Introduction
Ischemic heart disease within developed countries
has been associated with high rates of morbidity and mortality
(1). Heart failure following acute
myocardial infarction may arise from a marked loss of
cardiomyocytes due to the reduced regenerative ability of adult
cardiomyocytes; however, a previous study reported that cell-based
cardiac repair may serve as a novel treatment with promising
results (2). A variety of cell
types have previously been employed to investigate the underlying
mechanism of heart tissue repair (3). The ideal cell type for
transplantation constitutes a cardiomyocyte with natural
electrophysiological, structural and contractile properties.
Pluripotent stem cells (PSCs) as a source of cardiomyocytes may be
considered as a unique and infinite source of cells for the
development of regenerative medicine. Embryonic stem cells (ESCs)
have been reported to produce bona fide cardiomyocytes
(4,5); however, the use of ESCs in a clinical
setting has been associated with tumor formation, cellular
rejection and various ethical issues. To revert cardiomyocytes into
the induced (i)PSC form, four transcription factors may be employed
(6,7) to produce a source of autologous SCs;
the use of iPSCs may avoid the risk of immune rejection, however,
teratoma formation may occur.
In contrast to pluripotent reprogramming, direct
reprogramming of somatic cells, including easily accessible
adipocytes and dermal fibroblasts into therapeutic cell types
exhibiting tissue-specific transcription factor overexpression, may
serve as a valuable source of cells suitable for direct clinical
application (8–12). Brahma-associated factor 60c, a
chromatin remodeling factor, in combination with GATA binding
protein 4 (GATA4) and T-box transcription factor 5 (TBX5) was
reported to induce the trans-differentiation of the mouse
mesoderm intocardiac tissue (13).
It has been reported that postnatal cardiac or dermal fibroblasts
may be rapidly and efficiently reprogrammed into differentiated
cardiomyocyte-like cells, with a combination of three
cardiac-specific transcription factors, namely, GATA4,
myocyte-specific enhancer factor 2C (MEF2C), and TBX5 (GMT)
(9). Additionally, fibroblasts may
exhibit a cardiomyocyte-like phenotype via a combination of
microRNAs1, 133, 208 and 499, in vitro and in vivo
(14). These strategies exclude
the risk of teratoma formation as cells are not reverted to a
pluripotent stage; lineage reprogramming may be used to convert
cell fate in situ (15,16).
Progress towards cardiac reprogramming has been attained in
previous studies (17,18); however, the effects of induced
cardiomyocytes (iCMs) remain to be investigated. Further
investigation is required to identify unknown combinations of
transcription factors that exhibit greater potency in cardiac
reprogramming. Lineage reprogramming technology may convert
fibroblasts into iCMs; however, the percentage of fully
reprogrammed cardiomyocytes from fibroblasts was low in
vitro. Applying these findings to clinical practice is
dependent upon the improvement of cardiomyocyte enrichment, as
contaminated cell types may markedly attenuate drug responses and
other functional properties in vitro and in vivo
(19).
The aims of the present study were to investigate
the optimal combination of key cardiac transcription factors to
effectively reprogram mouse fibroblasts into iCMs and to determine
whether iCMs may be enriched via Percoll density centrifugation.
The combination of the four transcription factors, GMT with heart-
and neural crest derivatives-expressed protein 2 (HAND2; GMTH), was
compared with the combination of GMT within mouse fibroblasts. iCMs
reprogrammed from GMTH-transduced fibroblasts were successfully
enriched via Percoll density centrifugation. Enriched iCMs
constituted ≥72.4±5.5% α-MHC+ cells, which retained the
cardiac phenotype, and may serve as a valuable source of cells for
the development of cardiovascular disease research and clinical
treatment, with minimized risks of immune rejection and tumor
formation.
Materials and methods
Plasmid construction
pWPT lentiviral vectors (plasmid cat. no. 12255;
Addgene, Inc., Cambridge, MA, USA) were constructed using the
coding regions of mouse GATA4, MEF2C, TBX5 and
HAND2 genes, which were amplified via reverse
transcription-polymerase chain reaction (RT-PCR, RR014A; Takara
Biotechnology Co., Ltd., Dalian, China). The primers sequences used
are listed in Table I, and
denaturation at 94°C for 30 sec, annealing at 60°C for 30 sec,
extension at 72°C for 2 min, and 35 cycles were used. The sequences
were inserted into the BamHI/SalI or
MluI/SalI site of the pWPT plasmid. A Kozak sequence,
GCCACC, was inserted prior to the ATG codon; amplification of the
target cDNA fragment was achieved using a 5′-primer containing a
Kozak sequence and an ATG codon, and a 3′-primer with unique
restriction sites. Primer sequences were introduced into the
multiple cloning site of the pWPT lentiviral vector; a stop codon
at the 3′-end of the target sequence was omitted.
 | Table I.Primer sequences for reverse
transcription-polymerase chain reaction to amplify the candidate
genes. |
Table I.
Primer sequences for reverse
transcription-polymerase chain reaction to amplify the candidate
genes.
| Genes | Sequence
(5′-3′) |
|---|
| GATA4 | F:
GATCGGATCCGCCACCATGTACCAAAGCCTGGCCATGGC |
|
| R:
GATCGTCGACCGCGGTGATTATGTCCCCATGACT |
| MEF2C | F:
GATCGGATCCGCCACCATGGGGAGAAAAAAGATTCAGATTACG |
|
| R:
GATCGTCGACTGTTGCCCATCCTTCAGAGAG |
| TBX5 | F:
GATCGGATCCGCCACCATGGCCGATACAGATGAGGG |
|
| R:
GATCGTCGACGCTATTCTCACTCCACTCTGG |
| HAND2 | F:
GATCGGATCCGCCACCATGAGTCTGGTGGGGGGCTTTC |
|
| R:
GATCGTCGACCTGCTTGAGCTCCAGGGCCC |
Cell culture
The tail-tip fibroblasts (TTFs) used in the present
study were isolated from 10 male ICR mice (5 day-old). Briefly,
mice were anesthetized with 2% diethyl ether for 15 sec, and tails
were removed and then rinsed in ethanol followed by PBS. The tissue
was minced, transferred into a solution constituting 0.25% trypsin,
0.2% EDTA and 0.1% collagenase (Roche Diagnostics, Basel,
Switzerland), and incubated at 37°C for 1 h. Following
centrifugation at 500 × g at room temperature for 5 min, isolated
cells were resuspended in Dulbecco's modified Eagle's medium [DMEM;
containing 10% fetal bovine serum (FBS); Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA] and seeded onto 100 mm dishes.
TTFs of passages 2 to 3 were employed for cardiomyocyte induction.
293T cells (CRL-3216; American Type Culture Collection, Manassas,
VA, USA), which were used to produce lentiviruses, were maintained
in DMEM (containing 10% FBS; Gibco; Thermo Fisher Scientific,
Inc.). All procedures with experimental animals were approved by
the Institutional Animal Care and Use Committee of Chinese PLA
General Hospital (Beijing, China) and carried out according to the
guidelines of China Council on Animal Care and Use (permit no.
2014-X9-09).
