCC-3′; GAPDH, forward,
5′-CTTTGGTATCGTGGAAGGACTC-3′, and reverse,
5′-GTAGAGGCAGGGATGATGTTCT-3′. GAPDH was selected as an endogenous
reference gene to calculate the relative expression levels of Bcl-2
(14). All samples were analyzed
in triplicate and three independent experiments were performed.
ELISA
H1975/EGFR-TKI cells were seeded in 6-well plates at
a density of 5×105 cells per well, and were treated with
0, 250, 500 and 750 nmol/l TW37 for 24 h at 37°C. An ELISA assay
was performed in order to determine the expression levels of PIP3
in the cell supernatant according to the manufacturer's
instructions (Thermo Fisher Scientific, Inc.), finally, the samples
were examined and the absorbance was measured at a wavelength of
450 nm. A total of three independent experiments were
performed.
Western blot analysis
H1975/EGFR-TKI cells were treated with 0, 250, 500
and 750 nmol/l TW37 for 24 h. Following treatment,
radioimmunoprecipitation assay buffer, consisting of 50 mM Tris-Cl
(pH 7.4), 150 mM NaCl, 1% Triton X-100, 0.1% SDS and 1% sodium
deoxycholate, was used to extract total cellular protein. A
bicinchoninic acid assay kit (Thermo Fisher Scientific, Inc.) was
used to determine the protein concentration of sample extracts. The
samples (30 µg per lane) were separated on a 12% SDS-PAGE gel, and
transferred to a polyvinylidene difluoride membrane. The membrane
was blocked with 5% non-fat dry milk in TBS buffer containing 0.1%
Tween 20 for 2 h at room temperature. The membrane was subsequently
incubated with primary antibodies against Bcl-2, AKT, p-AKT (all
diluted at 1:1,000) and GAPDH (dilution, 1:2,000) at 4°C for 24 h.
This was followed by incubation with the secondary antibody
