Introduction
Asthma is a complex disease that involves a number
of genetic and environmental influences (1). The chronic inflammation in asthma is
characterized by eosinophilic recruitment, airway
hyperresponsiveness (AHR), goblet cell hyperplasia/metaplasia,
epithelial hypertrophy/hyperplasia, mucus hypersecretion, collagen
deposition, smooth muscle cell hypertrophy/hyperplasia and
subepithelial fibrosis (2,3). Worldwide ~300 million people suffer
from asthma, and this number is predicted to rise over the next
10–15 years to >400 million (4). It has been reported that chronic
airway inflammatory processes lead to the recruitment of activated
eosinophils and T helper 2 (Th2) lymphocytes to the site of injury
and an improper immune response to common allergens (5). Recurrent inflammation and subsequent
abnormalities in the tissue repair mechanisms may lead to
structural alterations in the airway wall that may develop the
clinically detectable features of epithelial injury, goblet cell
hyperplasia, subepithelial thickening, airway hyperplasia and
angiogenesis (6). Inflammatory
infiltrates in the airways that are characteristic of asthma may
affect the structural cells and lead to AHR (7). There are a number of different immune
cells in the infiltrates, including T lymphocytes that produce
cytokines, such as interleukin (IL)-4, IL-5 and IL-13, which serve
important roles in the pathogenesis of asthma (8–10).
Therefore, targeting these T lymphocytes may have the potential to
effectively treat asthma.
OX40 (also known as CD134) and its binding partner
OX40 ligand (OX40L; also known as CD252) are members of the tumor
necrosis factor (TNF)/TNF receptor superfamily and are expressed on
activated CD4 and CD8 T cells, and on a number of lymphoid and
non-lymphoid cells (11). OX40L is
mainly expressed by antigen-presenting cells (APCs), such as
dendritic cells, but is also expressed by B cells, macrophages and
Langerhans cells (12). Dendritic
cells in the airway express OX40L in response to epithelial
cell-derived thymic stromal lymphopoietin stimulation (TSLP)
(13). In vivo studies
using murine and nonhuman primate models of asthma have reported
that the inhibition of OX40L suppressed TSLP-mediated Th2
inflammation and reduced the number of OX40L+ dendritic
cells in the lungs (14).
OX40/OX40L interactions have been demonstrated to serve a central
role in numerous inflammatory and autoimmune disease development,
which suggested that they may be suitable candidates for clinical
intervention (15); however, the
effects and precise mechanisms of OX40/OX40L signaling in the
development of asthma remains unclear. Clarification of the
underlying mechanisms of the OX40/OX40L signaling in mediating
inflammation, immunoreactions or other cell functions in asthma may
lead to improved clinical treatment on asthma.
The present study examined the effects of OX40/OX40L
signaling on inflammation and T cell functions in a mouse asthma
model and investigated the possible underlying mechanisms. The aim
was to provide a new perspective and deeper understanding of the
etiology of asthma and to provide additional evidence for the
potential involvement of OX40/OX40L signaling in the development of
asthma.
Materials and methods
Reagents and antibodies
Murine interleukin (IL-) 4 (catalog no. BMS613),
IL-6 (catalog no. BMS603-2), IL-13 (catalog no. KMC2221), IL-17
(catalog no. BMS6001), tumor necrosis factor (TNF-)α (catalog no.
BMS607-3) and interferon (IFN-)γ (catalog no. 88-8314-77) ELISA
kits were purchased from Invitrogen (Thermo Fisher Scientific,
Inc., Waltham, MA, USA). Ovalbumin (OVA) was purchased from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). Neutralizing rat
anti-OX40L monoclonal antibody was purchased from Bio X Cell (West
Lebanon, NH, USA; catalog no. BE0033-1-25MG). Mouse recombinant
OX40L protein was purchased from R&D Systems, Inc.
(Minneapolis, MN, USA; catalog no. 1236-OX-025). Rabbit
anti-cleaved caspase 3 (Asp175), polyclonal antibody was purchased
from Abbexa, Ltd. (Cambridge, UK; catalog no. abx015533). Rabbit
anti-NF-κB polyclonal antibody (Aviva Systems Biology, San Diego,
CA, USA; catalog no. OAAI00072; phosphorylated (p-)Ser337).
Anti-GAPDH antibody was purchased from Beyotime Institute of
Biotechnology (Shanghai, China; catalog no. AF0006). Cell Counting
Kit-8 (CCK-8) was purchased from Dojindo Molecular Technologies,
Inc. (Kumamoto, Japan). Dead Cell Apoptosis kit with Annexin V
Alexa Fluor 488 & propidium iodide (PI) was purchased from
Thermo Fisher Scientific, Inc. (catalog no. V13241). Fluorescein
isothiocyanate-conjugated rat anti-CD4 monoclonal antibody was
purchased from LifeSpan BioSciences, Inc. (Seattle, WA, USA;
catalog no. LS-C62734-300). Phycoerythrin (PE-)conjugated goat
anti-OX40 polyclonal antibody was purchased from R&D Systems,
Inc. (catalog no. FAB1256P).
Experimental animals
Specific-pathogen-free female BALB/c mice (n=156;
age, 6–8 weeks; weight, 20–25 g) were obtained from Shanghai SLAC
Laboratory Animal Co. Ltd. (Shanghai, China), and were kept at
19–22°C and 40–75% relative humidity at all times in the animal
facility under specific-pathogen-free conditions. A 12-h light/dark
cycle was maintained during the course of the present study.
Animals were kept in groups of five and fed regular lab chow and
water ad libitum. All animal experiments performed in this
study conformed to the Guide for the Care and Use of Laboratory
Animals (16) and were approved by
the Institutional Animal Care and Use Committee of Soochow
University (Suzhou, China).
OX40+ T cell sorting
protocol
Murine CD4+ T cells were obtained from
mononuclear cells prepared from the bronchoalveolar lavage fluid
(BALF) of 18 OVA-challenged mice or the spleen of 6 BALB/c mice and
T cells were isolated using a Pan T Cell Isolation Kit II (Miltenyi
Biotec GmbH, Bergisch Gladbach, Germany; catalog no. 130-095-130)
according to the manufacturer's protocol (17) and collected in sterile PBS
containing 50% fetal calf serum (FCS). The purified T cells
(106 cells/ml) were then cultured in DMEM containing 10%
FCS in a 6-well plate with plate-bound anti-CD3 antibody (3 µg/ml;
R&D Systems, Inc.; catalog no. MAB4841-SP) and soluble
anti-CD28 antibody (10 µg/ml; R&D Systems, Inc.; catalog no.