Lentivirus production
Prior to transduction, 293T cells were seeded at
8×106 cells/100 mm dish. On the subsequent day, pWPT
lentiviral vectors were transduced into 293T cells using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) transfection reagent according to the
manufacturer's instructions. A total of 35 µl transfection reagent,
8 µg lentiviral expression plasmid, 4 µg psPAX2 and 2 µg pMD2.G
(all from Addgene, Inc.) were diluted in 3 ml Opti-MEM®
I medium (Thermo Fisher Scientific, Inc.), and then added dropwise
onto 293T cells of ~85% confluence; cells were maintained in 10 ml
fresh DMEM on 100 mm dishes. pWPT-GFP (Addgene, Inc.) was used as a
negative control. At 48 and 72 h following transduction, the viral
supernatants were collected, filtered using a 0.45 µm pore size
filter (EMD Millipore, Billerica, MA, USA) and concentrated 30-fold
with PEG-it™ Virus Precipitation Solution (System
Biosciences, Palo Alto, CA, USA). They were centrifuged at 1,500 ×
g at 4°C for 30 min, and subsequently resuspended in PBS.
Preparation of iCMs
Mouse TTFs were seeded onto gelatin-coated 100 mm
dishes at 1.5×104 cells/cm2. Following
initial cell culture, the culture medium was replaced with 10 ml
fresh DMEM containing 10% FBS and 10 µg/ml polybrene
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)-this was classed as
day 0. Prior to overnight incubation at 37°C, the same volume (500
µl) of supernatant containing each of the GMTH lentiviruses were
mixed and transferred to the dishes. In the control group, TTFs
were untransduced or transduced with green fluorescent protein
(GFP) lentivirus (Addgene, Inc.) On day 1, the medium of certain
plates of GMTH-transduced TTFs was replaced with 10 ml cardiac
inductive medium (CIM) constituting DMEM/medium199 (4:1), 10% FBS,
10% conditioned medium obtained from neonatal mouse cardiomyocyte
culture, 1% non-essential amino acids, vitamin mixture, 5% horse
serum, B-27, insulin-selenium-transferrin and sodium pyruvate
(Invitrogen; Thermo Fisher Scientific, Inc.). GMTH-transduced cells
were not treated with CIM as control. The medium was replaced every
2 days. Conditioned medium was filtered usinga 0.22 µm pore size
filter (EMD Millipore).
Percoll density centrifugation
TTFs (1.5×106) treated with CIM following
GMTH transduction were dissociated, resuspended and loaded onto a
discontinuous Percoll gradient on day 9. Percoll (GE Healthcare,
Chicago, IL, USA) was diluted in a buffer solution containing 150
mM NaCl and 20 mM 4-(2-hydroxyethyl)-1-piperazineethanesulphonic
acid. The gradient consisted of a 40.5% Percoll layer above a layer
of 58.5% Percoll; cell layers were observed following
centrifugation for 30 min at 1,500 × g at room temperature. Cells
of different layers were collected, washed with PBS and resuspended
in DMEM medium. They were then plated for immunostaining, or
collected for reverse transcription (RT)-qPCR analysis. The
fractionated cells were seeded onto chamber slides, cultured for an
additional few days and immunostained prior to immunocytochemical
analysis.
RT-semi (s)-qPCR and qPCR
Total RNA was isolated from cultured cells (TTFs,
TTFs + GMT and TTFs + GMTH) using TRIzol (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions. For
RT-sqPCR, cDNA was synthesized with RevertAid First Strand cDNA
Synthesis kit (Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. PCR analyses were performed with 2X
Taq PCR MasterMix (Tiangen Biotech Co., Ltd., Beijing, China)
according to the manufacturer's instructions. Thermocycling
condition were: Denaturation at 94°C for 30 sec, annealing at 58°C
for 30 sec, extension at 72°C for 30 sec, for 30 cycles. The
products were run in 1% agarose gel with ethidium bromide, and
images were captured with Gel Doc™ XR+Gel Documentation system
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). qPCR was performed
with the Rotor-Gene® 2000 system using the QuantiTect
SYBR Green one-step RT-PCR kit and QuantiTect Primer Assay (both
from Qiagen Co., Ltd., Shanghai, China) according to the
manufacturer's instructions. The thermocycling conditions were as
follows: 95°C for 15 sec, 60°C for 1 min, for 40 cycles. The
results were analyzed using the 2−ΔΔCq method (20) with GAPDH as a reference gene and
GFP-transduced TTF cells as a calibrator. Primer sequences are
presented in Table II.
 | Table II.Primer sequences for
semi-quantitative and quantitative polymerase chain reaction. |
Table II.
Primer sequences for
semi-quantitative and quantitative polymerase chain reaction.
| Gene | Forward sequence
(5′-3′) | Backward sequence
(5′-3′) |
|---|
| α-MHC |
ACCGTGGACTACAACAT |
CTTTCGCTCGTTGGGA |
| β-MHC |
ACCCCTACGATTATGCG |
GTGACGTACTCGTTGCC |
| ANF |
GGGGGTAGGATTGACAGGAT |
CAGAGTGGGAGAGGCAAGAC |
| NKX2.5 |
AGCAACTTCGTGAACTTTG |
CCGGTCCTAGTGTGGA |
| CTNT |
TGAGAGGAGGAAGGTGCTGG |
CGCGGGTCTTGGAGACTTTC |
| GJA1 |
CTTTCATTGGGGGAAAGGCG |
AGCGAAAGGCAGACTGTTCA |
| COL1A2 |
CACCCCAGCGAAGAACTCAT |
TCTCCTCATCCAGGTACGCA |
| GAPDH |
AACGACCCCTTCATTGAC |
TCCACGACATACTCAGCAC |
Immunocytochemistry
Immunostaining was performed as previously described
(21). Briefly, transduced and
untransduced TTF cells were rinsed with PBS and fixed with 4%
paraformaldehyde for 15 min at room temperature. The fixed cells
were washed with PBS three times and subsequently treated with 0.5%
Triton X-100 for 30 min at room temperature. Unspecific binding
sites were blocked by 5% bovine serum albumin (Sigma-Aldrich; Merck
KGaA) for 30 min at room temperature. The primary antibodies
against GATA4 (1:100, cat. no. sc-25310), MEF2C (1:100, cat. no.
sc-13268), TBX5 (1:100, cat. no. sc-376952), HAND2 (1:100, cat. no.
sc-9409), α-myosin heavy chain (MHC, 1:100, cat. no. sc-20641) (all
from Santa Cruz Biotechnology, Inc., Dallas, TX, USA), α-sarcomeric
actinin (1:100, cat. no. BM0003; Boster Biological Technology,
Wuhan, China) and cardiac troponin T (cTnT, 1:200, cat. no.
MAB-0374; Fuzhou Maixin Biotechnology, Fuzhou, China) were applied
without washing and incubated at 4°C overnight. Following washing
with PBS, cells were incubated with the secondary antibodies,
fluorescein isothioyanate (FITC)-labeled or phalloidin-tetramethyl
rhodamine-labeled goat anti-mouse immunoglobulin G (IgG, 1:50;
OriGene Technologies, Inc., Rockville, MD, USA) in the dark at room
temperature for 2 h. Nuclei were stained with 0.1 µg/ml DAPI
(Sigma-Aldrich; Merck KGaA) for 15 min at room temperature. Images
were captured by an inverted phase contrast fluorescence microscopy
(Olympus Corporation, Tokyo, Japan) and confocal laser scanning
microscopy (Zeiss GmbH, Jena, Germany).
Flow cytometric analysis
Cells were washed with PBS, dissociated with 0.25%
trypsin and 0.04% EDTA, and then filtered through a 35 µm
cell-strainer to remove cell clumps. The resulting single cell
suspensions were centrifuged at 300 × g for 5 min at room
temperature, resuspended in 1 ml PBS, pipetted into 1.5 ml
centrifuge tubes, further centrifuged at 400 × g for 5 min at room
temperature, and resuspended in 1 ml 4% paraformaldehyde at room
temperature for 10 min. Cells were collected by centrifugation at
400 × g for 5 min at room temperature and rinsed with PBS three
times, and were then resuspended in 0.5% Triton X-100 for 30 min at
room temperature. Subsequently, TTFs were stained with Anti-Myosin
Heavy Chain Alexa Fluor® 488 (1:100, cat. no.
