Introduction
Osteoarthritis (OA) is a degenerative disease of the
joints that may lead to progressive cartilage damage, osteophyte
formation and subchondral bone sclerosis, that may cause pain and
loss of movement (1). OA affects
>50% of people over the age of 65 and is a highly debilitating
disease (2). Although nonsteroidal
anti-inflammatory drugs have been used clinically to treat OA,
these agents ameliorate the clinical symptoms without suppressing
the progression of the disease (3). Therefore, investigation of safer and
better tolerated agents for the treatment of OA is required.
Previous studies have revealed that pro-inflammatory
cytokines, including interleukin (IL)-1β, serve a critical role in
the pathogenesis of OA (4–6). In response to IL-1β upregulation,
chondrocytes secrete matrix metalloproteinases (MMPs), which induce
chondrocyte apoptosis and inhibit extracellular matrix (ECM)
biosynthesis (7). IL-1β also
induced the expression of cyclooxygenase-2 (COX-2) and inducible
nitric oxide synthase (iNOS) (8),
which may lead to the elevated production of prostaglandin E2
(PGE2) and nitric oxide (NO), respectively.
Paeoniflorin (PF) is a monoterpene glycoside
extracted from the root of Paeonia lactiflora Pall. Previous
studies have indicated that PF has a protective effect on various
pathological states, including tumor growth, oxidative stress,
inflammation, and myocardial infarction (9–12).
Liu et al (13) reported
that PF pretreatment inhibited amyloid β1-42-induced production of
tumor necrosis factor α, IL-1β and IL-6 in rodent microglia.
Additionally, PF exhibits an anti-arthritic effect. It was reported
that PF reduced the levels of inflammatory cytokines and alleviated
rheumatoid arthritis in collagen-induced arthritis (14). However, it remains to be elucidated
whether PF affects inflammatory responses in OA chondrocytes.
Therefore, the objective of the present study was to investigate
the effects of PF on inflammatory responses in OA chondrocytes.
Materials and methods
Tissue collection and human articular
chondrocytes culture
Osteoarthritis cartilage tissues were obtained from
10 patients (all female; age, 63±5 years) with OA that underwent
total knee joint replacement between July and December 2014, at the
Affiliated Hospital of Shandong University (Jinan, China). The
present study was performed following a protocol approved by
Medical Ethical Committee of the Institute of Orthopedics, Xijing
Hospital, Fourth Military Medical University (Xi'an, China) and all
examinations were performed after written informed consent was
obtained from the patients.
Chondrocytes were isolated from cartilage as
previously described (15). The
whole articular cartilage was minced into pieces ~0.5
cm2. Following enzymatic digestion with 2 mg/ml pronase
in serum-free Dulbecco's modified Eagle's medium (DMEM; Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing 100
U/ml penicillin and 100 µg/ml streptomycin (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) for 2 h at 37°C in 5% CO2
atmosphere, the specimens were then digested with 0.2% (v/v)
collagenase I for 4 h at 37°C. Primary chondrocytes were cultured
at a density of 1×105 cells/ml in petri dishes in
monolayer culture for a period of 24 h at 37°C with 5%
CO2, and chondrocytes in passages 5 to 9 were used.
Cell viability
Cell viability was quantified by a Cell Counting
Kit-8 assay (CCK-8; Dojindo Molecular Technologies, Inc., Kumamoto,
Japan). Human chondrocytes (1×104 cells/well) in 96-well
plates were pretreated with 10, 50 and 100 µg/ml PF (Sigma-Aldrich;
Merck KGaA) for 2 h at 37°C and subsequently co-incubated in the
absence or presence of 10 ng/ml IL-1β (Sigma-Aldrich; Merck KGaA)
at room temperature for 24 h. Then, CCK-8 reagents were added and
incubated with the cells for 1 h at 37°C. The spectrophotometric
absorbance was quantified at 490 nm on a multifunctional microplate
reader (Tecan Group Ltd., Durham, NC, USA).
Nitric oxide (NO) and prostaglandin E2
(PGE2) quantification
NO levels in the culture medium were detected by
Griess reaction, as previously described (16). PGE2 levels were investigated using
a commercially available ELISA kit, according to the manufacturer's
protocol (KHL1701; Invitrogen; Thermo Fisher Scientific, Inc.).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from human chondrocytes using
TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.), according to
the manufacturer's protocol. Total RNA (5 µg) for each sample were
reverse transcribed into first strand cDNA using the iScript cDNA
synthesis kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA)
according to manufacturer's protocol for subsequent qPCR analysis.
qPCR was performed in a final volume of 10 µl, which contained 5 µl
of SsoFast™ EvaGreen Supermix (Bio-Rad Laboratories, Inc.), 1 µl of
cDNA (1:50 dilution) and 2 µl of the forward and reverse primers (1
mM). The following specific primer sequences were used: iNOS, sense
5′-TTTCCAAGACACACTTCACCA-3′, antisense 5′-ATCTCCTTTGTTACCGCTTCC-3′;
sense COX-2 5′-GAGAGATGTATCCTCCCACAGTCA-3′, antisense
5′-GACCAGGCACCAGACCAAAG-3′; and for β-actin, sense
5′-AGAAGGCTGGGGCTCATTTG-3′, antisense 5′-AGGGGCCATCCACAGTCTTC-3′.