(dilution, 1:5,000) at room temperature for 1 h. The membranes were
then incubated with ECL Plus reagent for 30 sec at room
temperature, and scanned using the BioSpectrum Imaging System (UVP,
Inc., Upland, CA, USA).
Statistical analysis
Data are presented as the mean ± standard deviation.
All statistical calculations were performed using SPSS version 11.7
software (SPSS, Inc., Chicago, IL, USA). One-way analysis of
variance followed by Bonferroni post hoc test and Student's t-test
were used to analyze differences between groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
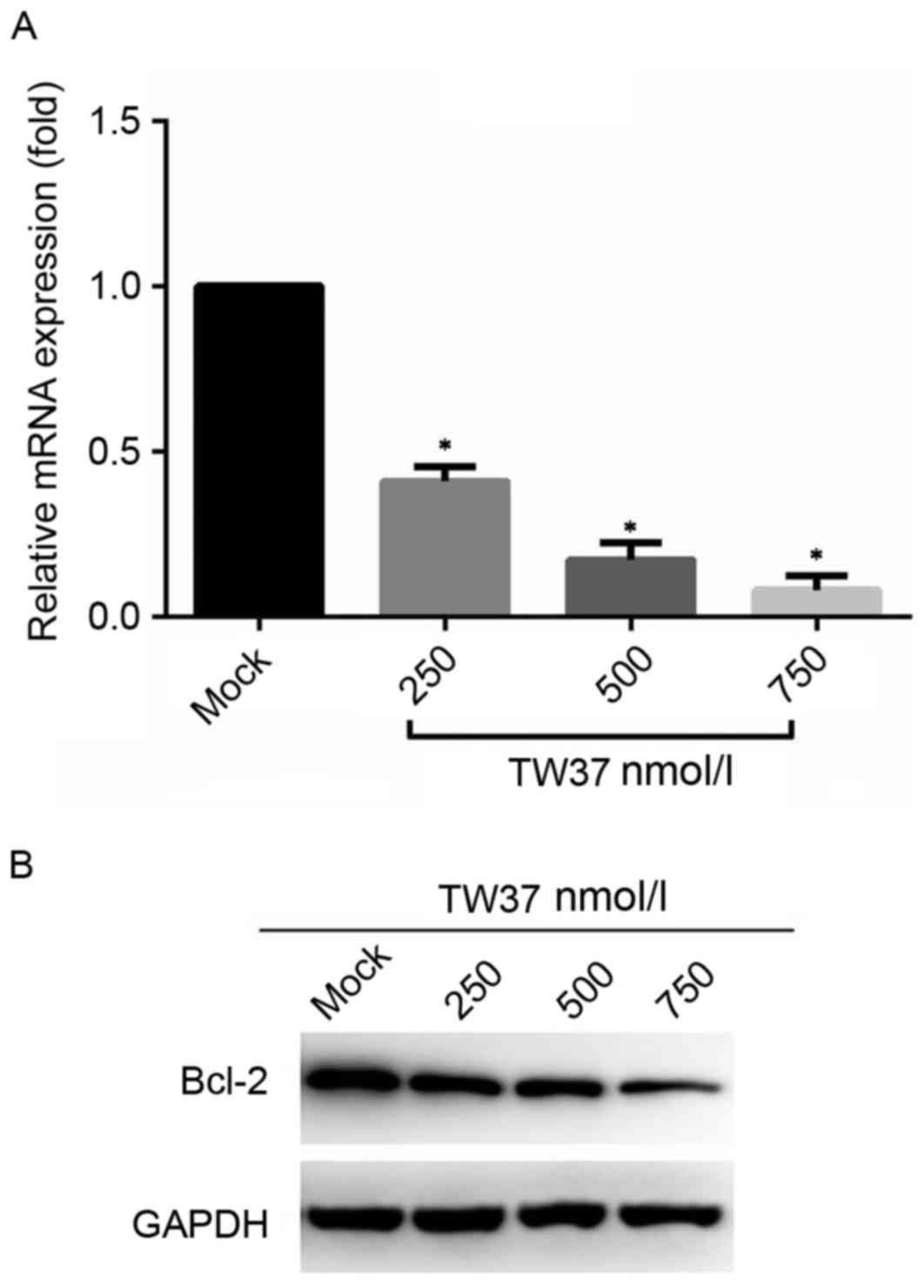
TW37 treatment reduces Bcl-2 mRNA and
protein expression levels
The mRNA and protein expression levels of Bcl-2 in
H1975/EGFR-TKI cells prior to and following treatment with TW37
were detected by RT-qPCR and western blot assays, respectively. The
results demonstrated that treatment with increasing concentrations
of TW37 for 24 h was associated with a significant decrease in
Bcl-2 mRNA (Fig. 1A) and a marked
decrease in protein (Fig. 1B)
expression levels when compared with the untreated control
group.
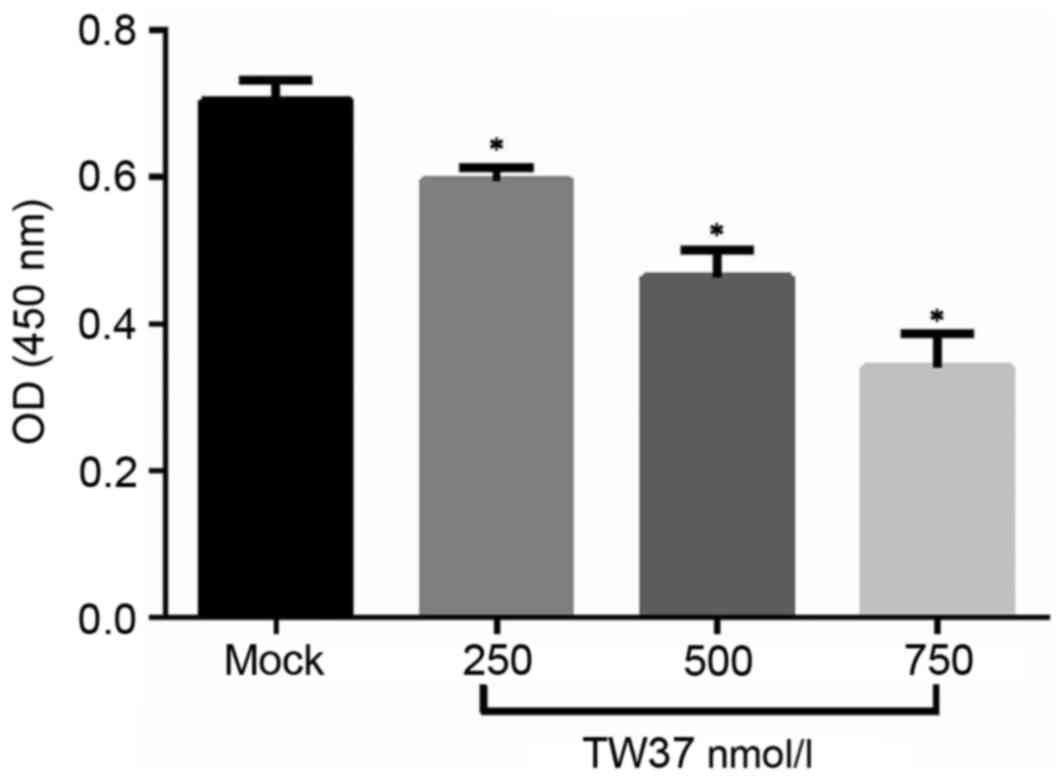
TW37 inhibited the proliferation of
H1975/EGFR-TKI cells
The effect of TW37 treatment on H1975/EGFR-TKI cell
proliferation was determined. Following treatment of H1975/EGFR-TKI
cells with 250, 500 and 750 nmol/l TW37 for 24 h, cell viability
was measured using an MTT assay. The results demonstrated that TW37
significantly reduced the viability of cells (Fig. 2). This indicates that TW37 may
serve an important role in inhibiting the proliferation of
H1975/EGFR-TKI cells in vitro.
