Introduction
Parkinson's disease (PD) is the second most common
neurodegenerative disease, and affects ~1% of individuals >65
years of age worldwide (1). It is
associated with dopamine (DA) depletion in the striatum caused by
the progressive loss of DA neurons in the substantia nigra pars
compacta (SNpc), which is considered to be the primary cause of the
motor symptoms of PD, which include rigidity, bradykinesia,
postural instability, gait disorder and tremor (2). In the last two decades, previous
studies have employed animal models to gain an improved
understanding of the PD process, as the availability of PD autopsy
materials is limited. Although numerous animal models of PD have
been employed in experimental studies concerning the mechanism of
PD and therapeutic strategies (3–6), the
unilateral 6-hydroxydopamine (6-OHDA) rat model, a neurotoxic
compound-induced rat model possessing PD-associated pathological
and behavioral features, is considered to be a classic animal model
in PD correlation research.
The 6-OHDA rat model of PD was previously
established by intracranial 6-OHDA injection into the SNpc, causing
loss of tyrosine hydroxylase (TH)-positive neurons in the SNpc
(4) and the TH-positive fibers in
the striatum (5). It was also
reported that death of TH+ fibers in the striatum
occurred prior to the TH+ neurons in the SNpc. This
phenomenon seems to be a duplication of the human PD progress
(6). Based on these studies, the
unilateral PD rat model is often established by injection of 6-OHDA
into one side of the striatum to reflect TH+ neurons
retrograde degeneration. Usually, the extent of the SNpc or
striatal lesion is determined by examining turning behavior induced
by amphetamine or apomorphine (APO). Although other behaviors,
determined by rotarod, cylinder and open field tests, have been
also observed in the model and used to determine the efficacy of
drugs as potential PD therapeutics (6–8).
However, there is a lack of evidence demonstrating a correlation
between these non-amphetamine or -APO induced behaviors and DA
neuron loss in the SNpc. Furthermore, different injection sites in
the striatum have been employed in different studies, which makes
interpreting behavioral and biochemical results from the rat model
more difficult. Therefore, it is important to formulate a standard
pattern of model establishment and phenotype analysis. In addition,
systematic investigation of the associations between behavior, DA
neuron loss and other pathological aspects during the course of PD
in animal models should be performed, which may improve the
evaluation of novel therapeutic approaches for PD.
In the present study, PD rat model was established
by 6-OHDA unilateral injection in the neostriatal region of the
striatum, and PD-associated behaviors and pathological features
were detected. In addition, correlation analysis was performed
between these phenotypes, and a potential intervention time-point,
in addition to a comprehensive evaluation index system, was
proposed for the assessment of PD therapeutic strategies using the
PD rat model.
Materials and methods
Animals
A total of 60 male Sprague-Dawley rats (age, 7
weeks; weight, 220–240 g; Beijing Vital River Lab Animal Technology
Co., Ltd., Beijing, China) were randomly assigned to the following
two groups: Model (n=38); and sham (n=22). Rats were housed on a
12-h light/dark cycle with free access to food and water at
22–25°C, relative humidity 40–60%, and habituated to the housing
conditions for 3 days prior to experiments. Every effort was made
to minimize suffering and stress. All experimental procedures were
approved by the Committee on Animal Care and Usage of Capital
Medical University (Beijing, China).
Surgical procedure
Rats were anesthetized with an intraperitoneal
injection of 6% chloral hydrate (350 mg/kg). Once anesthetized,
rats were fixed in a stereotaxic apparatus (David Kopf Instruments,
Tujunga, CA, USA) with a flat skull position. The skull was exposed
and a 1 mm burr hole was drilled to detect the cranial cavity. The
coordinates were as follows: Anteroposterior (AP), 0.8 mm from the
bregma; mediolateral (ML), 2.7 mm from the midline; and
dorsoventral (DV), −5.2 and −4.5, respectively, from the skull
(Fig. 1A) (9).
