Introduction
Breast cancer is a major life-threatening malignancy
and ranks as the second leading cause of mortality (1,2).
Triple-negative (TN) breast cancer accounts for ~15% of all
diagnosed breast cancers. TN breast cancer cells do not express
estrogen receptor, progesterone receptor or human epidermal growth
factor receptor (3,4). Drug-resistant TN breast cancers have
a poor prognosis, as they metastasize rapidly and are difficult to
treat (5). MDA-MB-231 TN breast
cancer cells are aggressive, invasive and resistant to a number of
anticancer agents (6). On this
basis, MDA-MB-231 cells provide an ideal in vitro model in
which to analyze the effects of anticancer treatments, such as with
tectorigenin (Tec). MCF-7 cells are an estrogen-responsive breast
cancer cell line, which was used to confirm the inhibitory effects
of Tec on cell proliferation, migration and invasion.
Chemotherapeutic drugs that inhibit the malignant characteristics
of MDA-MB-231 and MCF-7 cells would be the best drugs to treat
breast cancer.
Experience-based remedies such as Traditional
Chinese Medicines (TCMs) have been derived over many years of
clinical use in China. Most TCMs are extracted from at least one
medicinal herb and comprise multiple bioactive ingredients, which
suggested that TCMs may be a potential source for new anticancer
drugs (7). To date, a number of
naturally occurring phytochemicals have been reported that exhibit
antitumoral effects by inducing apoptosis and have received
considerable attention (1,2,4). Tec
is an effective component of the TCM that is derived from
Belamcanda chinensis (8).
Tec has been reported to exhibit beneficial effects in various
types of tumors, including osteosarcoma (9), ovarian cancer (10), lung carcinoma (11), hepatocellular carcinoma (12) and promyelocytic leukemia (13). In addition, Tec was revealed to
regulate adipogenic differentiation and adipocytokine secretion
through peroxisome proliferator-activated receptor-γ and IκB
kinase/nuclear factor-κB signaling (14). In addition, previous studies also
reported that Tec affects the proliferation of breast cancer cells
(15,16). However, the specific effects and
the underlying mechanisms of Tec on apoptosis and metastasis in
human breast cancer have not been elucidated.
Based on the present study results, it is
hypothesized that Tec may induce apoptosis in breast cancer cells
by downregulating the protein expression of matrix
metalloproteinases (MMPs), phosphorylated (p)-AKT and
mitogen-activated protein kinase (MAPK) signaling and by
upregulating the expression of cleaved caspase (CASP)-3 and cleaved
poly [ADP-ribose] polymerase (PARP) as MMPs, p-AKT, MAPK signaling,
cleaved CASP-3 and cleaved PARP have been linked to the apoptosis
or metastasis of breast cancer in previous reports (3,4,9,10).
Therefore, Tec may be a potential therapeutic drug for human breast
cancer.
Materials and methods
Main reagents
Tec was purchased from Sigma-Aldrich (Merck KGaA,
Darmstadt, Germany) and was dissolved in DMSO at a concentration of
200 mM and stored at −20°C. Primary antibodies against MMP2 (cat.
no. 87809), MMP9 (cat. no. 13667), BCL-2 (cat. no. 4223),
BCL-2-associated X (BAX; cat. no. 5023), p-AKT (cat. no. 4060),
total-AKT (cat. no. 4685), p-c-Jun N-terminal kinase (JNK; cat. no.
4668), total-JNK (cat. no. 9252), p-p38 (cat. no. 4511), p38 (cat.
no. 8690), p-extracellular signal-regulated kinase (ERK; cat. no.
4370), total-ERK (cat. no. 4695), cleaved CASP-3 (cat. no. 1050),
CASP-3 (cat. no. 14220), cleaved PARP (cat. no. 5625), PARP (cat.
no. 9532) and GAPDH (cat. no. 2118) and anti-rabbit IgG (H+L)
secondary antibody (DyLight™ 800 4X PEG conjugate; cat. no. 5151)
were purchased from Cell Signaling Technology, Inc. (Danvers, MA,
USA).
Cell lines and cell culture
Human breast cancer cell lines MDA-MB-231 and MCF-7,
and the human mesenchymal stem cell (hMSC) lines were purchased
from the Chinese Academy of Sciences (Shanghai, China) the
repository of ATCC cell lines in China. MDA-MB-231 and MCF-7 cell
lines were cultured in Dulbecco's modified Eagle's medium (DMEM;
Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.) and 1% penicillin-streptomycin solution (Gibco;
Thermo Fisher Scientific, Inc.), and containing <0.05% DMSO, at
37°C in a humidified atmosphere with 5% CO2. hMSCs were
cultured in α-minimum essential medium (α-MEM; Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% FBS and 1%
penicillin-streptomycin solution at 37°C in a humidified atmosphere
with 5% CO2. MDA-MB-231 and MCF-7 cells were used within
20 passages, and hMSCs were used within 5 passages.
Measurement of cell viability
Cell viability was measured using the Cell Counting
kit-8 (CCK-8), as previously described (17). Briefly, MDA-MB-231 and MCF-7 cells
(6,000 cells/well) were plated in a 96-well plate and incubated
overnight at 37°C without drug treatment. Subsequently, the cells
of the control group (0 µM group) were treated with 0.1% DMSO and
the cells in the Tec groups were treated with Tec at concentrations
of 0, 50, 100 or 200 µM and incubated at 37°C. The present study
used 0–200 µM Tec as the experimental dose according to a pretest
study and previously published studies (9,10).
Viability was examined every 24 h following treatment (that is, at
24, 48, 72 and 96 h) by incubating the cells with CCK-8 solution at
37°C for 2 h and measuring optical density (OD) at 490 nm using a
microplate reader (Thermo Electron Corporation; Thermo Fisher
Scientific, Inc.). Each condition included three replicate wells
with at least three independent repeats. Cell viability compared to
the control was calculated using the following equation: Cell
viability (%)=ODdrug-treated group/ODcontrol
group. The cell viability of hMSCs was detected using the
above methods at 72 and 96 h following Tec treatment. If there were
no effect on the cell viability of hMSCs at 72 and 96 h, 24 and 48
h should also demonstrate no effect. As a result, the inhibition of
proliferation in MCF-7 was more obvious than in MDA-MB-231 cells,
so these were selected to detect the apoptosis, cell cycle and
apoptosis-related genes of MDA-MB-231 cells in subsequent
experiments.
Apoptosis
Apoptosis was measured by flow cytometry using
Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI)
double-immunofluorescence staining kit (Thermo Fisher Scientific,
Inc.), according to the manufacturer's protocol. Briefly,
MDA-MB-231 cells (1×106 cells/well) were seeded in
6-well plates and harvested 24 h following treatment with 0, 100 or
200 µM Tec. As 50 µM was far less than IC50 at 24 h in
MDA-MB-231 cells, this concentration was removed. The cells were
vigorously pipetted and centrifuged at 500 × g for 5 min at 4°C and
the supernatants were discarded. Subsequently, the cells were
resuspended in 1X Annexin-binding buffer to verify drug-induced
apoptosis rates, and apoptotic events were indicated as a
combination of FITC+/PI− (early apoptotic)
and FITC+/PI+ (late apoptotic or dead), and
were analyzed using FlowJo version 7.6 (FlowJo LLC, Ashland, OR,
USA). The final results are expressed as the percentage of
FITC+ cells after subtracting the number of vehicle
cells. Data are presented as the mean ± standard deviation from at
least three independent experiments.
