Introduction
Osthole (C15H16O3),
or 7-methoxy-8-isopentenoxycoumarin, is also termed ‘She Chuang Zi
Su’ in China. As a traditional Chinese medicine, it has been widely
used in the treatment of various diseases. According to reports,
osthole exerts numerous positive effects, including antioxidant
(1), anti-hypertension (2), anti-arrhythmia (3), anticancer (4) and antitumor (5) activities. Lipopolysaccharide (LPS),
which is also termed bacterial endotoxin (6), is an important component of the cell
wall of Gram-negative bacterial cells. Previous studies have
demonstrated that LPS induces inflammatory responses in cells
(7–11). Therefore, the present study
employed LPS to establish an inflammatory model for the
investigation of the potential anti-inflammatory properties of
osthole. Inflammation of the central nervous system may lead to the
development of various diseases, including Alzheimer's disease,
multiple sclerosis and Parkinson's disease (12). Nerve cells are fragile cell types
and their regulatory ability is weak during inflammation and
oxidative stress. However, research concerning nerve inflammation
and their anti-inflammatory mechanisms is lacking.
Nuclear factor-κB (NF-κB) is an early-stage nuclear
transcription factor. It participates in the early stage of the
immune response and in each stage of inflammation, as NF-κB
regulates numerous factors that are associated with inflammation
(13). At rest, a complex between
NF-κB and NF-κB inhibitor (IκB) forms in the cytoplasm. If
stimulated, IκB becomes activated, which frees NF-κB and allows
NF-κB to transfer from the cytoplasm to the nucleus (14) to induce an inflammatory response in
the body. It has been previously verified that the NF-κB pathway is
closely associated with numerous diseases. For example, Brambilla
et al (15) demonstrated
that spinal cord injuries may be treated with drugs that inhibit
the NF-κB pathway, while Noort et al (16) reported that the activation of NF-κB
may be one cause of rheumatoid arthritis. Additionally, Duh et
al (17) confirmed that human
immunodeficiency virus type 1, which causes the pathogenesis of
acquired immune deficiency syndrome, is also associated with NF-κB.
These diseases are all associated with inflammation. Therefore, we
hypothesized that the activation of the NF-κB signaling pathway may
also be a major factor in the development of neurodegenerative
disease.
Nuclear factor erythroid 2-related factor 2 (Nrf2)
is an antioxidant response element. In normal conditions, Nrf2
combines with kelch-like ECH-associated protein 1 (Keap-1), and
this combined form of Nrf2 is degraded. Degradation effectively
controls the level of Nrf2 in cells (18). Heme oxygenase-1 (HO-1) is located
downstream of Nrf2 (19);
therefore, alterations in the levels of Nrf2 subsequently affect
the expression of HO-1. In the present study, we hypothesized that
osthole may protect cells against inflammation through the Nrf2 and
NF-κB pathways. The aim of the present study was to determine the
potential protective effect of osthole against inflammation in
cells and to investigate the underlying mechanisms.
Materials and methods
Reagents
Osthole (98%) was purchased from Sigma-Aldrich
(Merck KGaA, Darmstadt, Germany) and was dissolved in 0.1% dimethyl
sulfoxide (DMSO) to reach a concentration of 0.7 mg/ml and stored
at −20°C. The final concentration for DMSO used to dissolve the
drug was 0.1%; DMSO exhibited no effect on cell viability, as
demonstrated in a previous study (20). Dulbecco's modified Eagle's medium
(DMEM) was purchased from Hyclone (GE Healthcare Life Sciences,
Logan, UT, USA). LPS was purchased from Sigma-Aldrich (Merck KGaA).
The components of the cell lysis buffer were obtained from Beyotime
Institute of Biotechnology (Haimen, China). NF-κB p65,
phosphorylated (p)-NF-κB p65, Nrf2, HO-1, IκBα, p-IκBα, lamin B and
β-actin primary antibodies were obtained from Cell Signaling
Technology, Inc. (Danvers, MA, USA). MTT reagent and DMSO were
provided by Wuhan Boster Biological Technology, Co., Ltd. (Wuhan,
China). Fetal bovine serum (FBS) was obtained from Zhejiang
Tianhang Biotechnology Co., Ltd. (Huzhou, China). ELISA kits were
purchased from Cell Signaling Technology, Inc.