MAB4832-SP), as well as IL-2 (5 ng/ml; R&D Systems, Inc.;
catalog no. P04351), IL-4 (20 ng/ml; R&D Systems, Inc.; catalog
no. P07750), anti-IFN-γ antibody (10 µg/ml; R&D Systems, Inc.;
catalog no. MAB485-SP) and anti-IL-12 antibody (10 µg/ml; R&D
Systems, Inc.; catalog no. AF-419-SP) for 3 days at 37°C. Cells
were subsequently removed, washed and cultured with DMEM containing
10% FCS for a further 3-6 days at 37°C without further stimulation
(18). T cells were subsequently
processed for the sorting assay with phycoethryin-conjugated OX40
antibodies (10 µl/106 cells; R&D Systems, Inc.;
catalog no. FAB1256P) using flow cytometry to isolate
OX40+ T cells for further in vivo experiments.
Cells were analyzed with FlowJo software (version 7.6; FlowJo LLC,
Ashland, OR, USA).
Immunization and intervention
Protocols for immunization and intervention were as
previously described (19).
Briefly, 120 mice were immunized with an intraperitoneal injection
of OVA (100 µg; Sigma-Aldrich; Merck KGaA) and aluminum hydroxide
(2 mg; Pierce; Thermo Fisher Scientific, Inc.) in sterile saline on
days 1 and 8. On days 9–14 following the initial sensitization,
mice were challenged intranasally with 20 µg of 2% OVA in sterile
saline. In other experiments for intervention, 100 µg/kg of
recombinant murine OX40L protein, 12 mg/kg of neutralizing
anti-OX40L antibody or 5×106 isolated OX40+ T
cells or PBS (control) were injected intravenously through the tail
vein following anesthesia (20,21).
Cytokine and protein measurements in
bronchoalveolar lavage fluid (BALF)
BALF was collected by flushing 1 ml ice-cold PBS
back and forth three times through a tracheal cannula followed by
centrifugation at 2,000 × g at 4°C for 10 min, as previously
described (22). Protein
concentrations of IL-4, IL-6, IL-13, IL-17, TNF-α and IFN-γ in the
supernatant were measured using murine cytokine-specific ELISA kits
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. The concentration of IL-4, IL-6, IL-13,
IL-17, TNF-α and IFN-γ in the BALF were measured as markers of
inflammatory reaction using a microplate reader at 450 nm (Thermo
Fisher Scientific, Inc.; catalog no. 51119000).
Determination of eosinophils
Eosinophils were detected and counted as previously
described (23). Briefly, BALF was
performed by instilling 0.9% NaCl containing 0.6 mmol/l EDTA in two
separate 0.5 ml aliquots. The fluid was recovered by gentle suction
and placed on ice for immediate processing. An aliquot of ~0.5 ml
BALF was processed immediately for eosinophil count. BALF cell
suspensions were stained with PE-conjugated rat anti-mouse CCR3 at
4°C for 30 min (1:500; R&D Systems, Inc.; catalog no.
FAB1551T-100UG), followed by staining with fluorescein
isothiocyanate-conjugated anti-mouse CD16 (1:500; Antigenix America
Inc., Huntington Station, NY, USA; catalog no. RM160323) for 30 min
at 4°C in the dark. Samples stained with non-immunized rat IgG mAb
(1:100; R&D Systems, Inc.; catalog no. IC005P) for 30 min at
4°C were used as an isotype control. The percentage of eosinophils
(CCR3+CD16−) was calculated as the number of
eosinophils divided by the total number of cells in the BALF
sample, as determined by flow cytometry and were analyzed with
FlowJo software (version 7.6). All analyses were performed in a
blinded fashion. The remainder of the lavage fluid was centrifuged
at 1,000 × g for 10 min at 4°C and the supernatant was removed
aseptically and stored in individual aliquots at −80°C.
Western blot analysis
Cell pellets (1×107) from fresh BALF
collected from lung tissues was homogenized in 600 ml
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology; catalog no. P0013B) in the presence of protease
inhibitors for 30 min at 4°C. Protein concentration was determined
with the Bradford assay. Lysates were centrifuged at 9,000 × g for
15 min at 4°C. Samples (20 µg/lane) were boiled for 5 min at 100°C
and separated by 12% SDS-PAGE under denaturing conditions and
electroblotted to a polyvinylidene difluoride membrane (Bio-Rad
Laboratories, Inc., Hercules, CA). Membranes were blocked with PBS
and 5% non-fat dry milk for 12 h at 4°C and incubated with the
following antibodies for 1 h at room temperature: Anti-NF-κB
(1:500; Aviva Systems Biology; catalog no. OAAI00072; p-Ser337) and
anti-cleaved caspase 3 (1:500; Abbexa Ltd., Cambridge, UK; catalog
no. abx015533). Immunoblot assays were then washed and incubated
with a horseradish peroxidase-labeled secondary antibody (1:5,000
dilution; GE Healthcare Life Sciences, Shanghai, China; catalog
nos. RPN4301 and RPN4201) for 1 h at room temperature. Protein
bands were visualized using ECL Plus Enhanced Chemiluminescence
Reagent (GE Healthcare Life Sciences), according to the
manufacturer's protocol. Protein band intensity was determined
relative to GAPDH using ImageJ software (version 2.1.4.7; National
Institutes of Health, Bethesda, MD, USA).
Cell proliferation assay in vitro
The CCK-8 assay was used to evaluate proliferation
of isolated T cells. T cells were exposed to increasing
concentrations of OX40L protein (10, 50, 100 or 200 ng/ml) or 200
ng/ml of OX40L protein combined with 200 ng/ml of neutralizing
anti-OX40L antibody. Briefly, T cells (3×103 cells/well)
were seeded in each well of a 96-well plate and stimulated with
0.10 mg/ml phytohemagglutinin (Thermo Fisher Scientific, Inc.;
catalog no. 00-4977-93) for 24 h. Subsequently, the cells were
cultured in serum-free DMEM for 24 h starvation at 37°C. Cells were
treated with different concentrations of OX40L protein (10–200
ng/ml) or 200 ng/ml OX40L protein and 200 ng/ml neutralizing
anti-OX40L antibody (200 ng/ml) for 24 h at 37°C, and the
proliferative activity was determined by CCK-8 assay, according to
the manufacturer's protocol. CCK-8 (10 µl) was added to each well
followed by incubation for an additional 2–4 h at 37°C. When the
media changed from red to yellow, the absorbance value was detected
at a wavelength of 450 nm using a microplate reader (Thermo Fisher
Scientific, Inc.; catalog no. 51119000). The experiment was
performed at least three times.