Cell cycle analysis
To analyze the effects of Tec on cell cycle,
MDA-MB-231 cells (2×106 cells/dish) were seeded in 60 mm
culture dishes and treated with 0, 100 or 200 µM Tec for 24 h at
37°C. Cells were harvested, washed twice with PBS and fixed in 70%
ice-cold ethanol at −20°C for 2 h, followed by 2 washes with PBS.
Cells were resuspended in 300 µl PI/RNase staining buffer (BD
Biosciences, Franklin Lakes, NJ, USA) for 10 min at room
temperature and analyzed using a BD FACSCalibur flow cytometer (BD
Biosciences). The cell cycle distribution was analyzed with the
ModFit LT software version 3.0 (BD Biosciences). Data are presented
as the mean ± standard deviation from at least three independent
experiments.
Tumor cell migration and invasion
ability
Cell migration was analyzed using a Transwell assay
with 8 µm cell culture inserts (EMD Millipore, Billerica, MA, USA)
in 24-well plates. Untreated and Tec-treated MDA-MB-231 and MCF-7
cells (3×104 cells/well) were suspended in 100 µl of
serum-free DMEM and added to the upper Transwell chamber, whereas
the lower chamber was filled with 500 µl complete DMEM with 20%
FBS. Following incubation for 24 h at 37°C in a 5% CO2
atmosphere, the insert was washed with PBS and cells on the top
surface of the insert were removed by wiping with a cotton swab.
Cells that migrated to the bottom surface of the insert were fixed
with 4% paraformaldehyde at room temperature for 20 min, stained
with 0.1% crystal violet at room temperature for 20 min, and
examined using a light microscope (BX43; Olympus Corporation,
Tokyo, Japan). Cell counts were based on examination of five random
fields from the digital images (magnification, ×200) and reported
as the mean ± standard deviation. All assays were repeated three
times independently.
The invasion assay procedure was similar to that of
the cell migration assay, except that the Transwell membrane was
coated with 1:3 diluted Matrigel (BD Biosciences), and the cells
were incubated for 32 h at 37°C. All assays were repeated three
times independently.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
A total of 24 h post-treatment with different doses
of Tec, RNA was extracted from MDA-MB-231 cells (1×106
cells/well) using the TRIzol (Invitrogen; Thermo Fisher Scientific,
Inc.) method and RNA extracted according to the manufacturer's
protocol. The concentration and purity were measured by Nanodrop
2000 system (Thermo Fisher Scientific, Inc., Wilmington, DE, USA).
The iScript cDNA Synthesis kit (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) was used to synthesize cDNA using the reverse
transcription method according to the manufacturer's protocol. mRNA
expression levels were evaluated by qPCR using a SYBR Premix ex
Taq, Tli RNase H Plus kit (Takara Bio, Inc., Otsu, Japan) according
to the manufacturer's protocol. The thermocycling conditions were
as follows: 40 cycles of 95°C for 5 sec and 60°C for 34 sec. Primer
sequences are listed in Table I.
GAPDH was used as an internal control and for normalization of
expression. Data are presented as the mean ± standard deviation
from at least three independent experiments. Gene expression was
compared using the 2−ΔΔCq method (18).
 | Table I.Primer sequences used in reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primer sequences used in reverse
transcription-quantitative polymerase chain reaction.
| Gene | Primer sequence
(5′→3′) |
|---|
| MMP2 | F:
ATGCAGTGGGGGCTTAAGAA |
|
| R:
AAACAGGTTGCAGCTCTCCT |
| MMP9 | F:
TCTATGGTCCTCGCCCTGAA |
|
| R:
CATCGTCCACCGGACTCAAA |
| AKT1 | F:
GAAGGACGGGAGCAGGC |
|
| R:
CTCACGCGCTCCTCTCAG |
| AKT2 | F:
GCCACCATGAATGAGGTGAAT |
|
| R:
TCTCGTCTGGAGAATCCACG |
| AKT3 | F:
TTTTCTCTATTATTTGGGCTGAGTC |
|
| R:
CCCCTCTTCTGAACCCAACC |
| BCL-2 | F:
ATCTGGGCCACAAGTGAAGT |
|
| R:
GCTGATTCGACGTTTTGCCT |
| BAX | F:
AGAGGTCTTTTTCCGAGTGGC |
|
| R:
CAGGGACATCAGTCGCTTCAG |
| CASP-3 | F:
GCTCTGGTTTTCGGTGGGTG |
|
| R:
CTGAGGTTTGCTGCATCGAC |
| CASP-8 | F:
CTGGTCTGAAGGCTGGTTGT |
|
| R:
CAGGCTCAGGAACTTGAGGG |
| CASP-9 | F:
CAGGCTCAGGAACTTGAGGG |
|
| R:
TCGACAACTTTGCTGCTTGC |
| GAPDH | F:
AATGGGCAGCCGTTAGGAAA |
|
| R:
GCGCCCAATACGACCAAATC |
Western blot analysis
Protein extracts from MDA-MB-231 cells
(1×106 cells/well) subjected to various Tec treatments
were prepared using 120 µl radioimmunoprecipitation assay lysis
buffer (RIPA; Thermo Fisher Scientific, Inc.) supplemented with 1
mM phenylmethylsulfonyl fluoride and were quantified using a
Bicinchoninic Acid Protein Assay kit (Thermo Fisher Scientific,
Inc.). Lysates were diluted 5:1 with loading buffer and
heat-denatured at 99°C for 10 min. Equal amounts of protein (30 µg)
were resolved by SDS-PAGE using precast 7.5–12.5% gels (Thermo
Fisher Scientific, Inc., USA) at 80 V for 30 min and 120 V for 1 h.
Proteins were transferred onto an activated polyvinylidene fluoride
membrane by wet electrophoretic transfer (Bio-Rad Laboratories,
Inc.) for 2.5 h at a constant current of 250 mA. The membranes were
blocked for 1 h in TBS containing 0.05% Tween-20 (TBST) and 5%
nonfat milk powder and subsequently incubated overnight with
primary antibodies (all antibodies at 1:1,000) at 4°C. Following
three washes in TBST, the membranes were probed with the
corresponding secondary antibody (1:15,000) for 1 h at room
temperature. The membranes were washed in TBS, and the protein
bands were visualized using an Odyssey Infrared Imaging System
(LI-COR Biosciences, Lincoln, NE, USA). Positive immunoreactive
bands were densitometrically quantified and normalized to GAPDH.
Adobe Photoshop (Creative Suite 5; Adobe Systems, Inc., San Jose,
CA, USA) was used for densitometry. Data are presented as the mean
± standard deviation from at least three independent
experiments.
Statistical analysis
The Statistical Package for the Social Sciences
(SPSS) version 19.0 (IBM Corp., Armonk, NY, USA) was used to
analyze the data. Significant differences between experimental
groups and controls were assessed using the Student's t-test or
one-way analysis of variance and LSD test as appropriate. The data
are expressed as the mean ± standard deviation. P<0.05 was
considered to indicate a statistically significant difference.