53-6503-82; eBioscience; Thermo Fisher Scientific, Inc.) for 30 min
at 37°C, followed by three washes with PBS and centrifugation at
400 × g for 5 min at room temperature. The isotype control antibody
was FITC-conjugated mouse IgG1; TTF pellets were resuspended in 500
µl PBS to be detected with flow cytometer (FACSCalibur; BD
Biosciences, Franklin Lakes, NJ, USA) for flow cytometric analysis
(CellQuest Pro software version 5.1; BD Biosciences).
Statistical analyses
Results were repeated three times and expressed as
the mean ± standard deviation. Differences between groups were
examined by Student's t-test for statistical significance using
SPSS software version 4.0 (SPSS Inc., Chicago, IL, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
Generation of lentiviruses encoding
individual cardiac transcription factors
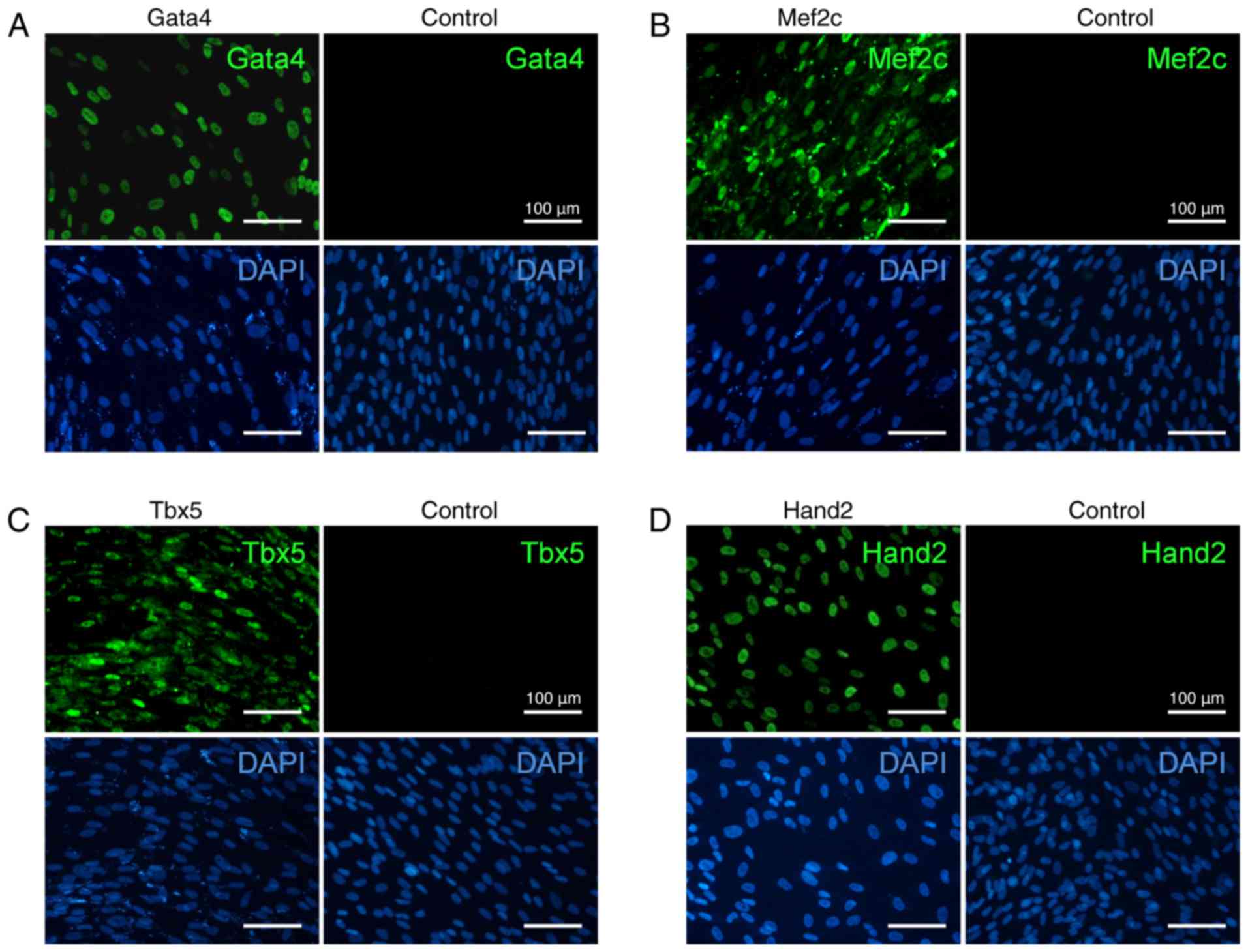
The generated individual lentiviruses efficiently
expressed each of the four cardiac-specific transcription factors
GATA4, MEF2C, TBX5 and HAND2 within mouse TTFs. Transduced or
untransduced TTFs were examined on day 2 post-transduction, and the
majority of transduced cells expressed cardiac transcription
factors. Immunofluorescence staining for GMTH proteins demonstrated
their nuclear localization (Fig.
1), and viral transduction efficiency was detected to be
>96%.
