Introduction
Despite advances in the treatment of coronary artery
disease, acute myocardial infarction (AMI) remains the leading
cause of human mortality worldwide (1). AMI is characterized by a sudden
reduction in blood and oxygen supply to the heart, irreversible
muscle damage and cardiomyocyte death, resulting in the formation
of an infarct zone containing nonfunctional myocytes, which are
remodeled into scar tissue (2).
The limited ability of the damaged heart to regenerate the damaged
myocardium leads to the progression of cardiac decompensation and
heart failure (3). AMI causes the
death of a majority of cardiomyocytes which are replaced by
fibrotic tissue, leading to cardiac malfunction. Unlike myocardial
muscle, fibrotic tissue fails to contract and therefore is unable
to pump blood to the whole body (4,5).
Therefore, a reduction in fibrosis and cardiomyocyte death is
necessary to repair the infarcted heart following AMI (6). Additionally, the death of
cardiomyocytes results in fibrous hyperplasia. Therapeutic
strategies for abating cardiomyocyte death are necessary to delay
or prevent the onset of fibrosis and heart failure following
AMI.
Recent studies have identified a linear association
between mitochondrial fate and cardiac dysfunction (7,8).
Mitochondria may be associated with cardiomyocyte survival and,
therefore, their contribution has a marked impact on the repair of
the infarcted heart post-AMI (9,10). A
growing body of evidence suggests that cardiomyocytes require
well-structured mitochondria to sustain the cardiac function in the
post-infarcted heart; this process requires well-orchestrated
mitophagy to maintain mitochondrial quantity and quality (11). However, an imbalance in the
mitochondrial degradation process results in alterations in
cellular homeostasis which, in turn, may aggravate cardiomyocyte
death and the development of cardiac dysfunction (12,13).
These previous studies indicated that mitophagy may be a potential
target for repairing the infarcted heart following AMI. In yeast
and mammals, three receptors have been identified to activate
mitophagy: Parkin, FUN14 domain-containing protein 1 (FUNDC1) and
BCL2/adenovirus E1B 19 kDa protein-interacting protein 3 (Bnip3).
Previous studies have established the role of FUNDC1 and Bnip3 in
cardiac injury (14,15). However, the role of Parkin in
chronic cardiac damage remains unclear. Parkin is activated by
serine/threonine-protein kinase PINK1 and primarily exerts its
influence in neurological disorders (16). Parkin is able to interact with
damaged mitochondria and traffic them to the lysosome (17). These previous data illustrate that
Parkin-mediated mitophagy is important for the cellular regulation
of steady-state mitochondrial turnover, and determines the number
of healthy mitochondria. Accordingly, the identification of a novel
drug to activate Parkin-mediated mitophagy may provide increased
beneficial effects for patients following AMI.
Liraglutide is an antidiabetic agent which reduces
hyperglycemia through increased glucose-dependent insulin
secretion, glucagon suppression, delayed gastric emptying and
appetite suppression (18–21). It has been demonstrated that
liraglutide is important for cardioprotection, including reducing
infarct size, improving the left ventricular ejection fraction and
reversing cardiac remodeling (22–24).
However, whether liraglutide protects against cardiomyocyte death
and repairs the post-infarction heart via mitophagy remains
unclear, in addition to the potential molecular links between
liraglutide and mitophagy.
NAD-dependent protein deacetylase sirtuin 1 (SIRT1)
is one of the seven mammalian homologs (SIRT1-SIRT7) of the yeast
silent information regulator 2 (25). SIRT1 is an
NAD+-dependent protein deacetylase which has been
reported to serve a number of roles in cells, including longevity,
apoptosis, DNA repair, inflammation and mitochondrial regulation
(26–28). The regulatory effect of SIRT1 on
the activation of mitophagy has gained attention, although the
underlying mechanisms have not been completely elucidated. Notably,
a previous study demonstrated that liraglutide was able to activate
SIRT1 to trigger autophagy, ameliorating non-alcoholic fatty liver
disease (29). These previous data
suggest a potential association between liraglutide and SIRT1.
Therefore, the present study posited and analyzed the following
three hypotheses: i) Whether liraglutide has the ability to repair
or improve the infarcted heart following AMI; ii) whether
Parkin-mediated mitophagy is responsible for the protective action
of liraglutide on the injured heart; and iii) whether liraglutide
regulates mitophagy via SIRT1 in cardiomyocytes.
Materials and methods
Ethics statement
The present study was performed in accordance with
the Declaration of Helsinki and the guidelines of the Ethics
Committee of No. 1 People's Hospital (Suzhou, China). All
experimental protocols were approved by the Ethics Committee of
No.1 People's Hospital. The experiments on animals were performed
in accordance with the Guide to the Care and Use of Experimental
Animals (Vol. 1, 2nd ed., 1993; and Vol. 2, 1984; Canadian Council
on Animal Care, Ottawa, ON, Canada; www.ccac.ca), or
the Guide for the Care and Use of Laboratory Animals (1996;
National Academy Press, Washington, DC, USA).
Myocardial infarction model
Male Sprague-Dawley rats (n=150, 8–10 weeks, weight,
250±10 g) were purchased from the Experimental Animal Center,
Academy of Military Medical Science and were housed under standard
laboratory conditions (27°C, 40–60% humidity, a 12-h light and dark
cycle). A commercial pellet diet and fresh drinking water were
given ad libitum. Rats were randomly divided into the following
groups, with n=6 in each: i) Sham group; ii) PBS; iii) low dose of
liraglutide; and iv) high dose of liraglutide. Rats were
intraperitoneally anaesthetized with sodium pentobarbital (30
mg/kg). The animals were subsequently incubated and ventilated with
a volume-regulated respirator during surgery. Subsequent to a left
lateral thoracotomy and pericardectomy, the left coronary artery
was identified and ligated with a 6.0 prolene suture. Freshly
prepared liraglutide (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) was administered via the caudal vein at doses of 0.09
mg/kg (low dose group) or 0.18 mg/kg (high dose group) for 28
successive days post-infarction. These concentrations of drugs were
selected as they were previously reported to be effective in
reducing cardiac damage (10).
Sample preparation and histological
analysis
The hearts were excised and rapidly frozen in
Optimal Cutting Temperature medium at −20°C (Agar Scientific, Ltd.,
Stansted, UK) for the preparation of frozen sections (4-µm
thickness). A total of 10 sections were prepared at 10 different
transversal levels at the site of tissue necrosis, equally
distributed from base to apex. Sirius red staining was performed at
room temperature in sections to quantify the cardiac fibrosis and
was observed using an inverted microscope (magnification, ×40;
BX51; Olympus Corp., Tokyo, Japan). The degrees of collagen fiber
accumulation in the infarcted area were evaluated by measuring the
fluorescence density of fibrotic region in the left ventricular
area, which was calculated using RS Image Pro, version 4.5 (Media
Cybernetics, Inc., Rockville, MD, USA). Terminal
deoxynucleotidyl-transferase-mediated dUTP nick end labeling
(TUNEL) staining was performed to assess cellular apoptosis
according to the protocol described below. The degree of apoptosis
was calculated as the number of TUNEL-positive cells per 500
cellular nuclei. The nuclei were stained with the chromatin dye
DAPI. Briefly, cells were fixed for 1 h in 4% (w/v)
paraformaldehyde at room temperature. Following labeling with TUNEL
for ~30 min at room temperature, the cells were exposed to DAPI in
the dark for 5 min. Then, the stained cells were observed using an
inverted microscope (magnification, ×100; BX51; Olympus Corp.). In
order to determine the inflammatory response, heart sections were
stained with anti-tumor necrosis factor (TNF) α
(1:1,000; Cell Signaling Technology, Inc., Danvers, MA, USA; cat.
no. 6945). In brief, samples were fixed with 4% paraformaldehyde
for 10 min at room temperature, permeabilized with 0.3% Triton
X-100 for 5 min, and blocked with 10% goat serum albumin
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) for
1 h at room temperature. Specimens were subsequently incubated with
transforming growth factor (TGF) β (1:1,000; Cell
Signaling Technology, Inc.; cat. no. 3711) overnight at 4°C, then
washed with PBS three times, and incubated with horseradish
peroxidase AffiniPure Goat Anti-Rabbit secondary antibody (1:2,000;
Beyotime Institute of Biotechnology, Haimen, China; cat. no. A0277)
for 45 min at room temperature. Images were captured using an
inverted microscope (magnification, ×40; BX51; Olympus Corp.).
Chronic hypoxia model in vitro
In vitro, chronic hypoxia of cardiomyocytes
was used to mimic the infarcted heart following AMI. Hypoxic
conditions were produced using fresh Hank's solution (Beyotime
Institute of Biotechnology) with 95% N2 and 5%
CO2. The dishes (1×106 cells) were placed
into a hypoxic incubator at 37°C, that was equilibrated with 95%
N2 and 5% CO2 and the actual oxygen
concentration was 0. Ambient O2 levels in the hypoxic
incubator were monitored with an O2 analyzer (series-2000; Alpha
Omega Instruments, Lincoln, RI, USA). Chronic hypoxia of
cardiomyocytes cell line H9C2 (purchased from the American Type
Culture Collection, Manassas, VA, USA) was performed for ~48 h. For
liraglutide treatment, H9C2 were treated with liraglutide (0–50 nM)
for 48 h in the presence of hypoxia. To activate SIRT1,
pretreatment with SRT1720 (SRT; 10 µM; Sigma-Aldrich; Merck KGaA)
was performed for ~4 h. To inhibit the SIRT1, Selisistat (10 µM;
Sigma-Aldrich; Merck KGaA) was used for 6 h. To suppress the role
of liraglutide, Exendin 9–39 (Ex9-39; 10 nM; Sigma-Aldrich; Merck
KGaA) was used to block the glucagon-like peptide 1 receptor
(GLP1R) under hypoxic conditions.
Immunofluorescence staining
The 1×106 cells were seeded into the
dishes then were washed in PBS and permeabilized for 10 min at 4°C
in a solution of 0.1% Triton X-100 and 0.1% sodium citrate in PBS.