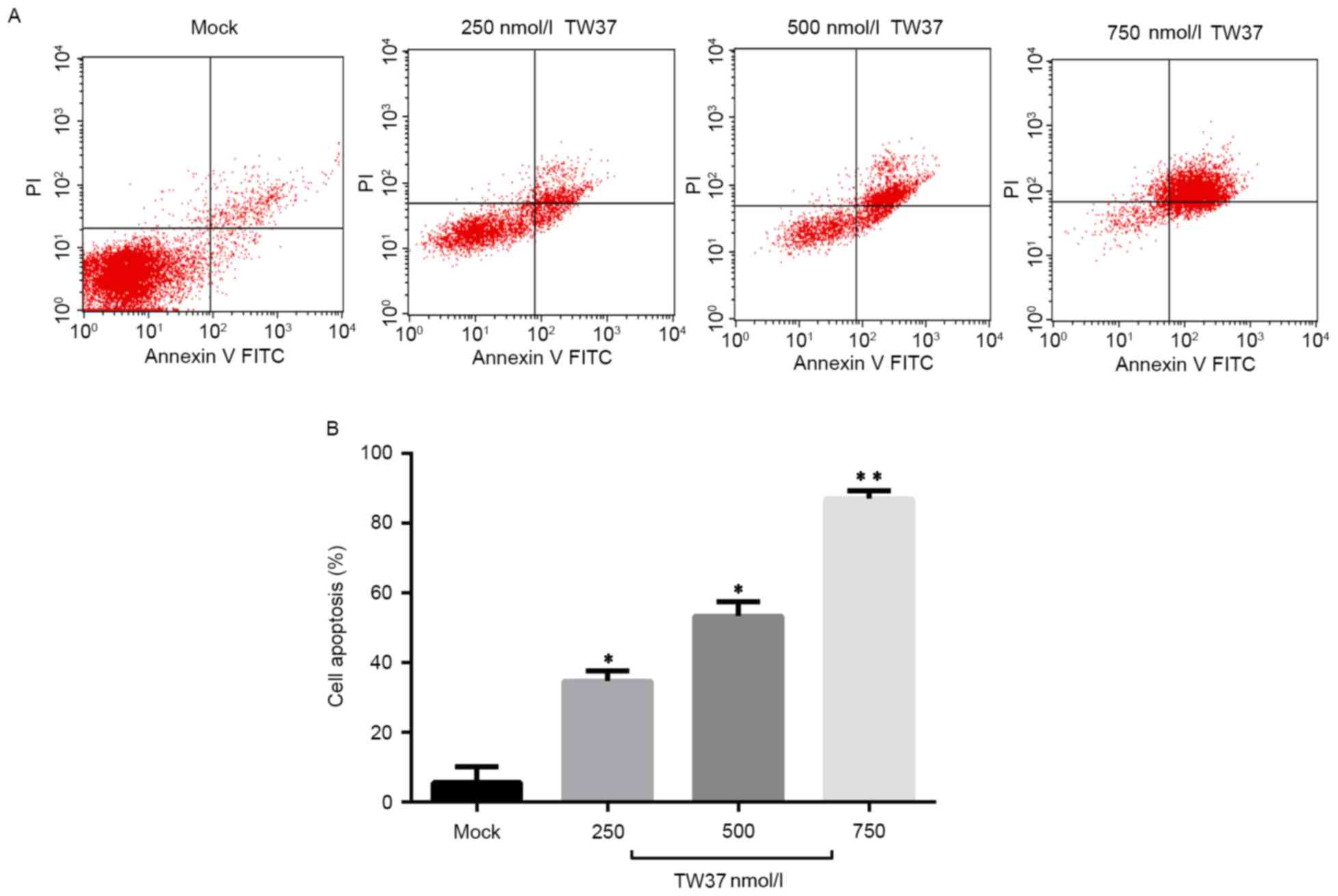
TW37 induced apoptosis of
H1975/EGFR-TKI cells
The effect of TW37 treatment on the level of
apoptosis in H1975/EGFR-TKI cells was next investigated.
