Introduction
Prostate cancer (PCa) is the most common malignancy
that is frequently diagnosed in males, and is a major cause of
cancer-associated mortality worldwide (1). Androgen deprivation therapy is the
most effective treatment for advanced PCa; however, most patients
with PCa develop castration-resistant prostate cancer (CRPC) due to
therapy-associated resistance (2,3).
Unfortunately, CRPC remains incurable (2) and the underlying mechanisms of CRPC
development are yet to be revealed.
The Hippo signaling pathway, which was originally
identified in fruit flies, is involved in tumor development via the
regulation of cell proliferation and apoptosis (3–7). The
core components of the Hippo signaling pathway within mammals
comprise protein kinases, including mammalian sterile 20-like 1 and
2, large tumor suppressor 1 and 2, and the adaptor proteins WW
domain-containing protein and Mps one binder 1. The core functions
of the Hippo signaling pathway serve tumor-suppressing roles via
yes-associated protein (YAP), and TAZ phosphorylation and
inactivation. YAP, which is a 65-kDa protein, is an effector
protein of the Hippo signaling pathway and is a transcriptional
co-activator of numerous transcription factors. Previous studies
have confirmed that YAP functions as an oncogenic protein in
mammalian cells via promoting cell growth (8–10).
Additionally, YAP overexpression has been associated with various
human cancers, including PCa, and breast, ovarian, lung, liver and
gastric cancer (11–17).
Increasing evidence has suggested the important
roles of YAP in regulating PCa cell behavior (18,19).
To the best our knowledge, no previous study has focused on the
role of YAP within human PCa DU145 cells. Therefore, the role of
YAP in human PCa DU145 cells and the underlying molecular
mechanisms were explored in the present study.
Materials and methods
Materials
The human PCa DU145 cells and RWPE-1 cells were
obtained from the American Type Culture Collection (Manassas, VA,
USA); serum-free keratinocyte medium (K-SFM) was obtained from
Gibco (Thermo Fisher Scientific, Inc., Waltham, MA, USA); the
RPMI-1640 medium, fetal bovine serum (FBS) and polyvinylidene
fluoride (PVDF) membrane were obtained from Sigma-Aldrich (Merck
KGaA, Darmstadt, Germany). Primary antibodies against connective
tissue growth factor (CTGF, cat. no. sc-14939 were purchased from
Santa Cruz Biotechnology, Inc., Dallas, TX, USA, while,
cysteine-rich angiogenic inducer 61 antibodies (CYR61, cat. no.
14476, 1:1,000), B-cell lymphoma 2 antibodies (Bcl-2, cat. no.
2872, 1:1,000), Bcl-2-associated X protein antibodies (Bax, cat.
no. 2774, 1:1,000), caspase 3 antibodies (cat. no. 9662, 1:1,000),
GAPDH antibodies (cat. no. 5174, 1:1,000) and secondary antibodies
(cat. no. 7076, 1:1,000) were obtained from Cell Signaling
Technology Inc., (Danvers, MA, USA); MTT and enhanced
chemiluminescence (ECL) Plus reagent were obtained from Eli Lilly
& Co., (Indianapolis, IN, USA); Annexin V-fluorescein
isothiocyanate (FITC) apoptosis detection kit was obtained from
Vazyme Biotech Co., (Nanjing, China). YAP-small interfering RNA
(YAP-siRNA, cat. no. sc-38637), control (Con)-siRNA (cat. no.
sc-37007) and the siRNA Transfection Reagent (cat. no. sc-29528)
were obtained from Santa Cruz Biotechnology, Inc.
Cell culture
Human PCa DU145 cells were cultured in RPMI-1640
supplemented with 10% FBS and 1% penicillin-streptomycin. RWPE-1
cells were cultured in 10 ml complete SFM containing 5 ng/ml
recombinant epidermal growth factor (CYT-217, ProSpec-Tany
TechnoGene Ltd., East Brunswick, NJ, USA), 50 µg/ml bovine
pituitary extract (CC023; macgene.bioon.com.cn/), 100 IU/ml penicillin and 100
IU/ml streptomycin. Cells were incubated in standard cell culture
conditions (5% CO2, 95% humidity) at 37°C. Human PCa
DU145 cells were passaged every 2–3 days, and RWPE-1 cells were
passaged every ~5 days.
Cell transfection
Human PCa DU145 cells were plated in a 6-well plate
1 day prior to transfection, the transfection assay was performed
once the cells reached 60–70% confluence. DU145 cells
(2×105 cells/well) were transfected with YAP-siRNA or
Con-siRNA (1 µg) using 30 µl transfection reagent plus and
incubated at 37°C for 6 h in 5% CO2 incubator, according
to the manufacturer's protocol. A total of 24 h following
transfection, the transfected DU145 cells were used for subsequent
experiments, and were collected for protein analysis after 72 h
incubation.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from human PCa DU145 and
RWPE-1 cells using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. To
determine the mRNA expression levels within the cells, RT was
performed using PrimeScript™ RT reagent kit (Takara Bio Inc., Otsu,
Japan) according to the manufacture's protocol. For qPCR,
SYBR® Premix Ex Taq™ II (Takara Bio Inc.) was
used and all reactions were performed in triplicate with the
following conditions: 95°C for 10 min, followed by 40 cycles at
95°C for 15 sec and 72°C for 30 sec, 78°C for 1.5 min for 35
cycles, after which samples were stored at 4°C. GAPDH was used as
an internal control. The 2−ΔΔCq method was performed to
analyze the relative amounts of each transcript (20). The qPCR primers are presented in
Table I.
 | Table I.Primer sequences for polymerase chain
reaction. |
Table I.