Then, samples were blocked with 10% goat serum albumin for 1 h at
room temperature and subsequently were incubated with primary
antibodies against overnight at 4°C (30). Following three rinses with PBS, the
secondary antibody, Alexa Fluor 488 donkey anti-rabbit antibody
(1:1,000; cat. no. A-21,206; Invitrogen; Thermo Fisher Scientific,
Inc.) was added to the samples for 1 h at room temperature. The
primary antibodies used in the present study were as follows:
Mitochondrial import receptor subunit TOM20 homolog (1:1,000;
Abcam, Cambridge, UK; cat. no. ab78547), lysosome-associated
membrane glycoprotein 1 (1:1,000; Abcam; cat. no. ab24170),
cytochrome-c (1:1,000; cyt-c; Abcam; cat. no. ab133504), Parkin
(1:1,000; Cell Signaling Technology, Inc.; cat. no. 2132) and Sirt1
(1:1,000; Cell Signaling Technology, Inc.; cat. no. 9475). Images
were taken using an inverted microscope (magnification, ×40; BX51;
Olympus Corp.).
Western blotting
Cells were washed with PBS and lysed in Laemmli
Sample Buffer (Bio-Rad Laboratories, Inc., Hercules, CA, USA), and
further homogenized with a rotor-stator homogenizer. Proteins were
isolated and concentrations were determined using the Bicinchoninic
Acid Protein Assay kit (Thermo Fisher Scientific, Inc.) (31). A mass of 20–80 µg proteins was
loaded on a 12–15% SDS-PAGE gel. Following electrophoresis,
proteins were transferred to a polyvinylidene fluoride western
blotting membrane (Roche Applied Science, Penzberg, Germany).
Membranes were blocked with 5% nonfat dried milk [in TBS-Tween 20
(TBST)] for 2 h at room temperature and incubated overnight at 4°C
with primary antibodies. The membrane was subsequently washed with
TBST (5 min; three times) and incubated with horseradish
peroxidase-conjugated secondary antibodies (Cell Signaling
Technology, Inc.) for 1 h at room temperature. Following washing
with TBST (5 min; three times), bands were detected using an
enhanced chemiluminescence substrate (Applygen Technologies, Inc.,
Beijing, China). Band intensities were normalized to the respective
internal standard signal intensity (β-actin antibody;
1:2,000; Abcam; cat. no. ab8224). The experiment was repeated three
times. The primaries antibodies used in the present study were
against the following proteins: Pro-caspase3 (1:1,000; Cell
Signaling Technology, Inc.; cat. no. 9662), cleaved caspase3
(1:1,000; Cell Signaling Technology, Inc.; cat. no. 9664),
baculoviral IAP repeat-containing protein 2 (c-IAP1; 1:2,000; Cell
Signaling Technology, Inc. cat. no. 7065), caspase9 (1:1,000;
Abcam; cat. no. ab32539), Parkin (1:1,000; Cell Signaling
Technology, Inc.; cat. no. 2132), microtubule-associated protein
light chain (LC) 3II (1:1,000; Cell Signaling Technology, Inc.;
cat. no. 3868), sequestome-1 (p62; 1:1,000; Abcam; cat. no.
ab56416), Beclin1 (1:1,000; Cell Signaling Technology, Inc.; cat.
no. 3495), autophagy protein 5 (Atg5; 1:1,000; Cell Signaling
Technology, Inc.; cat. no. 12,994), Sirt1 (1:1,000; Cell Signaling
Technology, Inc., cat. no. 9475), TGFβ (1:1,000; Cell
Signaling Technology, Inc.; cat. no. 3711), matrix
metalloproteinase (MMP) 9 (1:1,000; Cell Signaling Technology,
Inc.; cat. no. 13667) and poly (ADP ribose) polymerase 1 (PARP;
1:1,000; Cell Signaling Technology, Inc.; cat. no. 9532) The mean
densities of the bands were represented as the optical density in
units/mm2 and normalized to that of β-actin
(Quantity One, version 4.6.2; Bio-Rad Laboratories, Inc.).
Reactive oxygen species (ROS)
detection, JC-1 staining and adenosine 5′-triphosphate (ATP)
detection
Cells were incubated in serum-free Dulbecco's
modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific,
Inc.) containing dichloro-dihydro-fluorescein diacetate (10 µM) for
30 min (32). Cells were
subsequently washed with PBS. Flow cytometric analyses were
performed using a BD FACSCalibur™ flow cytometer (BD Biosciences,
Franklin Lakes, NJ, USA) using BD CellQuest Pro software (version
5.1; BD Biosciences).
The mitochondrial potential was assessed using the
probe JC-1, a sensitive fluorescent dye used to detect alterations
in mitochondrial potential. Following treatment, cells were
incubated with 10 mg/ml JC-1 for 10 min at 37°C in the dark and
monitored with a fluorescence microscope (magnification, ×100;
BX51; Olympus Corp.) (33).
Red-orange fluorescence was attributable to a potential-dependent
aggregation in the mitochondria. Green fluorescence, reflecting the
monomeric form of JC-1, appeared in the cytosol following
mitochondrial membrane depolarization.
The level of ATP in cells was determined using an
ATP Bioluminescence Assay kit (Beyotime Institute of Biotechnology)
Harvested cultured cells (1×106) were lysed with a PBS
(Sigma-Aldrich; Merck KGaA), followed by centrifugation at 10,000 ×
g for 2 min at 4°C. The level of ATP was determined by mixing 50 µl
supernatant with 50 µl luciferase reagent, which catalyzed the
light production from ATP and luciferin. The emitted light was
linearly compared with the ATP concentration and measured using a
microplate luminometer.
MTT and TUNEL assays
MTT experiments were performed in 96-well plates.
The cells were applied to the scaffold at a density of
104 cells/well. Following 2–3 days of culture, the
samples were washed 3 times with PBS, and 50 µl MTT was added to
each well. The samples were subsequently incubated for 4 h at 37°C
in a humid atmosphere containing 5% CO2. The MTT
solution was removed and 200 µl dimethyl sulfoxide was added to
each sample and incubated for 10 min. Following the addition of
Sorensen's buffer, the absorbance was determined at a wavelength of
570 nm (34).
A TUNEL assay to detect DNA fragmentation in the
cell nuclei was performed using an In Situ Cell Death
Detection kit (Roche Diagnostics GmbH, Mannheim, Germany) (35), according to the manufacturer's
protocol. DAPI was used to label the nuclei (at room temperature
for ~30 min). The results are expressed as apoptotic cells per
tubule cross-section.
ELISA analysis
Cells (1×106) in the treatment groups
were lysed, centrifuged for 10 min at 1,600 × g at 4°C, and the
supernatant (10 µl) was mixed with the pre-mixed reagent (according
to the manufacturer's protocol) using a vortex mixer. This mixture
was placed in a thermostatic water bath for 20 min at 37°C. The
mixture was incubated with the color-developing agent for 10 min at
room temperature. The absorbance value was determined at 560 nm
using a microplate reader. The superoxide dismutase (SOD),
glutathione (GSH) and malondialdehyde (MDA) levels were calculated
using the standard formula, according to the protocols of the
commercially-available ELISA kits (Beyotime Institute of
Biotechnology, Haimen, China; cat. nos. S0086, S0131 and S0058,
respectively).
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
Total RNA was isolated from cells using TRIzol™
reagent (Invitrogen; Thermo Fisher Scientific, Inc.). A total of 1
µg RNA from each sample was reverse-transcribed into cDNA using an
RT kit (Eurogentec, Liege, Belgium) (36). The qPCR was performed with primers
and matched probes from the Universal Fluorescence-labeled Probe
Library (Roche Diagnostics GmbH). Quantification of gene expression
was performed using an ABI PRISM 7500 Sequence Detection system
(Applied Biosystems; Thermo Fisher Scientific, Inc.) with
SYBR® Green (Beiijing Transgen Biotech Co., Ltd.,
Beijing, China). The relative mRNA expression levels were
normalized to that of β-actin using the
2−ΔΔCq method (12).
The thermocycling conditions were as follows: 95°C for 10 min,
followed by 40 cycles of 95°C for 15 sec and 72°C for 35 sec, for
PCR. The experiments were repeated three times with triplicates.
The primers used in the present study were as follows: Interleukin
(IL) 6, forward primer 5′-TCACCGATGTCTACCTGCTG-3′, reverse primer
5′-CACAGGGTTGAGCCAAAAGT-3′, C-C motif chemokine 2 (MCP1), forward
primer 5′-CTCAACATCATGAAGGTCTC-3′, reverse primer
5′-GGCATTCAGTTCCAGGTCAG-3′, and GAPDH, forward primer
5′-ACGACATAGACGGCATCCA-3′, reverse primer
5′-GCTGTGGTTCAGTTGTGGTG-3′.
Caspase3/9 activity and lactate
dehydrogenase (LDH) release assay
Caspase-3/9 activity kits (Beyotime Institute of
Biotechnology) were used, according to the manufacturer's protocols
(34). The relative caspase-3/9
activity was calculated from the ratio of treated cells to
untreated cells (1×106). The assays were repeated three
times.
LDH is a fairly stable enzyme that is released from
the cytosol into the culture medium as a consequence of cellular
integrity damage. Thus, we used an LDH assay (Beyotime Institute of
Biotechnology) to evaluate the presence of cell injury or damage.
The level of LDH released was expressed as a percentage of the
control group.
Small RNA interference assay
In order to evaluate the functional role of Parkin,
small interfering (si)RNA was used to reduce its expression. The
selective siRNA duplex (5′-GAGGAUGACAACGACAUAATT-3′, antisense,
5′-UUAUGUCGUUGUCAUCCUCTT-3′) and a nonspecific control duplex
(5′-UUCUCCGAACGUGUCACGUTT-3′; antisense,
5′-ACGUGACACGUUCGGAGAATT-3′). These were obtained from YangZhou
Ruibo Biotech Co., Ltd. (Yangzhou, China). Transfection of siRNA
into cells was performed using Lipofectamine® 2000
transfection reagent (Thermo Fisher Scientific, Inc.), according to
the manufacturer's protocol (37).