Cell culture
BV2 mouse microglial cells were obtained from the
Type Culture Collection of the Chinese Academy of Sciences
(Shanghai, China) and cultured in DMEM with 10% FBS and 1%
antibiotics (100 U/ml penicillin and streptomycin) (5). Cells were cultured at 37°C in a
humidified atmosphere of 5% CO2. The medium was changed
once a day and passage occurred when the cells grew on a
logarithmic scale.
Cell viability assay
BV2 cells in the logarithmic growth phase were added
to 96-well plates (7×103 cells/well) and cultured in a
cell culture box at 37°C for 12 h until the cells completely
attached to the wall. At 1 h prior to LPS (1 µg/ml) stimulation,
different concentrations of osthole (4, 7 and 10 µg/ml) were added
to each well at 37°C, and the activity of cells was detected at 24
h after stimulation with LPS. For the control group, an equivalent
volume of empty media was added. MTT reagent was added to all cell
groups and were subsequently cultivated in the incubator for 2–4 h
at 37°C, leading to the development of purple crystals. DMSO (150
µl/well) was added and shaken until all of the crystals
disappeared. The optical density of the 96-well plate was measured
at 570 nm.
Western blotting
BV2 cells were stimulated with LPS (1 µg/ml) at 1 h
after treatment with osthole (4, 7 and 10 µg/ml) in 6-well plates
(1×106 cells/well), and total protein samples were
collected at 24 h after the addition of LPS. Protein extraction was
performed in strict accordance with the specifications of the
Protein Extraction kit (cat. no. AR0103; Boster Biological
Technology, Pleasanton, CA, USA). Following removal of the medium,
the cells were washed with 4°C pre-cooled PBS three times.
Subsequently, 1 ml lysate was added, including 10 µl protease
inhibitors and 10 µl phosphorylase inhibitors. After 30 min, cells
from the 6-well plates were scraped, collected in a 1.5-ml
centrifuge tube and centrifuged for 5 min at 4°C and 1,000 × g. The
supernatant obtained following centrifugation was the protein, and
the protein concentration was detected with a BCA protein assay
kit. The protein (15 µg/lane) was concentrated in 10% of the
concentrated gel at 80 V for 30 min and separated by 12% SDS-PAGE
at 120 V for 70 min. After cutting the gel, the target protein was
transferred to polyvinylidene difluoride membranes. Membranes were
subsequently incubated with 5% bovine serum albumin for 2 h,
followed by incubation with primary antibodies, including NF-κB
p65, p-NF-κB p65, IκBα, p-IκBα (NF-κB pathway sampler kit; cat. no.
9936), Nrf2 (cat. no. 12721), HO-1 (cat. no. 5853), lamin B (cat.
no. 12255) and β-actin (cat. no. 3700) overnight at 4°C. All
antibodies were purchased from CST Biological Reagents Co., Ltd.
(Shanghai, China) and were added at 1:1,000 dilution. Prior to
incubation with secondary antibodies, the membrane was washed three
times with TBS-Tween-20 (0.1% Tween-20) for 10 min each time. After
45 min of incubation with anti-mouse IgG and anti-rabbit IgG
secondary antibodies (1:2,000; cat. nos. 7076 and 7074; CST
Biological Reagents Co., Ltd.) at room temperature, the washing
procedure was repeated. Protein expression was visualized using an
enhanced chemiluminescent reagent (Beyotime Institute of
Biotechnology) with ImageJ software (version 1.51k; National
Institutes of Health, Bethesda, MD, USA), and the membrane was
exposed to an X-ray film. Protein levels were detected in blots
according to β-actin or lamin B.
ELISA
BV2 cells were plated in 6-well plates
(1×106 cells/well) and cultured in a cell culture box
for 12 h until the cells completely attached to the wall.
Subsequently, cells were treated with various concentrations of
osthole (4, 7 and 10 µg/ml) for 1 h (5), followed by stimulation with LPS (1
µg/ml) for 6 h. ELISA was performed on cell culture medium to
determine the levels of tumor necrosis factor (TNF)-α (cat. no.