H1975/EGFR-TKI cells were treated with 250, 500 and 750 nmol/l TW37
for 24 h, and the level of apoptosis was measured using an Annexin
V-FITC apoptosis detection kit and quantified by flow cytometry
analysis. As shown in Fig. 3,
treatment of cells with 250, 500 and 750 nmol/l TW37 significantly
increased the level of apoptosis in a dose-dependent manner, when
compared with the untreated control.
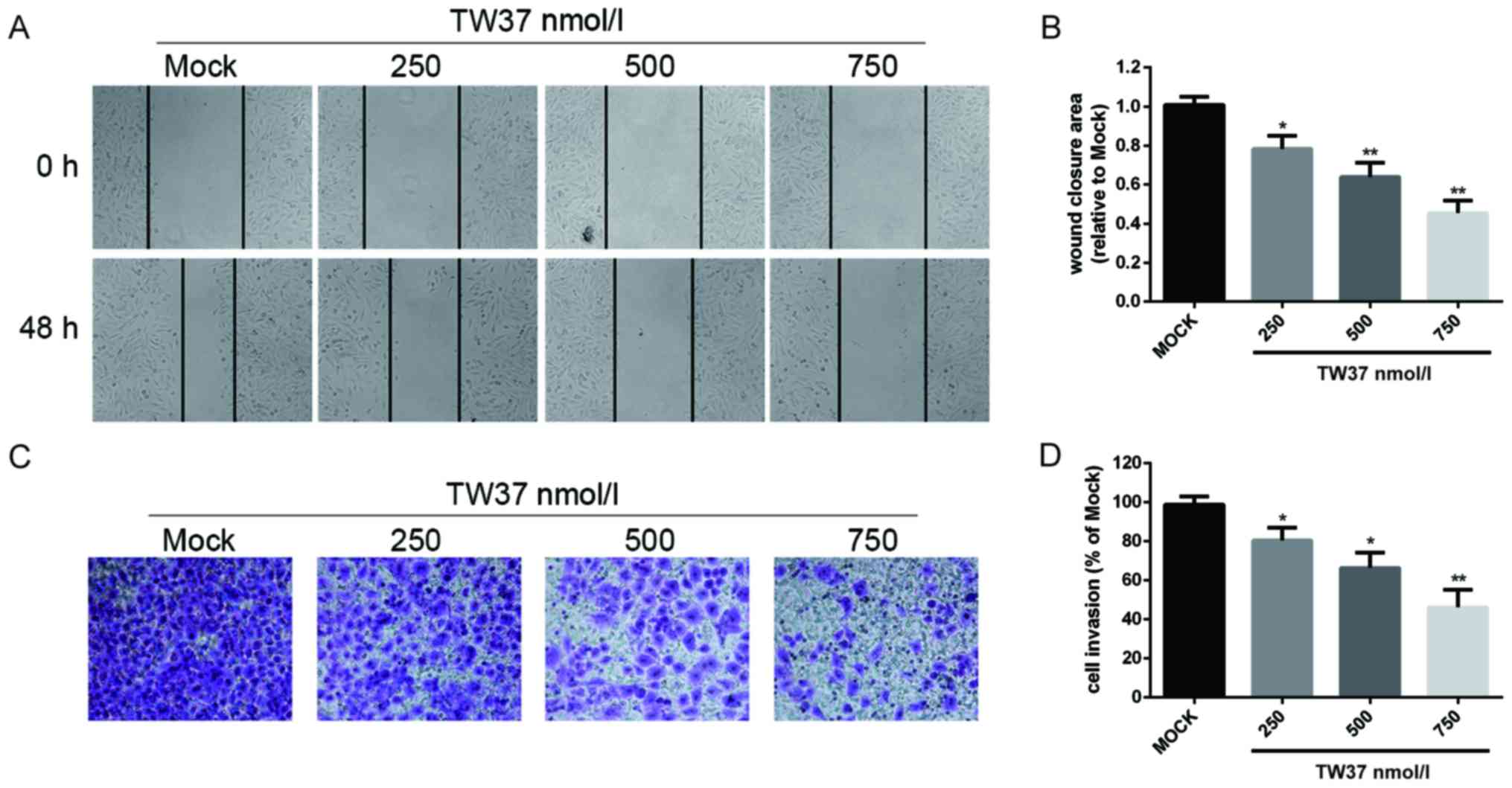
TW37 inhibited the migration and
invasion abilities of H1975/EGFR-TKI cells
In order to investigate the effect of TW37 on
H1975/EGFR-TKI cell migration, a scratch wound assay was performed.
The results demonstrated that treatment of H1975/EGFR-TKI cells
with 250, 500 and 750 nmol/l TW37 reduced cell migration ability in
a dose-dependent manner (Fig. 4).