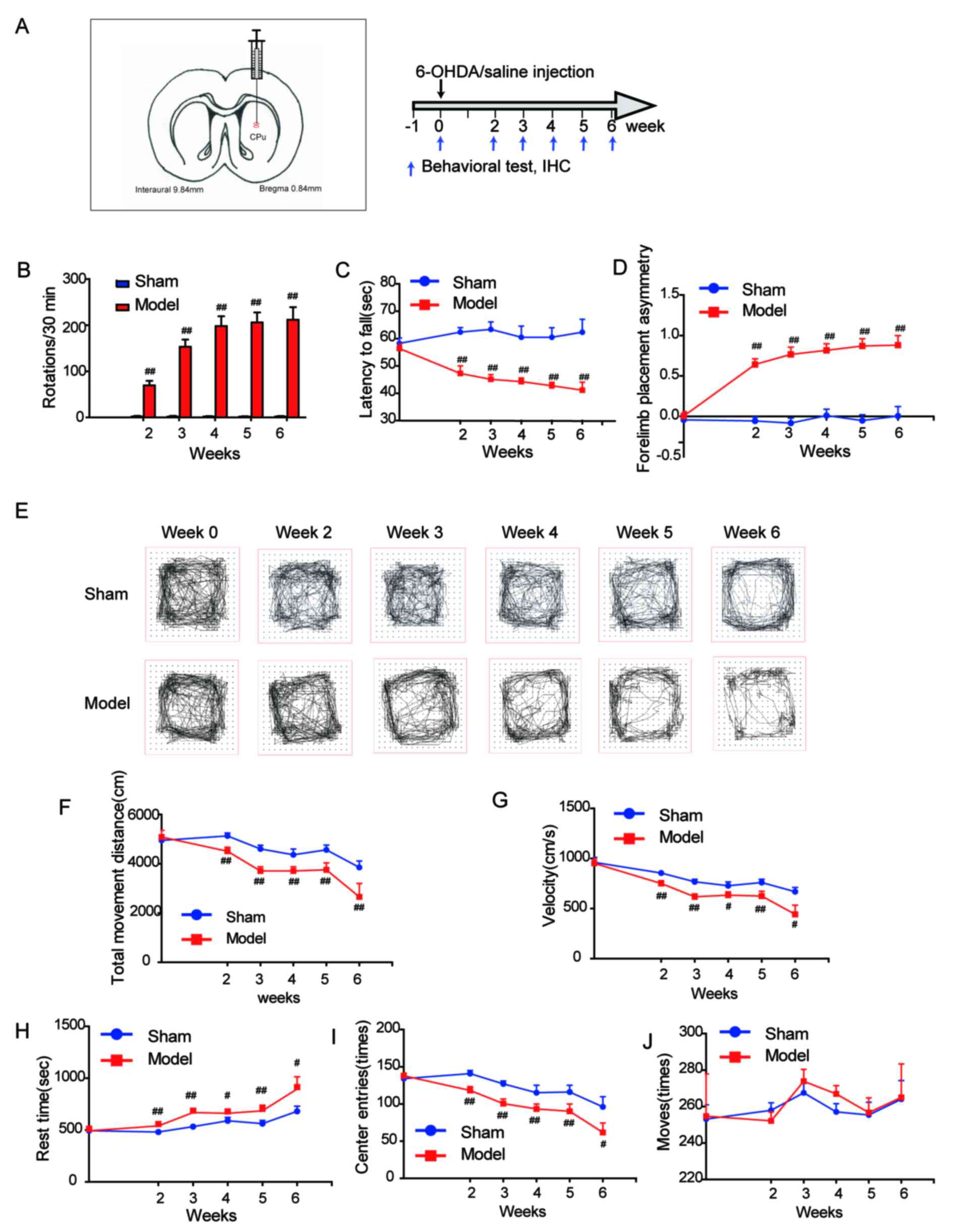 | Figure 1.Behavioral performance of the 6-OHDA
rat model of Parkinson's disease. (A) Stereotaxic coordinates of
the left striatum (anteroposterior=0.8 mm, mediolateral=2.7 mm and
dorsoventral=−5.2 and −4.5 mm) and the study time-line. Behavior of
rats was evaluated by (B) apomorphine-induced rotation, (C) rotarod
and (D) cylinder tests. (E) Representative graphs from open field
test. (F) Total movement distance, (G) velocity, (H) rest time, (I)
center entries and (J) moves were recorded during the open field
tests. Data are presented as the mean + standard error of the mean.
Week 0, n=38 and n=22 in model and sham groups, respectively; week
2, n=30 and n=22 in model and sham groups, respectively; week 3,
n=24 and n=18 in model and sham groups, respectively; week 4, n=18
and n=14 in model and sham groups, respectively; week 5, n=12 and
n=10 in model and sham groups, respectively; week 6, n=6 and n=6 in
model and sham groups respectively. #P<0.05;
##P<0.01 vs. sham. 6-OHDA, 6-hydroxydopamine; IHC,
immunohistochemistry. |
Subsequently, 6-OHDA [20 µg per rat in 4 µl saline
with 0.01% (w/v) ascorbic acid across two sites] was infused with
an infusion pump through a 10 µl Hamilton syringe at a constant
flow rate of 0.2 µl/min into the left striatum (2 µl was injected
at each coordinate). At the end of the infusion, the syringe was
left implanted for an additional 5 min per site and was then slowly
retracted. Sham-operated animals were submitted to the same
procedure except 4 µl vehicle [0.9% saline containing 0.01% (w/v)
ascorbic acid] was infused into the striatum instead of 6-OHDA.
During the surgery and recovery, animals were kept warm using a
heating pad.
APO-induced rotations
APO-induced rotation was recorded using a
multichannel rotometer system (RotoMax; AccuScan Instruments, Inc.,
Columbus, Ohio, USA), as described previously (10). Briefly, 2 weeks after surgery, all
animals were injected subcutaneously with APO hydrochloride (0.5
mg/kg; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and
individually placed in the test cylinder. Rats that rotated in
excess of 60 turns/30 min were considered to be the PD unilateral
models. The drug-induced rotation was reexamined at 3, 4, 5 and 6
weeks after surgery.
Rotarod test
The rotarod test was performed to evaluate motor
coordination and balance, as described previously (11). Briefly, rats were trained to stay
on the rotarod apparatus during a 5 min habituation trial (10 rpm)
of 2 consecutive days prior to the first testing day. Rats were
subsequently subjected to a total of four rotarod test sessions
with accelerating speeds (range, 4–40 rpm) over a period of 2 min
on weeks 0, 2, 3, 4, 5 and 6 after surgery. Each test session was
composed of two trials on the rotarod, with a maximum duration of 2
min per trial and a 20 min inter-trial interval. The time duration
of each animal staying on the rod was recorded as the latency to
fall, which was registered automatically by a trip switch under the
floor of each rotating drum. The best score achieved by each rat
was used for further analysis.
Cylinder test
Because unilateral injection of 6-OHDA can cause
limb impairment, the cylinder test was performed to investigate
spontaneous forelimb lateralization, taking advantage of the
natural exploratory instinct of rodents to a new environment
(12). The test was performed as
follows: Rats were placed individually inside a glass cylinder
(diameter, 22 cm; height, 26 cm) with two mirrors located behind
the cylinder at a 45° angle to allow 360° vision. The rats were
video recorded for 5 min after rats first touched the walls of the
cylinder with the impaired or unimpaired forelimb or both
simultaneously. Each individual rearing episode was counted by a
blinded researcher. The scores were calculated by the following
asymmetry ratio: Left-right/(right + left + both). Scores on the
forelimb asymmetry ratio range from −1 to 1. The positive ratio was
consistent with greater use of the unimpaired forelimb over the
impaired forelimb. By contrast, the negative asymmetry ratio
suggests greater use of the impaired forelimb compared with the
unimpaired forelimb. Therefore, a high positive ratio would be
consistent with a hemiparkinsonian lesion.