Results
Proliferation of breast cancer cells
and hMSCs following Tec treatment
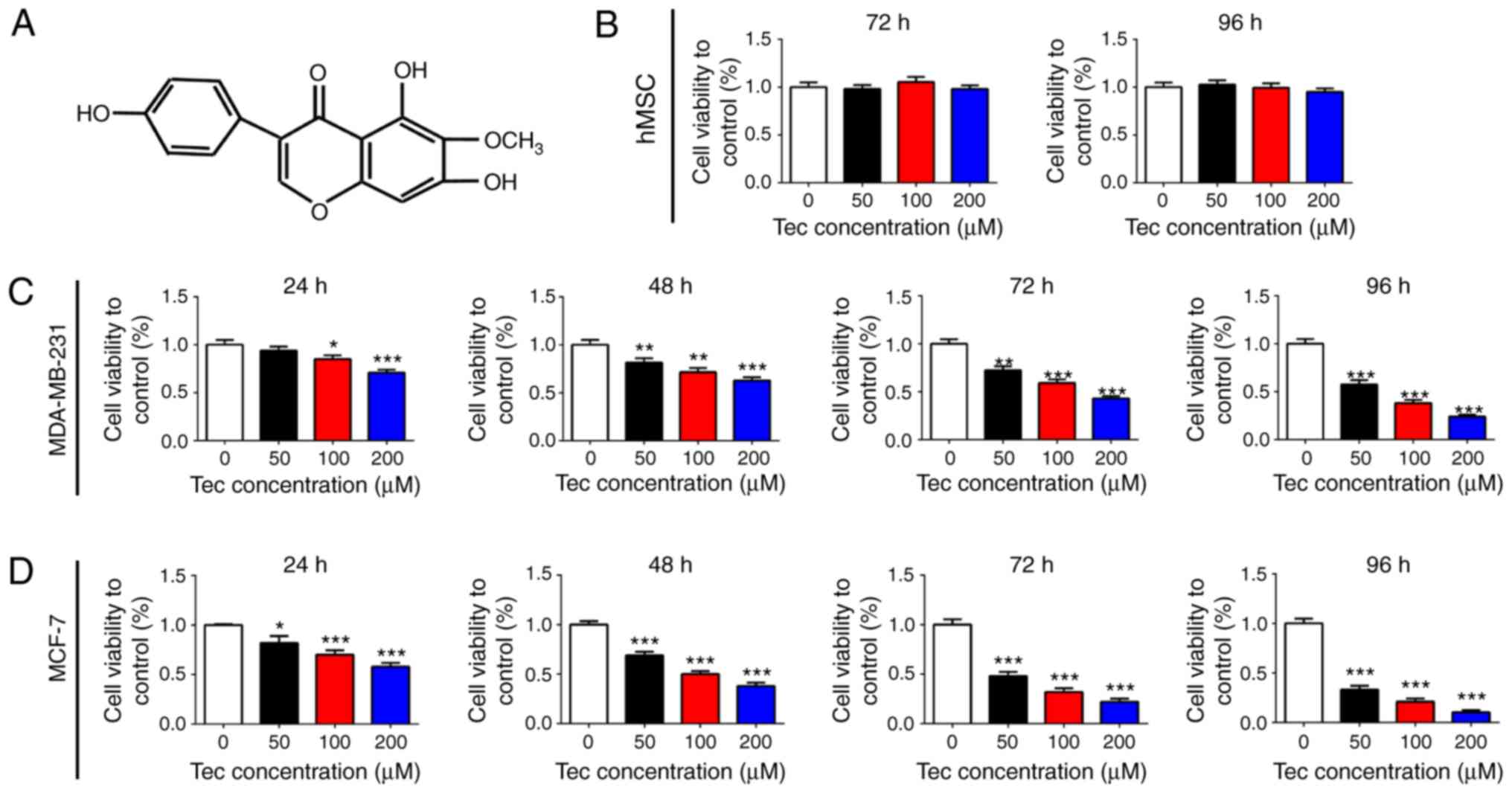
The structure of Tec is provided in Fig. 1A; molecular weight, 300.26. To
investigate the effects of Tec treatment on breast cancer cells, a
CCK-8 cell proliferation assay was performed on MDA-MB-231 and
MCF-7 cells; no significant differences were identified in hMSCs
following 72 or 96 h Tec treatment (Fig. 1B). The proliferation of MDA-MB-231
and MCF-7 cells was inhibited following treatment with Tec, and the
inhibitory effects of Tec on proliferation significantly increased
with the increasing Tec concentration in both cell lines, with the
strongest effects observed following 96 h treatment (Fig. 1C and D, respectively). The 50%
inhibitory concentration (IC50) was calculated (Table II). According to previous studies
(9,10) as well as the present
IC50 results, it was determined that the concentrations
of Tec at 0, 50, 100 and 200 µM were to be used in subsequent
experiments. These results indicated that Tec treatment inhibited
the proliferation of breast cancer cells in a dose- and
time-dependent manner.
 | Table II.IC50 calculated for
varying incubation periods for tectorigenin treatment on two breast
cancer cell lines. |
Table II.
IC50 calculated for
varying incubation periods for tectorigenin treatment on two breast
cancer cell lines.
|
| IC50
(µM) |
|---|
|
|
|
|---|
| Cell line | 24 h | 48 h | 72 h | 96 h |
|---|
| MDA-MB-231 | 417.4 | 283.9 | 184.2 | 77.4 |
| MCF-7 | 276.8 | 118.4 |
55.36 | 23.65 |
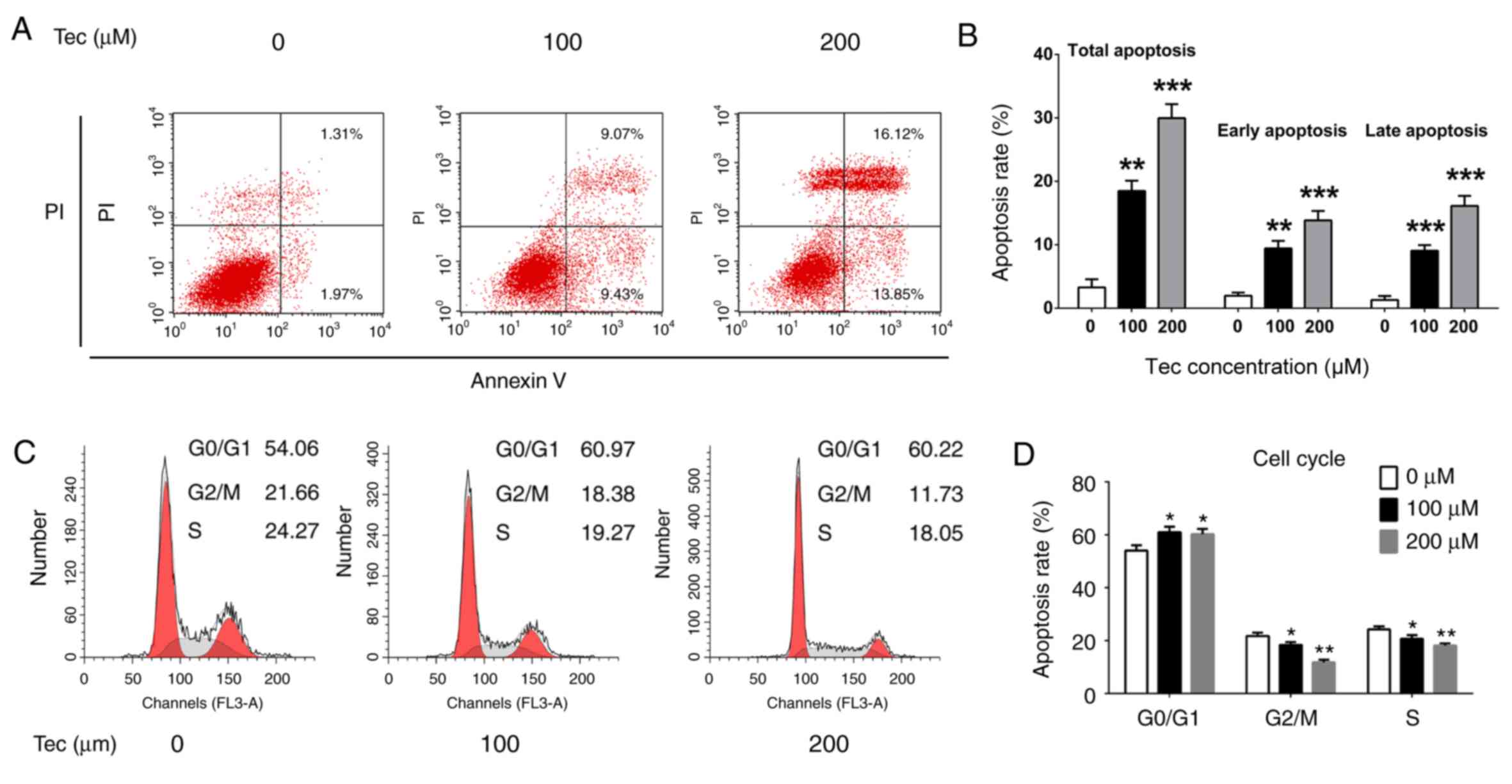
Tec-induced apoptosis and cell cycle
arrest of MDA-MB-231 cells
Flow cytometry was used to detect Tec-induced
apoptosis. Treatments with 100 and 200 µM Tec significantly