Flow cytometric analysis of cell
apoptosis
Mononuclear T cells were isolated from BALF
according to a previously described procedure with some
modifications (24). Briefly, T
cells (1×105 cells/test) were washed twice with PBS and
centrifuged at 1,000 × g for 5 min at room temperature. Each pellet
(~1×105 cells) of the T cells was then re-suspended in
400 µl PBS followed by incubation with 5 µl Annexin V and 1 µl
propidium iodide (PI; 1 mg/ml) for 15 min at room temperature.
Cells were subsequently analyzed using a flow cytometer without
washing the cells. Cells that were PI and Annexin V negative were
considered healthy cells, PI negative and Annexin V positive were
considered apoptotic and cells that were PI and Annexin V positive
were considered necrotic.
Statistical analysis
Data are presented as the mean ± standard error of
the mean. Statistical analyses were performed using SPSS 18.0
software package (SPSS Inc., Chicago, IL, USA). Comparisons among
multiple groups were performed using one-way analysis of variance
followed by the Bonferroni post-hoc test if the data were normally
distributed. P<0.05 was considered to indicate a statistically
significant difference.
Results
OX40/OX40L signaling effects on
eosinophil recruitment in lungs following sensitization and
challenge with OVA
The presence of eosinophils in the BALF and lungs is
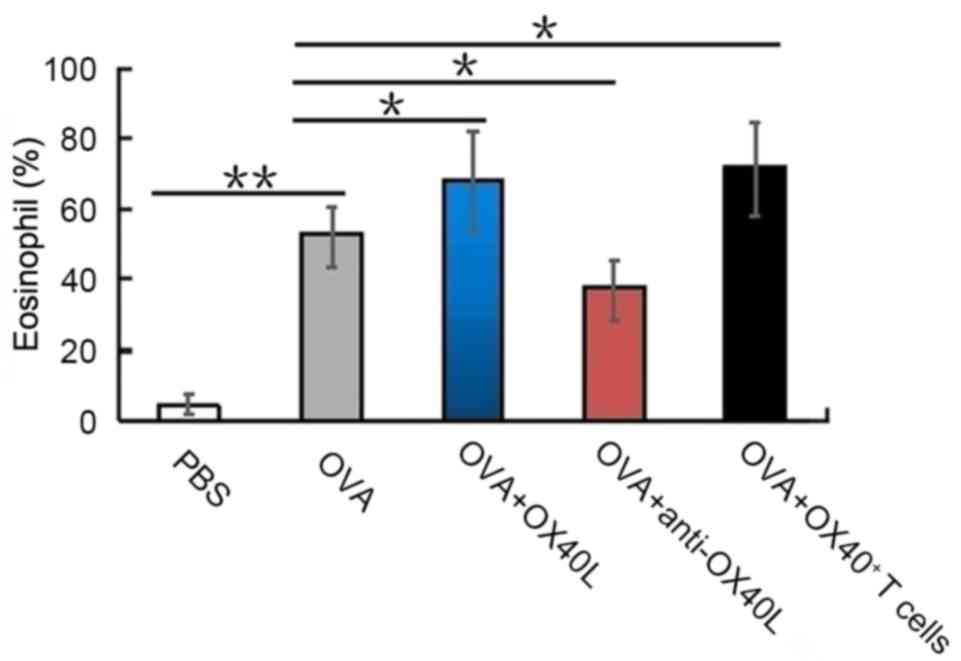
an important indicator of airway inflammation (25). OVA challenged mice had a
significantly higher eosinophil count (56±6.8%) compared with
PBS-treated control mice (4.4±3.6%; P<0.01); OX40L
protein-treated mice and OX40+ T cell-treated mice also
had a high eosinophil count (65±5.5 and 67±7.2%, respectively) in
the BALF following sensitization and challenge with OVA (Fig. 1), compared with those treated with
OVA alone. By contrast, eosinophils accounted for only 41±4.5% of
all cells in the BALF of mice treated with neutralizing anti-OX40L
antibody (Fig. 1). These results
suggested that OX40/OX40L signaling may serve an important role in
eosinophil recruitment into the lungs.
Effects of OX40/OX40L signaling on
cytokine expression levels in vivo
Mice challenged with OVA exhibited a significant
increase in IL-4, IL-6, IL-13, IL-17, TNF-α and IFN-γ expression
levels in BALF, compared with the levels in untreated mice
(P<0.05; Fig. 2). In mice
sensitized and challenged with OVA that were co-treated with
neutralizing anti-mouse OX40L antibody, this increase in IL-4,
IL-6, IL-13, IL-17, TNF-α and IFN-γ cytokine levels was
significantly suppressed (P<0.05 or P<0.01 vs. OVA; Fig. 2); whereas mice co-treated with
OX40L protein or OX40+ T cells exhibited a significant
increase in these cytokine levels in the BALF (P<0.05 or
P<0.01; Fig. 2), compared with
the levels the OVA group. These results suggested that OX40/OX40L
signaling may significantly increase the IL-4, IL-6, IL-13, IL-17,
TNF-α and IFN-γ cytokine levels in the BALF.