EK0527), interleukin (IL)-6 (cat. no. EK0411) and IL-1β (cat. no.
EK0394), according to the manufacturer's instructions (Boster
Biological Technology Co., Ltd.). Absorbance was determined at 450
nm.
Statistical analysis
Statistical analysis was performed with SPSS 17.0
(SPSS Inc., Chicago, IL, USA) with one-way analysis of variance.
Data are presented as the mean ± standard deviation of five
independent experiments. Statistically significant differences were
detected with one-way analysis of variance followed by Dunnet's
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
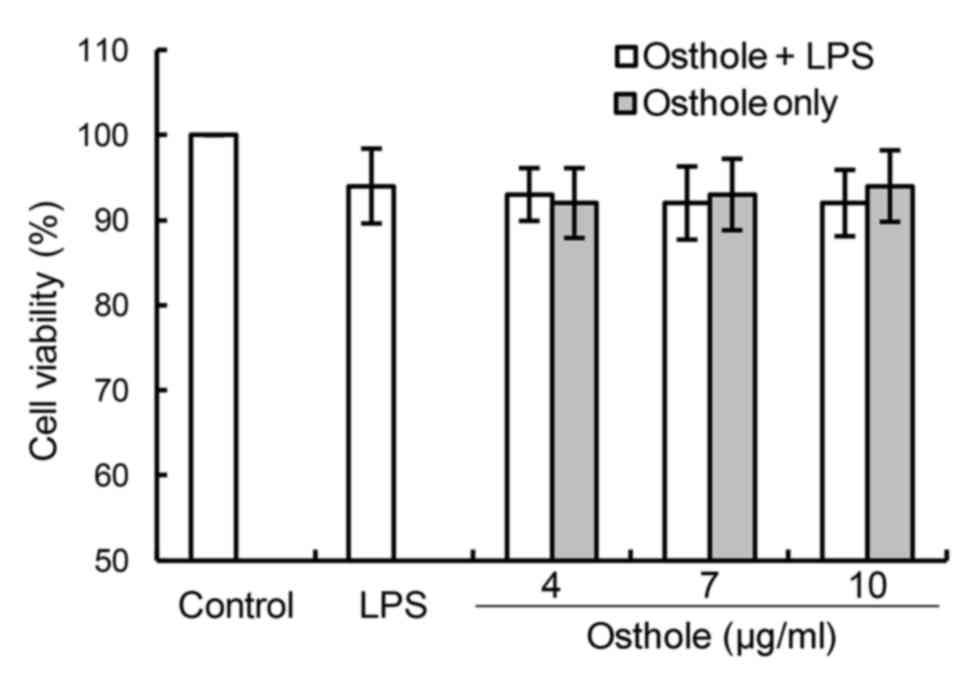
Effects of osthole on the viability of
BV2 cells
The results of the MTT assay, which was used to
detect the cell viability following incubation with LPS and/or
osthole for 24 h, are presented in Fig. 1. The results demonstrated that the
addition of various concentrations of osthole (4, 7 and 10 µg/ml)
to cells exhibited no cytotoxic effect, compared with the control
group. Therefore, these three concentrations were selected for
subsequent experiments.