increased the total apoptotic rates (18.5 and 29.97%, respectively)
compared with the apoptotic rate in untreated control cells (3.28%;
P<0.05; Fig. 2A and B);
quantitative data for early and late apoptosis rate were consistent
with this phenomenon (Fig. 2B).
The cell cycle was also examined by flow cytometry following PI
staining. Tec treatments significantly increased the proportion of
G0/G1-phase cells and significantly decreased the proportion of
S-phase and G2/M cells (Fig. 2C and
D), which suggested that Tec treatment may induce G0/G1-phase
arrest in MDA-MB-231 cells. These results indicated that the
inhibitory function of Tec may be through activating the apoptosis
pathway and G0/G1-phase arrest in MDA-MB-231 cells.
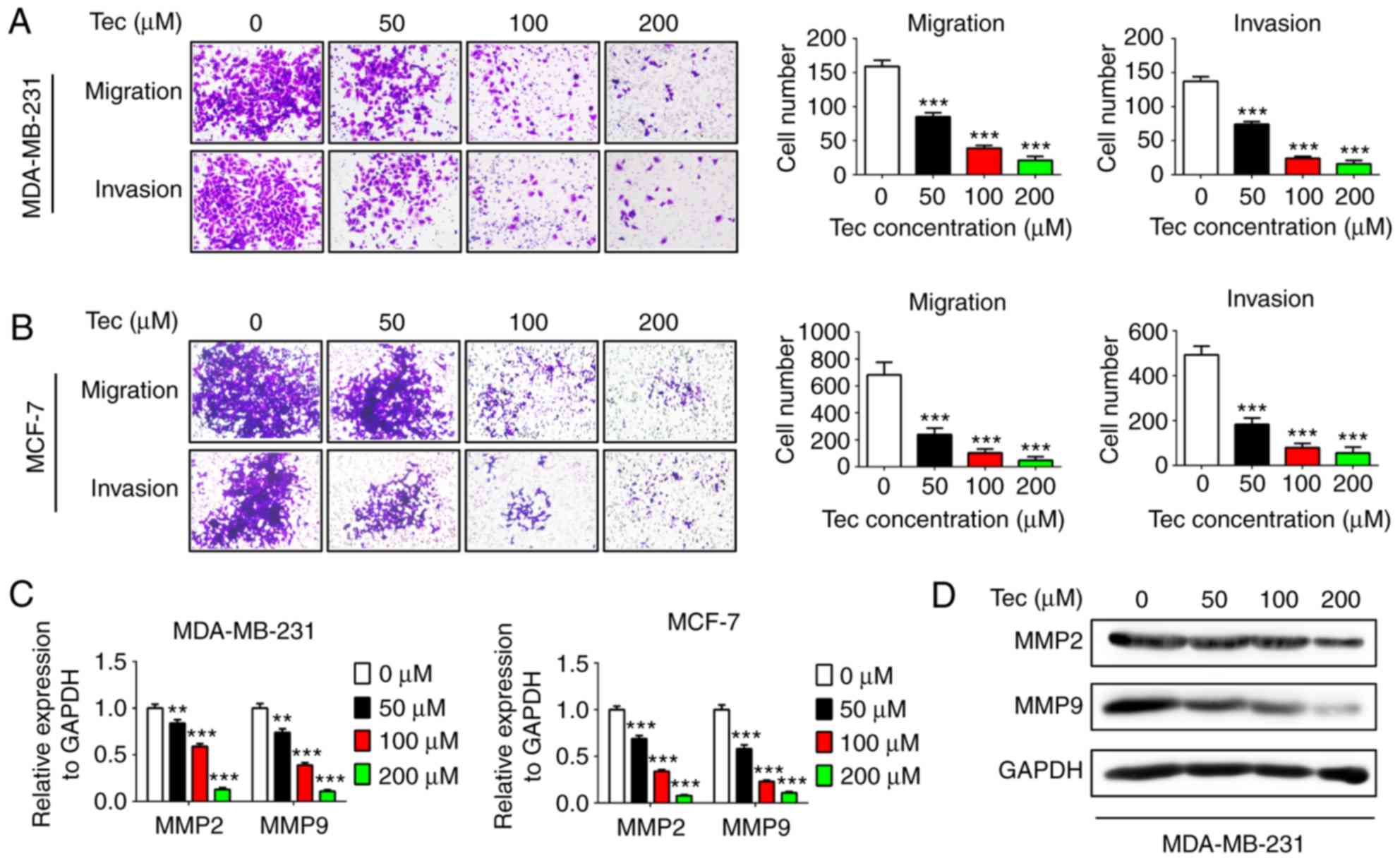
Tec treatment impairs migration and
invasion of breast cancer cells and inhibits the expression of
metastasis-related genes
Cell migration and invasion were examined using
Transwell and Matrigel assays, respectively, following treatment
with different concentrations of Tec. The results revealed that the
migratory and invasive abilities of MDA-MB-231 and MCF-7 cells were
inhibited by Tec in a dose-dependent manner (Fig. 3A and B, respectively). In addition,
the mRNA expression levels of MMP2 and MMP9 were inhibited by Tec
treatment in a dose-dependent manner in both MDA-MB-231 and MCF-7
cells (Fig. 3C). As the metastasis
ability of MDA-MB-231 is stronger than MCF-7 cells (1,4),
western blotting analysis was not performed for MMP expressions in
MCF-7 cells. The inhibitory effects of Tec on the protein
expression of MMP2 and MMP9 in MDA-MB-231 cells were confirmed
(Fig. 3D). These results indicated
that Tec may impair migration and invasion of breast cancer by
suppressing MMP2 and MMP9 expression.