Reprogramming of TTFs into
cardiac-like myocytes
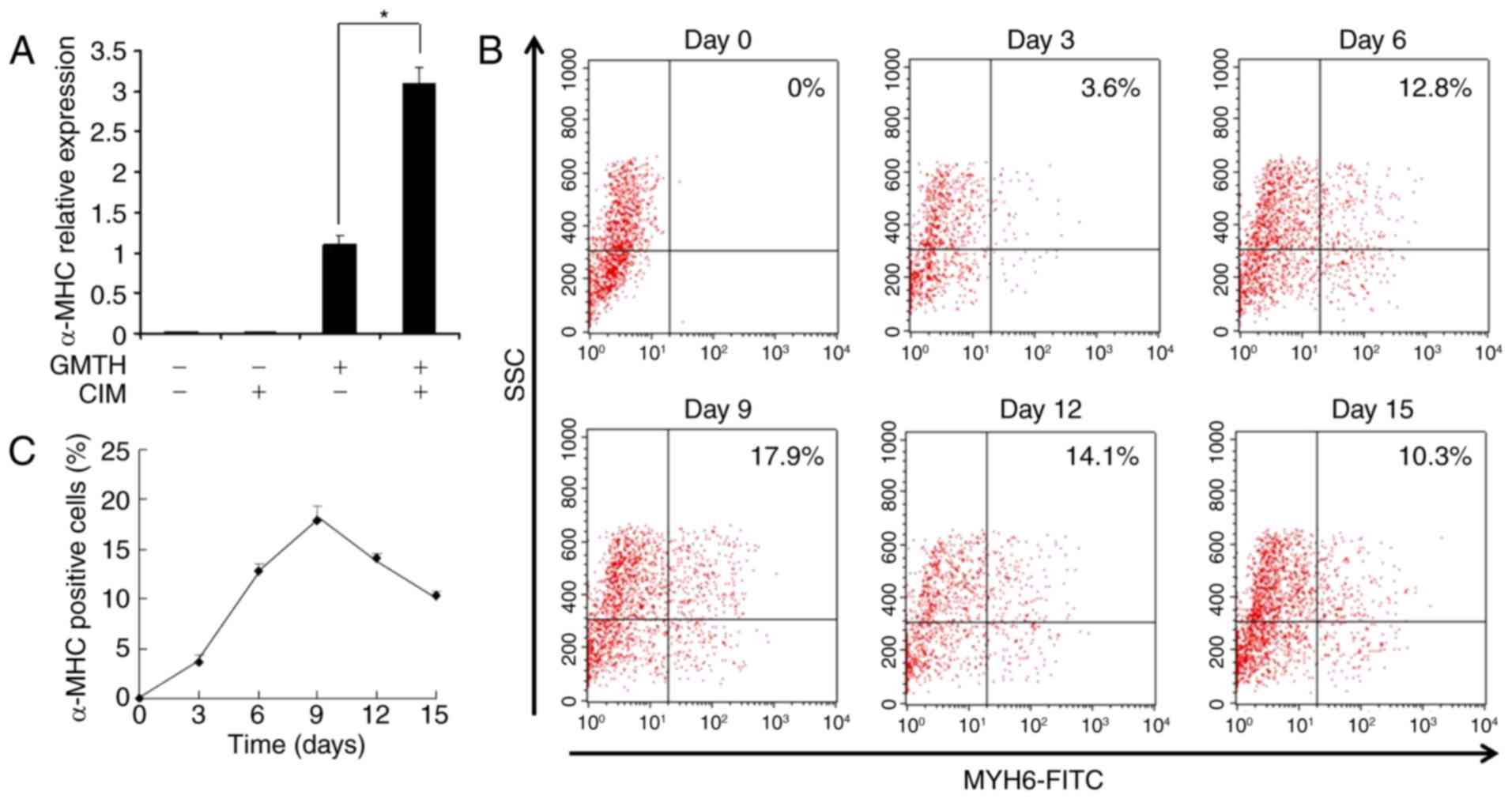
The effects of GMT were compared with those of GMTH
in the present study. RT-PCR revealed that cardiac-specific genes
were not expressed within the control TTFs compared with the GMT-
and GMTH-transduced TTFs. The expression of some cardiac-specific
genes within the GMT-transduced TTFs were detected; however, α-MHC
expression was not detected 14 days post-transduction. Conversely,
GMTH-transduced TTFs demonstrated increased expression levels of
cardiomyocyte markers, including α-MHC, β-MHC, atrial natriuretic
factor, NK2 homeobox 5 (Nkx2.5) and cTnT compared with GMT
transduction (Fig. 2A). Flow
cytometry analysis confirmed that ~3.6% of GMTH-transduced TTFs
were positive for α-MHC expression; however, α-MHC+ TTFs
were not detected two weeks following GMT-transduction (Fig. 2B). Additionally, immunofluorescence
staining indicated that some GMTH-transduced TTFs expressed α-MHC,
compared with in the control and GMT-transduced groups, in which no
positive cells were detected (Fig.
2C).
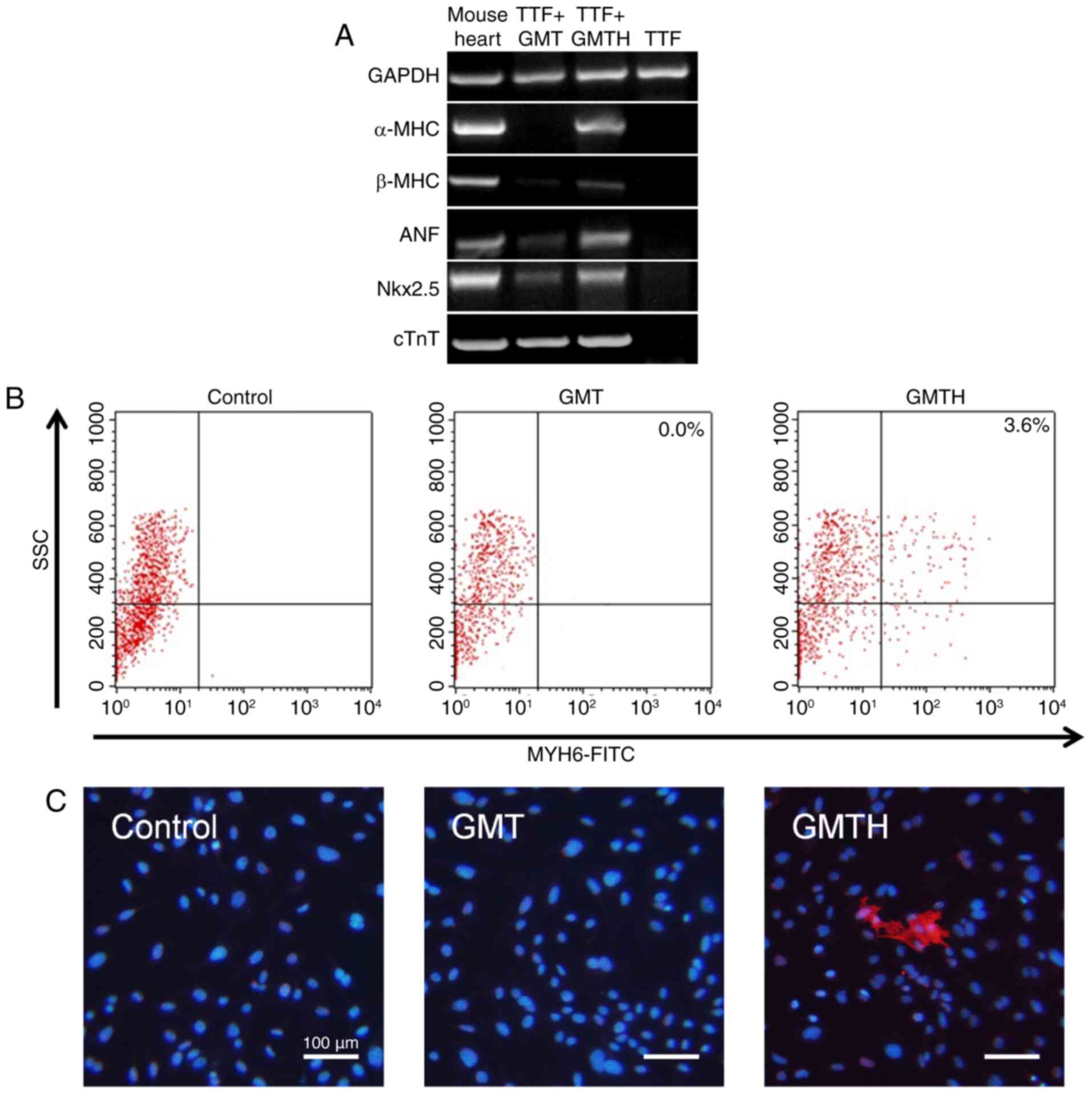 | Figure 2.Reprogramming of TTFs into
cardiac-like myocytes. (A) Semi-quantitative polymerase chain
reaction analysis of cardiac-specific genes in mouse heart tissue,
GMT-transduced TTFs, GMTH-transduced TTFs and the control TTFs two
weeks post-transduction. (B) Flow cytometric analysis of α-MHC
expression in TTFs only or those transduced with GMT or GMTH. (C)
Immunofluorescent staining of the control TTFs, GMT-infected and
GMTH-infected TTFs for α-MHC (red) and DAPI (blue). Scale bars, 100
µm. α-MHC, α-myosin heavy chain; GMT, GATA binding protein 4,
myocyte-specific enhancer factor 2C and T-box transcription factor
5; GMTH, GATA binding protein 4, myocyte-specific enhancer factor
2C, T-box transcription factor 5 and heart- and neural crest
derivatives-expressed protein 2; TTFs, tail-tip fibroblasts; ANF,
atrial natriuretic peptide; Nkx2.5, NK2 homeobox 5; cTnT, cardiac
troponin T; SSC, side scattered light. |
Optimization of cardiac reprogramming
by GMTH induction
To enhance the productivity of α-MHC+
cells, CIM was employed. CIM treatment stimulated the expression of
α-MHC within GMTH-transduced TTFs by 3-fold, when compared with in
untreated GMTH-transduced TTFs (Fig.