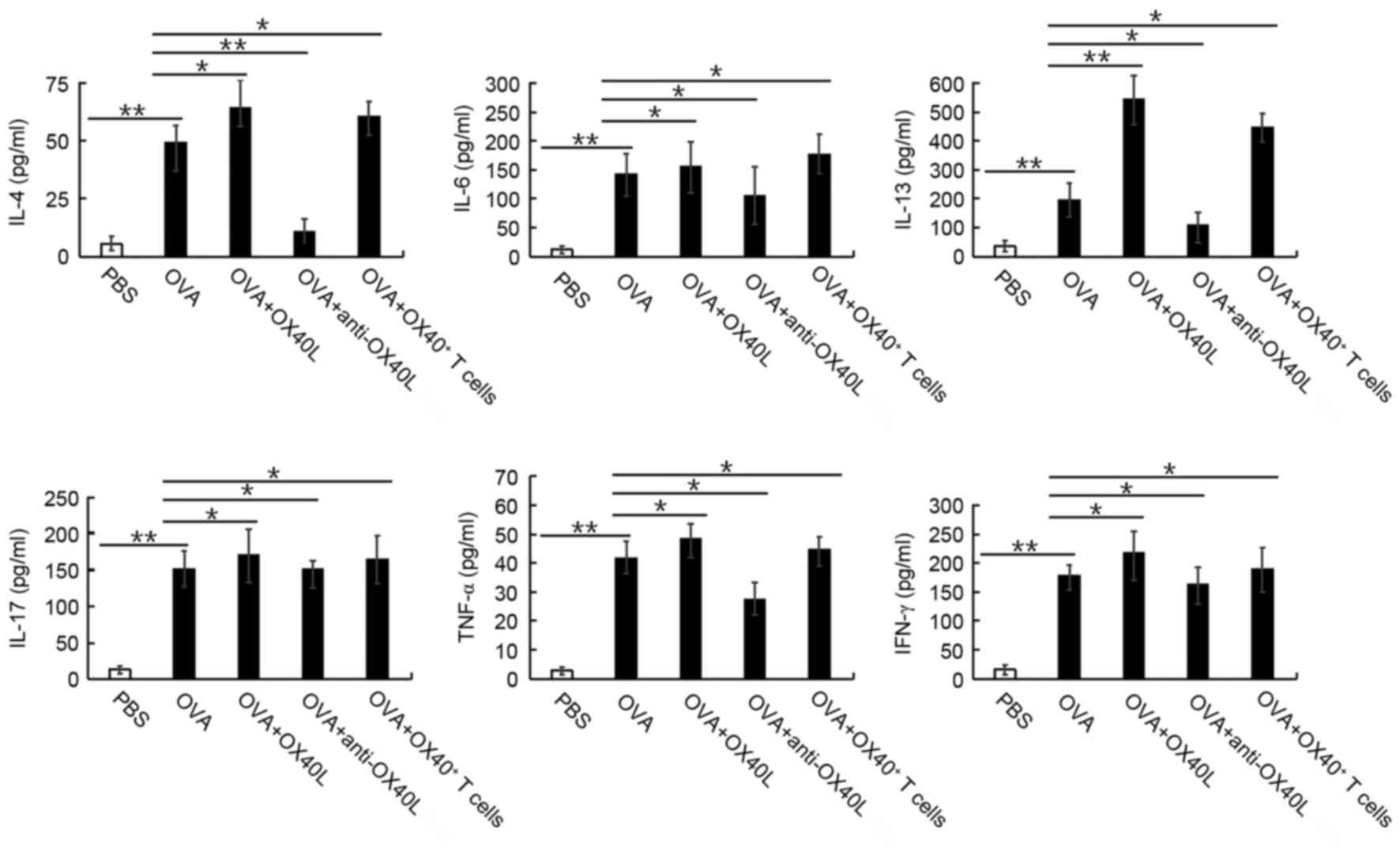 | Figure 2.Effects of OX40/OX40L signaling on
cytokine secretion in BALF in response to OVA treatment. Expression
levels of the cytokines IL-4, IL-6, IL-13, IL-17, TNF-α and IFN-γ
were measured by ELISA in the BALF of PBS treated mice and in mice
treated with OVA, OVA + OX40L protein, OVA + neutralizing
anti-OX40L antibody, and OVA + OX40+ T cells. Data are
shown as pg/ml ± SEM, n=6-8 mice/group; *P<0.05, **P<0.01.
BALF, bronchoalveolar lavage fluid; IFN, interferon; IL,
interleukin; OVA, ovalbumin; OX40L, OX40 ligand; TNF, tumor
necrosis factor. |
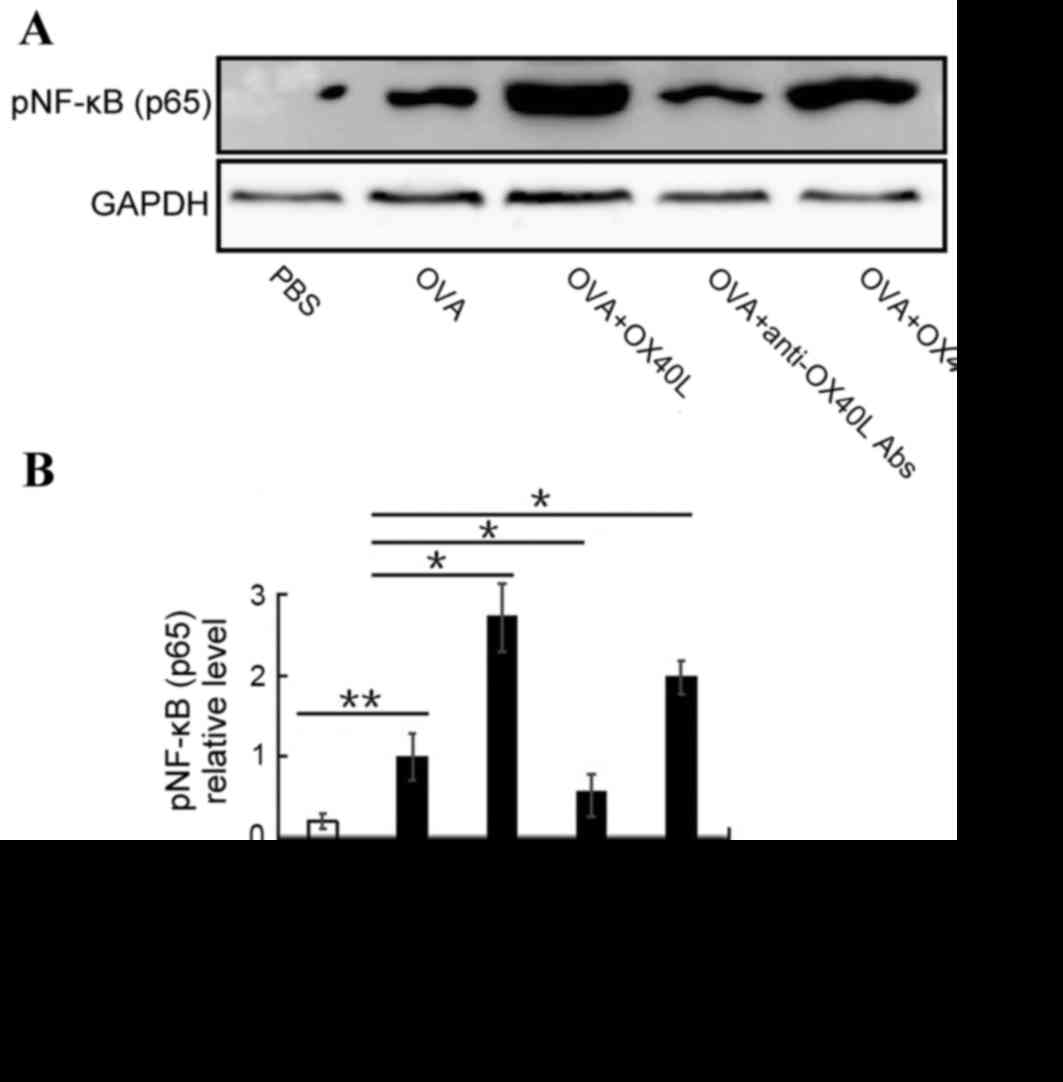
NF-κB activation promotes inflammatory cell
migration and proliferation, and mediates inflammatory factor
secretion (26). To determine
whether OX40/OX40L signaling is able to effect NF-κB activation,
the expression of activated NF-κB was detected in inflammatory
cells from the BALF. Activated NF-κB expression was significantly
increased in the OVA-challenged group compared with the PBS group
(P<0.01). Furthermore, pNF-κB expression was significantly
increased in OX40L protein or OX40+ T cell-treated mice
and significantly reduced in anti-OX40L antibody-treated mice
compared with mice in the OVA group (Fig. 3). These results indicated that
OX40/OX40L signaling may induce NF-κB activation and suggests that
OX40/OX40L signaling had a pro-inflammatory effect by regulating
the expression of IL-4, IL-6, IL-13, IL-17, TNF-α and IFN-γ through
NF-κB activation.