Apoptosis- and survival-related gene
expression
To investigate the molecular mechanisms of the
inhibitory effects of Tec in MDA-MB-231 cells, RT-qPCR was used to
examine the variation in apoptosis- and survival-related gene
expression levels. The mRNA expression levels of BCL-2, AKT1, AKT2
and AKT3 were reduced in Tec-treated MDA-MB-231 cells in a
dose-dependent manner (Fig. 4A-D,
respectively), whereas the expression levels of BAX, CASP-3, CASP-8
and CASP-9 were upregulated (Fig.
4E-H, respectively). These results suggested that these genes
may be potential downstream targets of Tec in breast cancer
treatment.
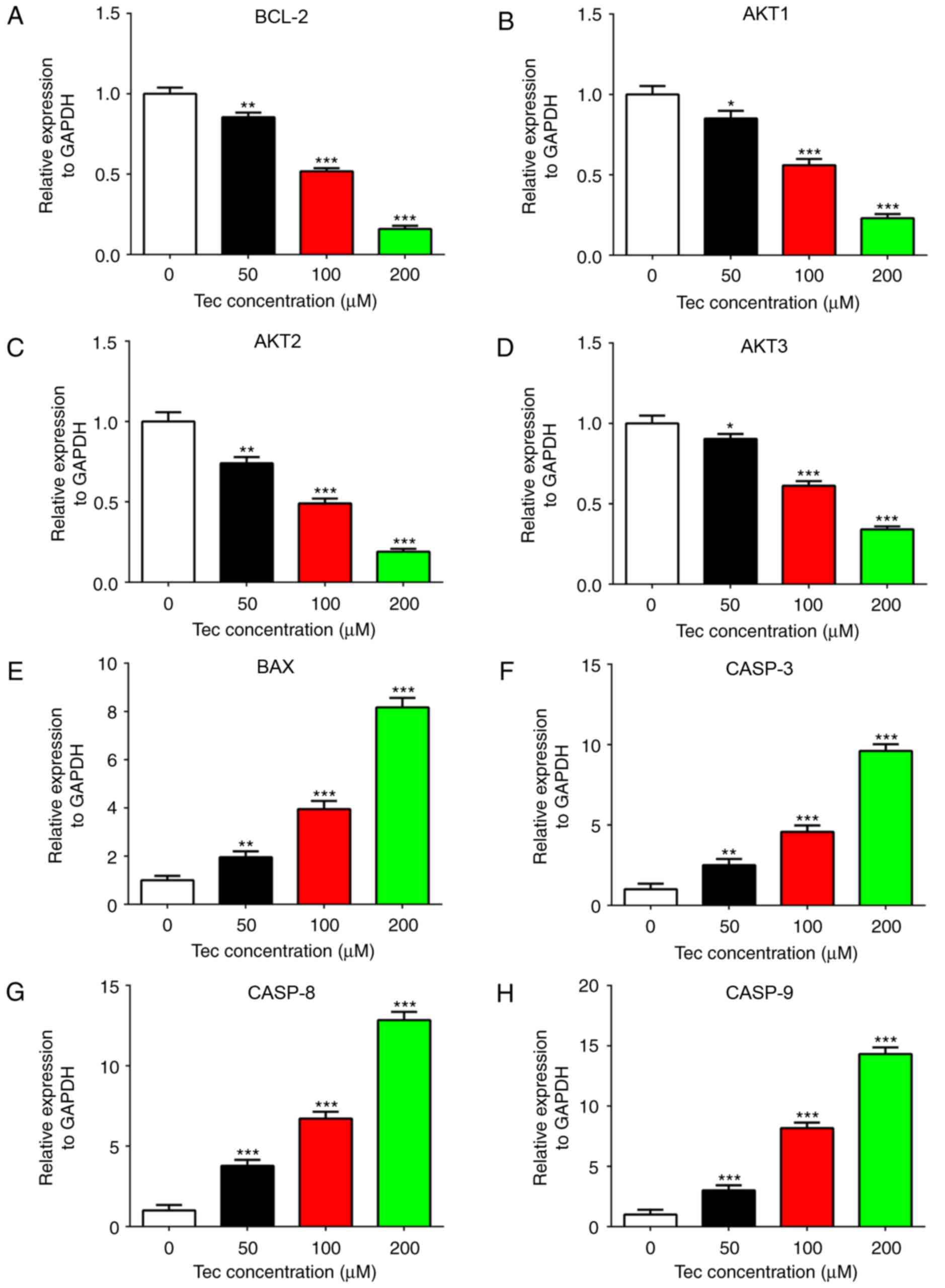 | Figure 4.Apoptosis- and survival-related gene
expression in MDA-MB-231 cells following Tec treatment. Reverse
transcription-quantitative polymerase chain reaction was used to
detect the mRNA expression levels of (A) BCL-2, (B) AKT1, (C) AKT2,
(D) AKT3, (E) BAX, (F) CASP-3, (G) CASP-8 and (H) CASP-9 in
Tec-treated MDA-MB-231 cells. Data are presented as the mean ±
standard deviation from at least three independent experiments;
*P<0.05, **P<0.01 and ***P<0.001 vs. DMSO treated control
(0 µM). BAX, BCL-2-associated X; CASP, caspase; Tec,
tectorigenin. |
Tec treatment regulates the expression
of apoptosis-related proteins and components of the MAPK signaling
pathway
Western blotting was used to analyze protein
expression levels of apoptosis-related proteins and components of
the MAPK signaling pathway in MDA-MB-231 cells following different
concentrations of Tec treatment. The results demonstrated that Tec
treatment inhibited the protein expression of MAPK signaling
proteins p-p38, p-JNK and p-ERK in (Fig. 5A and B). Tec treatment also
suppressed the expression of BCL-2 and p-AKT in a dose-dependent
manner (Fig. 5C and D). However,
Tec treatment led to increased expression levels of BAX, cleaved
PARP and cleaved CASP-3 in a dose-dependent manner (Fig. 5C-E). These data suggested that Tec
treatment induced apoptosis of breast cancer cells through
suppression of the MAPK pathway and by activation of CASP-3
dependent apoptosis.