Thermocycling conditions were as follows: 94°C for 5 min;
subsequently 35 cycles at 94°C for 1 min, 59°C for 15 sec and 72°C
for 30 sec; followed by 72°C for 10 min. qPCR data was calculated
by the 2−ΔΔCq method (17).
Western blotting
Chondrocytes were washed twice with ice-cold PBS and
sonicated with lysis buffer (Takara Biotechnology Co., Ltd.,
Dalian, China), the cell lysate supernatants were harvested by
centrifugation at 10,000 × g for 10 min at 4°C. The protein
concentrations of the cell supernatants were then quantified using
Bradford protein dye reagent (Bio-Rad Laboratories). Proteins (30
µg/lane) were separated by 12% SDS-PAGE and transferred onto
polyvinylidene difluoride membranes (EMD Millipore, Boston, MA,
USA). The membranes were incubated with appropriate primary
antibodies for iNOS (1:2,000; sc-7271), COX-2 (1:3,000; sc-19,999),
nuclear factor (NF)-κB p65 (1:1,500; sc-8008), inhibitor of κB
(IκBα) (1:3,000; sc-1643) and GAPDH (1:3,000; sc-59540) (Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) overnight at 4°C. The
membranes were subsequently washed with PBS containing 0.1% (v/v)
Tween 20 and incubated with horseradish peroxidase-conjugated
secondary antibodies (1:2,500; sc-2005; Santa Cruz Biotechnology,
Inc.) for 1 h at room temperature, followed by exposure using
enhanced chemiluminescence detection reagents (Gibco; Thermo Fisher
Scientific, Inc.). BandScan version 5.0 (Glyko, Inc., Novato, CA,
USA) was used for the quantification of all the proteins following
western blot analysis.
Enzyme-linked immunosorbent assay
(ELISA)
Culture medium was collected for ELISA assay. MMP-3
and MMP-13 levels were quantified using ELISA kits for human MMP3
and MMP13 (KAC1541 and EHMMP13; both from Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol.
Statistical analysis
The quantitative data are expressed as the mean ±
standard deviation. Statistical significance was assessed by the
one-way analysis of variance followed by Tukey's post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
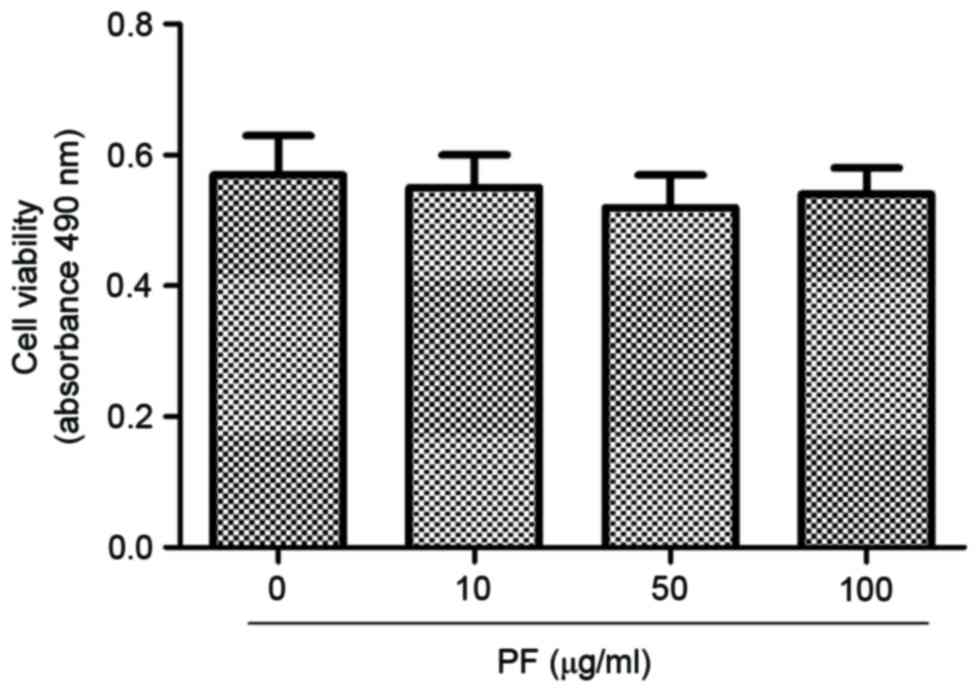
Effect of PF on viability of human OA
chondrocytes
Human OA chondrocytes were incubated with 0, 10, 50
and 100 µg/ml PF for 24 h, and the cell viability was determined
using a CCK-8 assay (Fig. 1). At
concentrations of 10, 50 and 100 µg/ml, PF induced no cytotoxic
effects in human OA chondrocytes.