Cultured cells were washed with Opti-Minimal Essential Medium
(Invitrogen; Thermo Fisher Scientific, Inc.) without serum or
antibiotics and seeded in 6-well plates to 30–40% confluence. The
transfection reagent and siRNA were diluted separately in
serum-free medium, mixed and incubated for 10 min at room
temperature to form the siRNA/lipid complex. This complex was added
to each well at a final concentration of 70 nM/well siRNA. At 48 h
post-transfection, cells were harvested to determine Parkin protein
expression levels by western blot analysis according to the
protocol described above.
Statistical analysis
All data are expressed as the mean ± standard
deviation. Statistical analyses were performed with SPSS software
(version 17.0; SPSS, Inc., Chicago, IL, USA). Statistical
significance between two groups was determined by Student's t-test.
Results for more than two groups were evaluated by one-way analysis
of variance with the least significant difference test. P<0.05
was considered to indicate a statistically significant
difference.
Results
Liraglutide reduces fibrosis,
inflammatory responses and cardiomyocyte apoptosis in the
post-infarcted heart
To examine the protective role of liraglutide in
repairing the infarcted heart, fibrosis and cardiomyocytes death
were assessed. Compared with the control group, the post-infarcted
heart (28 days following myocardial infarction) exhibited extensive
fibrosis, as evidenced by Sirius red staining (Fig. 1A and B). However, liraglutide had
the ability to reduce cardiac fibrosis in a dose-dependent manner.
To provide further evidence for the role of liraglutide in cardiac
fibrosis in the post-infarcted heart, the expression of TGFβ and
MMP9 was examined via western blotting. TGFβ and MMP9 are key
feature of fibrosis. Compared with the control group, the
post-infarcted heart exhibited more TGFβ and MMP9 expression
(Fig. 1C-E). However, these
phenotypic alterations were rescued by liraglutide in a
concentration-dependent manner.
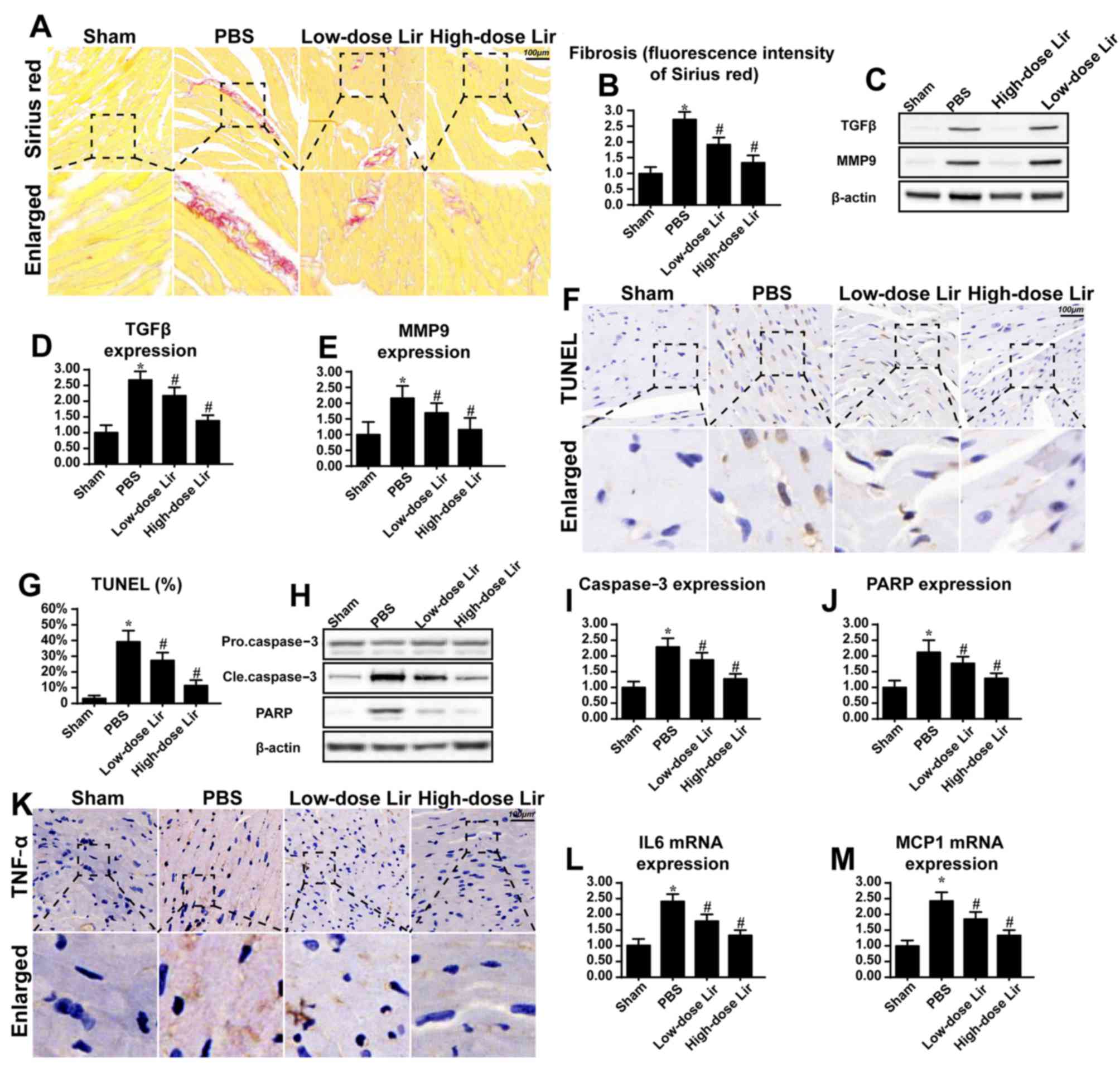 | Figure 1.Liraglutide repairs the infarcted
heart. Chronic heart damage was induced via myocardial infarction.
Freshly prepared liraglutide was administered via the caudal vein
at doses of 0.09 mg/kg (low-dose group) or 0.18 mg/kg (high-dose
group) for 28 successive days post-infarction. (A) Cardiac fibrosis
was evaluated via Sirius red staining and (B) the results were
quantified. Liraglutide was applied at high (0.18 mg/kg) and low
(0.09 mg/kg) dosages. (C) Western blotting was used to evaluate the
expression of (D) TGFβ and (E) MMP9, which are key feature of
cardiac fibrosis following myocardial infarction. (F) The TUNEL
assay was used to evaluate the apoptotic rate of heart 28 days
after post-infarction, and (G) the results were quantified. (H-J)
The alteration in apoptotic protein expression was assessed via (H)
western blotting in the heart tissue at 28 days post-infarction,
and the expression levels of (I) caspase3 and (J) PARP were
analyzed by densitometry. (K) Immunohistochemical analysis of TNFα
was applied to evaluate the cardiac inflammatory response. The
alteration in (L) IL-6 and (M) MCP1 mRNA expression was assessed.
Magnification, ×40. *P<0.05 vs. sham group;
#P<0.05 vs. PBS group. Lir, liraglutide; TUNEL,
terminal deoxynucleotidyl-transferase-mediated dUTP nick end
labeling; TGFβ, transforming growth factor-β;
MMP9, matrix metalloproteinase 9; Cle, cleaved; PARP, poly (ADP
ribose) phosphate polymerase 1; IL-6, interleukin-6; MCP1, C-C
motif chemokine 2; TNFα, tumor necrosis
factor-α. |
Since cardiomyocyte death is the primary reason for
the development of cardiac fibrosis and heart failure following
myocardial infarction, a TUNEL assay was used to observe
cardiomyocyte apoptosis. As presented in Fig. 1F and G, the post-infarcted heart
had an increased number of TUNEL-positive cells. However, treatment
with liraglutide was able to reduce the ratio of TUNEL-positive
cells. In order to provide further evidence for cellular death
during cardiac remodeling, apoptotic protein expression was
detected. Compared with the control group, caspase3 and its primary
cleavage target PARP were increased in the post-infarcted heart
(Fig. 1H-J), indicative of
myocardial death in the post-infarcted heart. However, these
alterations were reversed by treatment with liraglutide.
Apart from fibrosis and cellular death, the present
study additionally examined the inflammatory response which is
associated with cardiomyocyte death and fibrosis accumulation.
Compared with the control group, increased expression of TNFα was
observed in the myocardial tissue in the post-infarcted heart
(Fig. 1K). Additionally, the
transcriptional levels of IL6 and MCP1 were increased in the
post-infarcted heart (Fig. 1L-M).
However, treatment with liraglutide was able to repress the
expression of the inflammatory markers. These data suggested that
liraglutide had the ability to repair the damaged heart following
myocardial infarction.
Liraglutide sustains H9C2 survival by
abating mitochondrial apoptosis
To gain an insight into the mechanism through which
liraglutide maintained cardiomyocyte viability, a chronic hypoxia
model with H9C2 cells was used to mimic the infarcted heart, and
the molecular signaling responsible for the anti-apoptotic
properties of liraglutide was examined (Fig. 2). Following 48 h of hypoxia
stimulation, H9C2 apoptosis was detected via MTT and LDH release
assays. Chronic hypoxia reduced cell viability, as evidenced by a
reduced MTT value (Fig. 2A) and
increased LDH release (Fig. 2B).
However, treatment with liraglutide was able to inhibit the
apoptotic signaling in a dose-dependent manner. Considering that
the minimum protective concentration of liraglutide was 5 nM, this
concentration was used in the following experiments. To further
quantify H9C2 apoptosis under chronic hypoxia, a TUNEL assay was
used. It was observed that hypoxia increased the number of
TUNEL-positive cells (Fig. 2C and
D), which was inhibited by liraglutide. Since liraglutide, a
type of GLP1R agonist, exerts protective action on cells via GLP1R,
Ex9-39 was used to block the GLP1R. Following the blockade of
GLP1R, the protective effect of GLP1 on H9C2 apoptosis disappeared
(Fig. 2C and D). In order to
investigate whether mitochondrial apoptosis was involved in H9C2
apoptosis, cyt-c was assayed. In response to mitochondrial
apoptosis, cyt-c was released from the mitochondria into the
cytoplasm, where it interacts with caspase9 and activates caspase3.