3A); no effect was detected in control TTFs. The duration of
cardiomyocyte induction from TTFs was analyzed by flow cytometry.
α-MHC+ cells were detected as early as 3 days following
GMTH induction via CIM treatment, the number of α-MHC+
cells slowly increased to ≤18% at day 9 (Fig. 3B). The percentage of
α-MHC+ cells peaked at day 9 post-transduction, and
declined thereafter due to overgrowth of non-reprogrammed
fibroblasts (Fig. 3C).
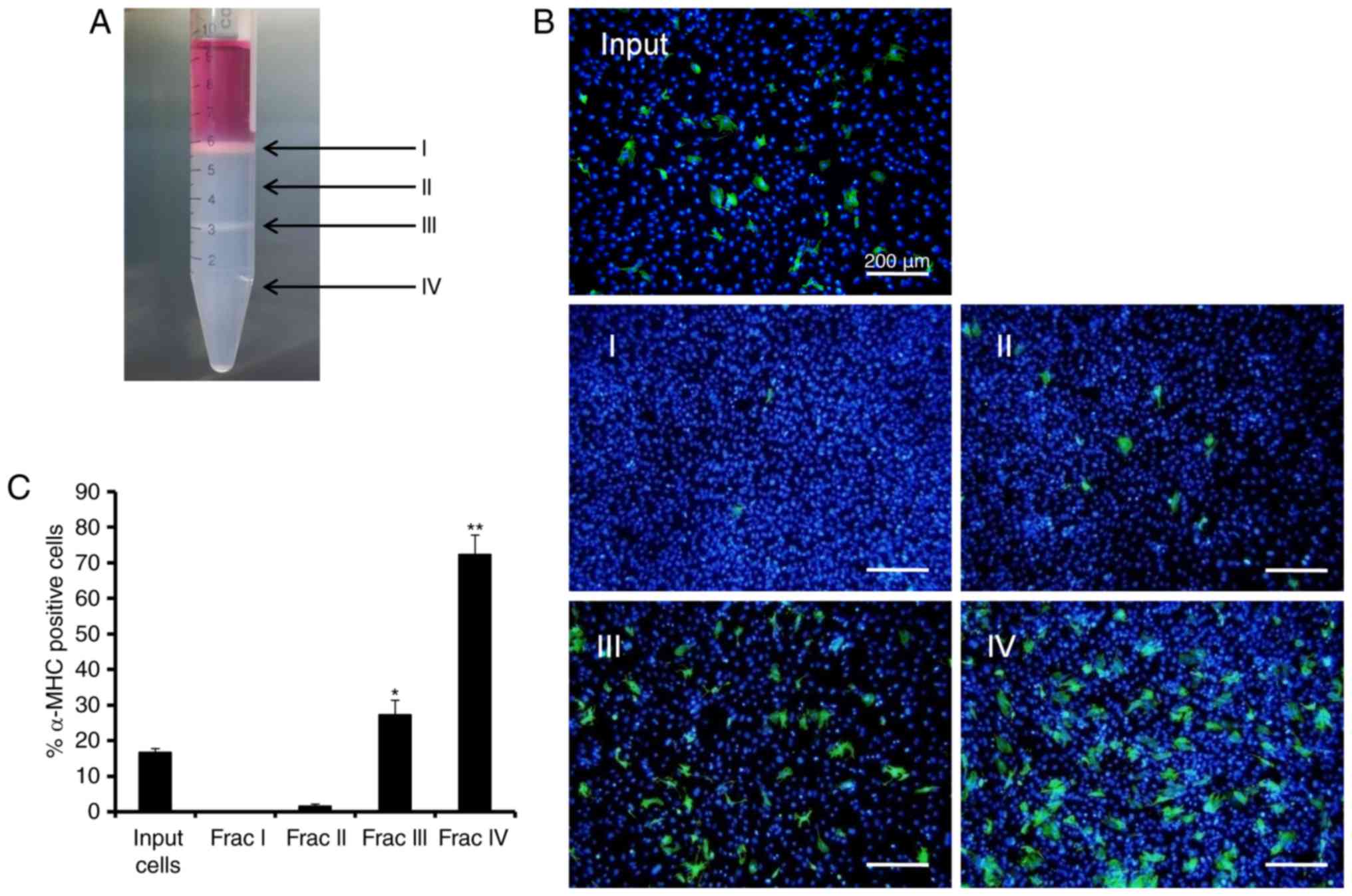
Enrichment of induced cardiomyocytes
using discontinuous Percoll gradients
CIM-treated TTFs at day 9 post-GMTH transduction
were collected and transferred to a discontinuous Percoll gradient
(40.5 over 58.5%). Two layers of cells were generated following
centrifugation (Fig. 4A). One
layer was observed above the layer of Percoll (fraction I), and the
second layer observed was between the two layers of Percoll
(fraction III). The input cells, and cells within the 40.5% Percoll
layer (fraction II) and the 58.5% Percoll layer (fraction IV) were
collected for α-MHC immunocytochemical staining (Fig. 4B). Quantitative detection of
triplicate samples revealed that fraction III contained 27.2±4.1%
α-MHC+ cells and fraction IV contained 72.4±5.5%
α-MHC+ cells, while fraction I and II contained only 0.1
to 1.5% α-MHC+ cells. When compared with the starting
input that constituted 14.7±1.1% α-MHC+ cells, fraction
IV revealed a 3.6-fold enrichment of α-MHC+ cells
(Fig. 4C). These results
demonstrated a marked enrichment of cardiomyocytes using the method
of discontinuous Percoll gradient centrifugation.
qPCR analysis of Percoll-enriched
cells
The GMTH-transduced cells were collected from
fractions III and IV following Percoll separation, and the cardiac
gene expressions were compared among TTFs, transduced and
unenriched cells, transduced and enriched cells, and neonatal mouse
cardiomyocytes. qPCR analysis of gene expression patterns revealed
that several cardiac-specific genes were upregulated; however, the
fibroblast marker gene, collagen type 1 a2 (COL1A2), was
inhibited in Percoll-enriched, GMTH transduced cells (Fig. 5F). Higher expressions of cardiac
genes myosin heavy chain 6 (MYH6), troponin 2
(TNNT2), NKX2-5, natriuretic peptide A (NPPA),
and gap junction α1 (GJA1) were observed in the enriched
cells when compared with transduced and unenriched cells; however,
they were not detected in TTFs by qPCR (Fig. 5A-E). These data indicated that iCMs
could be enriched from GMTH-transduced TTFs. Enriched iCMs had an
expression pattern similar to neonatal ventricular cardiomyocytes,
and full maturation may be a slow course that occurred over a few
of weeks.