Primer sequences for polymerase chain
reaction.
| Gene | Primer sequence
(5′-3′) |
|---|
| YAP-Forward |
ACGTTCATCTGGGACAGCAT |
| YAP-Reverse |
GTTGGGAGATGGCAAAGACA |
| CTGF-Forward |
TTGGCAGGCTGATTTCTAGG |
| CTGF-Reverse |
GGTGCAAACATGTAACTTTTGG |
| Cyr61-Forward |
CCCGTTTTGGTAGATTCTGG |
| Cyr61-Reverse |
GCTGGAATGCAACTTCGG |
| Bcl-2-Forward |
ATGTGTGTGGAGAGCGTCAA |
| Bcl-2-Reverse |
ACAGTTCCACAAAGGCATCC |
| Bax-Forward |
GGCCCACCAGCTCTGAGCAGA |
| Bax-Reverse |
GCCACGTGGGCGTCCCAAAGT |
| Caspase
3-Forward |
CTGGTTGGCGTCGCCTTG |
| Caspase
3-Reverse |
GAATCCACTGAGTTTTCAG |
| GAPDH-Forward |
CTTTGGTATCGTGGAAGGACTC |
| GAPDH-Reverse |
GTAGAGGCAGGGATGATGTTCT |
Western blot analysis
Total cellular protein from human PCa DU145 and
RWPE-1 cells was extracted using radioimmunoprecipitation assay
buffer (P0013B, Beyotime Biotechnology, Nanjing, China) and
SDS-PAGE analysis was performed to separate the total protein.
Protein concentration was determined by bicinchoninic protein assay
kit. Proteins (25 µg) were resolved by 10% sodium dodecyl sulfate
polyacrylamide gels and were then transferred to a polyvinylidene
membrane. The membrane was blocked with 5% skim milked for 1h at
room temperature. Subsequently, the membrane was blotted overnight
at 4°C with the following primary antibodies: CTGF (1:1,000
dilution), CYR61 (1:1,000 dilution), Bcl-2 (1:1,000 dilution), Bax
(1:1,000 dilution), caspase 3 (1:1,000 dilution) and GAPDH (1:2,000
dilution). The membrane was then incubated with horseradish
peroxidase-conjugated anti-mouse and anti-rabbit immunoglobulin G
secondary antibodies (1:5,000 dilution) at room temperature for 2
h. Protein bands were observed using enhanced chemiluminescence
Plus reagent prior to imaging and analysis.
MTT assay
Cell proliferation was detected using an MTT assay.
YAP-siRNA and Con-siRNA were transfected into DU145 cells.
Subsequently, log-phase human PCa DU145 cells were harvested with
0.25% trypsin and seeded in 96-well plates (5×103
cells/well). After 24 h incubation at 37°C with 5% CO2,
MTT was added to the cell culture medium and incubated for 4h at
37°C. Then DMSO was employed for formazan crystals dissolving.
Optical density was detected at 490 nm using a spectrophotometer.
Each experiment was repeated in triplicate and data were displayed
as the mean ± standard deviation.
Apoptosis analysis
To detect alterations in cell apoptosis, an Annexin
V-FITC apoptosis detection kit was utilized. DU145 cells were
transfected with YAP-siRNA or Con-siRNA for 24 h. Following
transfection, cells were rinsed with cold PBS. Cells
(5×105 cells/well) were then labeled with Annexin V-FITC
and propidium iodide, according to the manufacturer's protocol.
Flow cytometry (BD Biosciences, Franklin Lakes. NJ, USA) was used
for cell apoptosis analysis. Version 2.5 WinMDI (Purdue University
Cytometry Laboratories; www.cyto.purdue.edu/flowcyt/software/Catalog.htm) was
applied for data analysis. Each test was repeated in
triplicate.