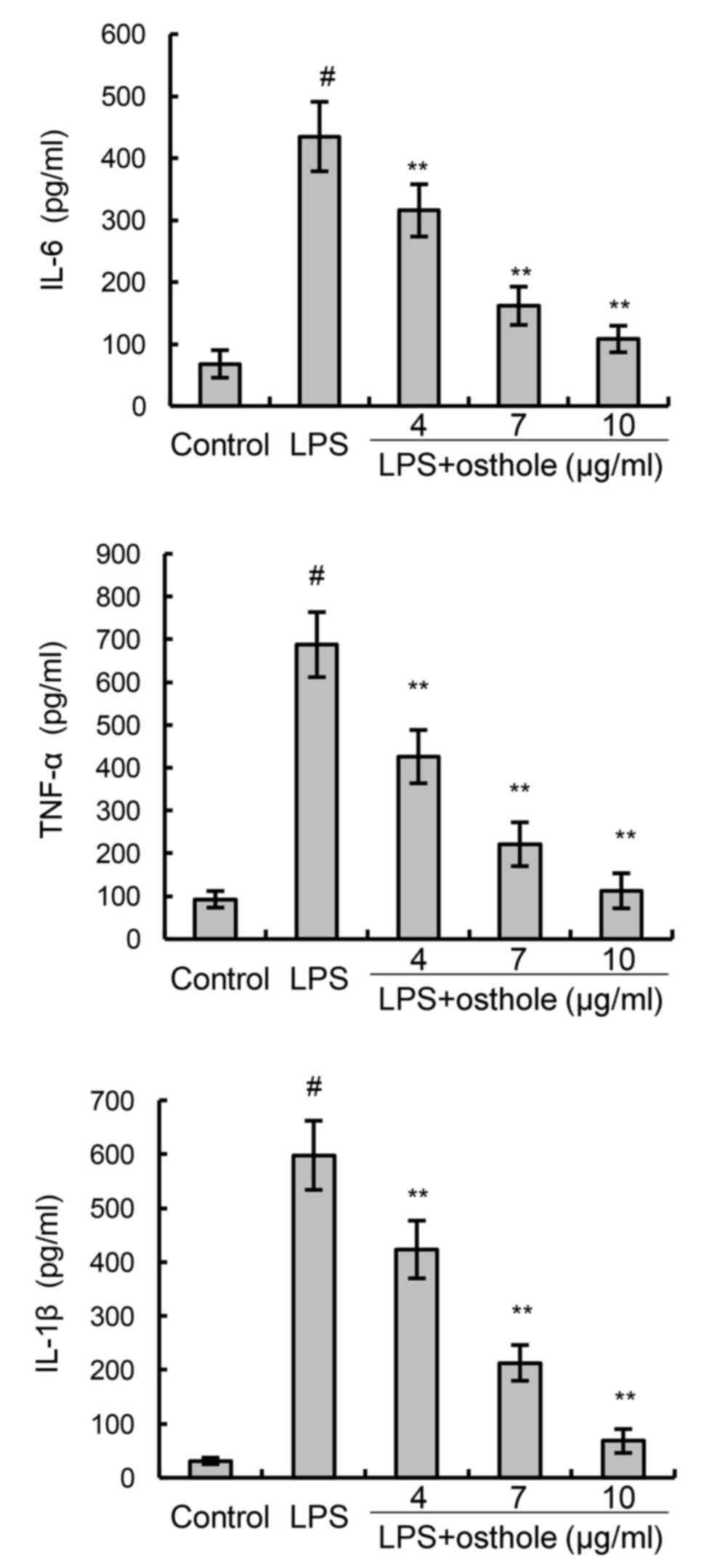
Effects of osthole on
inflammation-associated cytokines
To confirm the anti-inflammatory effects of osthole,
the present study assessed cytokine production by BV2 cells using
ELISA. The results demonstrated that the production of the
inflammatory mediators IL-6, TNF-α and IL-1β in the cells following
LPS stimulation was significantly increased, compared with the
control group (Fig. 2). However,
the LPS-induced increases in IL-6, TNF-α and IL-1β levels were
dose-dependently inhibited by treatment with osthole (Fig. 2).