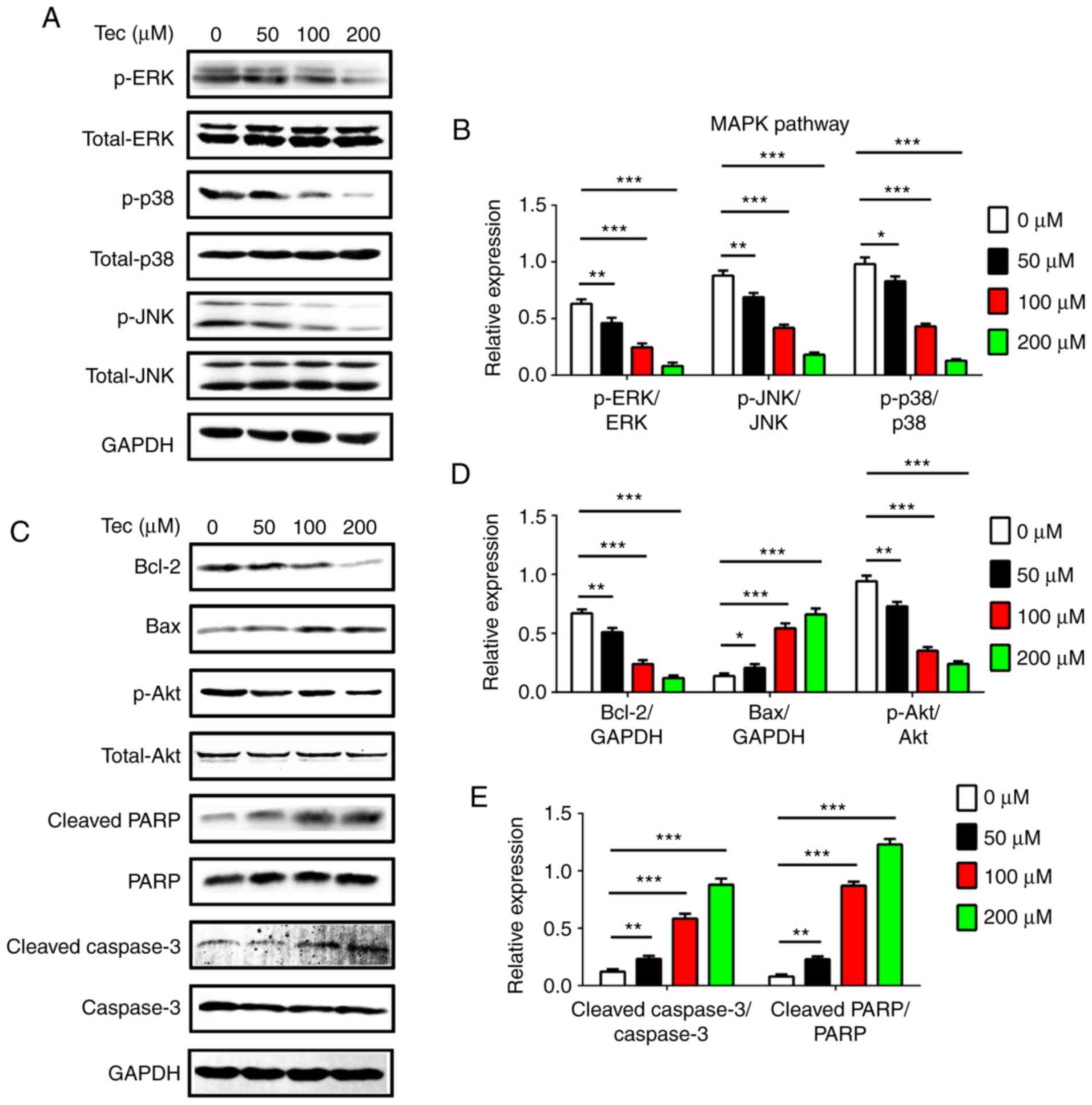 | Figure 5.Western blotting of MAPK pathway
components and apoptosis- and survival-related protein expression
levels following Tec-induced apoptosis in MDA-MB-231 breast cancer
cells. (A) Expression and (B) densitometric analysis of
p-ERK/total-ERK, p-JNK/total-JNK and p-p38/p38 in MDA-MB-231 cells
following Tec treatment. (C) Expression and (D and E) densitometric
analysis of BCL-2, BAX, p-AKT/total-AKT, cleaved PARP/PARP and
cleaved CASP-3/CASP-3 in MDA-MB-231 cells following Tec treatment.
Results are expressed as the ratio to: GAPDH for BCL-2 and BAX, or
total protein for p-AKT, p-ERK, p-JNK, p-p38, cleaved PARP and
cleaved CASP-3. Data are presented as the mean ± standard deviation
from at least three independent experiments; *P<0.05,
**P<0.01 and ***P<0.001 vs. DMSO treated control (0 µM). BAX,
BCL-2-associated X; CASP, caspase; ERK, extracellular
signal-regulated kinase; JNK, c-Jun N-terminal kinase; MAPK,
mitogen-activated protein kinase; p, phosphorylated; PARP, poly
[ADP-ribose] polymerase 1; Tec, tectorigenin. |
Discussion
Although the current first-line therapies for breast
cancer, which include surgery, radiotherapy and chemotherapy, have
allowed for great achievements in clinical control, poor prognosis
and serious side effects remain and must be addressed (19–24).
Another primary problem of treatments for breast cancer is a high
incidence of failure and relapse with drug resistance (4–6).
Therefore, an increasing number of studies are exploring more
efficient treatments for breast cancer, in which developing new
drug treatments is a primary goal (1). TCMs may offer a primary source for
the discovery of new anticancer drugs (25), and anticancer agents extracted from
Chinese herbs have attracted further attention. For example, one
recent study reported that Polyporus umbellatus including
its ingredients ergosta-4,6,8 (14), 22-tetraen-3-one and polyporusterone
A-G inhibited breast tumor cell proliferation and promoted
apoptosis by downregulating AKT expression (26). Another recent study reported that
emodin, a rhubarb-derived compound, inhibited breast cancer growth
and metastasis (27).
Tec is one of the bioactive components that is
purified from the Chinese herb Belamcanda chinensis that has
been investigated in the treatment of certain cancers; a number of
pharmacological effects have been identified (28,29),
which indicated that Tec may be an effective option for the
treatment of breast cancer. The function and underlying mechanisms
of Tec in breast cancer are not fully understood. To further
investigate its potential clinical application, the present study
examined the effects of Tec treatment on the proliferation,
apoptosis, cell cycle, migration and invasion ability in human
breast cancer cells in vitro.
The result demonstrated that up to Tec treatments
did not influence the proliferation of hMSCs, whereas culturing
MDA-MB-231 and MCF-7 cells with various concentrations inhibited
their proliferation. Tec treatment also induced MDA-MB-231 cell
apoptosis and G0/G1-phase arrest in a dose-dependent manner. The
migration and invasion of MDA-MB-231 and MCF-7 cells were also
inhibited by Tec in a dose-dependent manner probably through the
suppression of MMP2 and MMP9 expression. These data indicated that
Tec may be able to serve as an efficient chemotherapy drug for
breast cancer treatment. However, a number of previous studies have
reported that Tec stimulated the proliferation of MCF-7 and T-47D
human breast cancer cells (15,16).
The present study suspected that the reason for this discrepancy is
that a different number of cells used; for example, in the present
study the CCK-8 assay used 6,000 cells/well, whereas the previous
studies used 1×104 cells/well. Another possible reason
may be that the highest concentration of Tec in the previous
studies was 100 µM while the maximum concentration used in the
present study was 200 µM, and the inhibition of the proliferation
of tumor cells was not demonstrated in previous studies (15,16);
therefore, the inhibitory effects of Tec may not be obvious in
these studies. The inhibitory effects of Tec in other tumors of
epithelial origin, such as prostate cancer (30) and hepatocellular carcinoma
(12), have been confirmed in
other previous studies, and Tec treatment has now been confirmed to
inhibit the proliferation of breast cancer cells by the present
study.