Statistical analysis
All tests were performed in triplicate. Data are
displayed as the mean ± standard deviation. SPSS 17.0 statistical
software (SPSS, Inc., Chicago, IL, USA) was applied for all
statistical analyses. A Student's t-test was used to analyze the
difference between the two groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
YAP expression in human PCa DU145
cells
Firstly, the mRNA and protein expression levels of
YAP were detected in human PCa DU145 cells via RT-qPCR and western
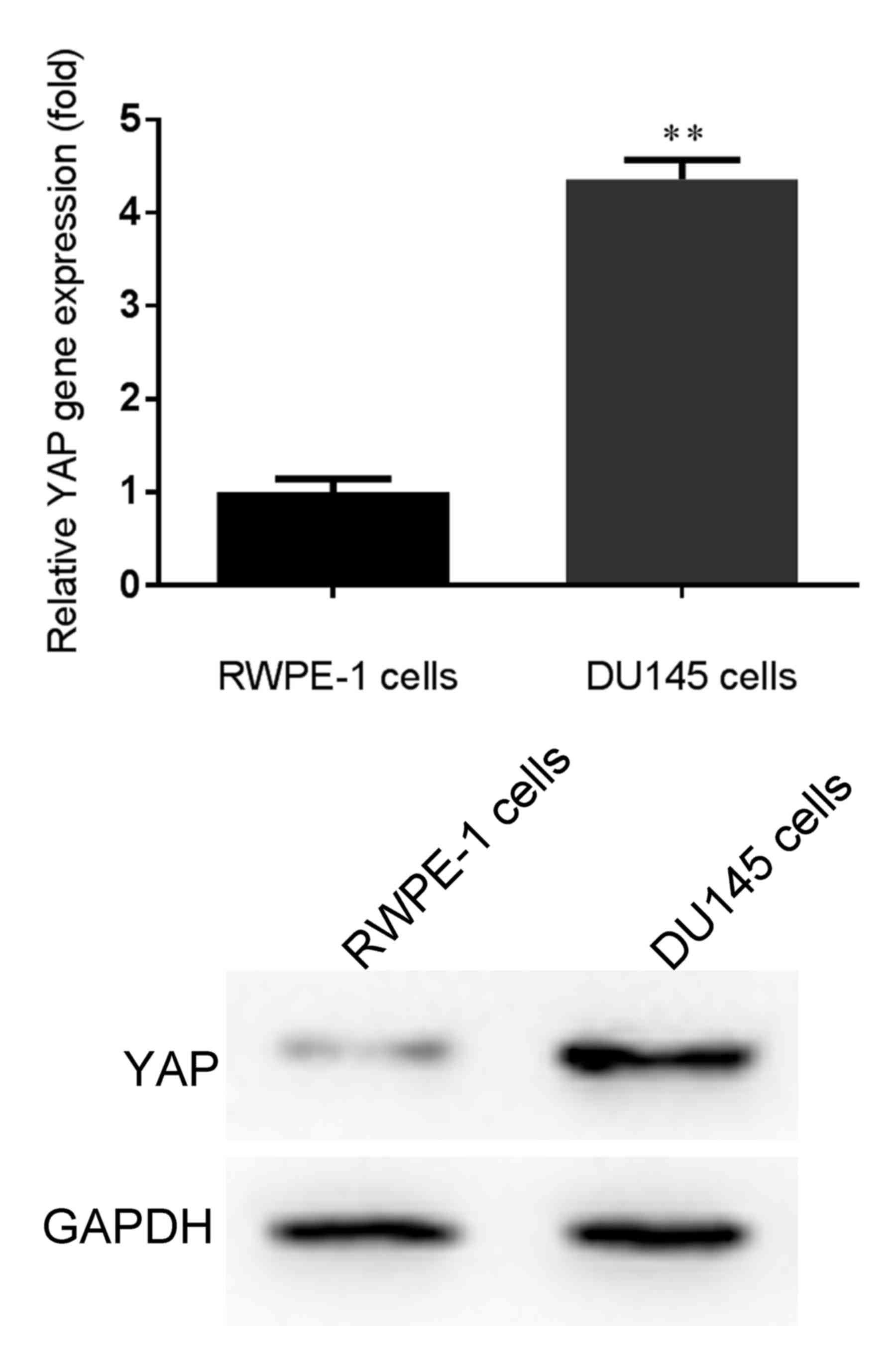
blotting, respectively. As demonstrated in Fig. 1, the mRNA and protein expression
levels of YAP were significantly higher in human PCa DU145 cells
compared with in normal prostate epithelial RWPE-1 cells. Based on
these results, the expression levels of YAP were significantly
increased in human PCa DU145 cells.