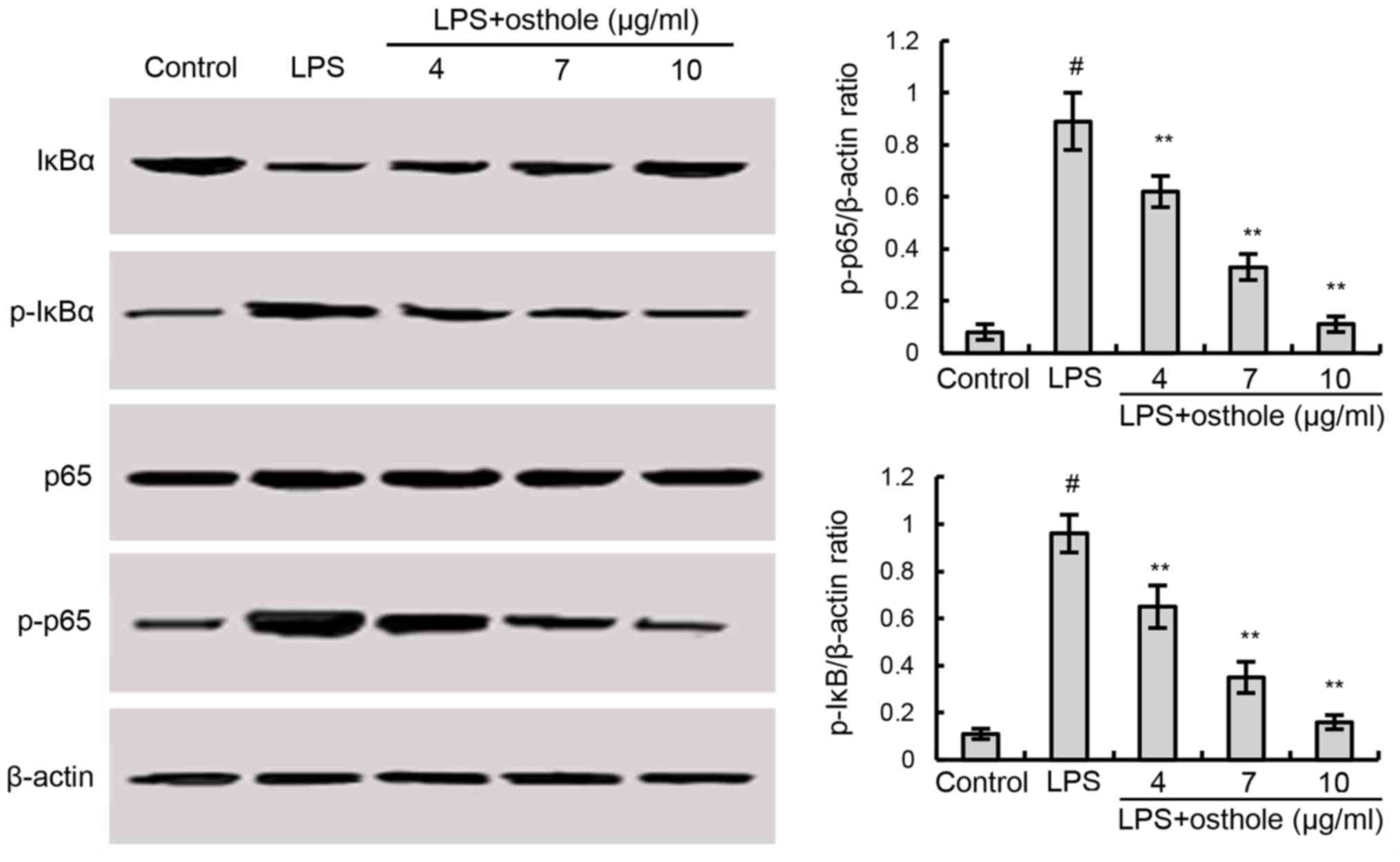
Effects of osthole on the expression
of NF-κB pathway-associated proteins
Members of the NF-κB family of proteins are
considered to be key factors in inflammation. To investigate the
effects of osthole on NF-κB activation induced by LPS, the present
study performed western blotting. As demonstrated in Fig. 3, following LPS treatment, BV2 cells
exhibited increased inflammation, as the expression of NF-κB
pathway-associated proteins, such as p-NF-κB p65 and p-IκBα, were
increased. However, osthole blocked LPS-induced NF-κB activation in
a dose-dependent manner (Fig.
3).