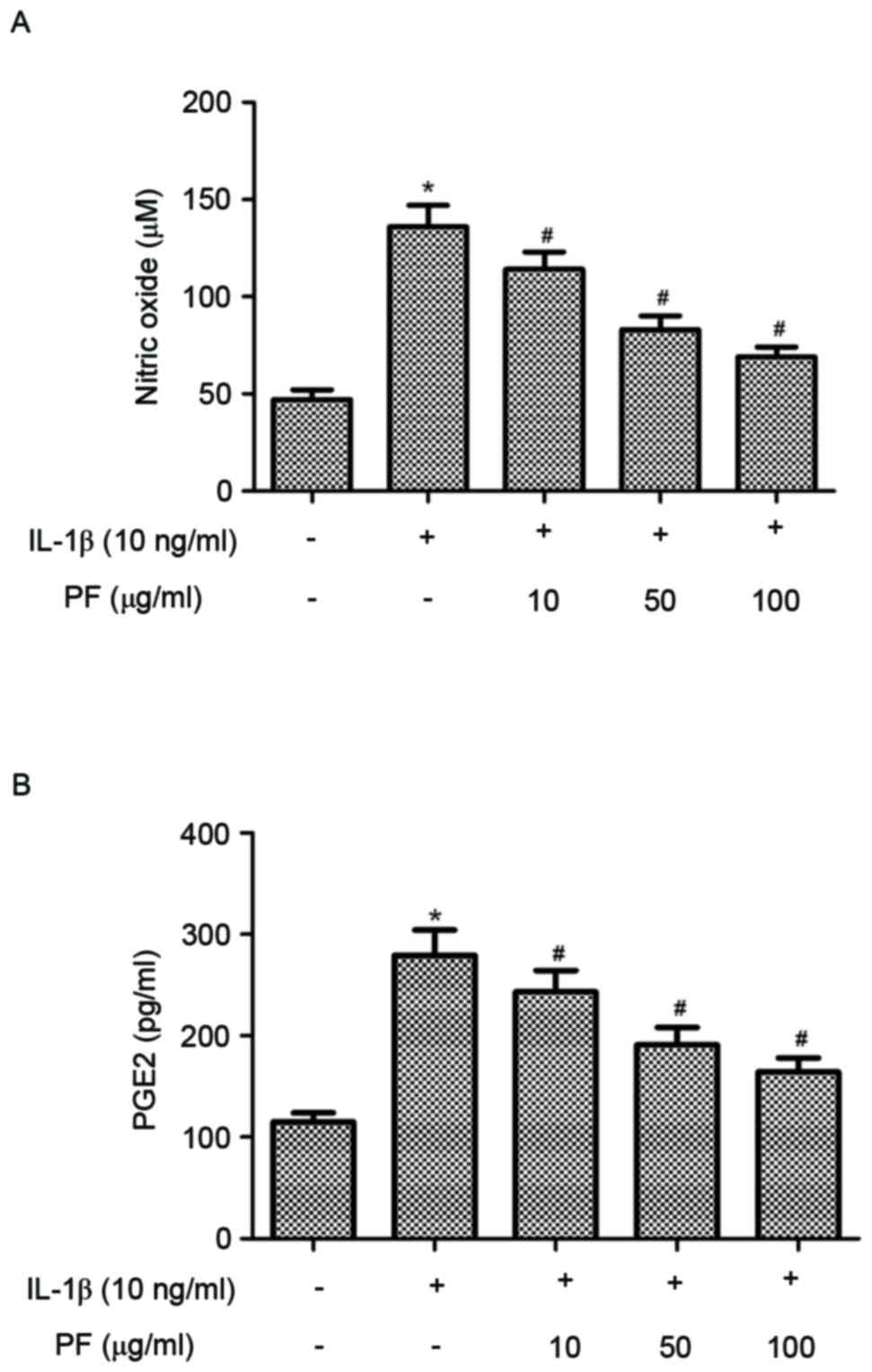
Effect of PF on NO and PGE2 production
in IL-1β-stimulated chondrocytes
Subsequently the effects of PF on NO and PGE2
production in IL-1β-stimulated human OA chondrocytes were
investigated. As indicated in Fig.
2, IL-1β significantly increased the production of NO and PGE2
compared with the control group (P<0.05). However, treatment
with PF significantly inhibited NO and PGE2 production induced by
IL-1β in human OA chondrocytes (P<0.05).