A transwell assay was performed to determine the effect of TW37 on
cell invasion ability. Treatment of cells with 250, 500 and 750
nmol/l TW37 for 24 h significantly reduced the number of
H1975/EGFR-TKI cells that migrated through the membrane in a
dose-dependent manner, when compared with the control group
(Fig. 4). These results suggested
that TW37 may inhibit the migration and invasion of H1975/EGFR-TKI
cells in vitro.
TW37 inhibited the AKT signaling
pathway in H1975/EGFR-TKI cells
In order to determine whether the AKT signaling
pathway may be involved in mediating the effects of TW37 on
H1975/EGFR-TKI cell growth, the effect of TW37 on the expression of
PIP3 and the phosphorylation of AKT was determined by ELISA and
western blot analyses, respectively. Following treatment of cells
with 250, 500 and 750 nmol/l TW37 for 24 h, the expression levels
of PIP3 in the culture supernatant were detected, and the
intracellular expression levels of p-AKT and AKT proteins were
measured. As shown in Fig. 5, TW37
significantly reduced the production of PIP3, and markedly reduced
the protein expression levels of p-AKT in a dose-dependent manner
when compared with untreated controls. These results indicated that
TW37 may inhibit AKT signaling pathway activation in H1975/EGFR-TKI
cells.
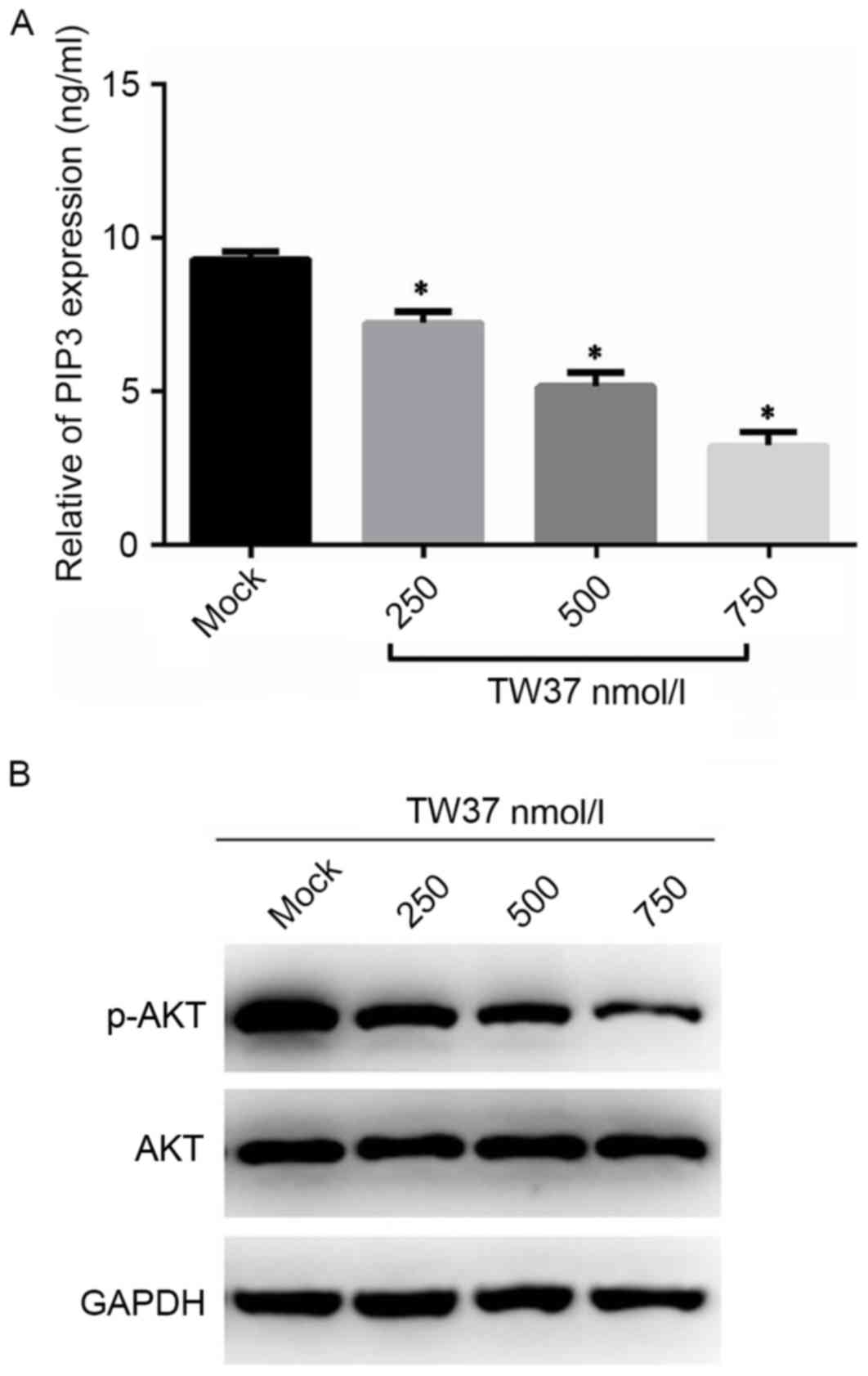 | Figure 5.Expression levels of AKT, p-AKT and
PIP3 in H1975/EGFR-TKI cells. H1975/EGFR-TKI cells were treated
with 0, 250, 500 and 750 nmol/l TW37 for 24 h, and the levels of
(A) PIP3 in the cell supernatant, and (B) intracellular AKT and
p-AKT were measured by enzyme-linked immunosorbent assay and
western blot analysis, respectively. Experiments were performed in
triplicate. *P<0.05 vs. Mock. AKT, AKT serine/threonine kinase
1; p-AKT, phosphorylated AKT; PIP3, plasma membrane intrinsic
protein 3; EGFR-TKI, epidermal growth factor receptor-tyrosine
kinase inhibitor; mock, untreated control. |
Discussion
As a proto-oncogene, Bcl-2 inhibits apoptosis and
serves important roles in the occurrence and development of tumors,
as well as the development of drug resistance during the treatment
of cancer (15). A recent study
demonstrated that high protein expression levels of Bcl-2 are
closely associated with resistance of tumor cells to chemotherapy
and radiotherapy (16). Qiao et
al (17) suggested that lung
cancer cells exhibited an enhancement of bcl-2 mediated
anti-apoptosis after treatment with chemotherapeutic drugs, thus
resulting in drug resistance. Since 2009, several studies have
observed high levels of Bcl-2 protein expression in a human lung
cancer cell line, which developed secondary resistance to gefitinib