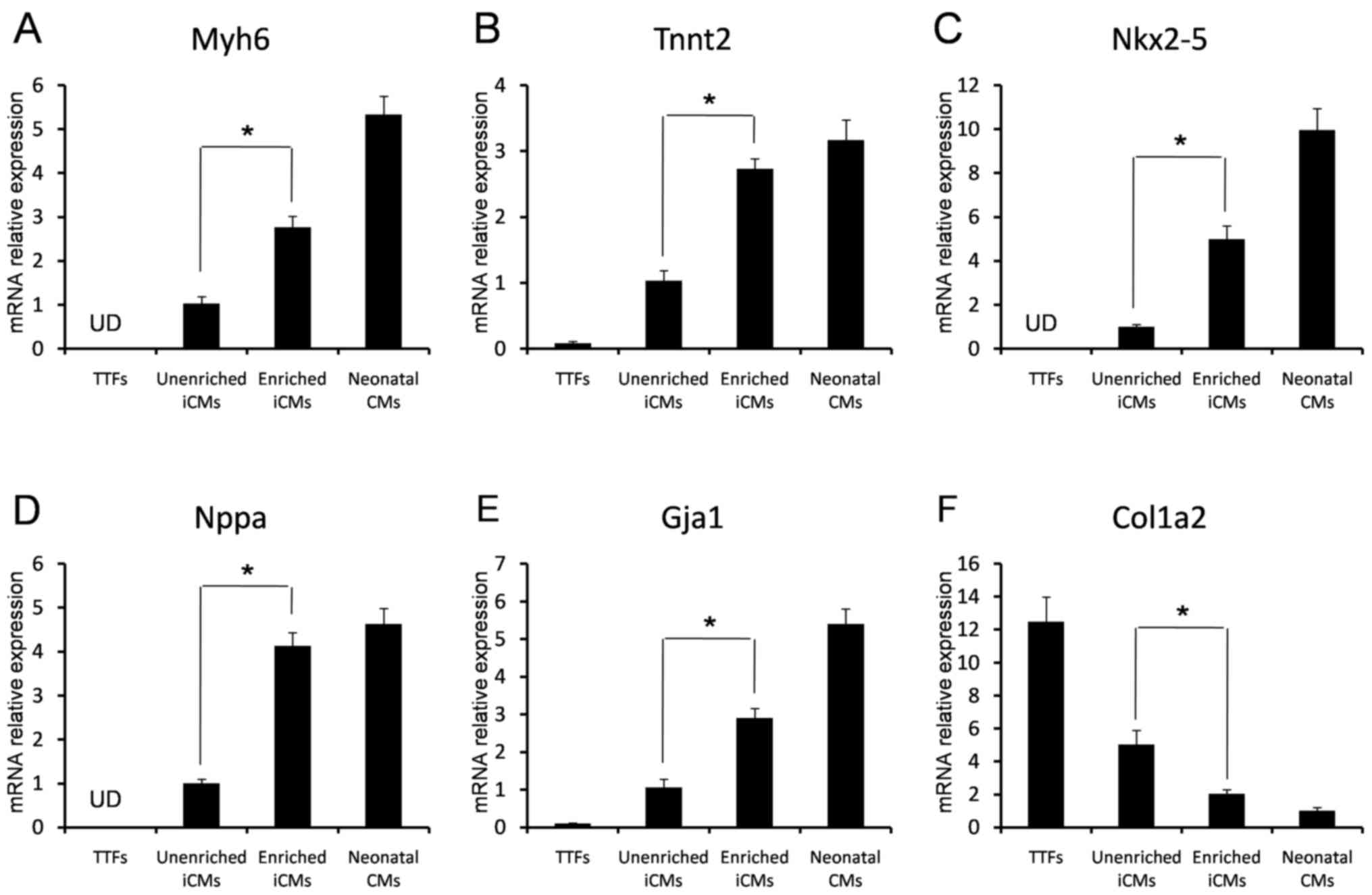 | Figure 5.Analysis of cardiac and fibroblast
gene expression by reverse transcription-qPCR. The (A) Myh6, (B)
Tnnt2, (C) Nkx2-5, (D) Nppa, (E) Gja1 and (F) Col1a2 relative mRNA
expression levels of TTFs, transduced and unenriched cells,
enriched cells from fractions III and IV, and neonatal mouse
cardiomyocytes, were determined by qPCR (n=3). *P<0.05. TTFs,
tail-tip fibroblasts; iCMs, induced cardiomyocytes; Myh6, myosin
heavy chain 6; Tnnt2, troponin 2; Nkx2-5, NK2 homeobox 5; Nppa,
natriuretic peptide A; Gja1, gap junction α1; Col1a2, collagen type
1 a2; qPCR, quantitative polymerase chain reaction. |
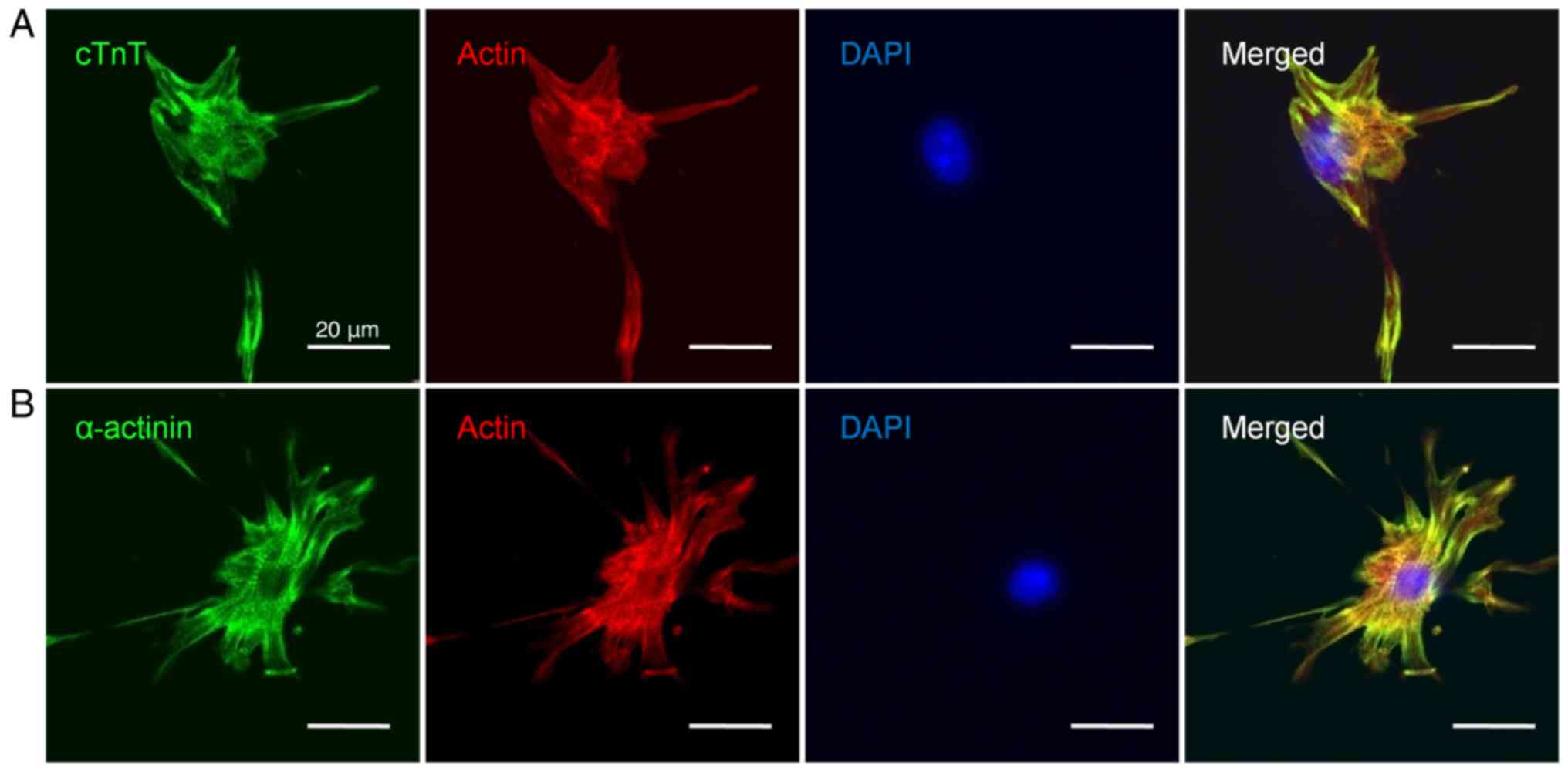
Immunocytochemical analysis of
Percoll-enriched cells
To characterize the Percoll-enriched cell
populations, immunocytochemistry was performed using antibodies
against cTnT and α-actinin. Confocal laser scanning microscopy
revealed that Percoll-enriched cells had strong immunofluorescence
for the sarcomeric proteins cTnT and α-actinin, and it confirmed
the extent of differentiation. The enriched cells exhibited typical
cardiomyocyte morphology and possessed distinct myofilaments. The
well-developed sarcomeric structures were observed by cTnT and
α-actinin immunocytochemistry (Fig.