Open field test
Open field tests were performed at 0, 2, 3, 4, 5 and
6 weeks after surgery, as described previously (13). Briefly, all behavioral procedures
were performed between 9:00 am and 3:00 pm, and silence in the room
was maintained for the duration of the test. Locomotor activity was
measured in automated activity chambers connected to an analyzer
that transmitted the number of beam breaks (activity data) to a
computer (VersaMon version 2.01; Accuscan Instruments, Inc.). The
rats were placed individually in the center of the chamber. Each
chamber consisted of an individual cage with a grid of infrared
beams mounted horizontally every 2.5 cm. Locomotor activity was
quantified as the number of beam interruptions (crossings)
registered by a computer and recorded as total movement distance
(cm), velocity (cm/sec), rest time (sec), center entries (times)
and moves (times) during the 30 min recording period. The open
field chambers were washed with 75% ethanol solution each time
prior to behavioral testing in order to eliminate odors left by the
previous rat.
Immunohistochemistry (IHC)
Following the behavioral tests each week, 10 animals
(n=6 for model and n=4 for sham) were anesthetized with 6% chloral
hydrate and perfused intracardially with warm 0.9% NaCl at room
temperature, followed by 200 ml cold 4% paraformaldehyde (PFA/0.1 M
PBS). The brains were rapidly removed from the skull following
decapitation and immersed in 4% paraformaldehyde for 24 h at 4°C.
Subsequently, the tissues were cryoprotected with 20 and 30%
sucrose sequentially, until they sank to the bottom of the tube.
Brains were subsequently embedded in optimal cutting temperature
medium at −80°C overnight. The coronal brain sections were obtained
using a microtome at 30 µm thickness for the striatum and 50 µm
thickness for the midbrain including the SNpc. IHC was performed on
free-floating sections, which were rinsed in 0.1 M PBS three times
for 5 min. Sections were then permeabilized with 0.3% Triton-X-100
for 30 min at room temperature and washed with 0.1 M PBS three
times for 5 min. Sections were treated with 3% hydrogen peroxide
for 30 min and washed with 0.1 M PBS three times for 5 min.
Subsequently, sections were blocked with 5% normal goat serum
(Vector Laboratories, Inc., Burlingame, CA, USA) in 0.1 M PBS for 1
h at room temperature, which was followed by incubation with
primary antibodies overnight at 4°C. The following primary
antibodies were used in the present study: Anti-TH (mouse; 1:5,000;
cat. no. T1299; Sigma-Aldrich; Merck KGaA; USA); anti-glial
fibrillary acidic protein (GFAP; mouse; 1:500; cat. no. MAB360; EMD
Millipore, Billerica, MA, USA); and anti-CD11b (Mac1; mouse; 1:500;
cat. no. MCA275G; Bio-Rad Laboratories, Inc., Hercules, CA, USA).
All antibodies were diluted in 5 ml 0.1 M PBS. Staining was
performed using Vectastain Universal Elite ABC kit (Vector
Laboratories, Inc. USA). Biotinylated anti-mouse IgG secondary
antibodies (1:200) were used to recognize primary antibodies at
37°C for 60 min, followed by washing three times and incubation
with a streptavidin-horseradish peroxidase complex (1:1,000) at
37°C for 30 min. The immunoreactivities were visualized by
3,3-diaminobenzidine (DAB) within 2 min. The sections were mounted,
coverslipped and dehydrated in a gradual concentration of
ethanol.
The number of TH-positive cells in the SNpc was
determined by stereological measurements using the optical
fractionator method in a computerized system (Stereo Investigator
software, version 8.0; Leica Microsystems GmbH, Wetzlar, Germany),
as previously described (14,15).
The sections were used for counting, including the entire SNpc from
the rostral tip of the pars compacta back to the caudal end of the
pars reticulate. Every eighth section throughout the entire SNpc
was counted, with a total of 6 sections for each animal. The
estimates of the total number of neurons were calculated according
to the optical fractionator formula, and coefficients of error
<0.10 were accepted. Both the injected and non-injected side of
the SNpc was quantified.
For measurement of TH fibers in the striatum,
briefly, high-resolution images were obtained from the sections
stained by TH using a ×1.25 (objective lens) Nikon light
microscope. The extent of striatal denervation was measured in
three sections per animal corresponding to +1.2, +0.6 and −0.2 mm
from the bregma, using Image-Pro Plus 6.0 (Media Cybernetics, Inc.,
Rockville, MD, USA). The entire striatum according to the
dorsoventral axis was divided into equal halves and the measured
values were corrected for nonspecific background staining by
subtracting values of the blank area. Data are presented as the
percentage of striatal densitometry with the non-injected
hemisphere corresponding to 100% for each individual rat.
The area occupied by Mac1- or GFAP-positive staining
was defined by densitometry using Image-Pro Plus 6.0. All the
analyses were performed by an investigator blind to different
samples. Data were normalized to the contralateral normal side.
Statistical analysis
Data was analyzed with SPSS 21.0 software (IBM
Corp., Armonk, NY, USA) and presented as the mean ± standard error
of the mean. Differences between the means of two groups were
analyzed using independent-samples Student's t-test or, when data
were not normally distributed, a nonparametric Mann-Whitney U test
was performed. For correlation analysis, the Pearson's correlation
coefficient, and subsequent linear regression, was determined.
P<0.05 was considered to indicate a statistically significant
difference.