mRNA and protein expression levels of
YAP in DU145 cells following cell transfection
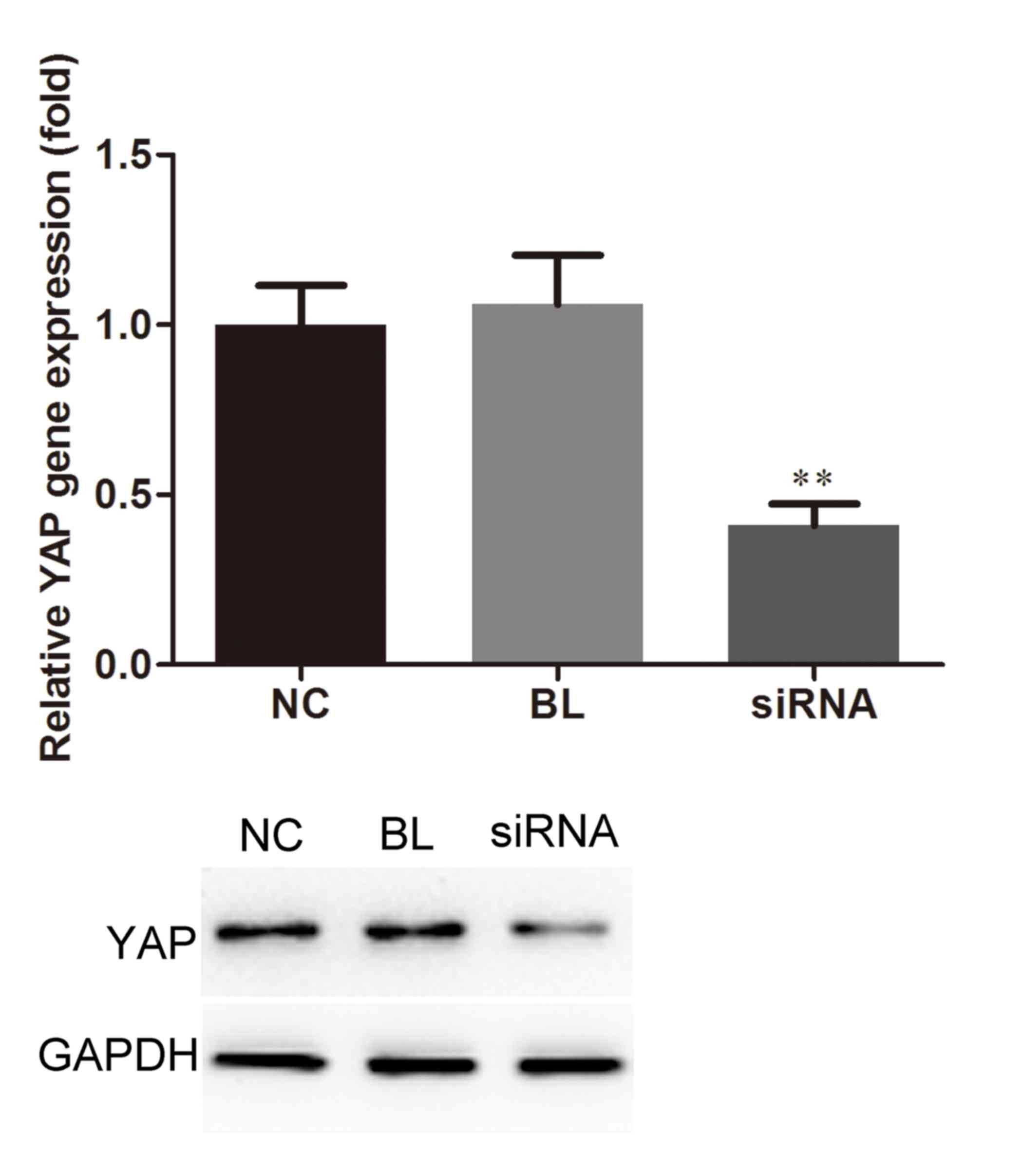
To investigate the role of YAP in human PCa DU145
cells, a stable YAP-silenced DU145 cell line was generated using
YAP-siRNA. The results of RT-qPCR, compared with the blank group,
indicated that the expression levels of YAP within
YAP-siRNA-transfected cells were markedly decreased, whereas
Con-siRNA did not affect YAP expression. Reduced YAP protein
expression was also revealed by western blotting in the YAP-siRNA
group (Fig. 2). These data
indicated that YAP-siRNA may effectively inhibit YAP
expression.
Downregulation of YAP reduces the
proliferative ability of DU145 cells
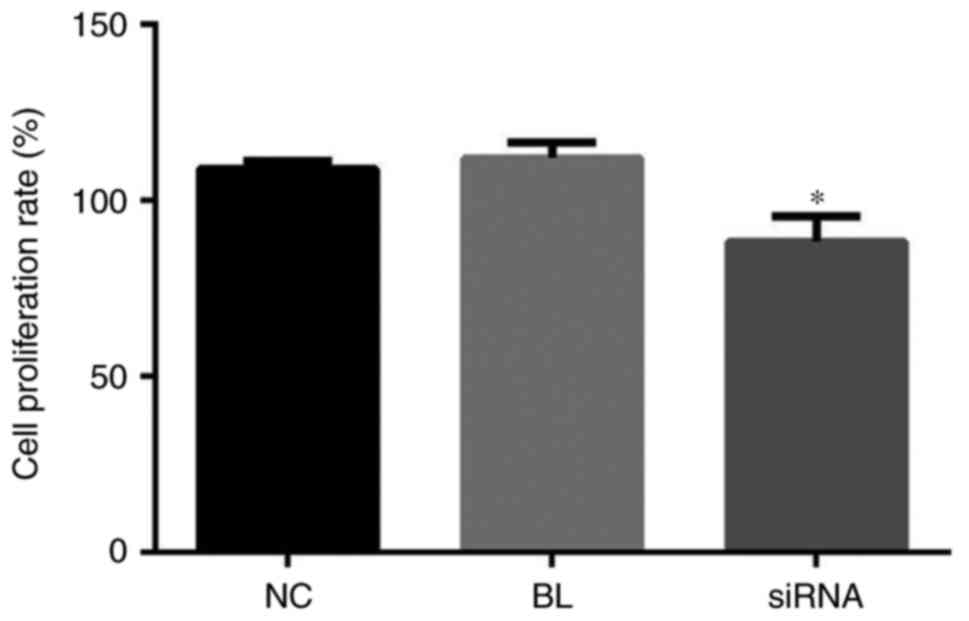
To investigate the effects of YAP expression on
DU145 cell proliferation, YAP-siRNA and Con-siRNA were transfected
into DU145 cells, and an MTT assay was performed. The results of
the present study demonstrated that compared with the blank and
control group, cell proliferation was markedly inhibited in
YAP-siRNA-transfected DU145 cells. Furthermore, colony formation
ability of cells was measured by MTT assay. The results indicated
that YAP knockdown significantly suppressed the DU145 cell
colony-forming ability (Fig. 3).