Therefore, immunofluorescence was used to observe the cyt-c
cellular location. In the normal cells, cyt-c was observed in the
cellular cytoplasm (Fig. 2G).
However, hypoxia induced the diffusion of cyt-c into the cytoplasm
and nucleus (Fig. 2G), indicating
the activation of mitochondrial apoptosis. By contrast, liraglutide
had the ability to reverse such alterations. Notably, the GLP1R
blocker was able to reverse the inhibitory action of liraglutide on
cyt-c diffusion.
 | Figure 2.Liraglutide reduces cardiomyocyte
death via inhibition of mitochondrial apoptosis. The hypoxia model
of H9C2 in vitro was used to mimic chronic cardiac injury.
H9C2 cells were cultured under hypoxic condition for ~48 h. (A) MTT
and (B) LDH assays were used to detect the cell viability.
Liraglutide was able to sustain H9C2 viability in a dose-dependent
manner under 48 h of hypoxia. *P<0.05 vs. Ctrl group;
#P<0.05 vs. Lir (0 nM) group. Since the minimum
concentration of liraglutide, which significantly promoted H9C2
viability under hypoxia was 5 nM, this concentration was used in
the following experiments. (C) A TUNEL assay was used to observe
the cellular apoptosis and (D) representative images are presented.
Ex9-39, a water-soluble GLP-1 receptor antagonist was used to
inhibit the action of liraglutide. *P<0.05 vs. Ctrl group;
#P<0.05 vs. hypoxia group; @P<0.05 vs.
hypoxia + Lir group. (E) Western blotting was used to detect
alterations in the expression of proteins associated with
mitochondrial apoptosis. (F) The protein expression of Caspase3 was
quantified. (G) Immunofluorescence analysis of cyt-c localization.
Chronic hypoxia induced the cyt-c leakage from mitochondria and
trafficking into the nucleus. The protein expression levels of (H)
caspase9, (I) Bax and (J) c-IAP1 were quantified. Liraglutide was
able to reduce the expression of apoptotic proteins, and this
effect was reversed by Ex9-39. *P<0.05 vs. Ctrl group;
#P<0.05 vs. hypoxia group; @P<0.05 vs.
hypoxia + Lir group. Lir, liraglutide; Ctrl, control; cle, cleaved;
LDH, lactate dehydrogenase; TUNEL, terminal
deoxynucleotidyl-transferas-mediated dUTP nick end labeling;
Ex9-39, exendin 9–39; Bax, apoptosis regulator BAX; c-IAP1,
baculoviral IAP repeat-containing protein 2; cyt-c, cytochrome
c. |
To provide further evidence for mitochondrial
apoptosis, alterations in the expression of proteins associated
with cyt-c leakage were assessed. As presented in Fig. 2F and H-J, hypoxia elevated the
expression of caspase3, caspase9 and Bax, and reduced the
expression of c-IAP1. However, liraglutide was able to promote
anti-apoptotic protein expression and limit pro-apoptotic protein
expression (Fig. 2F and H-J).
Notably, the anti-apoptotic effect of liraglutide was mediated by
GLP1R. These data indicated that liraglutide sustained H9C2
survival in the context of chronic hypoxia damage by abating
mitochondrial apoptosis.
Mitophagy is activated by liraglutide
via Parkin
As mitophagy is important for the maintenance of
mitochondrial homeostasis, it was hypothesized that liraglutide may
preserve mitochondrial balance via mitophagy (38). To validate this hypothesis, the
expression of mitophagy markers was examined. Compared with the
control group, hypoxia induced a downregulation of mitophagy, as
evidenced by decreased LC3II, p62, Beclin1 and Atg5 expression
(Fig. 3). However, liraglutide was
able to reverse the expression of mitophagy parameters, and this
effect was nullified by Ex9-39 (Fig.
3A, B and D-G). Notably, as Parkin is an important receptor in
mitophagy, alterations in Parkin expression were examined. It was
observed that hypoxia progressively reduced the expression of
Parkin (Fig. 3A and B), which was
rescued by treatment with liraglutide. However, with the blockade
of GLP1R, Parkin expression was decreased (Fig. 3A and B). These data indicated that
liraglutide triggered Parkin-associated mitophagy under
hypoxia.
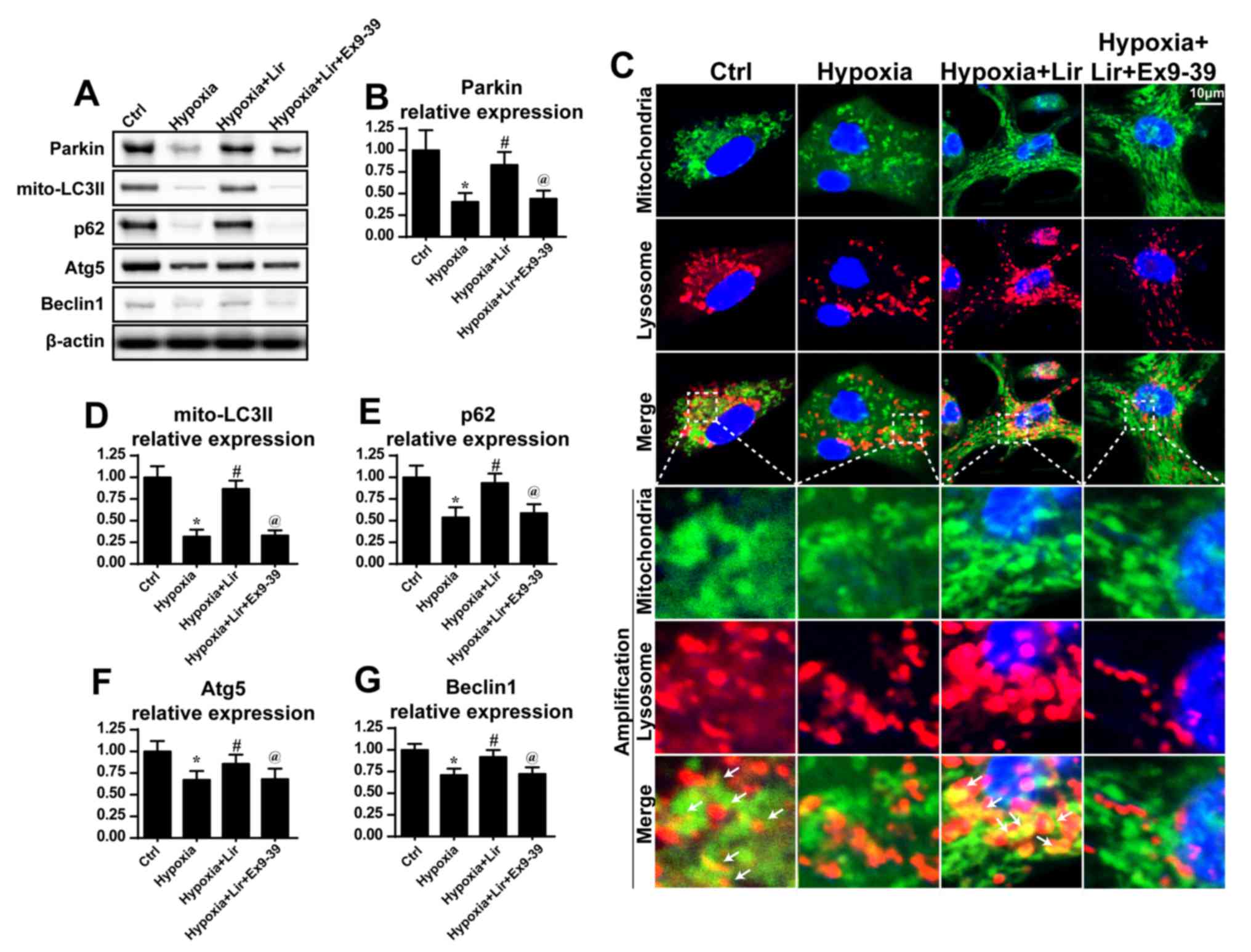 | Figure 3.Liraglutide activates mitophagy. (A)
Western blotting was used to measure the alterations in the
expression of mitophagy-associated proteins. (B) Parkin expression
was quantified. (C) Co-staining of mitochondria and lysosomes was
performed. In the enlarged panels, orange immunofluorescence
indicates mitophagy. Chronic hypoxic stimulation reduced the number
of foci of mitochondria and lysosomes. However, liraglutide was
able to increase the evidence of mitophagy, and this effect was
nullified by Ex9-39. (D) LC3II, (E) p62, (F) Atg5 and (G) Beclin-1
expression levels were quantified. *P<0.05 vs. Ctrl group;
#P<0.05 vs. hypoxia group; @P<0.05 vs.
hypoxia + Lir group. LC, microtubule-associated protein light
chain; p62, sequestome-1; Atg5, autophagy protein 5; Ctrl, control;
Lir, liraglutide; Ex9-39, exendin 9–39; mito, mitochondrial. |
Additionally, to provide direct evidence for the
role of liraglutide in the activation of mitophagy,
immunofluorescence analysis was used to observe the overlap of
mitochondria and lysosomes. As presented in Fig. 3C, in the control group, a number of
mitochondria were tagged with lysosomes. However, following chronic
hypoxic stimulation, the majority of mitochondria were separated
from lysosomes (Fig. 3C),
indicative of mitophagy inhibition. However, treatment with
liraglutide was able to reverse the interaction between
mitochondria and lysosomes, and contributed to the overlap of
mitochondria and lysosomes. However, with blockade of GLP1R,
mitophagy was inhibited despite treatment with liraglutide
(Fig. 3C). These data demonstrated
that liraglutide had the ability to reverse mitophagy activity
under chronic hypoxic conditions.
Loss of Parkin-associated mitophagy
inhibits the anti-apoptotic effect of liraglutide
To examine the consequences of mitophagy activation
under treatment with liraglutide, mitochondrial function was
evaluated. Since the generation of energy is the principal role of
mitochondria, the cellular ATP content was detected (39). As presented in Fig. 4A, hypoxia repressed ATP production
when compared with the control group. However, treatment with
liragutide reversed the effect on ATP content. To examine whether
Parkin-associated mitophagy was responsible for the protective role
of liraglutide on ATP production, siRNA was used to knock down the
expression of Parkin. Following silencing of Parkin in
liraglutide-treated H9C2 cells, the ATP content was decreased
(Fig. 4A). These data indicated
that Parkin-associated mitophagy was involved in
liraglutide-mediated ATP production.