Results
The behavior of PD rats
In the present study, the neostriatum was selected
as the injection site, which is illustrated in Fig. 1A. (AP=0.8 mm, ML=2.7 mm and DV=−5.2
and −4.5 mm). The experiment was performed according to the
schedule as presented in Fig. 1A.
At 2 weeks after surgery, the rats with >60 rotations/30 min
induced by APO were considered successful PD models. According to
the criteria, a total of 30 PD model rats were obtained.
In order to characterize the behaviors of the
hemiparkinsonian rat during the course of dopaminergic
nigrostriatal pathway degeneration, the current study observed
several behavioral parameters, including APO-induced rotation,
forelimb placement, motor coordination and locomotor activity from
weeks 2 to 6 following striatum injection. It was demonstrated in
Fig. 1B that, in the model group,
the number of APO-induced contralateral rotations during a 30 min
testing period was increased following surgery between weeks 2
(69.3±10.1 turns/30 min) and 6 (211.6±27.2 turns/30 min).
Strikingly, the rotations sharply increased from week 2 (69.3±10.1
turns/30 min) to week 3 (153.0±15.5 turns/30 min), after which the
number of turns remained quite stable at around 200 turns/30 min.
The number of turns/30 min did not change over the course of this
experiment in sham rats.
The motor coordination and balance skills in the PD
rats were assessed by the rotarod test. The mean time the model
group stayed on the accelerating rotarod was significantly shorter
compared with the sham group (P<0.01; Fig. 1C) between weeks 2 and 6 after
surgery. Notably, the latency to fall in the rotarod test was
decreased gradually across the whole experimental period in the
model group (week 2, 47.2±2.8 sec; week 6, 41.2±2.9 sec).
To appraise the forelimb placement of PD rats, the
cylinder test was performed. The ratio of forelimb placement
[(left-right)/(right + left + both)] of the model group was
significantly higher compared with the sham group (P<0.01;
Fig. 1D) during the experimental
period.
In addition, the open field test was performed to
examine locomotor activity and anxiety-associated behaviors
(8). Several parameters were
determined in the test, and significant decreases in total movement
distance (cm), velocity (cm/sec), center entries (times), and an
increase in rest time (sec), were observed in the model group
compared with the sham group (P<0.01; Fig. 1E-I). However, no significant
differences in the moves (times) between the model and sham group
were observed (Fig. 1J;
P>0.05).
IHC staining
TH is the rate-limiting enzyme in DA synthesis and
an established marker for DA neurons. IHC was used to determine the
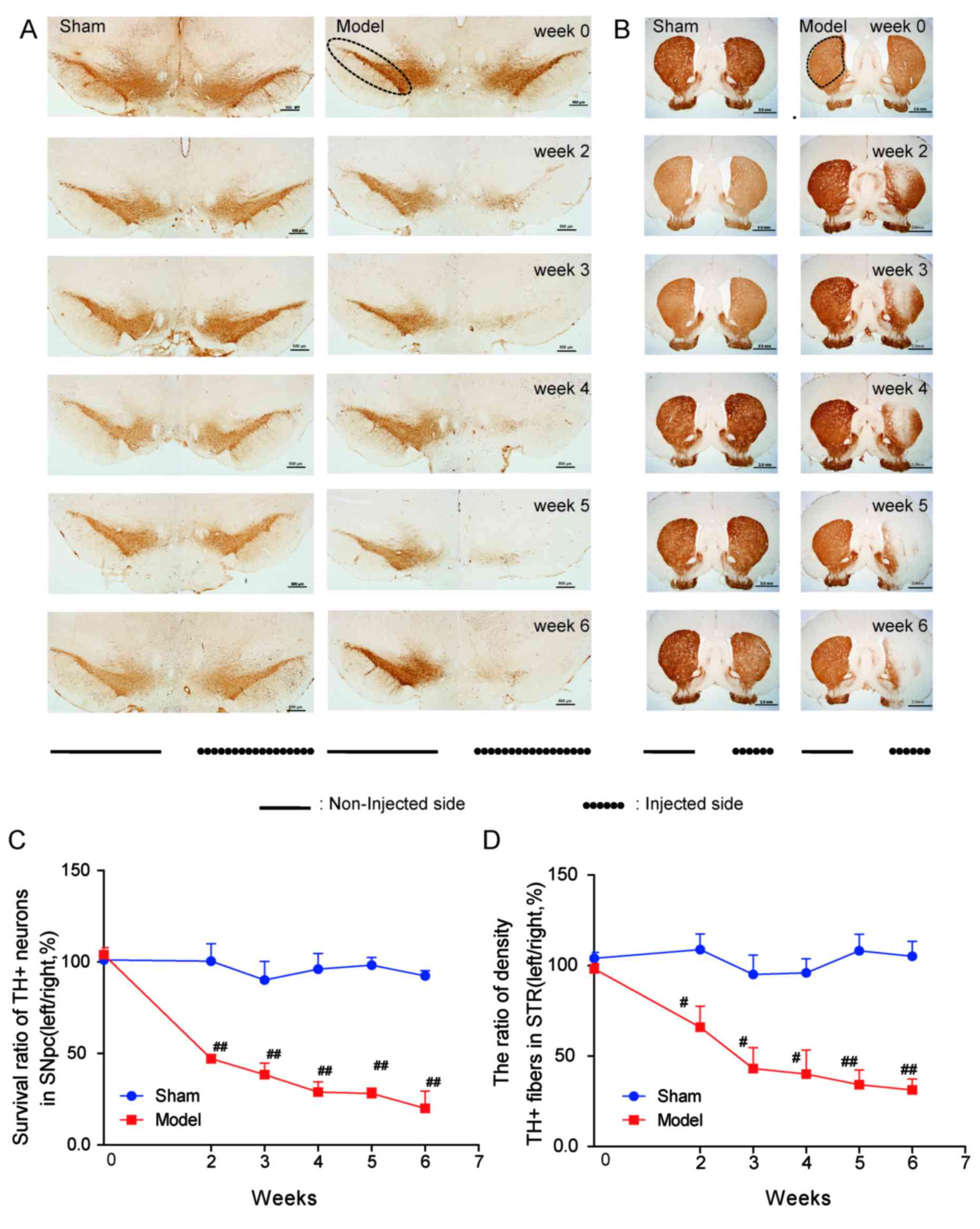
effects of 6-OHDA on TH+ neurons and fibers (Fig. 2). To confirm DA neuron deficiency,
stereological quantification of TH+ neurons in the SNpc
was performed in all groups at weeks 2–6 following surgery. As
anticipated, the number of surviving TH+ neurons in the
SNpc was significantly decreased in PD rats compared with sham rats
(P<0.01; Fig. 2A and C).