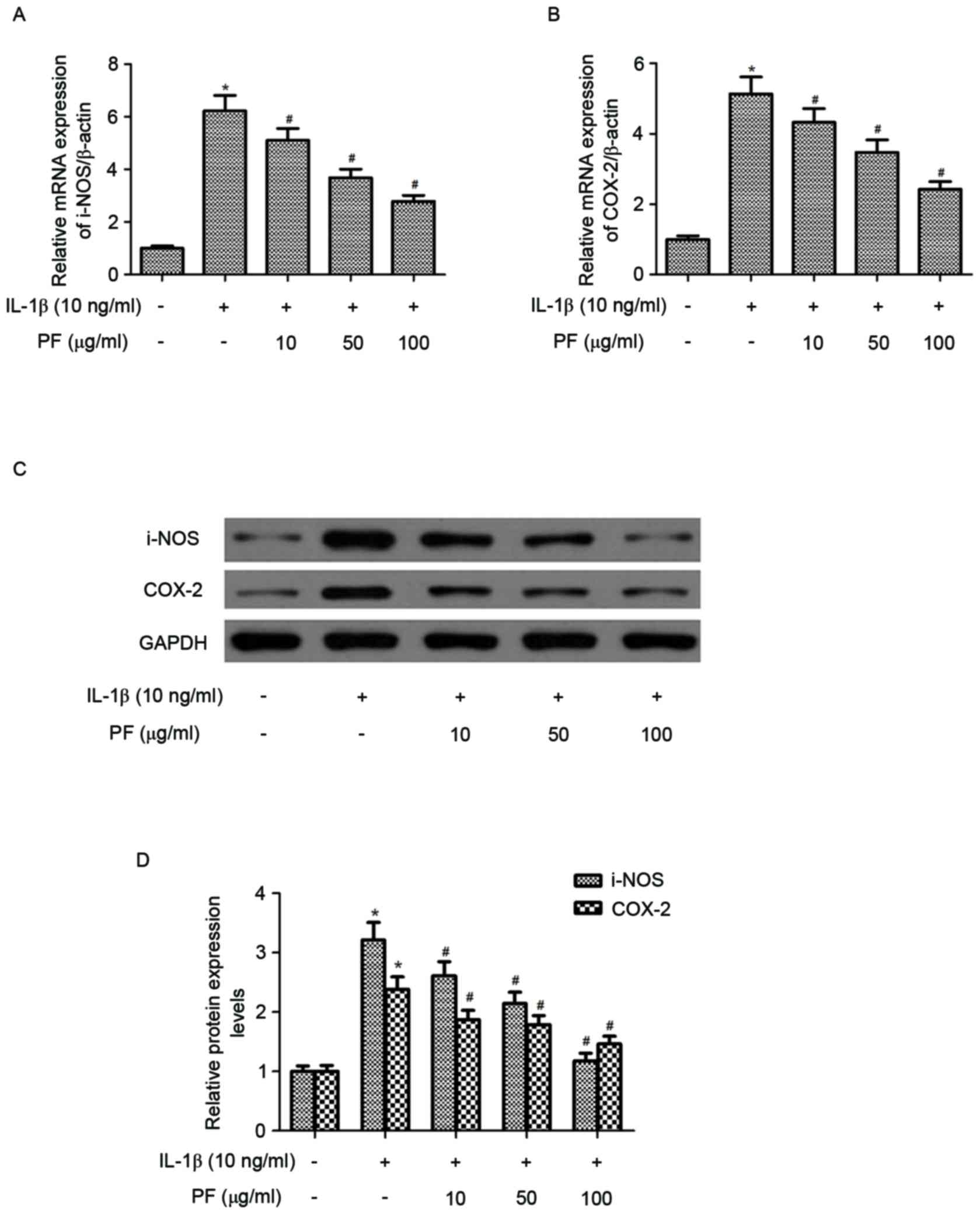
Effect of PF on iNOS and COX-2
expression levels in IL-1β-stimulated chondrocytes
The effect of PF on iNOS and COX-2 mRNA and protein
expression levels in IL-1β-induced chondrocytes were evaluated by
RT-qPCR and western blot analysis. As presented in Fig. 3A and B, IL-1β treatment
significantly increased the mRNA expression levels of iNOS and
COX-2 (P<0.05) compared with the control. However, treatment
with PF significantly inhibited IL-1β-induced expression of iNOS
and COX-2 in chondrocytes (P<0.05). Western blot analysis
demonstrated that PF also inhibited the protein expression levels
of iNOS and COX-2 induced by IL-1β in human OA chondrocytes
(P<0.05; Fig. 3C and D).