These results indicated that YAP downregulation reduced the
proliferation and colony-forming ability of DU145 cells.
Downregulation of YAP increases
apoptosis of DU145 cells
The observed growth inhibition in
YAP-siRNA-transfected DU145 cells was investigated using an Annexin
V-FITC apoptosis detection kit for cell apoptosis via flow
cytometry. The results revealed that YAP knockdown significantly
induced DU145 cell apoptosis compared with in the control group
(Fig. 4).
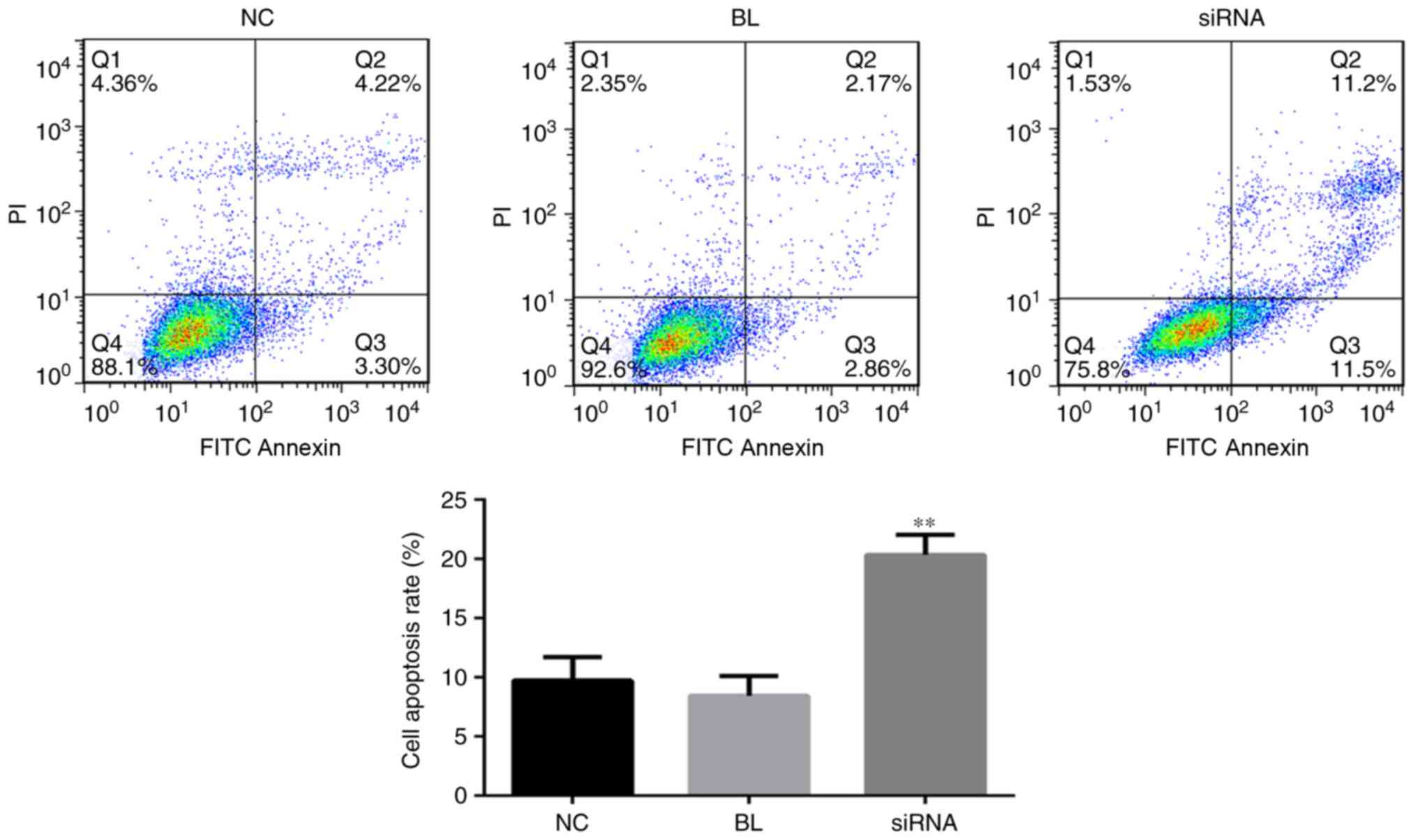 | Figure 4.YAP inhibition induces the apoptosis
of DU145 cells. A total of 24 h after DU145 cells were transfected
with YAP-siRNA or Con-siRNA, flow cytometry was performed to detect
cell apoptosis measuring the sum of cell percentage in quadrants Q2
and Q3. NC, cells transfected with Con-siRNA; BL, cells without any
treatment; siRNA, cells transfected with YAP-siRNA. All data are
presented as the mean ± standard deviation of three independent
experiments. **P<0.01. BL, blank; FITC, fluorescein
isothiocyanate; NC, negative control; PI, propidium iodide; siRNA,
small interfering RNA; YAP, yes-associated protein. |
Downregulation of YAP alters the
expression of CTGF, CYR61, Bcl-2, Bax and caspase 3
CTGF and CYR61 are downstream genes of YAP in the
Hippo signaling pathway, and are regulated by YAP proteins. It has
been reported that CTGF and CYR61 serve important roles in
promoting cell proliferation, migration and invasion in cancer
(21). Following transfection of
DU145 cells with YAP-siRNA, the mRNA and protein expression levels
of CTGF and CYR61 genes were significantly reduced compared with in
Con-siRNA-transfected cells (Fig.