Furthermore, the survival of TH+ neurons (from 47% in
week 2 to 20% in week 6) in the model group in SNpc descended
progressively across the experimental period. Consistently,
TH+ fiber density in the striatum of the model group was
lower compared with the sham group (P<0.01; Fig. 2B and D).
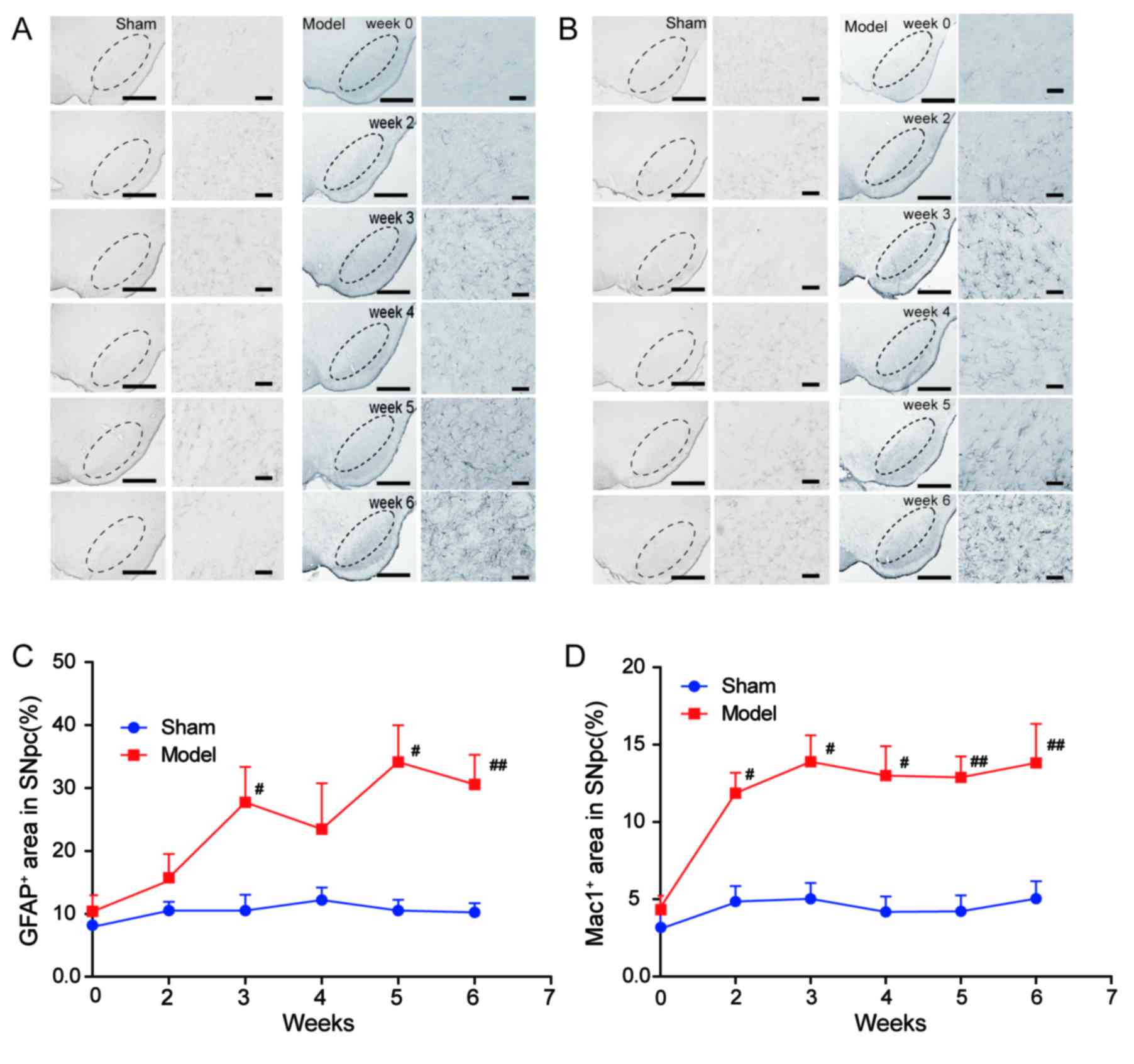
As glial reactivity is considered to be a crucial
event in the process of 6-OHDA toxicity in the PD model (16), the present study also observed the
morphological alterations of glial cells activated by 6-OHDA
(Fig. 3). The population of
astrocytes and microglia in the rat brain were presented as the
percentage of area occupied by GFAP+ (specific for
astrocyte) and Mac1+ (specific for microglia) cells in
the ipsilateral SNpc, respectively, at all time-points. There was a
significant increase in the area occupied by GFAP+ cells
in the SNpc of the model group compared with the sham group
(P<0.01; Fig. 3A and C) at
weeks 3, 5, 6. The area occupied with Mac1+ cells began
to increase at week 2 in the SNpc of the model group compared with
the sham group, and the microglial activation persisted at weeks 3,
4, 5 and 6 (P<0.01; Fig. 3B and
D).