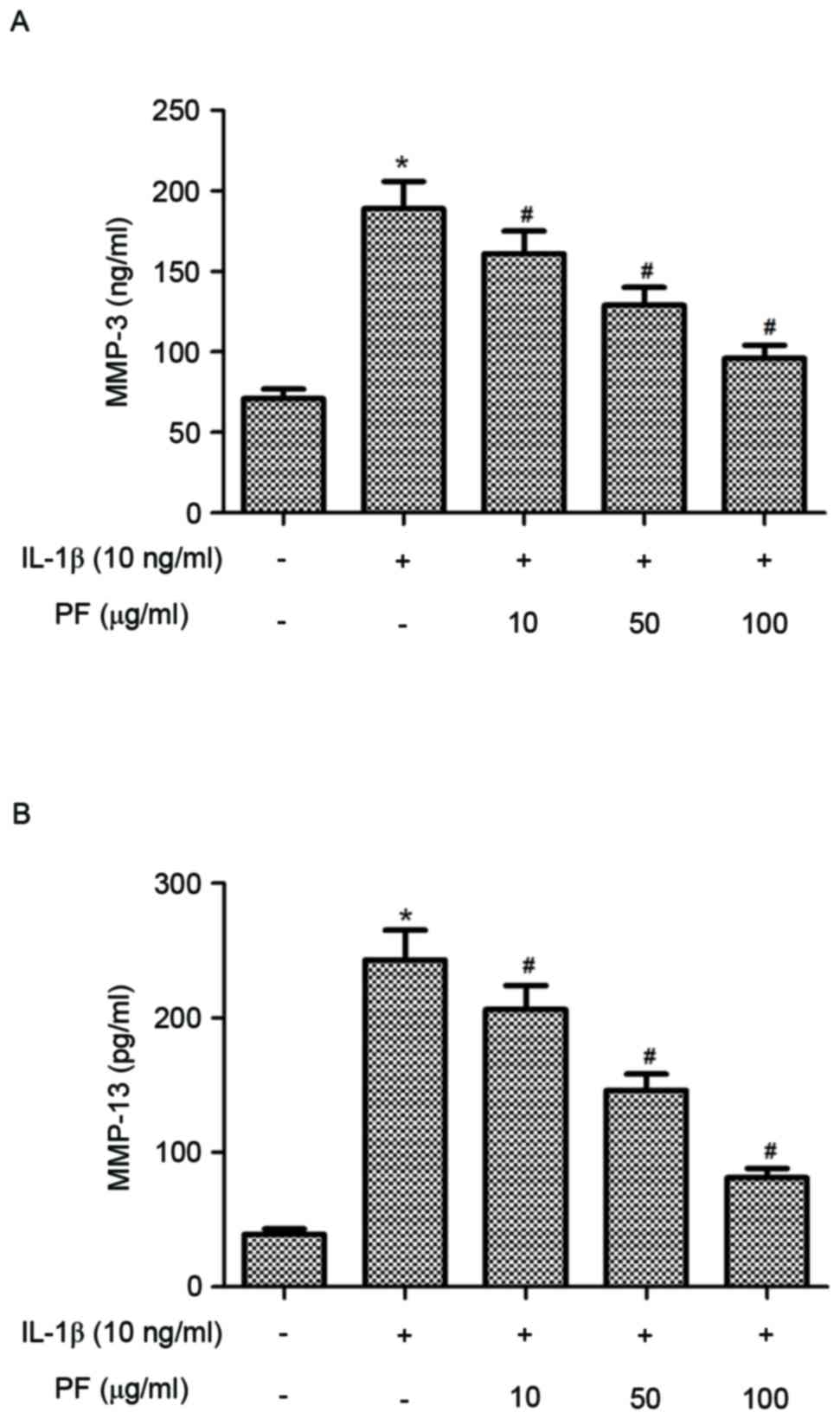
Effect of PF on production of MMPs in
IL-1β-stimulated chondrocytes
It is established that MMPs perform a critical role
in bone remodeling and OA; therefore, the present study
investigated the effect of PF on MMP-3 and MMP-13 production in
IL-1β-induced chondrocytes. As presented in Fig. 4, chondrocytes stimulated with IL-1β
exhibited significantly increased levels of MMP-3 and MMP-13
compared with the control group (P<0.05). However, treatment of
chondrocytes with PF significantly reduced the production of MMP-3
and MMP-13 induced by IL-1β in human OA chondrocytes
(P<0.05).