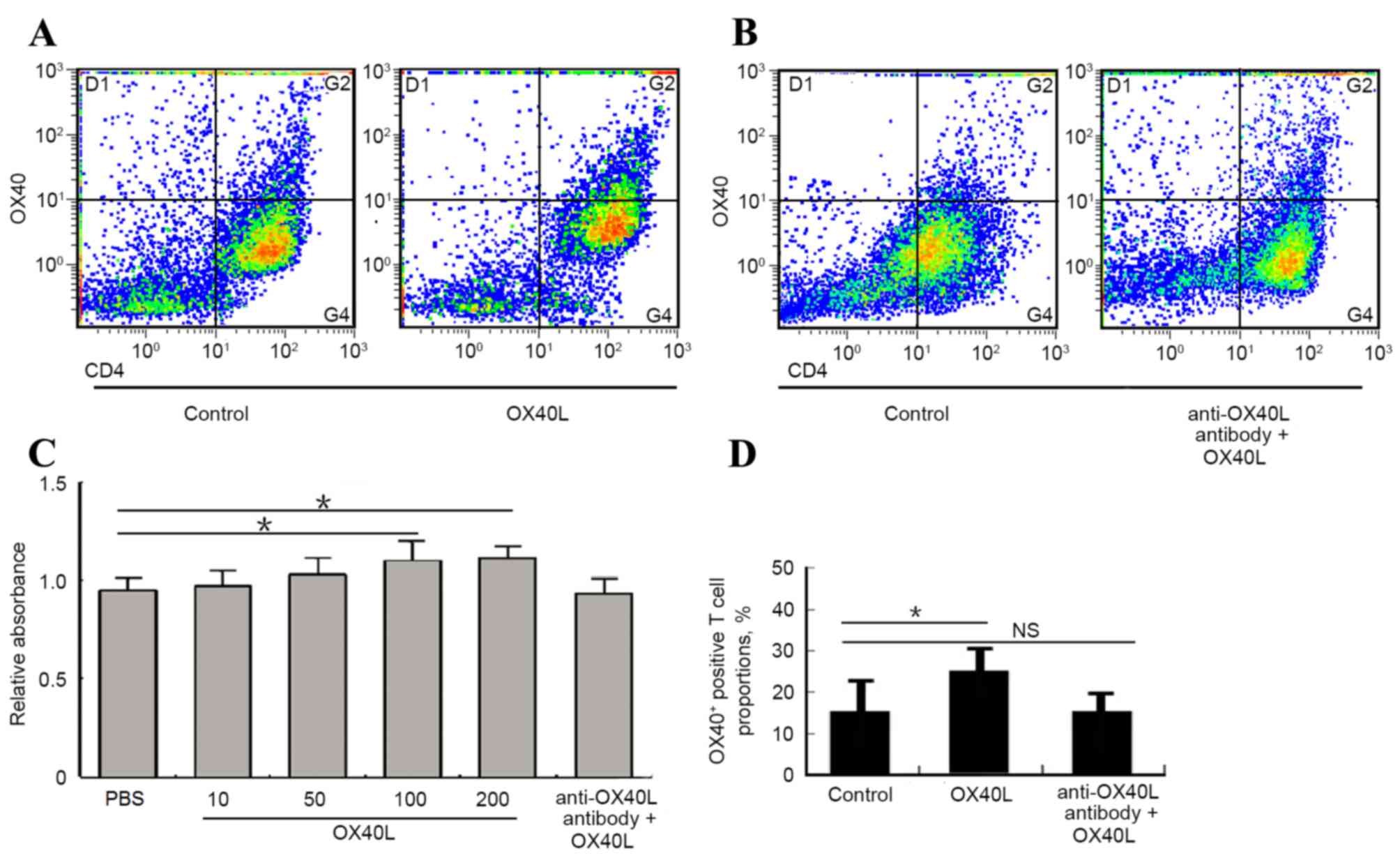
Effects of OX40/OX40L signaling on
proliferation and polarization of CD4+ T cells in
vitro
It has been previously reported that OX40L promoted
the number of OX40+ CD4 T cells in an asthma model
(27), which indicated that
OX40/OX40L signaling may serve an important role in the process of
CD4+ T cell polarization, which may eventually affect
the progression of asthma. The present study examined the effects
of OX40/OX40L signaling on CD4 T cell proliferation and
polarization in vitro. CD4+ T cells isolated from
mouse spleen cultured with 100 or 200 ng/ml OX40L protein exhibited
a significant increase in proliferation, whereas no change in
proliferation was detected between the OX40L protein and
neutralizing anti-OX40L antibody co-culture group and the
PBS-treated control group (Fig.
4A). Furthermore, OX40L treatment significantly promoted the
number of OX40+ T cells, whereas co-treatment with
neutralizing anti-OX40L antibody suppresses OX40+ T cell
number (Fig. 4B-D). These data
indicated that OX40/OX40L signaling may be involved in
CD4+ T cell proliferation and polarization, and thus
influences T cell bio-function in asthma pathology.
OX40/OX40L signaling effects on T cell
apoptosis in lungs following sensitization and challenge with
OVA
To further explore the mechanism underlying
OX40/OX40L signaling involved in asthma process, the expression
levels of apoptosis-related protein cleaved caspase-3 were examined
in isolated T cells from PBS-treated, OX40L protein-treated or
neutralizing anti-OX40L antibody-treated mice. Western blot
analysis demonstrated that the relative protein expression level of
cleaved-caspase-3 was significantly increased in anti-OX40L
antibody-treated mice (0.87±0.11) and decreased in OX40L
protein-treated mice (0.36±0.18) compared with PBS-treated mice
(0.51±0.17; P<0.05 Fig. 5A and
B). Similar results were obtained by flow cytometric analyses
for apoptosis (Fig. 5C and D).