The molecular mechanisms of Tec treatment on breast
cancer cells were explored. Breast cancer cells often express
continued active survival and signaling pathways, such as AKT and
MAPK signaling, as well as gene mutation, rearrangements and
chromosomal translocation in other signaling pathways (31–33).
The survival-signaling pathway serves an important role in
proliferation, tumorigenesis, anti-apoptosis and drug resistance
(34,35). The more constitutively active the
survival signaling pathways are in breast cancer, the poorer is the
prognosis (9). Apoptosis is an
important mode of cell death during tumor chemotherapy. The caspase
pathway is usually involved in the process of apoptosis, and CASP-3
is the main apoptogenic protein downstream of the mitochondrial
apoptosis signaling pathways (17); it cleaves various key cellular
substrates, therefore resulting in apoptosis (36). Cleavage of PARP is another defining
characteristic of apoptosis, and serves a pivotal role in it
(37). Heavy DNA damage usually
results from apoptosis stimuli, which elicits a major increase in
PARP activity, which rapidly depletes cellular levels of
nicotinamide-adenine dinucleotide (NAD)+ and ATP
(38). Efforts to resynthesize
NAD+ increase ATP consumption, which leads to an energy
crisis that results in cell apoptosis (39). Members of the BCL-2 protein family
are key regulators of apoptosis, and the BCL-2/BAX ratio is a key
determinant of apoptosis through the mitochondrial pathway
(40). The present results
indicated that the expression of BCL-2 was downregulated, whereas
BAX expression was upregulated in breast cancer cells following
treatment with Tec. These data also demonstrated that Tec treatment
enhanced CASP-3, CASP-8, CASP-9 and PARP activity in a
dose-dependent manner, which indicated that the mitochondrial
apoptosis signaling pathway may be involved in breast cancer cell
apoptosis induced by Tec. The MAPK and AKT signaling pathways are
two main pathways that regulate cell proliferation in breast cancer
(31–35). The present results demonstrated
that both AKT and MAPK signal transduction pathways were suppressed
simultaneously in Tec-treated breast cancer cells. It was
hypothesized that Tec, by inhibiting the activation of AKT and MAPK
pathways and promoting activation of the caspase family, may create
a more effective response in antitumor therapy, as the inhibitor
simultaneously targets three pathways in breast cancer cells. Tec
may be more effective compared with specific inhibitors that have a
single target for the treatment of breast cancer because drug
resistance frequently emerges under single target treatments due to
hyperactivation of alternative signaling pathways. In the present
study, Tec treatment exhibited multi-targeted characteristics, and
it is expected that resistance to Tec in breast cancer may occur
rarely, response to Tec may be higher and the response duration may
be longer.
Results from the present study have demonstrated the
role of Tec in restricting proliferation, migration and invasion,
and inducing apoptosis in human breast cancer cells. It was also
revealed that Tec may indirectly or directly affect the vitality of
AKT survival signaling, MAPK signaling and caspase-related
apoptosis signaling, which are key regulators of cell survival and
apoptosis during breast cancer treatment. However, further
investigations should be conducted to better determine the in-depth
molecular mechanisms controlling Tec-mediated effects on cell
survival and apoptosis-related signaling pathways. In addition,
animal experiments should be constructed to verify the therapeutic
effect of Tec in vivo, and the present results should be
verified by patient treatment trials.
In conclusion, the present study revealed the
biological response of human breast cancer cells to a novel
traditional Chinese herb Tec. It is suggested that Tec may induce
apoptosis in breast cancer cells by downregulating the protein
expression of p-AKT, BCL-2 and the MAPK pathway, and by
upregulating the expression of cleaved CASP-3, cleaved PARP and
BAX. In addition, Tec may be a potential therapeutic drug for human
breast cancer. However, future work needs to be performed in
additional cell lines to confirm these results for this molecule to
be used in the clinic.
Acknowledgements
The present study was supported by The Health System
‘Outstanding Young Talent’ Cultivation Plan of Shanghai Jinshan
District (grant no. JSYQ201620 to Xiangdong Kong), The Science and
Technology Innovation Fund Projects of Shanghai Jinshan District
(grant no. 2015-3-24 to Xiangdong Kong) and The Medical Subject
Construction Fund Project of Shanghai Jinshan District (grant no.
JSZK2015B06 to Ming Wu).
References
|
1
|
Kwak JH, Park JY, Lee D, Kwak JY, Park EH,
Kim KH, Park HJ, Kim HY, Jang HJ, Ham J, et al: Inhibitory effects
of ginseng sapogenins on the proliferation of triple negative
breast cancer MDA-MB-231 cells. Bioorg Med Chem Lett. 24:5409–5412.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang P, Du X, Xiong M, Cui J, Yang Q, Wang
W, Chen Y and Zhang T: Ginsenoside Rd attenuates breast cancer
metastasis implicating derepressing microRNA-18a-regulated Smad2
expression. Sci Rep. 6:337092016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jia Y, Wang X, Liu Q, Leung AW, Wang P and
Xu C: Sonodynamic action of hypocrellin B triggers cell apoptosis
of breast cancer cells involving caspase pathway. Ultrasonics.
73:154–161. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Qiao H, Wang TY, Yan W, Qin A, Fan QM, Han
XG, Wang YG and Tang TT: Synergistic suppression of human breast
cancer cells by combination of plumbagin and zoledronic acid in
vitro. Acta Pharmacol Sin. 36:1085–1098. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
He J, Du L, Bao M, Zhang B, Qian H, Zhou Q
and Cao Z: Oroxin A inhibits breast cancer cell growth by inducing
robust endoplasmic reticulum stress and senescence. Anti-cancer
Drugs. 27:204–215. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liedtke C, Mazouni C, Hess KR, André F,
Tordai A, Mejia JA, Symmans WF, Gonzalez-Angulo AM, Hennessy B,
Green M, et al: Response to neoadjuvant therapy and long-term
survival in patients with triple-negative breast cancer. J Clin
Oncol. 26:1275–1281. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Qu Z, Cui J, Harata-Lee Y, Aung TN, Feng
Q, Raison JM, Kortschak RD and Adelson DL: Identification of
candidate anti-cancer molecular mechanisms of compound kushen
injection using functional genomics. Oncotarget. 7:66003–66019.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang Y, He L, Yue S, Huang Q, Zhang Y and
Yang J: Characterization and evaluation of a self-microemulsifying
drug delivery system containing tectorigenin, an isoflavone with
low aqueous solubility and poor permeability. Drug Deliv.
24:632–640. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Guo Y, Chen YH, Cheng ZH, Ou-Yang HN, Luo
C and Guo ZL: Tectorigenin inhibits osteosarcoma cell migration
through downregulation of matrix metalloproteinases in vitro.
Anticancer Drugs. 27:540–546. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang YI, Lee KT, Park HJ, Kim TJ, Choi YS,
Shih Ie M and Choi JH: Tectorigenin sensitizes paclitaxel-resistant
human ovarian cancer cells through downregulation of the Akt and
NFκB pathway. Carcinogenesis. 33:2488–2498. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Amin A, Mokhdomi TA, Bukhari S, Wani SH,
Wafai AH, Lone GN, Qadri A and Qadri RA: Tectorigenin ablates the
inflammation-induced epithelial-mesenchymal transition in a
co-culture model of human lung carcinoma. Pharmacol Rep.