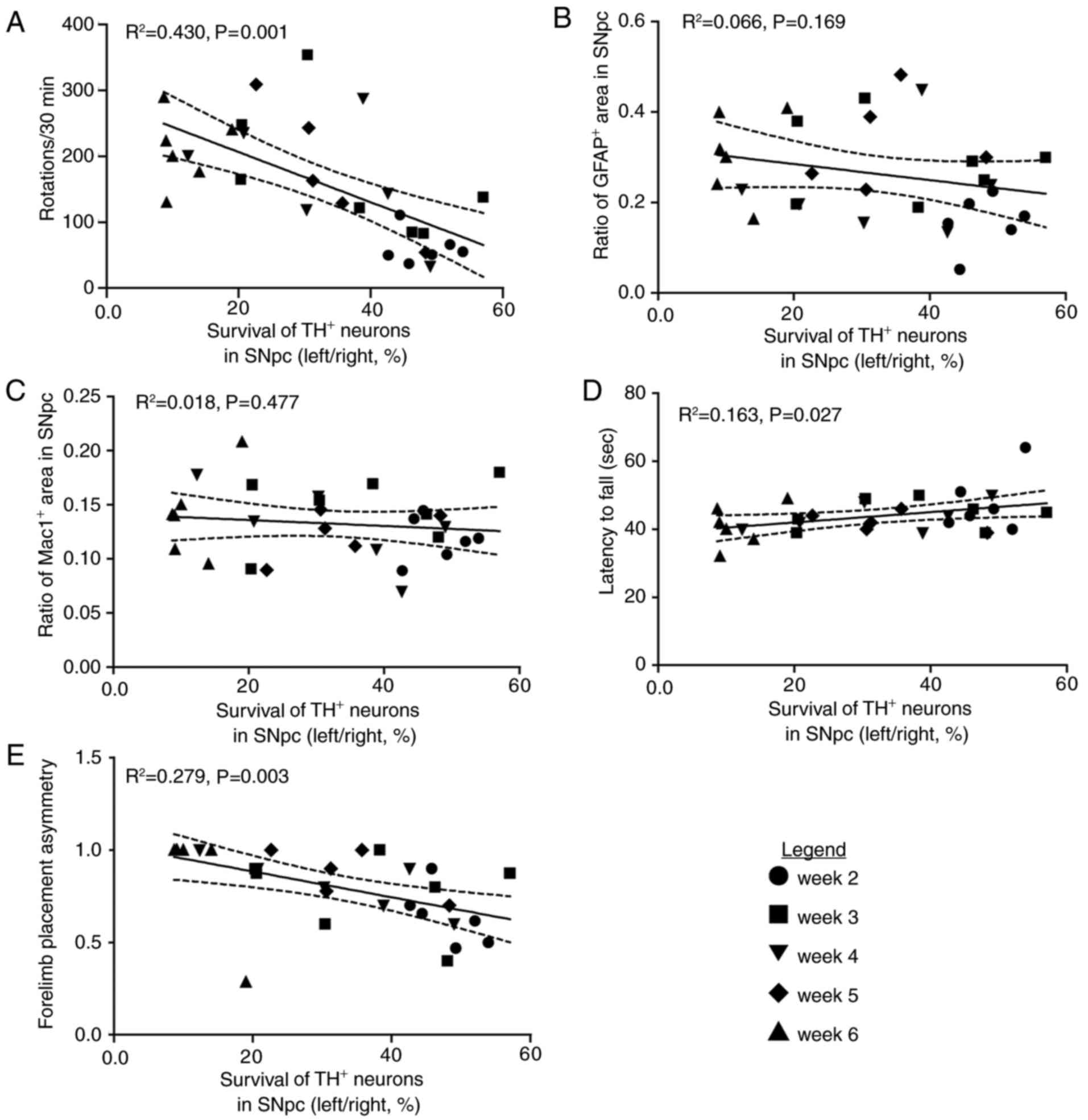
Correlation between morphological and
behavioral measures
In order to investigate the association between the
survival of nigral DA neurons and these behavioral tests in the PD
rat model, linear regression analysis was performed. The survival
of nigral DA neurons within the SNpc was correlated with
APO-induced rotations (R2=0.430, P=0.001; Fig. 4A), but not correlated with either
the area of GFAP+ staining (R2=0.066,
P=0.169; Fig. 4B) or the area of
Mac1+ staining (R2=0.018, P=0.477; Fig. 4C) in the SNpc. The latency to fall
of the rotarod test (R2=0.163, P=0.027; Fig. 4D) and the paw preference
(asymmetry) in the cylinder test (R2=0.279, P=0.003;
Fig. 4E) were strongly correlated
with the survival of DA neurons in the SNpc, indicating that these
two behavioral tests together with APO-induced rotations may be an
applicable evaluation index for PD-associated behaviors induced by
6-OHDA.
Discussion
The customary model of PD may be established by the
injection of 6-OHDA into one of the following three targets: SNpc,
medial forebrain bundle or the neostriatal region. The latter is
established to be an appropriate site for establishment of a slow
progressive PD animal model (7).
Although the 6-OHDA lesion PD rat or mouse model has been widely
used for decades (17–20), the present study, to the best of
our knowledge, was the first to demonstrate, over a long time
period, the correlation between progressive nigrostriatal
neurodegeneration and the degree of APO-induced and
non-drug-induced behavioral impairments, however, no correlation
was observed with morphological glial reactivity in the SNpc of the
rat model. The present study indicates relatively stable
time-points for therapeutic interventions and also provides a
system for evaluation of the PD rat model even when APO is not
available.