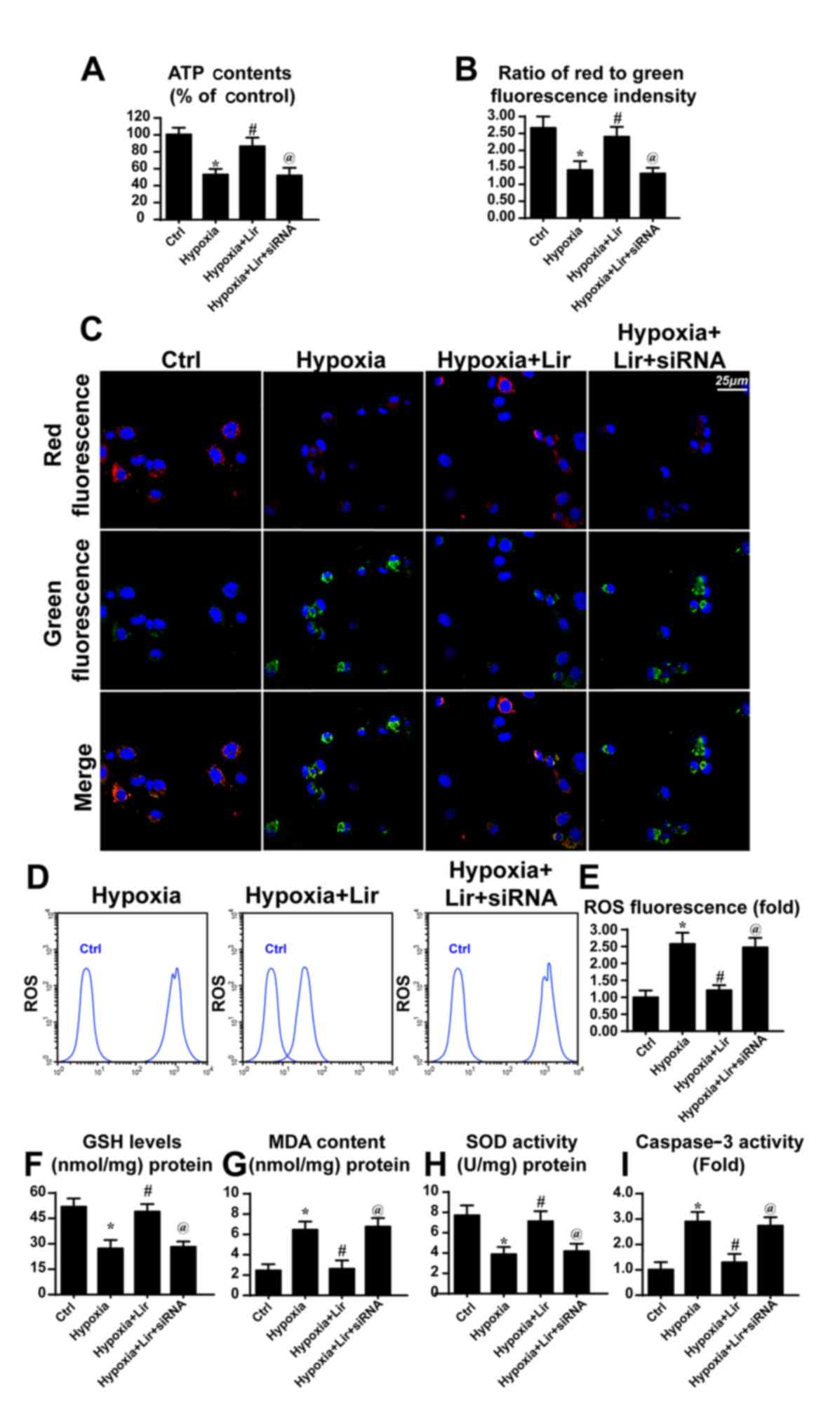 | Figure 4.Liraglutide protects the
mitochondrial function via Parkin-associated mitophagy. siRNA was
used to knock down the expression of Parkin in liraglutide-treated
H9C2 cells. (A) ATP production was detected, and liraglutide
preserved the ATP concentration in a Parkin-dependent manner. (B)
Mitochondrial potential was detected via JC-1 staining and (C)
representative images are presented. Red fluorescence indicates the
normal or healthy mitochondria and green fluorescence indicates the
damaged mitochondrial potential. (D) Cellular ROS was detected via
flow cytometry and (E) the results were analyzed. Alterations in
the expression of cellular antioxidant factors were detected. (F)
GSH, (G) MDA and (H) SOD concentrations were detected via ELISA
analysis. (I) Caspase activity detection was used to demonstrate
the role of Parkin-associated mitophagy in cellular apoptosis.
*P<0.05 vs. Ctrl group; #P<0.05 vs. hypoxia group;
@P<0.05 vs. hypoxia + Lir group. ATP, adenosine
5′-triphosphate; siRNA, small interfering RNA; ROS, reactive oxygen
species; Lir, liraglutide; Ctrl, control; GSH, glutathione; MDA,
malondialdehyde; SOD, superoxide dismutase. |
ATP generation results from mitochondrial membrane
potential (38). Mitochondria
convert the proton gradient of mitochondrial membrane potential to
chemical energy. Accordingly, the alteration in membrane potential
was investigated. Through JC1 staining, it was observed that
hypoxia reduced the mitochondrial membrane potential, as revealed
by increased green fluorescence (Fig.
4B and C). However, liraglutide had the ability to maintain the
membrane potential and this effect was reversed by Parkin
knockdown. Aside from the collapse of mitochondrial membrane
potential, it was observed that hypoxia induced excessive cellular
oxidative stress, as evidenced by elevated ROS (Fig. 4D and E). Liraglutide had the
ability to repress ROS release, whereas this effect was neutralized
by Parkin silencing (Fig. 4D and
E). As a consequence of ROS overproduction, a marked decline in
the expression of antioxidant factors was observed, including GSH
and SOD (Fig. 4F-H). By contrast,
the expression of MDA, the lipid peroxide, was increased.
Liraglutide had the ability to reverse the effect on GSH and SOD,
although it repressed MDA in a Parkin-dependent manner (Fig. 4F-H). In order to examine whether
mitophagy was associated with cellular death under chronic hypoxia,
caspase3 activity was detected. As presented in Fig. 4I, the caspase3 activity was
elevated under treatment with hypoxia and was reduced in response
to liraglutide application. Notably, loss of Parkin increased
caspase3 activity despite treatment with liraglutide. These data
indicated that Parkin-associated mitophagy was responsible for the
beneficial effects of liraglutide on mitochondria involving energy
metabolism, mitochondrial membrane potential, cellular oxidative
stress, redox biology and cellular apoptosis.
SIRT1 is activated by liraglutide and
contributes to Parkin-dependent mitophagy
The molecular mechanism through which liraglutide
regulated Parkin-dependent mitophagy was investigated in the
present study (Fig. 5). SIRT1 has
been identified to be a novel factor for mitochondrial homeostasis
by sustaining mitochondrial membrane potential, reducing oxidative
injury and promoting mitochondrial biogenesis (40). Based on this, it was hypothesized
that SIRT1 may be the upstream signaling molecule for
Parkin-dependent mitophagy. Western blotting was performed in the
infarcted heart to examine the alterations in SIRT1 expression
(Fig. 5A and B). It was observed
that SIRT1 expression was downregulated in the infarcted heart.
However, treatment with liraglutide was able to could increase
SIRT1 expression in a dose dependent manner (Fig. 5A and B). Similarly, the
downregulation of SIRT1 was also observed in H9C2 cells under
chronic hypoxia (Fig. 5C-E), and
this tendency was reversed by liraglutide treatment.
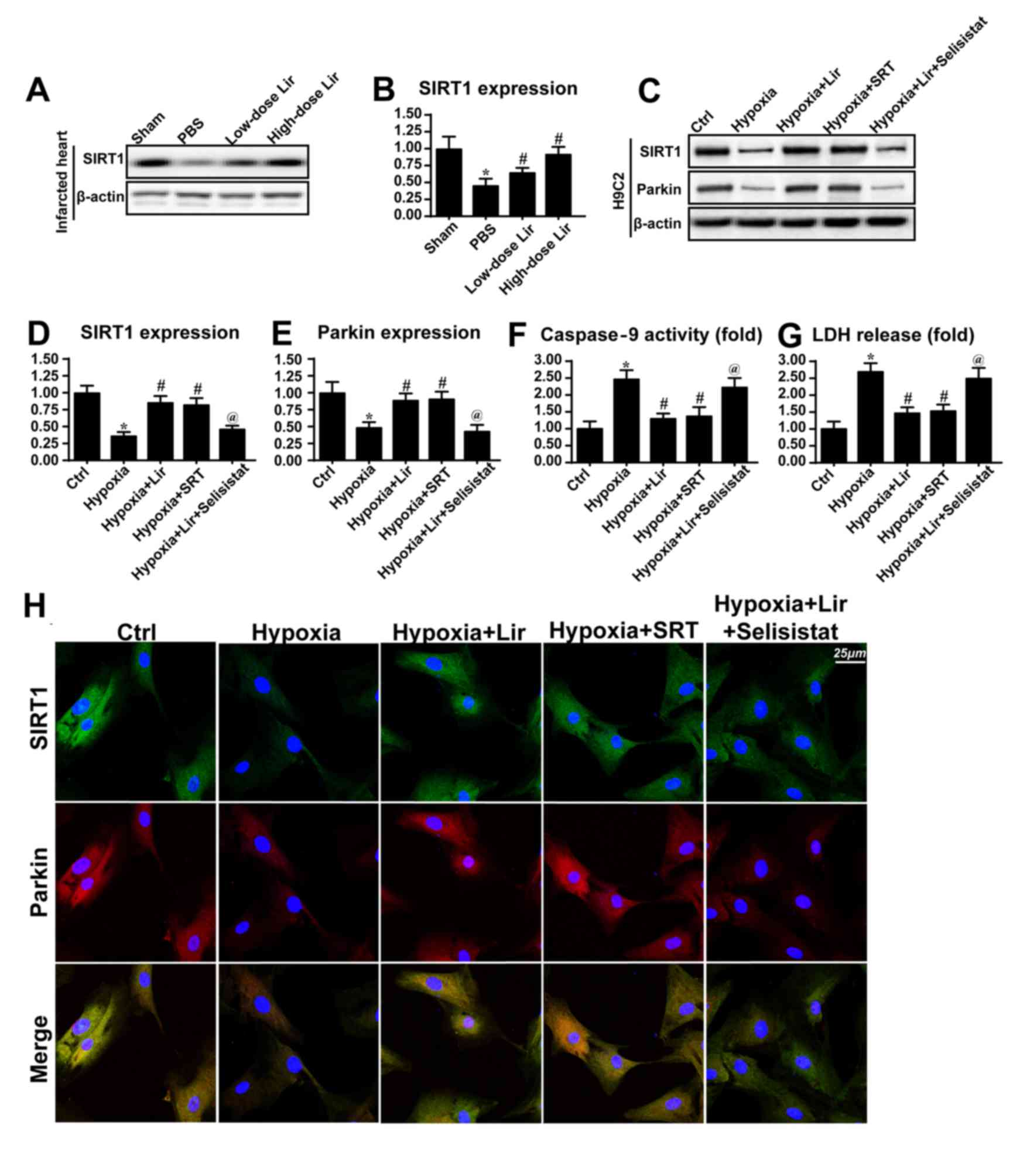 | Figure 5.Liraglutide activates Parkin via the
SIRT1 pathway. (A) Western blotting and (B) densitometric analysis
was used to detect the alterations in SIRT1 in the infarcted heart.