67:382–387. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jiang CP, Ding H, Shi DH, Wang YR, Li EG
and Wu JH: Pro-apoptotic effects of tectorigenin on human
hepatocellular carcinoma HepG2 cells. World J Gastroenterol.
18:1753–1764. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee KT, Sohn IC, Kim YK, Choi JH, Choi JW,
Park HJ, Itoh Y and Miyamoto K: Tectorigenin, an isoflavone of
Pueraria thunbergiana Benth., induces differentiation and apoptosis
in human promyelocytic leukemia HL-60 cells. Biol Pharm Bull.
24:1117–1121. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li QY, Chen L, Yan MM, Shi XJ and Zhong
MK: Tectorigenin regulates adipogenic differentiation and
adipocytokines secretion via PPARγ and IKK/NF-κB signaling. Pharm
Biol. 53:1567–1575. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Monthakantirat O, De-Eknamkul W, Umehara
K, Yoshinaga Y, Miyase T, Warashina T and Noguchi H: Phenolic
constituents of the rhizomes of the Thai medicinal plant Belamcanda
chinensis with proliferative activity for two breast cancer cell
lines. J Nat Prod. 68:361–364. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Umehara K, Nemoto K, Matsushita A, Terada
E, Monthakantirat O, De-Eknamkul W, Miyase T, Warashina T, Degawa M
and Noguchi H: Flavonoids from the heartwood of the Thai medicinal
plant Dalbergia parviflora and their effects on
estrogenic-responsive human breast cancer cells. J Nat Prod.
72:2163–2168. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Han XG, Li Y, Mo HM, Li K, Lin D, Zhao CQ,
Zhao J and Tang TT: TIMP3 regulates osteosarcoma cell migration,
invasion and chemotherapeutic resistances. Tumour Biol.
37:8857–8867. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fang C, Wang FB, Li Y and Zeng XT:
Down-regulation of miR-199b-5p is correlated with poor prognosis
for breast cancer patients. Biomed Pharmacother. 84:1189–1193.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chlebowski RT, Pan K and Col NF: Ovarian
suppression in combination endocrine adjuvant therapy in
premenopausal women with early breast cancer. Breast Cancer Res
Treat. 161:185–190. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kong X, Li Z and Li X: GSTP1, GSTM1 and
GSTT1 polymorphisms as predictors of response to chemotherapy in
patients with breast cancer: A meta-analysis. Cancer Chemother
Pharmacol. 78:1163–1173. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun S, Wang F, Dou H, Zhang L and Li J:
Preventive effect of zoledronic acid on aromatase
inhibitor-associated bone loss for postmenopausal breast cancer
patients receiving adjuvant letrozole. Onco Targets Ther.
9:6029–6036. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Meng L, Xu Y, Xu C and Zhang W: Biomarker
discovery to improve prediction of breast cancer survival: Using
gene expression profiling, meta-analysis and tissue validation.
Onco Targets Ther. 9:6177–6185. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fleisher B, Clarke C and Ait-Oudhia S:
Current advances in biomarkers for targeted therapy in
triple-negative breast cancer. Breast Cancer (Dove Med Press).
8:183–197. 2016.PubMed/NCBI
|
|
25
|
Wang X, Xia X, Leung AW, Xiang J, Jiang Y,
Wang P, Xu J, Yu H, Bai D and Xu C: Ultrasound induces cellular
destruction of nasopharyngeal carcinoma cells in the presence of
curcumin. Ultrasonics. 51:165–170. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tan XL, Guo L and Wang GH: Polyporus
umbellatus inhibited tumor cell proliferation and promoted tumor
cell apoptosis by down-regulating AKT in breast cancer. Biomed
Pharmacother. 83:526–535. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Iwanowycz S, Wang J, Hodge J, Wang Y, Yu F
and Fan D: Emodin inhibits breast cancer growth by blocking the
tumor-promoting feedforward loop between cancer cells and
macrophages. Mol Cancer Ther. 15:1931–1942. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kapoor S: Tectorigenin and its inhibitory
effects on tumor growth in systemic malignancies. Immunopharmacol
Immunotoxicol. 35:5332013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ha le M, Que do TN, Huyen do TT, Long PQ
and Dat NT: Toxicity, analgesic and anti-inflammatory activities of
tectorigenin. Immunopharmacol Immunotoxicol. 35:336–340. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Thelen P, Scharf JG, Burfeind P,
Hemmerlein B, Wuttke W, Spengler B, Christoffel V, Ringert RH and
Seidlová-Wuttke D: Tectorigenin and other phytochemicals extracted
from leopard lily Belamcanda chinensis affect new and established
targets for therapies in prostate cancer. Carcinogenesis.
26:1360–1367. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ho JY, Hsu RJ, Liu JM, Chen SC, Liao GS,
Gao HW and Yu CP: MicroRNA-382-5p aggravates breast cancer
progression by regulating the RERG/Ras/ERK signaling axis.
Oncotarget. 8:22443–22459. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang D, Wu P, Wang H, Zhu L, Zhao W and Lu
Y: SIN1 promotes the proliferation and migration of breast cancer
cells by Akt activation. Biosci Rep. 36:e004242016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang H, Jia XH, Chen JR, Yi YJ, Wang JY,
Li YJ and Xie SY: HOXB4 knockdown reverses multidrug resistance of
human myelogenous leukemia K562/ADM cells by downregulating P-gp,
MRP1 and BCRP expression via PI3K/Akt signaling pathway. Int J
Oncol. 49:2529–2537. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Aghazadeh S and Yazdanparast R:
Mycophenolic acid potentiates HER2-overexpressing SKBR3 breast
cancer cell line to induce apoptosis: Involvement of AKT/FOXO1 and
JAK2/STAT3 pathways. Apoptosis. 21:1302–1314. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang Z, Liu L, Liu C, Cao S, Zhu Y and
Mei Q: TIPE2 suppresses the tumorigenesis, growth and metastasis of
breast cancer via inhibition of the AKT and p38 signaling pathways.
Oncol Rep. 36:3311–3316. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Din TA, Seeni A, Yusoff MS, Shamsuddin S
and Jaafar H: PF4-Induced apoptosis in breast cancer in vivo study:
The role of the caspases family. Pathology. 48 Suppl 1:S1222016.
View Article : Google Scholar
|
|
37
|
Lazebnik YA, Kaufmann SH, Desnoyers S,
Poirier GG and Earnshaw WC: Cleavage of poly(ADP-ribose) polymerase
by a proteinase with properties like ICE. Nature. 371:346–347.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Soldani C and Scovassi AI:
Poly(ADP-ribose) polymerase-1 cleavage during apoptosis: An update.
Apoptosis. 7:321–328. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bhaskara VK, Panigrahi M, Challa S and
Babu PP: Comparative status of activated ERK1/2 and PARP cleavage
in human gliomas. Neuropathology. 25:48–53. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sharifi S, Barar J, Hejazi MS and Samadi
N: Roles of the Bcl-2/Bax ratio, caspase-8 and 9 in resistance of
breast cancer cells to paclitaxel. Asian Pac J Cancer Prev.
15:8617–8622. 2014. View Article : Google Scholar : PubMed/NCBI
|