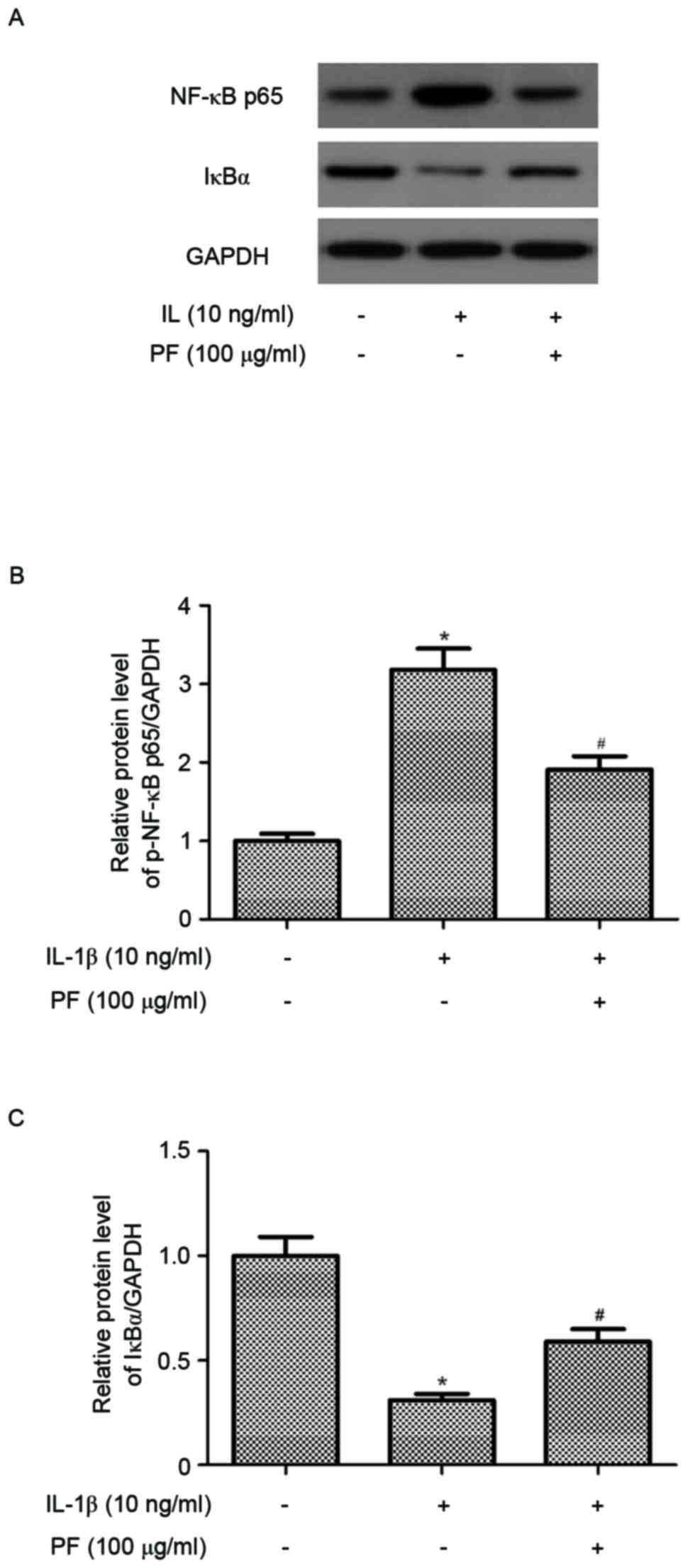
Effect of PF on NF-κB activation in
IL-1β-stimulated chondrocytes
In order to further understand the anti-inflammatory
mechanisms of PF, the present study examined the level of NF-κB p65
expression in IL-1β-stimulated chondrocytes. As indicated in
Fig. 5, the protein expression
level of NF-κB p65 in the nucleus of chondrocytes was significantly
increased by IL-1β compared with the untreated control (P<0.05).
However, PF significantly suppressed the expression of NF-κB p65
(P<0.05; Fig. 5B).
Additionally, PF inhibited IL-1β-induced IκB-α degradation in human
OA chondrocytes (P<0.05; Fig.
5C).
Discussion
Inflammatory cytokines serve important roles in the
pathogenesis of OA. The results of the present study indicate that
pretreatment with PF inhibited the production of NO and PGE2
induced by IL-1β, and the expression of iNOS and COX-2 in
chondrocytes. Pretreatment with PF also significantly inhibited the
IL-1β-stimulated production of MMP-3 and MMP-13 in chondrocytes.
Furthermore, PF inhibited the expression of NF-κB p65 and IκB-α
degradation induced by IL-1β in OA chondrocytes.
NO is an inflammatory mediator, which is produced by
iNOS. PGE2 is also an inflammatory factor, which is elevated by
COX-2. Increased serum levels of NO and PGE2 have been previously
observed in patients with OA (18,19).
Additionally, IL-1β has been identified as a potent inducer of iNOS
and COX-2 in cultured chondrocytes in vitro (8). The present study determined that PF
inhibited the production of NO and PGE2 induced by IL-1β and the
expression of iNOS and COX-2 in chondrocytes. These results are
supported by Guo et al (20), that determined treatment with PF
reduced the protein expression levels of iNOS and COX2 and
5-lipoxidase in the brain of transient middle cerebral artery
occlusion rats (20). The results
of the present study suggest that the inhibition of NO and PGE2 by
PF may be associated with the regulation of the expression levels
of iNOS and COX-2 in chondrocytes.
Previous studies indicated that an excess of MMPs
may have an important role in the progression of OA, as they are
proteolytic enzymes, in OA progression they may be associated with
their ability to degrade the ECM (20–23).
MMP-3 and MMP-13 are important for degrading collagens,
proteoglycans and other ECM macromolecules in chondrocytes. MMP-3
may aggravate inflammation via the activation of various pro-MMPs
and cleaves extracellular components (24). MMP-13 may also degrade the ECM,
including the cartilage-specific component type II collagen during
the progression of OA (25). The
present study determined that PF may inhibit IL-1β-induced
production of MMP-3 and MMP-13 in human OA chondrocytes. Therefore,
the results of the present study revealed that PF exerted an
anti-arthritic effect via inhibition of the MMP-3 and MMP-13
expression levels.
Among multiple pathways and mediators influencing
the development of OA, NF-κB transcription factor has a prominent
role (16,26,27).