Liraglutide was able to sustain the expression level of SIRT1 in a
dose-dependent manner. In vitro, total proteins were
isolated and analyzed via (C) western blotting to measure the
alterations in (D) SIRT1 and (E) Parkin expression. SRT, the
activator of SIRT1, was applied in the control group under hypoxia
conditions. SRT elevated the expression levels of SIRT1 and Parkin
in the context of hypoxic stimulation. Selisistat, the inhibitor of
SIRT1, was used in the liraglutide-treated cells. Selisistat
blocked the promotive effect of liraglutide on SIRT1 and Parkin
expression. (F) Caspase9 activity and (G) LDH release assays were
used to detect cell viability and mitochondrial apoptosis,
respectively. (H) Co-staining of SIRT1 and Parkin. Liraglutide
enhanced the level of Parkin expression via SIRT1. Blockade of
SIRT1 inhibited the role of liraglutide on Parkin. By contrast,
activation of SIRT1 was able to reverse the alteration in Parkin
expression under hypoxic condition. *P<0.05 vs. Ctrl group;
#P<0.05 vs. hypoxia group; @P<0.05 vs.
hypoxia + Lir group. SIRT1, NAD-dependent protein deacetylase
sirtuin-1; SRT, SRT1720; Lir, liraglutide; Ctrl, control; LDH,
lactate dehydrogenase. |
To demonstrate whether SIRT1 was involved in
Parkin-dependent mitophagy, SRT (10 µM for 4 h), an activator of
SIRT1, was used to reverse the decrease in SIRT1 expression under
treatment with hypoxia. The SIRT1 inhibitor Selisistat (10 µM for 6
h) was applied to reduce SIRT1 expression in liraglutide-treated
cells. As presented in Fig. 5C-E,
activation of SIRT1 under hypoxic conditions was able to reverse
the effect on Parkin expression, consistent with the results from
the liraglutide group. However, in the liraglutide-treated cells,
Selisistat not alleviated the SIRT1 expression and repressed Parkin
expression (Fig. 5C-E). In order
to provide further evidence for the regulatory role of SIRT1 in
Parkin, co-staining for SIRT1 and Parkin was performed. It was
observed that hypoxia reduced the level of SIRT1 and Parkin
expression (Fig. 5H). However,
these alterations were rescued by treatment with liraglutide or SRT
(Fig. 5H). By contrast, following
inhibition of SIRT1 with Selisistat, the beneficial effect of
liraglutide on SIRT1 and Parkin disappeared.
Caspase9 activity and LDH release assays were
performed to investigate whether SIRT1 was involved in
mitochondrial protection and cellular survival. It was observed
that caspase9 activity and LDH content were increased in the
hypoxia group (Fig. 5F and G).
However, treatment with liraglutide or SRT application was able to
abate the caspase9 activity and LDH release (Fig. 5F and G). Notably, following the
blockade of SIRT1 with Selisistat, caspase9 activity and LDH
content were increased despite treatment with liraglutide. These
data illustrated that SIRT1 was the upstream molecule for
Parkin-dependent mitophagy, and that SIRT1 contributed to
mitochondrial protection and cellular survival. The beneficial role
of liraglutide in the post-infarcted heart through regulation of
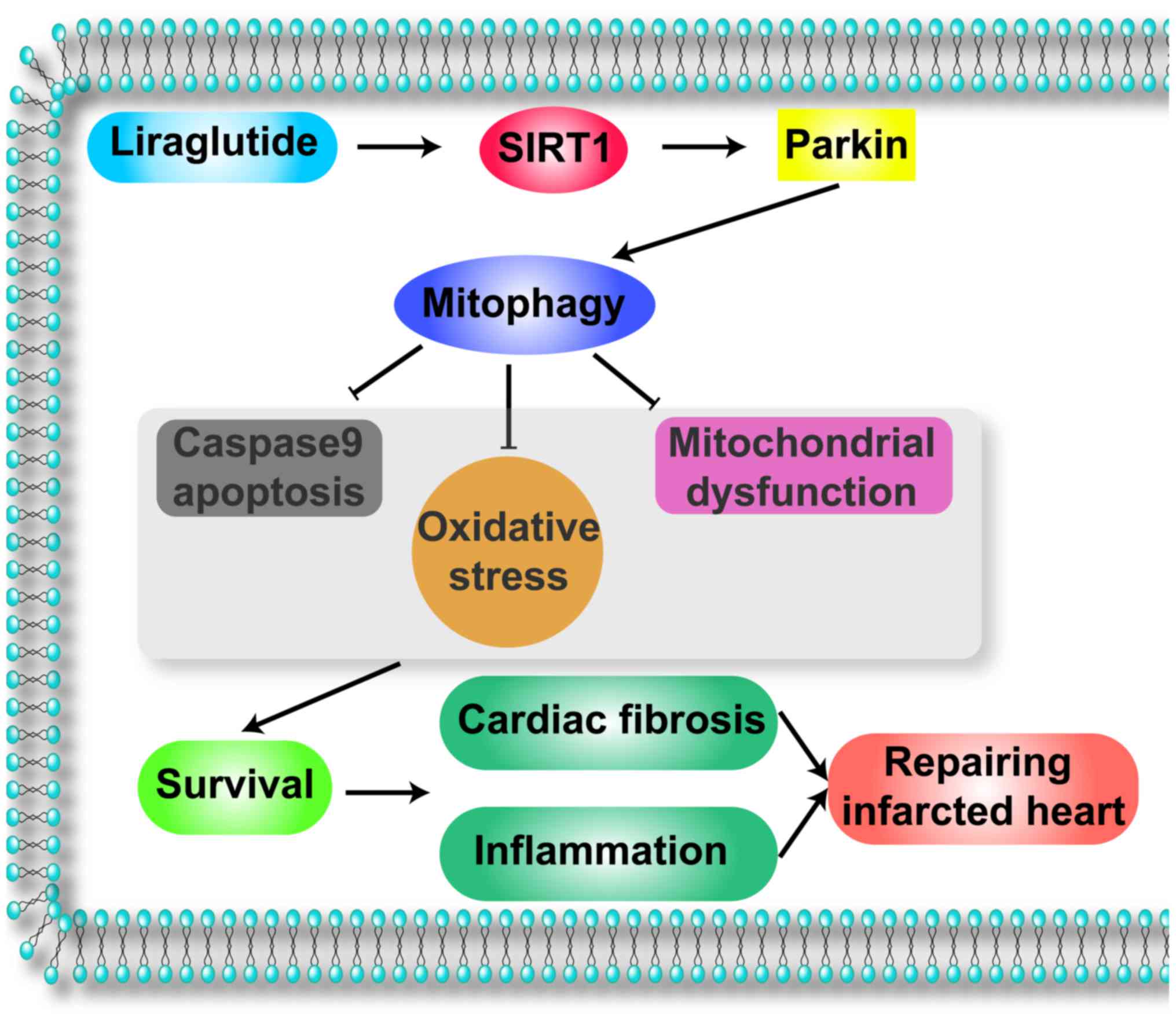
SIRT1-mediated mitophagy was summarized in Fig. 6.
Discussion
In the present study, it was observed that: i)
Liraglutide had the ability to repair the post-infarcted heart; ii)
liraglutide reduced fibrosis, the inflammatory response and
myocardial death; iii) mechanistically, chronic hypoxic stimulation
caused cardiomyocyte death via mitochondrial apoptosis, as
evidenced by reduced mitochondrial membrane potential, cyt-c
leakage and increased pro-apoptotic protein expression; iv)
liraglutide inhibited mitochondrial apoptosis via mitophagy; v)
liraglutide elevated SITR1 expression which increased Parkin,
leading to mitophagy activation; vi) protective mitophagy reversed
cellular ATP production, reduced cellular oxidative stress and
balanced the redox response; and vii) the beneficial effect of
liraglutide on cardiomyocyte apoptosis was dependent on GLP1R. To
the best of our knowledge, this is the first study to describe the
role of liraglutide in the post-infarcted heart, Parkin-dependent
mitophagy and the SIRT1 signaling pathway.
Liraglutide is a novel GLP-1 receptor agonist that
acts as a hypoglycemic agent by activating the GLP1R. A previous
study has reported that liraglutide holds cardioprotective
properties, and that this cardioprotective effect is independent of
its hypoglycemic action (21).