Discussion
OX40/OX40L signaling serves a key role in the
development, differentiation and physiological functions of T cells
and other immunological cells (28). The expression of OX40 is
upregulated on activated T cells (29), whereas it is constitutively
expressed on T regulatory cells (30,31).
This difference in expression is consistent with the previously
hypothesized role of OX40/OX40L interactions in the propagation of
the immune response and in initial T cell priming (32). OX40L is predominantly expressed in
APCs, such as B cells, macrophages, microglia, dendritic cells and
endothelial cells (33–39). Signaling through OX40/OX40L
interactions during effector T cell responses has been previously
reported to enhance T cell survival (40,41),
cytokine production (42) and
increase the number of memory CD4+ T cells (43). Experimental models of autoimmunity
and inflammation have indicated the potential role for OX40/OX40L,
as inhibiting the interaction between OX40 and OX40L attenuates
disease progression or severity (44,45).
Owing to this important role in the pathological process of
numerous diseases, the present study examined the effects and the
mechanisms of OX40/OX40L signaling in experimentally induced
asthma.
A number of previous in vivo studies
(15,46–48)
have demonstrated a pathogenic role for OX40/OX40L signaling in
autoimmune diseases, and the disruption of this axis was reported
to be beneficial for prevention and treatment (49). However, whether OX40/OX40L serves
pathogenic roles in human asthma requires further exploration. Our
previous study demonstrated that OX40L was overexpressed by myeloid
APCs in peripheral blood and BALF in a mouse asthma model (27). Another previous study reported that
the number of OX40L-expressing myeloid APCs was positively
correlated with disease activity, as assessed by intrafluid
expression of inflammatory factors (50). In the present study, the results
indicated that OX40/OX40L signaling promoted CD4+ T cell
proliferation and polarization into CD4+OX40+
T cells, as evidenced by the significantly increased proportion of
CD4+OX40+ T cells present when OX40L was
administered to isolated T cells from experimental mice in
vitro. Conversely, CD4+ T cell proliferation was
suppressed in cells co-treated with the neutralizing anti-mouse
OX40L antibody. These observations suggest that OX40/OX40L
interactions may have activated CD4+OX40+ T
cells and this event may represent a crucial component for
affecting the progression of experimental asthma (51).
Although OX40/OX40L signaling inhibition was
demonstrated to reduce inflammation, as determined by its effect on
IL-4, IL-6, IL-13, IL-17, TNF-α and IFN-γ expression in peripheral
blood and bronchoalveolar lavage fluid, there are still many signal
transduction processes that may be involved in the pathogenesis of
asthma progression, which makes these types of investigations very
complex. Previous studies have focused on NF-κB signaling, which is
necessary for the proliferation and migration of cells, the
expression of multiple cytokines and the inflammatory response
(52). The present study examined
activated NF-κB expression in asthma lung tissues by western blot
analysis and demonstrated that activated NF-κB expression was
elevated in OX40L protein- and CD4+OX40+ T
cell-treated groups, and it was decreased in anti-OX40L antibody
treated group, suggesting that OX40/OX40L interactions may affect
asthma progression through the NF-κB pathway. Song et al
(53) reported that the activation
of NF-κB1 by OX40 contributes to antigen-driven T cell expansion
and survival. Burrows et al (54) demonstrated that OX40 blockade
inhibits house dust mite-driven allergic lung inflammation in mice
and allergic responses in humans in vitro. The effects of
OX40/OX40L on T cell function and the inflammatory response through
the NF-κB pathway demonstrated in these previous reports is
consistent with the results of the present study.
Inflammatory responses are important factors for
asthma-induced lung injury (55–57).
During the acute pathological process, inflammatory cells
(including neutrophils and macrophages) are recruited into lung
lesions, where they are induced to secret pro-inflammatory
cytokines to further accelerate inflammatory responses and promote
asthma (58). Therefore, effective
blocking or inhibiting of the inflammatory responses may be a
fundamental treatment strategy for asthma. OX40/OX40L interaction
is a promoting factor in pathological processes of asthma (59). Consistently, OX40/OX40L
interactions in the present study were also demonstrated to promote
inflammatory cell infiltration and facilitated pro-inflammatory
cytokine expression. A clear association has been demonstrated
between an experimental model of asthma and the regulation of the
inflammatory response by OX40/OX40L (60,61).
In conclusion, although it requires further
exploration, OX40/OX40L signaling may be a prospective intervention
target for inhibiting CD4+OX40+ T cell
activation, inflammatory factor secretion and inflammatory cell
infiltration into BALF in clinical settings of asthma therapy.
Results from the present study revealed that the neutralizing
anti-mouse OX40L antibody was an efficient inhibitor of
CD4+OX40+ T cell production in OVA-induced
asthma, and this inhibiting effect may further impact IL-4, IL-6,
IL-13, IL-17, TNF-α and IFN-γ expression, reduce inflammatory
responses and thus alleviate asthma progression. Targeting of the
OX40/OX40L axis may provide therapeutic effects in the treatment of
asthma.
Acknowledgements
This study was supported by The National Natural
Science Foundation of China (grant nos. 81300026 and 31600736), The
Science and Education of Public Health Project for Young Medical
Talents of Jiangsu Province (grant nos. QNRC2016747 and
QNRC2016718), The Societal and Developmental Project of Suzhou
(grant no. SS201630), The Suzhou Key Laboratory for Respiratory
Medicine (grant no. SZS201617) and The Clinical Medical Center of
Suzhou (grant no. Szzx201502).
References
|
1
|
Blume C and Davies DE: In vitro and ex
vivo models of human asthma. Eur J Pharm Biopharm. 84:394–400.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kumar RK and Foster PS: Modeling allergic
asthma in mice: Pitfalls and opportunities. Am J Respir Cell Mol
Biol. 27:267–272. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Saw S, Kale SL and Arora N: Serine
protease inhibitor attenuates ovalbumin induced inflammation in
mouse model of allergic airway disease. PLoS One. 7:e411072012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hamid Q and Tulic M: Immunobiology of
asthma. Annu Rev Physiol. 71:489–507. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Paul WE and Zhu J: How are T(H)2-type
immune responses initiated and amplified? Nat Rev Immunol.