treatment (18,19). This indicated that high expression
levels of Bcl-2 may be associated with the development of secondary
drug resistance to EGFR-TKI. Bcl-2 is a key downstream factor of
EGFR, RAS and additional signaling pathways (20). As a target for the treatment and
reversal of drug resistance, Bcl-2 has recently gained significant
interest. A previous study suggested that synthetic peptides and
adenovirus-mediated targeted therapies inhibit the abnormal
expression of Bcl-2 in tumor cells, thus effectively suppressing
tumor cell growth, and reversing the resistance of cancer cells to
chemotherapeutic drugs (21). TW37
is known to inhibit the growth of a variety of tumor cells,
including breast, pancreatic and prostate cancer cells (12,13).
TW37 inhibits Bcl-2 expression and induces S-phase cell cycle
arrest by regulating several important cell cycle-associated genes
(12,13).
The aim of the present study was to investigate the
effect of TW37 on H1975/EGFR-TKI cell growth and to explore the
underlying mechanisms. First, the mRNA and protein expression
levels of Bcl-2 in H1975/EGFR-TKI cells prior to and following
treatment with TW37 was measured by RT-qPCR and western blot
analyses, respectively. The results suggested that TW37 may
effectively inhibit the expression of Bcl-2. The effect of TW37 on
H1975/EGFR-TKI cell growth was then determined using an MTT assay.
The results indicated that TW37 significantly inhibited the
proliferation of H1975/EGFR-TKI cells in a dose-dependent manner.
This confirmed that TW37 demonstrated an anti-proliferative effect
on H1975/EGFR-TKI cells.
A number of previous studies have reported the
pro-apoptotic role of TW37 in various cancers (12,22–24).
The results of the current study are therefore consistent with
those of previous studies. The present study suggested that the
rate of H1975/EGFR-TKI cell apoptosis increased in a dose-dependent
manner. The balance of cell survival and apoptosis signaling
pathways is critical for determining the fate of cells, and
multiple genes and molecules are involved in the regulation of this
balance (25). Bcl-2 family
proteins serve key roles in regulating cell apoptosis (26), and the results of the present study
indicated that TW37 reduced the expression levels of the
anti-apoptotic protein Bcl-2, as well as induced apoptosis of
H1975/EGFR-TKI cells.
A previous study indicated that TW37 inhibited the
migration and invasion of pancreatic cancer cells (27). Concordant with these findings, the
results of the current study suggested that TW37 inhibited the
migration and invasion of H1975/EGFR-TKI cells in a dose-dependent
manner.