In the current study, the injection site of the
striatum was selected as it is the neostriatum area in the rat that
is the specific affected field in the human brain that results in
the PD-associated motor deficits and the progression of PD
pathology (7). It is widely
accepted that with the neurotoxin-induced neuronal degeneration,
the DA receptors on the injected side of the striatum become
relatively hypersensitive to stimulation by its ligands. APO, a DA
agonist, stimulates the receptors causing a higher activity in the
striatum on the injected side relative to the non-injected side,
ultimately inducing a rotation of the body turning contralateral
from the lesioned side (7). The PD
model is well-recognized by the characteristic rotational behavior
of rodents administrated with dopaminomimetics. This drug-induced
rotational behavior is associated with the degree of the
nigrostriatal TH+ protein loss (7). Thus, the APO-induced rotations (60
turns/30 min) is considered the criterion to identify the rat model
of PD in this experiment.
In addition, to thoroughly assess the 6-OHDA rat
model of PD, other non-drug-induced behavioral tests, including
cylinder, rotarod and open field tests, were investigated as
symptomatic parkinsonian signs of the rat model. The number of
APO-induced contralateral rotations during a 30 min testing period
sharply increased between weeks 2 and 3 following surgery, whereas
rotation number remained stable between weeks 4 and 6. This
phenomenon was consistent with the results of cylinder and rotarod
tests (Fig. 1). Previous studies
have demonstrated that 6-OHDA injection into the striatum led to a
protracted retrograde DA neuron-specific degeneration, which
usually occurs after 1–3 weeks (10,21–23).
In the present study, the survival of DA neurons in the SNpc of the
rat model decreased progressively (Fig. 2). When linear regression analysis
was performed, APO-induced rotations exhibited a negative
correlation with the survival of DA neurons, which was also
positively correlated with latency to fall from rotarod and
negatively correlated with paw preference (asymmetry) on the
cylinder test. Therefore, we hypothesized that the 6-OHDA model may
be evaluated by one or all of the tests that exhibited a
significant correlation with the survival of DA neurons, according
to experimental conditions.
For the open field test, several parameters,
including total movement distance (cm), velocity (cm/sec), rest
time (sec), moves (times) and center entries (times), were observed
as these parameters were reported to represent the locomotor
activity and anxiety behaviors of PD rats (7,24).
Locomotor activity has also been reported to be associated with
alterations in DA transmitter function, for example, decreased DA
levels may lead to reduced locomotion (7). In the present study, a reduced level
of locomotor activity was observed in the PD rats. Therefore, it is
clear that the spontaneous movements associated with alterations in
DA transmitter function of PD rats may be estimated using the open
field test.
In terms of the association between PD-associated
pathology and behavior, the results indicate that the extent of
nigral DA neuron loss in the SNpc may be predicted through both
drug-induced rotations and non-drug-induced movement behaviors.
Consistently, the TH+ fiber density in the striatum of
the PD rat model also decreased in a slow progressive pattern
(Fig. 2). Consistent with the
results of other studies that employed injection of 6-OHDA into the
striatum, where striatal terminal damage was observed within 1 day
of injection and SNpc neuron loss was minimal until 1 week later,
reaching a maximum within 2–3 weeks (10,21,25),
the present study demonstrated a slowly developing partial lesion
of the nigrostriatal pathway induced by 6-OHDA. Compared with other
studies (18,25), a little difference in the
time-point of the lesion of nigral neuron was observed, i.e., it
exhibited swift changes from week 2 to week 3 maintained kept a
stable state from week 3 to week 6. The detectable discrepancy may
result from diverse injection sites in the striatum.
Glial responses are considered to exhibit dual
effects in the process of neurodegeneration. The activation of
glial cells may benefit DA neurons at early stages of PD, however,
the excessive continued activation of glia was reported to be
harmful to the neurons (16,26,27).
In the present study, the activation of astrocytes and microglia
was observed throughout the impairment of the nigrostriatal pathway
in the SNpc (Fig. 3). Notably,
although the continued lesion of DA neurons was observed during the
period of observation, the survival of TH+ neurons
within the SNpc was not correlated with the glial activation in the
SNpc. Considering the crucial role of glial responses, particularly
microglial activation in PD pathogenesis (16), the correlation data in the present
study indicate that the abnormal activation of glia cells was not
linked with loss of dopaminergic neurons induced by 6-OHDA, and
other pathways such as cytokines or inflammatory factors may also
be involved in the process, which may not be associated with
morphological microglial activation (28).
In conclusion, it is of great importance to
formulate a standard index of key characteristics of the 6-OHDA
lesion PD model for assessing novel drugs, developing novel
treatment strategies and understanding the nature of the pathogenic
processes of PD. The results of the current study may be useful in
appraising the potential of novel treatment strategies for PD and
in investigating the mechanisms associated with functional recovery
of dopaminergic neurons in the PD brain.
Acknowledgements
The present study was supported by the Chinese
National Basic Research Program (grant no. 2011CB504100), the
Important National Science and Technology Specific Project (grant
no. 2011ZX09102-003-01) and the Projects Under Beijing Municipality
(grant no. IDHT20140514). The authors thank Ms Min Sun and Ms
Xiaoli Gong (Department of Neurobiology, Capital Medical
University, Beijing, China) for their technical support.
References
|
1
|
Redgrave P, Vautrelle N and Reynolds JN:
Functional properties of the basal ganglia's re-entrant loop
architecture: Selection and reinforcement. Neuroscience.
198:138–151. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Su X and Federoff HJ: Immune responses in
Parkinson's disease: Interplay between central and peripheral
immune systems. Biomed Res Int. 2014:2751782014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jackson-Lewis V, Blesa J and Przedborski
S: Animal models of Parkinson's disease. Parkinsonism Relat Disord.
18 Suppl 1:S183–S185. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ungerstedt U: 6-Hydroxy-dopamine induced
degeneration of central monoamine neurons. Eur J Pharmacol.