10:225–235. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Busse WW and Lemanske RF Jr: Asthma. N
Engl J Med. 344:350–362. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Holgate ST: Pathogenesis of asthma. Clin
Exp Allergy. 38:872–897. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Medoff BD, Thomas SY and Luster AD: T cell
trafficking in allergic asthma: The ins and outs. Annu Rev Immunol.
26:205–232. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schuijs MJ, Willart MA, Hammad H and
Lambrecht BN: Cytokine targets in airway inflammation. Curr Opin
Pharmacol. 13:351–361. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Koshy S, Huq R, Tanner MR, Atik MA, Porter
PC, Khan FS, Pennington MW, Hanania NA, Corry DB and Beeton C:
Blocking KV1.3 channels inhibits Th2 lymphocyte function and treats
a rat model of asthma. J Biol Chem. 289:12623–12632. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Webb GJ, Hirschfield GM and Lane PJ: OX40,
OX40L and autoimmunity: A comprehensive review. Clin Rev Allergy
Immunol. 50:312–332. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang YH and Liu YJ: Thymic stromal
lymphopoietin, OX40-ligand, and interleukin-25 in allergic
responses. Clin Exp Allergy. 39:798–806. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu YJ: Thymic stromal lymphopoietin and
OX40 ligand pathway in the initiation of dendritic cell-mediated
allergic inflammation. J Allergy Clin Immunol. 120:238–244. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Seshasayee D, Lee WP, Zhou M, Shu J, Suto
E, Zhang J, Diehl L, Austin CD, Meng YG, Tan M, et al: In vivo
blockade of OX40 ligand inhibits thymic stromal lymphopoietin
driven atopic inflammation. J Clin Invest. 117:3868–3878. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Croft M, So T, Duan W and Soroosh P: The
significance of OX40 and OX40L to T-cell biology and immune
disease. Immunol Rev. 229:173–191. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
National Research and Council (US), .
Committee for the update of the guide for the care and use of
laboratory animals: Guide for the care and use of laboratory
animals. 8th. Washington (DC): National Academies Press (US);
2011
|
|
17
|
Wu Q, Tang Y, Hu X, Wang Q, Lei W, Zhou L
and Huang J: Regulation of Th1/Th2 balance through OX40/OX40L
signalling by glycyrrhizic acid in a murine model of asthma.
Respirology. 21:102–111. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Salek-Ardakani S, Song J, Halteman BS,
Jember AG, Akiba H, Yagita H and Croft M: OX40 (CD134) controls
memory T helper 2 cells that drive lung inflammation. J Exp Med.
198:315–324. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee MY, Lee JA, Seo CS, Ha H, Lee NH and
Shin HK: Protective effects of Mentha haplocalyx ethanol extract
(MH) in a mouse model of allergic asthma. Phytother Res.
25:863–869. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Malmström V, Shipton D, Singh B,
Al-Shamkhani A, Puklavec MJ, Barclay AN and Powrie F: CD134L
expression on dendritic cells in the mesenteric lymph nodes drives
colitis in T cell-restored SCID mice. J Immunol. 166:6972–6981.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Haley KJ, Ciota A, Contreras JP, Boothby
MR, Perkins DL and Finn PW: Alterations in lung collectins in an
adaptive allergic immune response. Am J Physiol Lung Cell Mol
Physiol. 282:L573–L584. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang M, Fei X, Zhang GQ, Zhang PY, Li F,
Bao WP, Zhang YY and Zhou X: Role of neutralizing anti-murine
interleukin-17A monoclonal antibody on chronic ozone-induced airway
inflammation in mice. Biomed Pharmacother. 83:247–256. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Arestides RS, He H, Westlake RM, Chen AI,
Sharpe AH, Perkins DL and Finn PW: Costimulatory molecule OX40L is
critical for both Th1 and Th2 responses in allergic inflammation.
Eur J Immunol. 32:2874–2880. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li YY, Chang JW, Chou WC, Liaw CC, Wang
HM, Huang JS, Wang CH and Yeh KY: Zoledronic acid is unable to
induce apoptosis, but slows tumor growth and prolongs survival for
non-small-cell lung cancers. Lung Cancer. 59:180–191. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lucas CD, Dorward DA, Sharma S, Rennie J,
Felton JM, Alessandri AL, Duffin R, Schwarze J, Haslett C and Rossi
AG: Wogonin induces eosinophil apoptosis and attenuates allergic
airway inflammation. Am J Respir Crit Care Med. 191:626–636. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Carrero R, Cerrada I, Lledó E, Dopazo J,
García-García F, Rubio MP, Trigueros C, Dorronsoro A, Ruiz-Sauri A,
Montero JA and Sepúlveda P: IL1β induces mesenchymal stem cells
migration and leucocyte chemotaxis through NF-κB. Stem Cell Rev.
8:905–916. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lei W, Zeng DX, Zhu CH, Liu GQ, Zhang XQ,
Wang CG, Wang Q and Huang JA: The upregulated expression of
OX40/OX40L and their promotion of T cells proliferation in the
murine model of asthma. J Thorac Dis. 6:979–987. 2014.PubMed/NCBI
|
|
28
|
Kow NY and Mak A: Costimulatory pathways:
Physiology and potential therapeutic manipulation in systemic lupus
erythematosus. Clin Dev Immunol. 2013:2459282013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gramaglia I, Weinberg AD, Lemon M and
Croft M: OX40 ligand: A potent costimulatory molecule for
sustaining primary CD4 T cell responses. J Immunol. 161:6510–6517.
1998.PubMed/NCBI
|
|
30
|
Takeda I, Ine S, Killeen N, Ndhlovu LC,
Murata K, Satomi S, Sugamura K and Ishii N: Distinct roles for the
OX40-OX40 ligand interaction in regulatory and nonregulatory T
cells. J Immunol. 172:3580–3589. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Valzasina B, Guiducci C, Dislich H,
Killeen N, Weinberg AD and Colombo MP: Triggering of OX40 (CD134)
on CD4(+)CD25+ T cells blocks their inhibitory activity: A novel
regulatory role for OX40 and its comparison with GITR. Blood.
105:2845–2851. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ekkens MJ, Liu Z, Liu Q, Whitmire J, Xiao
S, Foster A, Pesce J, VanNoy J, Sharpe AH, Urban JF and Gause WC:
The role of OX40 ligand interactions in the development of the Th2
response to the gastrointestinal nematode parasite Heligmosomoides
polygyrus. J Immunol. 170:384–393. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Miura S, Ohtani K, Numata N, Niki M, Ohbo
K, Ina Y, Gojobori T, Tanaka Y, Tozawa H, Nakamura M, et al:
Molecular cloning and characterization of a novel glycoprotein,
gp34, that is specifically induced by the human T-cell leukemia
virus type I transactivator p40tax. Mol Cell Biol. 11:1313–1325.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Baum PR, Gayle RB III, Ramsdell F,
Srinivasan S, Sorensen RA, Watson ML, Seldin MF, Baker E,
Sutherland GR, Clifford KN, et al: Molecular characterization of
murine and human OX40/OX40 ligand systems: Identification of a
human OX40 ligand as the HTLV-1-regulated protein gp34. EMBO J.