5).
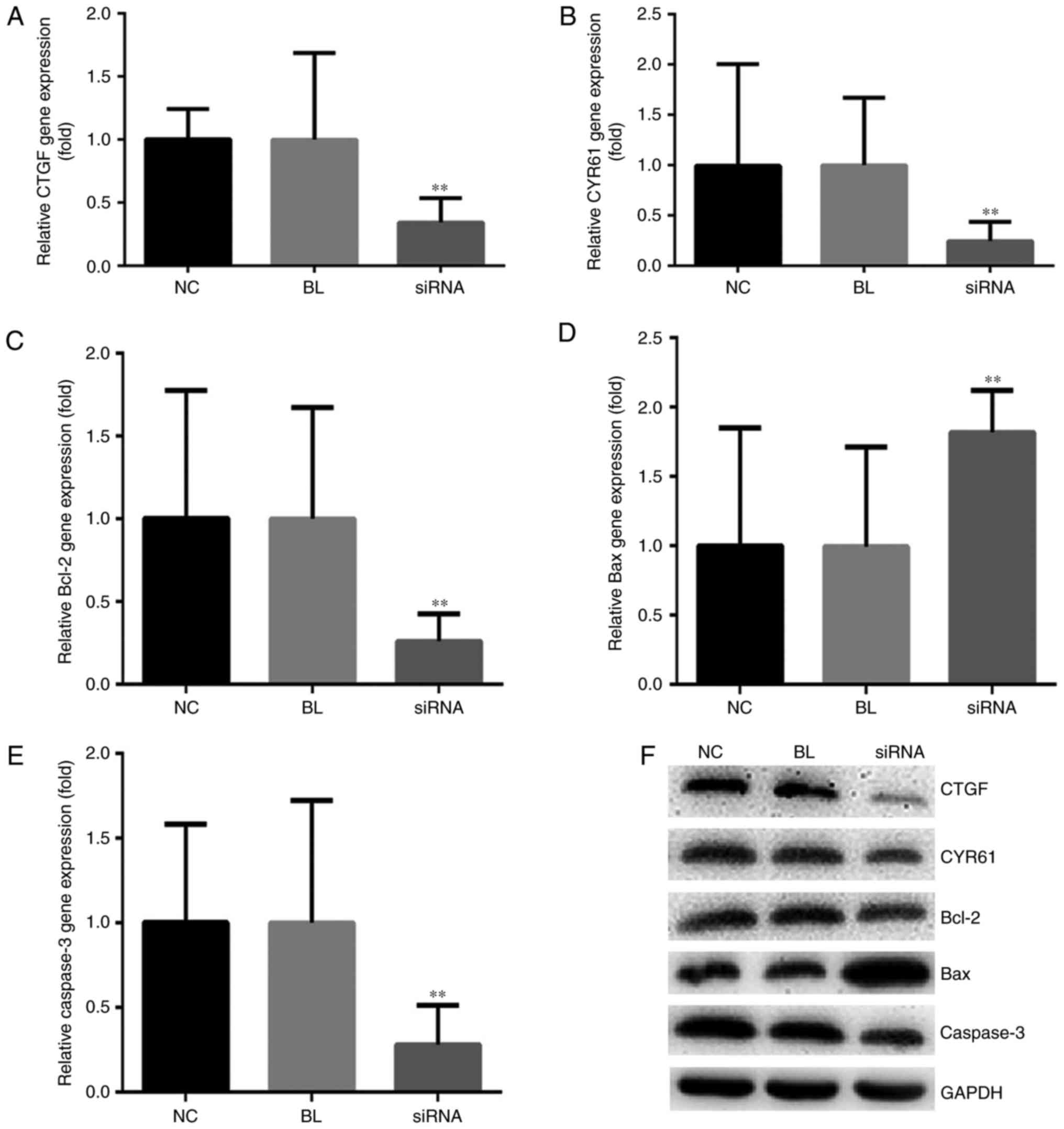 | Figure 5.YAP inhibition alters the expression
of related genes in DU145 cells. DU145 cells were transfected with
YAP-siRNA or Con-siRNA. A total of 24 h after transfection, CTGF,
CYR61, Bcl-2, Bax and caspase 3 expression levels were measured
using (A-E) reverse transcription-quantitative polymerase chain
reaction and (F) western blotting, respectively. NC, cells
transfected with Con-siRNA; BL, cells without any treatment; siRNA,
cells transfected with YAP-siRNA. **P<0.01. Bax, B-cell lymphoma
2-associated X protein; Bcl-2, B-cell lymphoma 2; CTGF, connective
tissue growth factor; CYR61, cysteine-rich angiogenic inducer 61;
siRNA, small interfering RNA; YAP, yes-associated protein. |
Western blotting and RT-qPCR were performed to
further explore the mechanism of YAP knockdown-induced cell
apoptosis through the expression levels of the apoptosis-associated
proteins Bax, Bcl-2 and caspase 3. The results of the present study
suggested that the Bcl-2/Bax ratio was markedly lower in DU145
cells transfected with YAP-siRNA compared with in cells transfected
with Con-siRNA. As expected, the expression levels of caspase 3
were notably decreased following YAP-siRNA transfection (Fig. 5).