It has been reported that IL-1β may activate NF-κB via triggering
of IκBα degradation, leading to the translocation of NF-κB p65 from
cytoplasm to nucleus to regulate the expression of various
inflammation-associated genes (28). Additionally, it was previously
reported that NF-κB may stimulate the expression level of enzymes,
such as iNOS and COX-2 in chondrocytes and the products of these
enzymes may contribute to the pathogenesis of OA (29). The NF-κB inhibitor has been
identified to reduce IL-1β-induced MMP-3 and MMP-13 production in
human chondrocytes (30). The
present study determined that PF inhibited the expression of NF-κB
p65 and IκB-α degradation induced by IL-1β in chondrocytes. The
results of the current study are partly supported by Wu et
al (31), that determined that
PF may suppress NF-κB activation through modulation of IκB-α in
human gastric carcinoma cells. In a recent study, PF was also
identified to block nuclear translocation of NF-κB p65 in
lipopolysaccharide-stimulated RAW264.7 mouse macrophage cells
(32). These previous results
combined with the present study suggest that PF may inhibit
IL-1β-induced expression of inflammatory mediators by suppressing
the activation of the NF-κB signaling pathway in human OA
chondrocytes.
In conclusion, the results of the present study
suggest that PF may inhibit the expression of inflammatory
mediators in IL-1β-induced OA chondrocytes by suppressing the
activation of the NF-κB signaling pathway. Therefore, PF may be a
potential therapeutic agent for the future treatment of OA.
References
|
1
|
Buckwalter JA, Mankin HJ and Grodzinsky
AJ: Articular cartilage and osteoarthritis. Instr Course Lect.
54:465–480. 2005.PubMed/NCBI
|
|
2
|
Jakobsson U and Hallberg IR: Pain and
quality of life among older people with rheumatoid arthritis and/or
osteoarthritis: A literature review. J Clin Nurs. 11:430–443. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Richette P, Latourte A and Frazier A:
Safety and efficacy of paracetamol and NSAIDs in osteoarthritis:
Which drug to recommend? Expert Opin Drug Saf. 14:1259–1268. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Loeser RF: Molecular mechanisms of
cartilage destruction: Mechanics, inflammatory mediators, and aging
collide. Arthritis Rheum. 54:1357–1360. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhou PH, Liu SQ and Peng H: The effect of
hyaluronic acid on IL-1beta-induced chondrocyte apoptosis in a rat
model of osteoarthritis. J Orthop Res. 26:1643–1648. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Goldring SR and Goldring MB: The role of
cytokines in cartilage matrix degeneration in osteoarthritis. Clin
Orthop Relat. 427 Suppl:S27–S36. 2004. View Article : Google Scholar
|
|
7
|
Mengshol JA, Vincenti MP, Coon CI,
Barchowsky A and Brinckerhoff CE: Interleukin-1 induction of
collagenase 3 (matrix metalloproteinase 13) gene expression in
chondrocytes requires p38, c-jun N-terminal kinase and nuclear
factor kappaB: Differential regulation of collagenase 1 and
collagenase 3. Arthritis Rheum. 43:801–811. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chabane N, Zayed N, Afif H, Mfuna-Endam L,
Benderdour M, Boileau C, Martel-Pelletier J, Pelletier JP, Duval N
and Fahmi H: Histone deacetylase inhibitors suppress
interleukin-1beta-induced nitric oxide and prostaglandin E2
production in human chondrocytes. Osteoarthritis Cartilage.
16:1267–1274. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang H, Zhou H, Wang CX, Li YS, Xie HY,
Luo JD and Zhou Y: Paeoniflorin inhibits growth of human colorectal
carcinoma HT 29 cells in vitro and in vivo. Food Chem Toxicol.
50:1560–1567. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wankun X, Wenzhen Y, Min Z, Weiyan Z, Huan
C, Wei D, Lvzhen H, Xu Y and Xiaoxin L: Protective effect of
paeoniflorin against oxidative stress in human retinal pigment
epithelium in vitro. Mol Vis. 17:3512–3522. 2011.PubMed/NCBI
|
|
11
|
Cao W, Zhang W, Liu J, Wang Y, Peng X, Lu
D, Qi R, Wang Y and Wang H: Paeoniflorin improves survival in
LPS-challenged mice through the suppression of TNF-α and IL-1β
release and augmentation of IL-10 production. Int Immunopharmacol.