6). Noncardiac cells expressed SMA or vimentin, which was
indicative of the existence of fibroblasts or smooth muscle cells
in the Percoll-enriched populations (data not shown).
Discussion
Direct cardiomyocyte reprogramming via the
transduction of cardiac transcription factors may be considered as
a potential strategy for cardiac regeneration (22). The present study reported that GMTH
may facilitate the conversion of mouse TTFs to cardiomyocytes in
vitro. In addition, the enrichment of iCMs via Percoll density
centrifugation produced a population containing ~72%
α-MHC+ cells; enriched iCMs exhibited cardiomyocyte
marker expression profiles similar to natural cardiomyocytes, with
developed sarcomeric structures. The findings of the present study
may provide a novel cell progenitor valuable to cardiovascular
disease research and clinical treatment, with minimized risks of
immune rejection and tumor formation.
A previous study reported that GMT-mediated
induction may reprogram murine cardiac fibroblasts and TTFs into
cardiomyocyte-like cells in vitro (9). However, the results of the present
study demonstrated that GMT-mediated induction within TTFs induced
the expression of a subset of cardiac genes without inducing the
cardiac-specific gene, α-MHC, supporting previous evidence
(17). The addition of HAND2 to
GMT induced the expression of α-MHC and other cardiac-specific
genes the effect of HAND2 on direct cardiomyocyte reprogramming was
investigated previously; ectopic expression of HAND2 was
demonstrated to enhance the efficiency of reprogramming mammalian
fibroblasts to express a cardiac-like profile (16,23).
GMTH has been reported to serve as crucial cardiac transcription
factors during the early stages of heart development (24,25).
GATA4 has been identified as a ‘pioneer’ factor, which may open
chromatin structure in cardiac loci (26); GATA4 facilitates the binding of
Mef2c, Tbx5 and Hand2 to corresponding binding sites, which may
result in the complete activation of cardiac reprogramming. In
addition, HAND2, a basic helix-loop-helix transcription factor, has
been considered to be an important mediator of cardiomyocyte
generation. Within mice, the loss of HAND2 function has been
associated with hypoplasia of the right ventricle and outflow tract
(27), indicating that
cardiomyocyte development may be derived from the second heart
field (SHF) and may be enhanced by HAND2. In addition, the deletion
of HAND2 function from the SHF via tissue-specific deletion of a
conditional allele may influence the survival of this progenitor
population (28). HAND2
exhibitsimportant and instructive effects on promoting
cardiomyocyte generation by interfering with specification and
proliferation processes (29–31).
The results of the present study indicated that
cardiomyocyte reprogramming may be markedly induced by CIM
treatment, which contains particular cytokines and small molecules
that may induce cardiogenesis by modulating the activities of the
bone morphogenetic protein (BMP), transforming growth factor β
(TGFβ), wingless-related integration site (Wnt), Notchor Hedgehog
signaling pathways (32).
In the present study, CIM treatment was associated
with the induction of cardiomyocyte reprogramming. The presence of
particular cytokines and small molecules within CIM may modulate
the activities of the BMP, TGFβ, Wnt, Notchor Hedgehog pathways
(32). Reprogramming factors may
induce the progenitor cell population, which may result in a
variety of cell types as these progenitor cells regress into
epigenetically-stable stages (33–35).
Fully differentiated cell types may be obtained via the
overexpression of a variety of lineage-specific transcription
factors. The effects of BMP4 on direct cardiac differentiation is
yet to be investigated; however, BMP4 may serve an important role
in the induction of cardiogenesis from nascent precursors during a
key developmental stage (36,37).
Following optimization of the reprogramming
conditions and determination of the enrichment time, iCMs were
predominantly identified in fractions III and IV using a
discontinuous Percoll gradient similar to the separation of
ESC-derived and natural ventricular cardiomyocytes as observed in
previous studies (38,39). qPCR analysis revealed that
increased expression levels of cardiac-specific genes, including
MYH6, TNNT2, NKX2-5, NPPA and GJA1 were
detected within Percoll-enriched iCMs. In addition, the expression
levels of the fibroblast marker gene COL1A2 was
downregulated. Confocal laser scanning microscopy analysis revealed
that the enriched iCMs exhibited typical cardiomyocyte morphology
and formed characteristic sarcomeric structures. Additionally,
Percoll-enriched iCMs exhibited an expression profile similar to
that of neonatal mouse ventricular cardiomyocytes (9).
The present study demonstrated that a
cardiomyocyte-enriched population may be obtained from TTFs
transduced with a combination of cardiac-specific transcription
factors (GMTH), and enriched iCMs may be applied to cell
transplantation, cardiac tissue engineering and drug discovery,
amongst other developments. However, numerous challenges remain in
transcription factor-based cardiac reprogramming; future
investigations may focus on the cardiac reprogramming of human
autologous cell sources and transplantation into the infarcted
heart for cardiac repair.
Acknowledgements
The present study was supported in part by the
National Key R&D Program of China (grant no. 2017YFC1104701),
the National Basic Science and Development Program (973 Program;
grant nos. 2012CB518103, 2012CB518105 and 2013CB127304), the 863
Projects of Ministry of Science and Technology of China (grant nos.
2013AA020105 and 2012AA020502), the National Natural Science
Foundation of China (grant nos. 81121004, 81230041, 31100705,
30901564 and 81101883) and the Beijing Novel Program (grant nos.
2008B53 and 2009A038), and the China Postdoctoral Science
Foundation (grant nos. 2013M542517 and 2015T81099).
Glossary
Abbreviations
Abbreviations:
|
iPSCs
|
induced pluripotent stem cells
|
|
GMTH
|
Gata4, Mef2c, Tbx5 and Hand2
|
|
GMT
|
Gata4, Mef2c and Tbx5
|
|
iCMs
|
induced cardiomyocytes
|
|
ESCs
|
embryonic stem cells
|
|
TTFs
|
tail-tip fibroblasts
|
|
CIM
|
cardiac inductive medium
|
|
SHF
|
second heart field
|
|
BMP
|
bone morphogenetic protein
|
References
|
1
|
Malliaras K and Marbán E: Cardiac cell
therapy: Where we've been, where we are, and where we should be
headed. Br Med Bull. 98:161–185. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zimmermann WH: Remuscularizing failing
hearts with tissue engineered myocardium. Antioxid Redox Signal.
11:2011–2023. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Laflamme MA and Murry CE: Regenerating the
heart. Nat Biotechnol. 23:845–856. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zimmermann WH and Eschenhagen T: Embryonic
stem cells for cardiac muscle engineering. Trends Cardiovasc Med.