Discussion
The major findings of the present study included the
high expression of YAP in human PCa DU145 cells compared with in
normal prostate epithelial RWPE-1 cells. Silencing of the YAP gene
in DU145 cells was associated with reduced proliferative ability
and a significant increase in apoptosis. The expression levels of
tumor-associated genes, including CTGF, CYR61, Bcl-2, BAX and
caspase 3, were also altered in DU145 cells when YAP expression was
knocked down. These findings suggested that YAP may function as an
oncogene in human PCa.
An imbalance between cell proliferation and
apoptosis may result in cancer (22). The Hippo signaling pathway serves
important roles in the regulation of tumor cell and tissue growth.
YAP, which is a core component of the Hippo-YAP signaling pathway,
has been confirmed to be associated with tumor metastasis, grade
and stage (23,24). It has also been reported that the
function of YAP may be dysregulated in numerous cancers, serving
oncogenic and tumor suppressive roles; previous studies have also
indicated that YAP depletion may inhibit the growth and metastasis
of tumor cells (25–30).
In the present study, YAP expression was detected in
human PCa DU145 cells, and the effects of YAP knockdown on the
proliferative ability and apoptosis of DU145 cells were determined
in vitro. The results suggested that YAP was highly
expressed in DU145 cells. However, the proliferative ability of
cells was reduced and cell apoptosis was increased following YAP
downregulation. The underlying mechanism of the regulation of cell
behavior via YAP knockdown was investigated through the detection
of tumor-associated genes. CYR61 and CTGF are matricellular
proteins involved in numerous physiological and pathological
processes, including carcinogenesis, which serve critical roles in
regulating tumor cell growth (31). Following YAP knockdown in DU145
cells, decreased expression levels of CTGF and CYR61 were detected.
Furthermore, the expression levels of apoptosis-associated proteins
(Bax, Bcl-2 and caspase 3) were analyzed. The data revealed that
the Bcl-2/Bax ratio was markedly reduced in YAP-silenced DU145
cells and the expression levels of caspase 3 were significantly
decreased compared with in the control group.
In conclusion, these results indicated that YAP
knockdown suppressed the proliferation, and induced apoptosis of
human PCa DU145 cells. Therefore, the YAP gene may be associated
with tumorigenesis and the development of PCa which may serve as a
potential future treatment target.
Acknowledgements
The authors would like to thank Professor Ming Chen
and Professor Shuqiu Chen (Zhongda Hospital Affiliated to Southeast
University, Nanjing, Jiangsu, China), and their team, for help with
the experiments and data collection.
References
|
1
|
Foley C and Mitsiades N: Moving beyond the
androgen receptor (AR): Targeting AR-interacting proteins to treat
prostate cancer. Horm Cancer. 7:84–103. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen Y, Sawyers CL and Scher HI: Targeting
the androgen receptor pathway in prostate cancer. Curr Opin
Pharmacol. 8:440–448. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhao B, Li L, Lei Q and Guan KL: The
Hippo-YAP pathway in organ size control and tumorigenesis: An
updated version. Genes Dev. 24:862–874. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pan D: The hippo signaling pathway in
development and cancer. Dev Cell. 19:491–505. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Park HW and Guan KL: Regulation of the
Hippo pathway and implications for anticancer drug development.
Trends Pharmacol Sci. 34:581–589. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Harvey KF, Zhang X and Thomas DM: The
Hippo pathway and human cancer. Nat Rev Cancer. 13:246–257. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Johnson R and Halder G: The two faces of
Hippo: Targeting the Hippo pathway for regenerative medicine and
cancer treatment. Nat Rev Drug Discov. 13:63–79. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bertini E, Oka T, Sudol M, Strano S and
Blandino G: YAP: At the crossroad between transformation and tumor
suppression. Cell Cycle. 8:49–57. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Overholtzer M, Zhang J, Smolen GA, Muir B,
Li W, Sgroi DC, Deng CX, Brugge JS and Haber DA: Transforming
properties of YAP, a candidate oncogene on the chromosome 11q22
amplicon. Proc Natl Acad Sci USA. 103:pp. 12405–12410. 2006;
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zender L, Spector MS, Xue W, Flemming P,
Cordon-Cardo C, Silke J, Fan ST, Luk JM, Wigler M, Hannon GJ, et
al: Identification and validation of oncogenes in liver cancer
using an integrative oncogenomic approach. Cell. 125:1253–1267.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Steinhardt AA, Gayyed MF, Klein AP, Dong
J, Maitra A, Pan D, Montgomery EA and Anders RA: Expression of
Yes-associated protein in common solid tumors. Hum Pathol.