Previous studies have demonstrated that the application of
liraglutide for 3 months reduced myocardial injury and increased
cardiac function in patients with AMI (20,24).
These findings suggested that liraglutide may have the ability to
repair the infarcted heart. However, little evidence is available
to explain the mechanism underlying this. The present study
demonstrated that treatment with liraglutide for 1 month following
myocardial infarction reduced cardiac fibrosis, inflammatory
responses and cardiomyocyte death. These observations may explain
the protective effect of liraglutide on the infarcted heart.
Notably, liraglutide was previously demonstrated to improve
hypertension, dyslipidemia, vascular endothelial dysfunction and
other cardiovascular disease risk factors that serve as the
pathological foundations of AMI (19,22,41).
These beneficial actions of liraglutide may be involved in the
improvement of the post-infarcted heart. Considering that few
strategies are accessible to delay and repair the post-infarcted
heart, the use of liraglutide may bring further clinical benefits
for patients with AMI.
In the present study, it was determined that the
SIRT1/Parkin/mitophagy pathway was the primary mechanism of defense
that was enhanced by liraglutide to preserve cardiomyocyte survival
in a state of chronic hypoxia. Mitochondria are the center of
energy metabolism and are the regulators of cellular signaling
(40,42). Previous studies have demonstrated
that mitochondrial dynamics, particularly mitophagy, serve an
important role in reducing myocardial and endothelial cell damage
(13,43). Mitophagy is a selective form of
general autophagy in which mitochondria are specifically targeted
for degradation at the autophagolysosome (44). Through mitophagy, the damaged
mitochondria are separated, leading to the balance of mitochondrial
quality and quantity (45,46). Notably, the process of mitophagy
relies on a growing cadre of ‘mitophagy adaptors’ or ‘mitophagy
receptors’. There are three receptors which have been identified to
be associated with mitophagy activation: FUNDC1, Bnip3 and Parkin
(47,48). In the present study, it was
observed that Parkin-associated mitophagy was inactivated under
chronic hypoxia treatment. Notably, a previous study suggested that
acute hypoxia, including ischemia reperfusion injury, may activate
mitophagy (11). Brief ischemia or
hypoxia may trigger mitophagy to consume damaged mitochondria and
sustain cellular homeostasis. However, long-term hypoxia is the
inhibitory signal for mitophagy, according to the present data and
a previous study (49). Previous
studies have demonstrated that mitophagy activity is downregulated
during the progression of cardiac malfunction following AMI
(50–52), which is consistent with the results
of the present study. Therefore, identifying a means by which to
activate mitophagy is important for the repair of the infarcted
heart. In the present study, liraglutide activated mitophagy via
the SIRT1/Parkin pathway. SIRT1 is one of the seven mammalian
homologs (SIRT1-SIRT7) of yeast silent information regulator 2.
SIRT1 is an NAD+-dependent protein deacetylase. SIRT1
serves a number of roles in cells, including longevity, apoptosis,
DNA repair, inflammation and mitochondrial regulation (25). As an important protein in cellular
metabolism, the regulatory effect of SIRT1 on mitochondrial
dynamics has gained much attention. In the present study, it was
demonstrated that SIRT1 was responsible for Parkin upregulation and
mitophagy activation. These findings enrich the current
understanding of the role of SIRT1 in mitochondrial
homeostasis.
As a consequence of mitophagy activation in the
present study, cellular oxidative and mitochondrial damage was
rescued. Considering that excessive oxidative injury and
mitochondrial energy disorders are the principal feature of cardiac
failure following AMI (53,54),
mitophagy may be a potential therapeutic target for restoring
mitochondrial homeostasis and cardiac energy metabolism. Notably,
previous studies have indicated that excessive mitophagy may shift
cellular survival towards death signaling. It has been argued that
excessive mitophagy may consume the majority of mitochondria,
leading to the loss of the majority of the mitochondrial mass
(13,55). As a consequence of mitochondrial
loss, cells fail to generate sufficient ATP to fuel the cellular
biological functions, including contraction, division and
mobilization. Therefore, it was suggested that moderate mitophagy
activity is required to maintain cellular homeostasis. However,
whether excessive mitophagy is harmful for myocardial structure and
function remains unclear. Further studies are required to examine
this question.
In conclusion, the results of the present study
illustrated the important role of liraglutide in repairing the
infarcted heart via mitophagy. Liraglutide amplified the
SIRT1/Parkin pathway leading to the activation of protective
mitophagy, which was the essential element of mitochondrial
protection. Mitophagy reduced cellular oxidative stress, alleviated
mitochondrial damage and abated cellular apoptosis under chronic
hypoxic conditions. The present findings elucidated the mechanism
underling cardiac dysfunction. In addition, the results of the
present study identified a convenient and effective method of
repairing the infarcted heart. Therefore, the enhancement of
mitophagy by liraglutide may be a practical and efficient adjuvant
to the treatment of cardiac failure following AMI.
Acknowledgements
The authors of the present study would like to thank
the Institute of Basic Medicine Science of Suzhou No. 1 People's
Hospital (Suzhou, China). The present study was supported by grants
from the Jiangsu Medical and Health Science and Technology
Development Plan (grant no. 2016WS0259).
References
|
1
|
Bae S, Park M, Kang C, Dilmen S, Kang TH,
Kang DG, Ke Q, Lee SU, Lee D and Kang PM: Hydrogen
peroxide-responsive nanoparticle reduces myocardial
ischemia/reperfusion injury. J Am Heart Assoc. 5:2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stähli BE, Gebhard C, Duchatelle V,
Cournoyer D, Petroni T, Tanguay JF, Robb S, Mann J, Guertin MC,
Wright RS, et al: Effects of the P-selectin antagonist inclacumab
on myocardial damage after percutaneous coronary intervention
according to timing of infusion: Insights from the SELECT-ACS
trial. J Am Heart Assoc. 5:2016. View Article : Google Scholar
|
|
3
|
Han D, Huang W, Li X, Gao L, Su T, Li X,
Ma S, Liu T, Li C, Chen J, et al: Melatonin facilitates
adipose-derived mesenchymal stem cells to repair the murine
infarcted heart via the SIRT1 signaling pathway. J Pineal Res.
60:178–192. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
de Souza P, Guarido KL, Scheschowitsch K,
da Silva LM, Werner MF, Assreuy J and da Silva-Santos JE: Impaired
vascular function in sepsis-surviving rats mediated by oxidative
stress and Rho-Kinase pathway. Redox Biol. 10:140–147. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhu R, Sun H, Yu K, Zhong Y, Shi H, Wei Y,
Su X, Xu W, Luo Q, Zhang F, et al: Interleukin-37 and dendritic
cells treated with interleukin-37 plus troponin I ameliorate
cardiac remodeling after myocardial infarction. J Am Heart Assoc.
5:2016. View Article : Google Scholar
|
|
6
|
Nishikido T, Oyama J, Shiraki A, Komoda H
and Node K: Deletion of apoptosis inhibitor of macrophage
(AIM)/CD5L attenuates the inflammatory response and infarct size in
acute myocardial infarction. J Am Heart Assoc. 5:e0028632016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Koenitzer JR, Bonacci G, Woodcock SR, Chen
CS, Cantu-Medellin N, Kelley EE and Schopfer FJ: Fatty acid
nitroalkenes induce resistance to ischemic cardiac injury by
modulating mitochondrial respiration at complex II. Redox Biol.
8:1–10. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
He B, Zhao Y, Xu L, Gao L, Su Y, Lin N and
Pu J: The nuclear melatonin receptor RORa is a novel endogenous
defender against myocardial ischemia/reperfusion injury. J Pineal
Res. 60:313–326. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Quijano C, Trujillo M, Castro L and
Trostchansky A: Interplay between oxidant species and energy
metabolism. Redox Biol. 8:28–42. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hu SY, Zhang Y, Zhu PJ, Zhou H and Chen
YD: Liraglutide directly protects cardiomyocytes against
reperfusion injury possibly via modulation of intracellular calcium
homeostasis. J Geriatr Cardiol. 14:57–66. 2017.PubMed/NCBI
|
|
11
|
Zhang W, Ren H, Xu C, Zhu C, Wu H, Liu D,
Wang J, Liu L, Li W, Ma Q, et al: Hypoxic mitophagy regulates
mitochondrial quality and platelet activation and determines
severity of I/R heart injury. Elife. 5:2016. View Article : Google Scholar
|
|
12
|
Zhou H, Li D, Zhu P, Hu S, Hu N, Ma S,
Zhang Y, Han T, Ren J, Cao F and Chen Y: Melatonin suppresses
platelet activation and function against cardiac
ischemia/reperfusion injury via PPARγ/FUNDC1/mitophagy pathways. J
Pineal Res. 63:2017. View Article : Google Scholar :
|
|
13
|
Zhou H, Zhang Y, Hu S, Shi C, Zhu P, Ma Q,
Jin Q, Cao F, Tian F and Chen Y: Melatonin protects cardiac
microvasculature against ischemia/reperfusion injury via
suppression of mitochondrial fission-VDAC1-HK2-mPTP-mitophagy axis.
J Pineal Res. 63:2017. View Article : Google Scholar :
|
|
14
|
Zhou H, Zhu P, Guo J, Hu N, Wang S, Li D,
Hu S, Ren J, Cao F and Chen Y: Ripk3 induces mitochondrial
apoptosis via inhibition of FUNDC1 mitophagy in cardiac IR injury.
Redox Biol. 13:498–507. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu H, Wei H, Sehgal SA, Liu L and Chen Q:
Mitophagy receptors sense stress signals and couple mitochondrial
dynamic machinery for mitochondrial quality control. Free Radic
Biol Med. 100:199–209. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mouton-Liger F, Jacoupy M, Corvol JC and
Corti O: PINK1/Parkin-dependent mitochondrial surveillance: From
Pleiotropy to Parkinson's disease. Front Mol Neurosci. 10:1202017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
McWilliams TG and Muqit MM: PINK1 and
Parkin: Emerging themes in mitochondrial homeostasis. Curr Opin
Cell Biol. 45:83–91. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rodriguez V, Weiss MC, Weintraub H,
Goldberg IJ and Schwartzbard A: Cardiovascular disease leads to a
new algorithm for diabetes treatment. J Clin Lipidol. 11:1126–1133.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Levin PA, Nguyen H, Wittbrodt ET and Kim
SC: Glucagon-like peptide-1 receptor agonists: A systematic review
of comparative effectiveness research. Diabetes Metab Syndr Obes.