5:107–110. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Blandini F, Armentero MT and Martignoni E:
The 6-hydroxydopamine model: News from the past. Parkinsonism Relat
Disord. 14 Suppl 2:S124–S129. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee CS, Sauer H and Bjorklund A:
Dopaminergic neuronal degeneration and motor impairments following
axon terminal lesion by instrastriatal 6-hydroxydopamine in the
rat. Neuroscience. 72:641–653. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bové J and Perier C: Neurotoxin-based
models of Parkinson's disease. Neuroscience. 211:51–76. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Meredith GE and Kang UJ: Behavioral models
of Parkinson's disease in rodents: A new look at an old problem.
Mov Disord. 21:1595–1606. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Paxinos G and Watson C: The Rat Brain in
Stereotaxic Coordinates. Academic Press; 2005
|
|
10
|
Przedborski S, Levivier M, Jiang H,
Ferreira M, Jacksonlewis V, Donaldson D and Togasaki DM:
Dose-dependent lesions of the dopaminergic nigrostriatal pathway
induced by intrastriatal injection of 6-hydroxydopamine.
Neuroscience. 67:631–647. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Monville C, Torres EM and Dunnett SB:
Comparison of incremental and accelerating protocols of the rotarod
test for the assessment of motor deficits in the 6-OHDA model. J
Neurosci Methods. 158:219–223. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Landers MR, Kinney JW and van Breukelen F:
Forced exercise before or after induction of 6-OHDA-mediated
nigrostriatal insult does not mitigate behavioral asymmetry in a
hemiparkinsonian rat model. Brain Res. 1543:263–270. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yuan HL, Li B, Xu J, Wang Y, He Y, Zheng Y
and Wang XM: Tenuigenin protects dopaminergic neurons from
inflammation-mediated damage induced by the lipopolysaccharide. CNS
Neurosci Ther. 18:584–590. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
van der Perren A, Macchi F, Toelen J,
Carlon MS, Maris M, de Loor H, Kuypers DR, Gijsbers R, Van den
Haute C, Debyser Z and Baekelandt V: FK506 reduces
neuroinflammation and dopaminergic neurodegeneration in an
α-synuclein-based rat model for Parkinson's disease. Neurobiol
Aging. 36:1559–1568. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sanchez-Guajardo V, Febbraro F, Kirik D
and Romero-Ramos M: Microglia acquire distinct activation profiles
depending on the degree of alpha-synuclein neuropathology in a rAAV
based model of Parkinson's disease. PLoS One. 5:e87842010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
McGeer PL and McGeer EG: Glial reactions
in Parkinson's disease. Mov Disord. 23:474–483. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mendez JS and Finn BW: Use of
6-hydroxydopamine to create lesions in catecholamine neurons in
rats. Neurosurg. 42:166–173. 1975. View Article : Google Scholar
|
|
18
|
Stott SR and Barker RA: Time course of
dopamine neuron loss and glial response in the 6-OHDA striatal
mouse model of Parkinson's disease. Eur J Neurosci. 39:1042–1056.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schlachetzki JC, Marxreiter F,
Regensburger M, Kulinich A, Winner B and Winkler J: Increased
tyrosine hydroxylase expression accompanied by glial changes within
the non-lesioned hemisphere in the 6-hydroxydopamine model of
Parkinson's disease. Restor Neurol Neurosci. 32:447–462.
2014.PubMed/NCBI
|
|
20
|
Walsh S, Finn DP and Dowd E: Time-course
of nigrostriatal neurodegeneration and neuroinflammation in the
6-hydroxydopamine-induced axonal and terminal lesion models of
Parkinson's disease in the rat. Neuroscience. 175:251–261. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sauer H and Oertel WH: Progressive
degeneration of nigrostriatal dopamine neurons following
intrastriatal terminal lesions with 6-hydroxydopamine: A combined
retrograde tracing and immunocytochemical study in the rat.
Neuroscience. 59:401–415. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Marti MJ, Saura J, Burke RE, Jackson-Lewis
V, Jiménez A, Bonastre M and Tolosa E: Striatal 6-hydroxydopamine
induces apoptosis of nigral neurons in the adult rat. Brain Res.
958:185–191. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Marti MJ, James CJ, Oo TF, Kelly WJ and
Burke RE: Early developmental destruction of terminals in the
striatal target induces apoptosis in dopamine neurons of the
substantia nigra. J Neurosci. 17:2030–2039. 1997.PubMed/NCBI
|
|
24
|
Prut L and Belzung C: The open field as a
paradigm to measure the effects of drugs on anxiety-like behaviors:
A review. Eur J Pharmacol. 463:3–33. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Blandini F, Levandis G, Bazzini E, Nappi G
and Armentero MT: Time-course of nigrostriatal damage, basal
ganglia metabolic changes and behavioural alterations following
intrastriatal injection of 6-hydroxydopamine in the rat: New clues
from an old model. Eur J Neurosci. 25:397–405. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mosley RL, Benner EJ, Kadiu I, Thomas M,
Boska MD, Hasan K, Laurie C and Gendelman HE: Neuroinflammation,
oxidative stress and the pathogenesis of Parkinson's disease. Clin
Neurosci Res. 6:261–281. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vivekanantham S, Shah S, Dewji R, Dewji A,
Khatri C and Ologunde R: Neuroinflammation in Parkinson's disease:
Role in neurodegeneration and tissue repair. Int J Neurosci.
125:717–725. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Depino AM, Earl C, Kaczmarczyk E, Ferrari
C, Besedovsky H, del Rey A, Pitossi FJ and Oertel WH: Microglial
activation with atypical proinflammatory cytokine expression in a
rat model of Parkinson's disease. Eur J Neurosci. 18:2731–2742.
2003. View Article : Google Scholar : PubMed/NCBI
|