As one of the factors of the mitogen-activated
protein kinase signaling pathway, AKT serves an important role in
the regulation of cell proliferation, apoptosis and migration of
cancer cells (28,29). The results of the present study
indicated that TW37 significantly decreased levels of p-AKT in
H1975/EGFR-TKI cells in a dose-dependent manner. This suggests that
TW37 may mediate its anti-cancer effects on H1975/EGFR-TKI cells by
reducing AKT phosphorylation.
In conclusion, the current study is the first to
demonstrate that TW37 significantly inhibits the proliferation,
migration and invasion of H1975/EGFR-TKI cells, as well as induces
apoptosis. These effects were associated with the inhibition of AKT
phosphorylation. The authors hypothesize that TW37 may reverse the
resistance of NSCLC cells to EGFR-TKI treatment, and may therefore
serve crucial roles in improving the efficacy of drug therapy in
the treatment of patients with NSCLC.
Acknowledgements
The present study was supported by the Shenzhen
Commission on Technology and Innovation (grant no.
JCYJ20150403091443290).
References
|
1
|
Révész D, Engelhardt EG, Tamminga JJ,
Schramel FMNH, Onwuteaka-Philipsen BD, van de Garde EMW, Steyerberg
EW, Jansma EP, De Vet HCW and Coupé VMH: Decision support systems
for incurable non-small cell lung cancer: A systematic review. BMC
Med Inform Decis Mak. 17:17–144. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang L, Parkin DM, Li LD, Chen YD and Bray
F: Estimation and projection of the national profile of cancer
mortality in China: 1991–2010. Br J Cancer. 90:2157–2166. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Swami U, Shah U and Goel S: Eribulin in
non-small cell lung cancer: Challenges and potential strategies.
Expert Opin Investig Drugs. 26:495–508. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Berhoune M, Banu E, Scotte F, Prognon P,
Oudard S and Bonan B: Therapeutic strategy for treatment of
metastatic non-small cell lung cancer. Ann Pharmacother.
42:1640–1652. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang L, Li J, Hu J, Li D, Wang X, Zhang
R, Zhang H, Shi M and Chen H: Cigarette smoke extract induces
EGFR-TKI resistance via promoting EGFR signaling pathway and ROS
generation in NSCLC cell lines. Lung Cancer. 109:109–116. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Inoue A, Kobayashi K, Maemondo M, Sugawara
S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I,
et al: Updated overall survival results from a randomized phase III
trial comparing gefitinib with carboplatin-paclitaxel for
chemo-naïve non-small cell lung cancer with sensitive EGFR gene
mutations (NEJ002). Ann Oncol. 24:54–59. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mitsudomi T, Morita S, Yatabe Y, Negoro S,
Okamoto I, Tsurutani J, Seto T, Satouchi M, Tada H, Hirashima T, et
al: Gefitinib versus cisplatin plus docetaxel in patients with
non-small-cell lung cancer harbouring mutations of the epidermal
growth factor receptor (WJTOG3405): An open label, randomised phase
3 trial. Lancet Oncol. 11:121–128. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rosell R, Carcereny E, Gervais R,
Vergnenegre A, Massuti B, Felip E, Palmero R, Garcia-Gomez R,
Pallares C, Sanchez JM, et al: Erlotinib versus standard
chemotherapy as first-line treatment for European patients with
advanced EGFR mutation-positive non-small-cell lung cancer
(EURTAC): A multicentre, open-label, randomised phase 3 trial.
Lancet Oncol. 13:239–246. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sequist LV, Yang JC, Yamamoto N, O'Byrne
K, Hirsh V, Mok T, Geater SL, Orlov S, Tsai CM, Boyer M, et al:
Phase III study of afatinib or cisplatin plus pemetrexed in
patients with metastatic lung adenocarcinoma with EGFR mutations. J
Clin Oncol. 31:3327–3334. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pao W and Chmielecki J: Rational,
biologically based treatment of EGFR-mutant non-small-cell lung
cancer. Nat Rev Cancer. 10:760–774. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Su JC, Lin KL, Chien CM, Tseng CH, Chen
YL, Chang LS and Lin SR: Naphtho[1,2-b]furan-4,5-dione inactivates
EGFR and PI3K/Akt signaling pathways in human lung adenocarcinoma
A549 cells. Life Sci. 86:207–213. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang Z, Azmi AS, Ahmad A, Banerjee S, Wang
S, Sarkar FH and Mohammad RM: TW-37, a small-molecule inhibitor of
Bcl-2, inhibits cell growth and induces apoptosis in pancreatic
cancer: Involvement of Notch-1 signaling pathway. Cancer Res.