39:1582–1589. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fernandez-L A, Northcott PA, Dalton J,
Fraga C, Ellison D, Angers S, Taylor MD and Kenney AM: YAP1 is
amplified and up-regulated in hedgehog-associated medulloblastomas
and mediates Sonic hedgehogdriven neural precursor proliferation.
Genes Dev. 23:2729–2741. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu MZ, Yao TJ, Lee NP, Ng IO, Chan YT,
Zender L, Lowe SW, Poon RT and Luk JM: Yes-associated protein is an
independent prognostic marker in hepatocellular carcinoma. Cancer.
115:4576–4585. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim
J, Xie J, Ikenoue T, Yu J, Li L, et al: Inactivation of YAP
oncoprotein by the Hippo pathway is involved in cell contact
inhibition and tissue growth control. Genes Dev. 21:2747–2761.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang X, George J, Deb S, Degoutin JL,
Takano EA, Fox SB; AOCS Study group, ; Bowtell DD and Harvey KF:
The Hippo pathway transcriptional co-activator, YAP, is an ovarian
cancer oncogene. Oncogene. 30:2810–2822. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang J, Xu ZP, Yang YC, Zhu JS, Zhou Z
and Chen WX: Expression of Yes-associated protein in gastric
adenocarcinoma and inhibitory effects of its konckdown on gastric
cancer cell proliferation and metastasis. Int J Immunopathol
Pharmacol. 25:583–590. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang W, Nandakumar N, Shi Y, Manzano M,
Smith A, Graham G, Gupta S, Vietsch EE, Laughlin SZ, Wadhwa M, et
al: Downstream of mutant KRAS, the transcription regulator YAP is
essential for neoplastic progression to pancreatic ductal
adenocarcinoma. Sci Sig. 7:ra422014. View Article : Google Scholar
|
|
18
|
Zhang L, Yang S, Chen X, Stauffer S, Yu F,
Lele SM, Fu K, Datta K, Palermo N, Chen Y and Dong J: The Hippo
pathway effector YAP regulates motility, invasion, and
castration-resistant growth of prostate cancer cells. Mol Cell
Biol. 35:1350–1362. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sheng X, Li WB, Wang DL, Chen KH, Cao JJ,
Luo Z, He J, Li MC, Liu WJ and Yu C: YAP is closely correlated with
castration-resistant prostate cancer, and downregulation of YAP
reduces proliferation and induces apoptosis of PC-3 cells. Mol Med
Rep. 12:4867–4876. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Planque N and Perbal B: A structural
approach to the role of CCN (CYR61/CTGF/NOV) proteins in
tumourigenesis. Cancer Cell Int. 3:152003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lamar JM, Stern P, Liu H, Schindler JW,
Jiang ZG and Hynes RO: The Hippo pathway target, YAP, promotes
metastasis through its TEAD-interaction domain. Proc Natl Acad Sci
USA. 109:pp. E2441–E2450. 2012; View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dong J, Feldmann G, Huang J, Wu S, Zhang
N, Comerford SA, Gayyed MF, Anders RA, Maitra A and Pan D:
Elucidation of a universal size-control mechanism in Drosophila and
mammals. Cell. 130:1120–1133. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhao B, Li L, Wang L, Wang CY, Yu J and
Guan KL: Cell detachment activates the Hippo pathway via
cytoskeleton reorganization to induce anoikis. Genes Dev. 26:54–68.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yu FX, Zhao B, Panupinthu N, Jewell JL,
Lian I, Wang LH, Zhao J, Yuan H, Tumaneng K, Li H, et al:
Regulation of the Hippo-YAP pathway by G-Protein-Coupled receptor
signaling. Cell. 150:780–791. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li W, Wang L, Katoh H, Liu R, Zheng P and
Liu Y: Identification of a tumor suppressor relay between the FOXP3
and the Hippo pathways in breast and prostate cancers. Cancer Res.
71:2162–2171. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cordenonsi M, Zanconato F, Azzolin L,
Forcato M, Rosato A, Frasson C, Inui M, Montagner M, Parenti AR,
Poletti A, et al: The Hippo transducer TAZ confers cancer stem
cell-related traits on breast cancer cells. Cell. 147:759–772.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xia Y, Chang T, Wang Y, Liu Y, Li W, Li M
and Fan HY: Correction: YAP promotes ovarian cancer cell
tumorigenesis and is indicative of a poor prognosis for ovarian
cancer patients. PLoS One. 11:e01527122016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ehmer U and Sage J: Control of
proliferation and cancer growth by the Hippo signaling pathway. Mol
Cancer Res. 14:127–140. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhou Z, Zhu JS, Gao CP, Li LP, Zhou C,
Wang H and Liu XG: siRNA targeting YAP gene inhibits gastric
carcinoma growth and tumor metastasis in SCID mice. Oncol Lett.
11:2806–2814. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cheng TY, Wu MS, Hua KT, Kuo ML and Lin
MT: Cyr61/CTGF/Nov family proteins in gastric carcinogenesis. World
J Gastroenterol. 20:1694–1700. 2014. View Article : Google Scholar : PubMed/NCBI
|