10:123–139. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen WR, Tian F, Chen YD, Wang J, Yang JJ,
Wang ZF, Da Wang J and Ning QX: Effects of liraglutide on no-reflow
in patients with acute ST-segment elevation myocardial infarction.
Int J Cardiol. 208:109–114. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen WR, Chen YD, Tian F, Yang N, Cheng
LQ, Hu SY, Wang J, Yang JJ, Wang SF and Gu XF: Effects of
liraglutide on reperfusion injury in patients with
ST-segment-elevation myocardial infarction. Circ Cardiovasc
Imaging. 9:2016. View Article : Google Scholar :
|
|
22
|
Paneni F and Lüscher TF: Cardiovascular
protection in the treatment of type 2 diabetes: A review of
clinical trial results across drug classes. Am J Med. 130:S18–S29.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen WR, Shen XQ, Zhang Y, Chen YD, Hu SY,
Qian G, Wang J, Yang JJ, Wang ZF and Tian F: Effects of liraglutide
on left ventricular function in patients with non-ST-segment
elevation myocardial infarction. Endocrine. 52:516–526. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen WR, Hu SY, Chen YD, Zhang Y, Qian G,
Wang J, Yang JJ, Wang ZF, Tian F and Ning QX: Effects of
liraglutide on left ventricular function in patients with
ST-segment elevation myocardial infarction undergoing primary
percutaneous coronary intervention. Am Heart J. 170:845–854. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang W, Huang Q, Zeng Z, Wu J, Zhang Y
and Chen Z: Sirt1 inhibits oxidative stress in vascular endothelial
cells. Oxid Med Cell Longev. 2017:75439732017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
D'Onofrio N, Servillo L, Giovane A, Casale
R, Vitiello M, Marfella R, Paolisso G and Balestrieri ML:
Ergothioneine oxidation in the protection against high-glucose
induced endothelial senescence: Involvement of SIRT1 and SIRT6.
Free Radic Biol Med. 96:211–222. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mayo JC, Sainz RM, González Menéndez P,
Cepas V, Tan DX and Reiter RJ: Melatonin and sirtuins: A ‘not-so
unexpected’ relationship. J Pineal Res. 62:2017
|
|
28
|
Zhang C, Qu S, Wei X, Feng Y, Zhu H, Deng
J, Wang K, Liu K, Liu M, Zhang H and Xiao X: HSP25 down-regulation
enhanced p53 acetylation by dissociation of SIRT1 from p53 in
doxorubicin-induced H9c2 cell apoptosis. Cell Stress Chaperones.
21:251–260. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tong W, Ju L, Qiu M, Xie Q, Chen Y, Shen
W, Sun W, Wang W and Tian J: Liraglutide ameliorates non-alcoholic
fatty liver disease by enhancing mitochondrial architecture and
promoting autophagy through the SIRT1/SIRT3-FOXO3a pathway. Hepatol
Res. 46:933–943. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Prieto-Domínguez N, Ordóñez R, Fernández
A, Méndez-Blanco C, Baulies A, Garcia-Ruiz C, Fernández-Checa JC,
Mauriz JL and González-Gallego J: Melatonin-induced increase in
sensitivity of human hepatocellular carcinoma cells to sorafenib is
associated with reactive oxygen species production and mitophagy. J
Pineal Res. 61:396–407. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ho HY, Lin CW, Chien MH, Reiter RJ, Su SC,
Hsieh YH and Yang SF: Melatonin suppresses TPA-induced metastasis
by downregulating matrix metalloproteinase-9 expression through
JNK/SP-1 signaling in nasopharyngeal carcinoma. J Pineal Res.
61:479–492. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang Y, Zhou H, Wu W, Shi C, Hu S, Yin T,
Ma Q, Han T, Zhang Y, Tian F and Chen Y: Liraglutide protects
cardiac microvascular endothelial cells against
hypoxia/reoxygenation injury through the suppression of the
SR-Ca(2+)-XO-ROS axis via activation of the
GLP-1R/PI3K/Akt/survivin pathways. Free Radic Biol Med. 95:278–292.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xu S, Pi H, Zhang L, Zhang N, Li Y, Zhang
H, Tang J, Li H, Feng M, Deng P, et al: Melatonin prevents abnormal
mitochondrial dynamics resulting from the neurotoxicity of cadmium
by blocking calcium-dependent translocation of Drp1 to the
mitochondria. J Pineal Res. 60:291–302. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Quintana C, Cabrera J, Perdomo J, Estévez
F, Loro JF, Reiter RJ and Quintana J: Melatonin enhances
hyperthermia-induced apoptotic cell death in human leukemia cells.
J Pineal Res. 61:381–395. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pariente R, Pariente JA, Rodríguez AB and
Espino J: Melatonin sensitizes human cervical cancer HeLa cells to
cisplatin-induced cytotoxicity and apoptosis: Effects on oxidative
stress and DNA fragmentation. J Pineal Res. 60:55–64. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kitsati N, Mantzaris MD and Galaris D:
Hydroxytyrosol inhibits hydrogen peroxide-induced apoptotic
signaling via labile iron chelation. Redox Biol. 10:233–242. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lin YW, Lee LM, Lee WJ, Chu CY, Tan P,
Yang YC, Chen WY, Yang SF, Hsiao M and Chien MH: Melatonin inhibits
MMP-9 transactivation and renal cell carcinoma metastasis by
suppressing Akt-MAPKs pathway and NF-κB DNA-binding activity. J
Pineal Res. 60:277–290. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ursini F, Maiorino M and Forman HJ: Redox
homeostasis: The Golden Mean of healthy living. Redox Biol.
8:205–215. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Du K, Ramachandran A and Jaeschke H:
Oxidative stress during acetaminophen hepatotoxicity: Sources,
pathophysiological role and therapeutic potential. Redox Biol.
10:148–156. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rizzo NR, Hank NC and Zhang J: Detecting
presence of cardiovascular disease through mitochondria respiration
as depicted through biophotonic emission. Redox Biol. 8:11–17.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Singh AK and Singh R: SAVOR-TIMI to
SUSTAIN-6: A critical comparison of cardiovascular outcome trials
of antidiabetic drugs. Expert Rev Clin Pharmacol. 10:429–442. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Mailloux RJ and Treberg JR: Protein
S-glutathionlyation links energy metabolism to redox signaling in
mitochondria. Redox Biol. 8:110–118. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Caja S and Enríquez JA: Mitochondria in
endothelial cells: Sensors and integrators of environmental cues.
Redox Biol. 12:821–827. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang W, Siraj S, Zhang R and Chen Q:
Mitophagy receptor FUNDC1 regulates mitochondrial homeostasis and
protects the heart from I/R injury. Autophagy. 13:1080–1081. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Qiu S, Zhu S, Xu S, Han Y, Liu W and Zuo
J: Molecular dynamics simulations of human E3 ubiquitin ligase
Parkin. Mol Med Rep. 16:4561–4568. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Shida M, Kitajima Y, Nakamura J,
Yanagihara K, Baba K, Wakiyama K and Noshiro H: Impaired mitophagy
activates mtROS/HIF-1α interplay and increases cancer
aggressiveness in gastric cancer cells under hypoxia. Int J Oncol.
48:1379–1390. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Díaz-Casado ME, Lima E, García JA,
Doerrier C, Aranda P, Sayed RK, Guerra-Librero A, Escames G, López
LC and Acuña-Castroviejo D: Melatonin rescues zebrafish embryos
from the parkinsonian phenotype restoring the
parkin/PINK1/DJ-1/MUL1 network. J Pineal Res Res Pineal Res.
61:96–107. 2016. View Article : Google Scholar
|
|
48
|
Mendivil-Perez M, Soto-Mercado V,
Guerra-Librero A, Fernandez-Gil BI, Florido J, Shen YQ, Tejada MA,
Capilla-Gonzalez V, Rusanova I, Garcia-Verdugo JM, et al: Melatonin
enhances neural stem cell differentiation and engraftment by
increasing mitochondrial function. J Pineal Res. 63:2017.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Bravo-San Pedro JM, Kroemer G and Galluzzi
L: Autophagy and mitophagy in cardiovascular disease. Circ Res.
120:1812–1824. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Nan J, Zhu W, Rahman MS, Liu M, Li D, Su
S, Zhang N, Hu X, Yu H, Gupta MP and Wang J: Molecular regulation
of mitochondrial dynamics in cardiac disease. Biochim Biophys Acta.
1864:1260–1273. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Vásquez-Trincado C, García-Carvajal I,
Pennanen C, Parra V, Hill JA, Rothermel BA and Lavandero S:
Mitochondrial dynamics, mitophagy and cardiovascular disease. J
Physiol. 594:509–525. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Shires SE and Gustafsson ÅB: Mitophagy and
heart failure. J Mol Med (Berl). 93:253–262. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Akkafa F, Halil Altiparmak I, Erkus ME,
Aksoy N, Kaya C, Ozer A, Sezen H, Oztuzcu S, Koyuncu I and Umurhan
B: Reduced SIRT1 expression correlates with enhanced oxidative
stress in compensated and decompensated heart failure. Redox Biol.
6:169–173. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kozlov AV, Lancaster JR Jr, Meszaros AT
and Weidinger A: Mitochondria-meditated pathways of organ failure
upon inflammation. Redox Biol. 13:170–181. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Dhingra A, Jayas R, Afshar P, Guberman M,
Maddaford G, Gerstein J, Lieberman B, Nepon H, Margulets V, Dhingra
R and Kirshenbaum LA: Ellagic acid antagonizes Bnip3-mediated
mitochondrial injury and necrotic cell death of cardiac myocytes.
Free Radic Biol Med. 112:411–422. 2017. View Article : Google Scholar : PubMed/NCBI
|