69:2757–2765. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ashimori N, Zeitlin BD, Zhang Z, Warner K,
Turkienicz IM, Spalding AC, Teknos TN, Wang S and Nör JE: TW-37, a
small-molecule inhibitor of Bcl-2, mediates S-phase cell cycle
arrest and suppresses head and neck tumor angiogenesis. Mol Cancer
Ther. 8:893–903. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Oltersdorf T, Elmore SW, Shoemaker AR,
Armstrong RC, Augeri DJ, Belli BA, Bruncko M, Deckwerth TL, Dinges
J, Hajduk PJ, et al: An inhibitor of Bcl-2 family proteins induces
regression of solid tumours. Nature. 435:677–681. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee HW, Choi YW, Han JH, Kim JH, Jung JH,
Jeong SH, Kang SY, Choi JH, Oh YT, Park KJ, et al: Expression of
excision repair cross-complementation group 1 protein predicts poor
outcome in advanced non-small cell lung cancer patients treated
with platinum-based doublet chemotherapy. Lung Cancer. 65:377–382.
2012. View Article : Google Scholar
|
|
17
|
Qiao GB, Zeng WS, Peng LJ, et al:
Overexpression of bcl-2 and sensitivity to Platinum agents in non
small cell lung cancer. J Clin Oncol. 24 Suppl:410S–411S. 2013.(In
Chinese).
|
|
18
|
Ma J, Zhu W and Zhou Q: Expression and
Significance of bag-1, bcl-2 in Non-small cell lung cancer and the
correlation with Multi-drug resistance. Zhongguo Fei Ai Za Zhi.
12:1089–1094. 2009.(In Chinese). PubMed/NCBI
|
|
19
|
Koty PP, Zhang H, Franklin WA, Yousem SA,
Landreneau R and Levitt ML: In vivo expression of p53 and Bcl-2 and
their role in programmed cell death in premalignant and malignant
lung lesions. Lung Cancer. 35:155–163. 2012. View Article : Google Scholar
|
|
20
|
Su JC, Lin KL, Chien CM, Tseng CH, Chen
YL, Chang LS and Lin SR: Naphtho[1,2-b]furan-4,5-dione inactivates
EGFR and PI3K/Akt signaling pathways in human lung adenocarcinoma
A549 cells. Life Sci. 86:207–213. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Song JH, Kandasamy K, Zemskova M, Lin YW
and Kraft AS: The BH3 mimetic ABT-737 induces cancer cell
senescence. Cancer Res. 71:506–515. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zeitlin BD, Joo E, Dong Z, Warner K, Wang
G, Nikolovska-Coleska Z, Wang S and Nör JE: Antiangiogenic effect
of TW37, a small-molecule inhibitor of Bcl-2. Cancer Res.
66:8698–8706. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zeitlin BD, Spalding AC, Campos MS,
Ashimori N, Dong Z, Wang S, Lawrence TS and Nör JE: Metronomic
small molecule inhibitor of Bcl-2 (TW-37) is antiangiogenic and
potentiates the anti-tumor effect of ionizing radiation. Int J
Radiat Oncol Biol Phys. 78:879–887. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang H, Zhang Z, Wei X and Dai R:
Small-molecule inhibitor of Bcl-2 (TW-37) suppresses growth and
enhances cisplatin-induced apoptosis in ovarian cancer cells. J
Ovarian Res. 8:32015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Matsuzawa A, Nishitoh H, Tobiume K, Takeda
K and Ichijo H: Physiological roles of ASK1-mediated signal
transduction in oxidative stress- and endoplasmic reticulum
stress-induced apoptosis: Advanced findings from ASK1 knockout
mice. Antioxid Redox Signal. 4:415–425. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vogler M, Walter HS and Dyer MJS:
Targeting anti-apoptotic BCL2 family proteins in haematological
malignancies-from pathogenesis to treatment. Br J Haematol.
178:364–379. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang Z, Song W, Aboukammad M, Mohammad M,
Wang G, Banerjee S, Kong D, Wang S, Sarkar FH and Mohammad RM:
TW-37, a small-molecule inhibitor of Bcl-2, inhibits cell growth
and invasion in pancreatic cancer. Int J Cancer. 123:958–966. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zheng Q, Wang B, Gao J, Xin N, Wang W,
Song X, Shao Y and Zhao C: CD155 knockdown promotes apoptosis via
AKT/Bcl-2/Bax in colon cancer cells. J Cell Mol Med.
16–Aug;2017.(Epub ahead of print). View Article : Google Scholar
|
|
29
|
Liang Z, Wang X, Xu X, Xie B, Ji A, Meng
S, Li S, Zhu Y, Wu J, Hu Z, et al: MicroRNA-608 inhibits
proliferation of bladder cancer via AKT/FOXO3a signaling pathway.
Mol Cancer. 16:962017. View Article : Google Scholar : PubMed/NCBI
|