17:134–140. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
He W, Ye L, Li S, Liu H, Wu B, Wang Q, Fu
X, Han W and Chen Z: Construction of vascularized cardiac tissue
from genetically modified mouse embryonic stem cells. J Heart Lung
Transplant. 31:204–212. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Takahashi K, Tanabe K, Ohnuki M, Narita M,
Ichisaka T, Tomoda K and Yamanaka S: Induction of pluripotent stem
cells from adult human fibroblasts by defined factors. Cell.
131:861–872. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Takahashi K and Yamanaka S: Induction of
pluripotent stem cells from mouse embryonic and adult fibroblast
cultures by defined factors. Cell. 126:663–676. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou Q, Brown J, Kanarek A, Rajagopal J
and Melton DA: In vivo reprogramming of adult pancreatic exocrine
cells to beta-cells. Nature. 455:627–632. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ieda M, Fu JD, Delgado-Olguin P, Vedantham
V, Hayashi Y, Bruneau BG and Srivastava D: Direct reprogramming of
fibroblasts into functional cardiomyocytes by defined factors.
Cell. 142:375–386. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vierbuchen T, Ostermeier A, Pang ZP,
Kokubu Y, Südhof TC and Wernig M: Direct conversion of fibroblasts
to functional neurons by defined factors. Nature. 463:1035–1041.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sekiya S and Suzuki A: Direct conversion
of mouse fibroblasts to hepatocyte-like cells by defined factors.
Nature. 475:390–393. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Szabo E, Rampalli S, Risueño RM, Schnerch
A, Mitchell R, Fiebig-Comyn A, Levadoux-Martin M and Bhatia M:
Direct conversion of human fibroblasts to multilineage blood
progenitors. Nature. 468:521–526. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Takeuchi JK and Bruneau BG: Directed
transdifferentiation of mouse mesoderm to heart tissue by defined
factors. Nature. 459:708–711. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jayawardena TM, Egemnazarov B, Finch EA,
Zhang L, Payne JA, Pandya K, Zhang Z, Rosenberg P, Mirotsou M and
Dzau VJ: MicroRNA-mediated in vitro and in vivo direct
reprogramming of cardiac fibroblasts to cardiomyocytes. Circ Res.
110:1465–1473. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Qian L, Huang Y, Spencer CI, Foley A,
Vedantham V, Liu L, Conway SJ, Fu JD and Srivastava D: In vivo
reprogramming of murine cardiac fibroblasts into induced
cardiomyocytes. Nature. 485:593–598. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Song K, Nam YJ, Luo X, Qi X, Tan W, Huang
GN, Acharya A, Smith CL, Tallquist MD, Neilson EG, et al: Heart
repair by reprogramming non-myocytes with cardiac transcription
factors. Nature. 485:599–604. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen JX, Krane M, Deutsch MA, Wang L,
Rav-Acha M, Gregoire S, Engels MC, Rajarajan K, Karra R, Abel ED,
et al: Inefficient reprogramming of fibroblasts into cardiomyocytes
using Gata4, Mef2c, and Tbx5. Circ Res. 111:50–55. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chien KR, Yi BA, Xu H and Mummery CL:
Cardiomyocyte reprogramming and the new age of cellular alchemy. J
Mol Cell Cardiol. 53:311–313. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Braam SR, Passier R and Mummery CL:
Cardiomyocytes from human pluripotent stem cells in regenerative
medicine and drug discovery. Trends Pharmacol Sci. 30:536–545.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
He W, Ye L, Li S, Liu H, Wang Q, Fu X, Han
W and Chen Z: Stirred suspension culture improves embryoid body
formation and cardiogenic differentiation of genetically modified
embryonic stem cells. Biol Pharm Bull. 35:308–316. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Doppler SA, Deutsch MA, Lange R and Krane
M: Direct reprogramming-the future of cardiac regeneration? Int J
Mol Sci. 16:17368–17393. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nam YJ, Song K, Luo X, Daniel E, Lambeth
K, West K, Hill JA, DiMaio JM, Baker LA, Bassel-Duby R and Olson
EN: Reprogramming of human fibroblasts toward a cardiac fate. Proc
Natl AcadSci USA. 110:pp. 5588–5593. 2013; View Article : Google Scholar
|
|
24
|
Olson EN: Gene regulatory networks in the
evolution and development of the heart. Science. 313:1922–1927.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Srivastava D: Making or breaking the
heart: From lineage determination to morphogenesis. Cell.
126:1037–1048. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cirillo LA, Lin FR, Cuesta I, Friedman D,
Jarnik M and Zaret KS: Opening of compacted chromatin by early
developmental transcription factors HNF3 (FoxA) and GATA-4. Mol
Cell. 9:279–289. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Srivastava D, Thomas T, Lin Q, Kirby ML,
Brown D and Olson EN: Regulation of cardiac mesodermal and neural
crest development by the bHLH transcription factor, dHAND. Nat
Genet. 16:154–160. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tsuchihashi T, Maeda J, Shin CH, Ivey KN,
Black BL, Olson EN, Yamagishi H and Srivastava D: Hand2 function in
second heart field progenitors is essential for cardiogenesis. Dev
Biol. 351:62–69. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yelon D, Ticho B, Halpern ME, Ruvinsky I,
Ho RK, Silver LM and Stainier DY: The bHLH transcription factor
hand2 plays parallel roles in zebrafish heart and pectoral fin
development. Development. 127:2573–2582. 2000.PubMed/NCBI
|
|
30
|
Schoenebeck JJ, Keegan BR and Yelon D:
Vessel and blood specification override cardiac potential in
anterior mesoderm. Dev Cell. 13:254–267. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schindler YL, Garske KM, Wang J, Firulli
BA, Firulli AB, Poss KD and Yelon D: Hand2 elevates cardiomyocyte
production during zebrafish heart development and regeneration.
Development. 141:3112–3122. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Efe JA, Hilcove S, Kim J, Zhou H, Ouyang
K, Wang G, Chen J and Ding S: Conversion of mouse fibroblasts into
cardiomyocytes using a direct reprogramming strategy. Nat Cell
Biol. 13:215–222. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Meshorer E, Yellajoshula D, George E,
Scambler PJ, Brown DT and Misteli T: Hyperdynamic plasticity of
chromatin proteins in pluripotent embryonic stem cells. Dev Cell.
10:105–116. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hochedlinger K and Plath K: Epigenetic
reprogramming and induced pluripotency. Development. 136:509–523.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Artyomov MN, Meissner A and Chakraborty
AK: A model for genetic and epigenetic regulatory networks
identifies rare pathways for transcription factor induced
pluripotency. PLoS Comput Biol. 6:e10007852010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cohen ED, Tian Y and Morrisey EE: Wnt
signaling: An essential regulator of cardiovascular
differentiation, morphogenesis and progenitor self-renewal.
Development. 135:789–798. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Klaus A and Birchmeier W: Developmental
signaling in myocardial progenitor cells: A comprehensive view of
Bmp- and Wnt/beta-catenin signaling. PediatrCardiol. 30:609–616.
2009.
|
|
38
|
Xu C, Police S, Rao N and Carpenter MK:
Characterization and enrichment of cardiomyocytes derived from
human embryonic stem cells. Circ Res. 91:501–508. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
E LL, Zhao YS, Guo XM, Wang CY, Jiang H,
Li J, Duan CM and Song Y: Enrichment of cardiomyocytes derived from
mouse embryonic stem cells. J Heart Lung Transplant. 25:664–674.
2006. View Article : Google Scholar : PubMed/NCBI
|