11:172–178. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nizamutdinova IT, Jin YC, Kim JS, Yean MH,
Kang SS, Kim YS, Lee JH, Seo HG, Kim HJ and Chang KC: Paeonol and
paeoniflorin, the main active principles of Paeonia albiflora,
protect the heart from myocardial ischemia/reperfusion injury in
rats. Planta Med. 74:14–18. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu H, Wang J, Wang J, Wang P and Xue Y:
Paeoniflorin attenuates Aβ1-42-induced inflammation and chemotaxis
of microglia in vitro and inhibits NF-κB- and VEGF/Flt-1 signaling
pathways. Brain Res. 1618:149–158. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu D, Chen J, Zhu H, Xiong XG, Liang QH,
Zhang Y, Zhang Y, Wang Y, Yang B and Huang X: UPLC-PDA
determination of paeoniflorin in rat plasma following the oral
administration of Radix Paeoniae Alba and its effects on rats with
collagen-induced arthritis. Exp Ther Med. 7:209–217. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cheng AW, Stabler TV, Bolognesi M and
Kraus VB: Selenomethionine inhibits IL-1β inducible nitric oxide
synthase (iNOS) and cyclooxygenase 2 (COX2) expression in primary
human chondrocytes. Osteoarthritis Cartilage. 19:118–125. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Marcu KB, Otero M, Olivotto E, Borzí RM
and Goldring MB: NF-kappaB signaling: Multiple angles to target OA.
Curr Drug Targets. 11:599–613. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Salvatierra J, Escames G, Hernandez P,
Cantero J, Crespo E, Leon J, Salvatierra D, Acuña-Castroviejo D and
Vives F: Cartilage and serum levels of nitric oxide in patients
with hip osteoarthritis. J Rheumatol. 26:2015–2017. 1999.PubMed/NCBI
|
|
19
|
Salvemini D, Misko TP, Masferrer JL,
Seibert K, Currie MG and Needleman P: Nitric oxide activates
cyclooxygenase enzymes. Proc Natl Acad Sci USA. 90:pp. 7240–7244.
1993; View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guo RB, Wang GF, Zhao AP, Gu J, Sun XL and
Hu G: Paeoniflorin protects against ischemia-induced brain damages
in rats via inhibiting MAPKs/NF-κB-mediated inflammatory responses.
PLoS One. 7:e497012012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tetlow LC, Adlam DJ and Woolley DE: Matrix
metalloproteinase and proinflammatory cytokine production by
chondrocytes of human osteoarthritic cartilage: Associations with
degenerative changes. Arthritis Rheum. 44:585–594. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dean DD, Martel-Pelletier J, Pelletier JP,
Howell DS and Woessner JF Jr: Evidence for metalloproteinase and
metalloproteinase inhibitor imbalance in human osteoarthritic
cartilage. J Clin Invest. 84:678–685. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mahmoud RK, El-Ansary AK, El-Eishi HH,
Kamal HM and El-Saeed NH: Matrix metalloproteinases MMP-3 and MMP-1
levels in sera and synovial fluids in patients with rheumatoid
arthritis and osteoarthritis. Ital J Biochem. 54:248–257.
2005.PubMed/NCBI
|
|
24
|
Wu JJ, Lark MW, Chun LE and Eyre DR: Sites
of stromelysin cleavage in collagen types II IX, X, and XI of
cartilage. J Biol Chem. 266:5625–5628. 1991.PubMed/NCBI
|
|
25
|
Aigner T, Fundel K, Saas J, Gebhard PM,
Haag J, Weiss T, Zien A, Obermayr F, Zimmer R and Bartnik E:
Large-scale gene expression profiling reveals major pathogenetic
pathways of cartilage degeneration in osteoarthritis. Arthritis
Rheum. 54:3533–3544. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Scanzello CR and Goldring SR: The role of
synovitis in osteoarthritis pathogenesis. Bone. 51:249–257. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Roman-Blas JA and Jimenez SA: NF-kappaB as
a potential therapeutic target in osteoarthritis and rheumatoid
arthritis. Osteoarthritis Cartilage. 14:839–848. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Newton R, Kuitert LM, Bergmann M, Adcock
IM and Barnes PJ: Evidence for involvement of NF-kappaB in the
transcriptional control of COX-2 gene expression by IL-1beta.
Biochem Biophys Res Commun. 237:28–32. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Stuhlmeier KM: The anti-rheumatic gold
salt aurothiomalate suppresses interleukin-1beta-induced hyaluronan
accumulation by blocking HAS1 transcription and by acting as a
COX-2 transcriptional repressor. J Biol Chem. 282:2250–2258. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liacini A, Sylvester J, Li WQ and
Zafarullah M: Inhibition of interleukin-1-stimulated MAP kinases,
activating protein-1 (AP-1) and nuclear factor kappa B (NF-kappaB)
transcription factors down-regulates matrix metalloproteinase gene
expression in articular chondrocytes. Matrix Biol. 21:251–262.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu H, Li W, Wang T, Shu Y and Liu P:
Paeoniflorin suppress NF-kappaB activation through modulation of
IkappaB alpha and enhances 5-fluorouracil-induced apoptosis in
human gastric carcinoma cells. Biomed Pharmacother. 62:659–666.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang J, Dou W, Zhang E, Sun A, Ding L,
Wei X, Chou G, Mani S and Wang Z: Paeoniflorin abrogates
DSS-induced colitis via a TLR4-dependent pathway. Am J Physiol
Gastrointest Liver Physiol. 306:G27–G36. 2014. View Article : Google Scholar : PubMed/NCBI
|