13:3992–4001. 1994.PubMed/NCBI
|
|
35
|
Stüber E, Neurath M, Calderhead D, Fell HP
and Strober W: Cross-linking of OX40 ligand, a member of the
TNF/NGF cytokine family, induces proliferation and differentiation
in murine splenic B cells. Immunity. 2:507–521. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Stüber E and Strober W: The T cell-B cell
interaction via OX40-OX40L is necessary for the T cell-dependent
humoral immune response. J Exp Med. 183:979–989. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ohshima Y, Tanaka Y, Tozawa H, Takahashi
Y, Maliszewski C and Delespesse G: Expression and function of OX40
ligand on human dendritic cells. J Immunol. 159:3838–3848.
1997.PubMed/NCBI
|
|
38
|
Brocker T, Gulbranson-Judge A, Flynn S,
Riedinger M, Raykundalia C and Lane P: CD4 T cell traffic control:
In vivo evidence that ligation of OX40 on CD4 T cells by
OX40-ligand expressed on dendritic cells leads to the accumulation
of CD4 T cells in B follicles. Eur J Immunol. 29:1610–1616. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Imura A, Hori T, Imada K, Ishikawa T,
Tanaka Y, Maeda M, Imamura S and Uchiyama T: The human OX40/gp34
system directly mediates adhesion of activated T cells to vascular
endothelial cells. J Exp Med. 183:2185–2195. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rogers PR, Song J, Gramaglia I, Killeen N
and Croft M: OX40 promotes Bcl-xL and Bcl-2 expression and is
essential for long-term survival of CD4 T cells. Immunity.
15:445–455. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kinnear G, Wood KJ, Marshall D and Jones
ND: Anti-OX40 prevents effector T-cell accumulation and CD8+ T-cell
mediated skin allograft rejection. Transplantation. 90:1265–1271.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Flynn S, Toellner KM, Raykundalia C,
Goodall M and Lane P: CD4 T cell cytokine differentiation: The B
cell activation molecule, OX40 ligand, instructs CD4 T cells to
express interleukin 4 and upregulates expression of the chemokine
receptor, Blr-1. J Exp Med. 188:297–304. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Vu MD, Clarkson MR, Yagita H, Turka LA,
Sayegh MH and Li XC: Critical, but conditional, role of OX40 in
memory T cell-mediated rejection. J Immunol. 176:1394–1401. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Murata K, Nose M, Ndhlovu LC, Sato T,
Sugamura K and Ishii N: Constitutive OX40/OX40 ligand interaction
induces autoimmune-like diseases. J Immunol. 169:4628–4236. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ueno H and Blanco P: OX40/OX40L axis: Not
a friend in autoimmunity. Oncotarget. 6:21779–21780. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gough MJ and Weinberg AD: OX40 (CD134) and
OX40L. Adv Exp Med Biol. 647:94–107. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hori T: Roles of OX40 in the pathogenesis
and the control of diseases. Int J Hematol. 83:17–22. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Redmond WL and Weinberg AD: Targeting OX40
and OX40L for the treatment of autoimmunity and cancer. Crit Rev
Immunol. 27:415–436. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Park BS, Hong GU and Ro JY: Foxp3(+)-Treg
cells enhanced by repeated low-dose gamma-irradiation attenuate
ovalbumin-induced allergic asthma in mice. Radiat Res. 179:570–583.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Obermeier F, Schwarz H, Dunger N, Strauch
UG, Grunwald N, Schölmerich J and Falk W: OX40/OX40L interaction
induces the expression of CXCR5 and contributes to chronic colitis
induced by dextran sulfate sodium in mice. Eur J Immunol.
33:3265–3274. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kay AB: The role of T lymphocytes in
asthma. Chem Immunol Allergy. 91:59–75. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yang T, Zhang X, Wang M, Zhang J, Huang F,
Cai J, Zhang Q, Mao F, Zhu W, Qian H and Xu W: Activation of
mesenchymal stem cells by macrophages prompts human gastric cancer
growth through NF-κB pathway. PLoS One. 9:e975692014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Song J, So T and Croft M: Activation of
NF-kappaB1 by OX40 contributes to antigen-driven T cell expansion
and survival. J Immunol. 180:7240–7248. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Burrows KE, Dumont C, Thompson CL, Catley
MC, Dixon KL and Marshall D: OX40 blockade inhibits house dust mite
driven allergic lung inflammation in mice and in vitro allergic
responses in humans. Eur J Immunol. 45:1116–1128. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Robinson DP, Hall OJ, Nilles TL, Bream JH
and Klein SL: 17β-estradiol protects females against influenza by
recruiting neutrophils and increasing virus-specific CD8 T cell
responses in the lungs. J Virol. 88:4711–4720. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Jandl K, Stacher E, Bálint Z, Sturm EM,
Maric J, Peinhaupt M, Luschnig P, Aringer I, Fauland A, Konya V, et
al: Activated prostaglandin D2 receptors on macrophages enhance
neutrophil recruitment into the lung. J Allergy Clin Immunol.
137:833–843. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Jeon J, Kim Y, Kim H, Kang JS and Lee WJ:
Anti-inflammatory effect of alloferon on ovalbumin-induced asthma.
Immune Netw. 15:304–312. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Bouchard JC, Beal DR, Kim J, Vaickus LJ
and Remick DG: Chemokines mediate ethanol-induced exacerbations of
murine cockroach allergen asthma. Clin Exp Immunol. 172:203–216.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Farres MN, Sabry MK, Ahmed EE, Elkady HM
and Mohamed NA: OX40 ligand: A potential costimulatory molecule in
atopic asthma. J Asthma. 51:573–577. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Kaur D and Brightling C: OX40/OX40 ligand
interactions in T-cell regulation and asthma. Chest. 141:494–499.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Lei W, Zhu CH, Zeng da X, Wang Q, Zhang
XQ, Chen YB, Mu CY and Huang JA: SOX40L: An important inflammatory
mediator in adult bronchial asthma. Ann Acad Med Singapore.
41:200–204. 2012.PubMed/NCBI
|