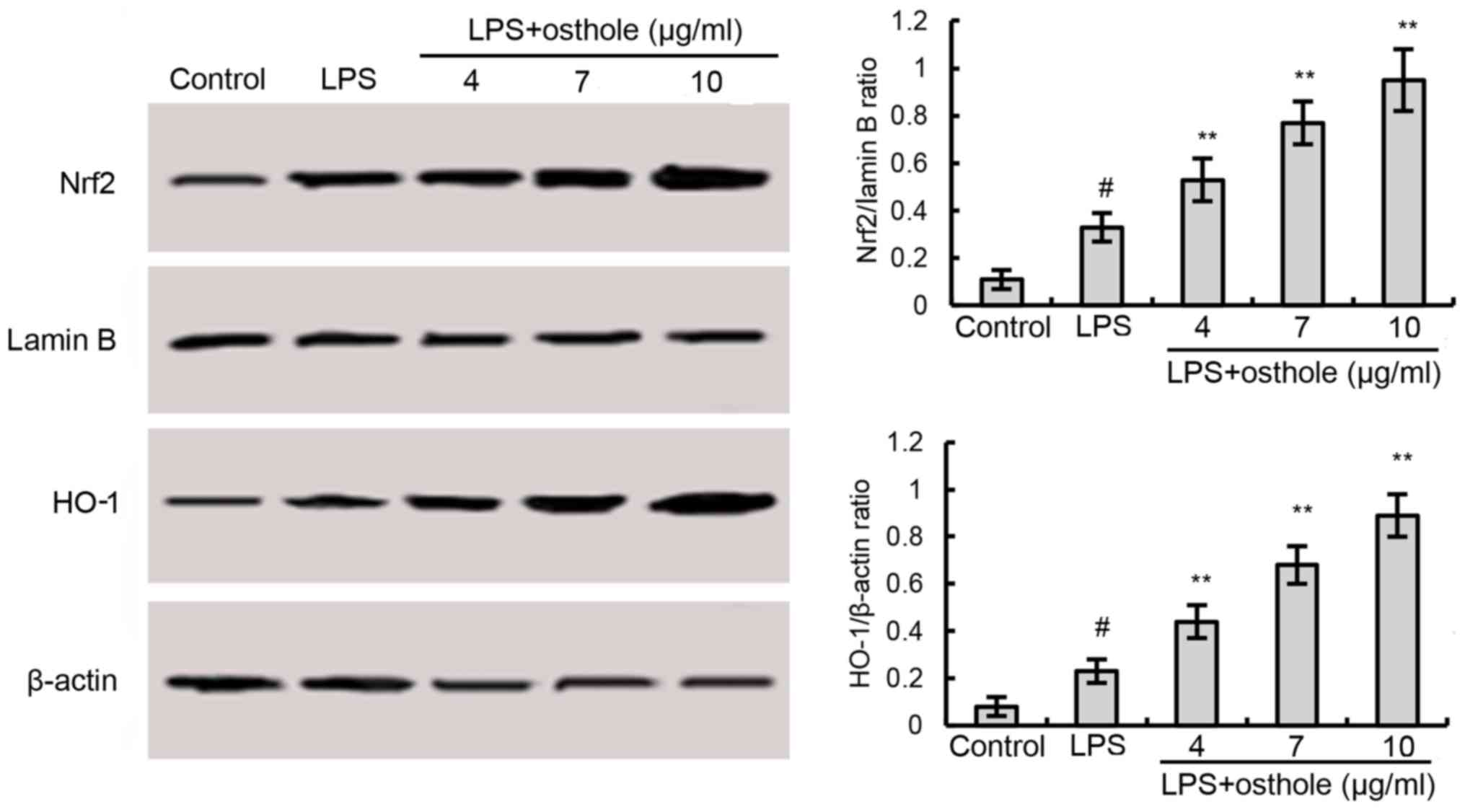
Effects of osthole on the protein
expression of Nrf2 and HO-1
Previous studies have reported that Nrf2 is involved
in the regulation of antioxidant responses and inflammation
(21–24). Western blotting demonstrated that
the protein expression of Nrf2 and HO-1, which lies downstream of
Nrf2, was further upregulated in osthole-treated BV2 microglia,
compared with cells exposed to LPS alone (Fig. 4). These results indicated that
osthole may induce the activation of the Nrf2 pathway.
Discussion
With increasing research on traditional Chinese
medicine and its effective components, an increasing number of
traditional Chinese medicines have been recognized worldwide.
Traditional Chinese medicine and its effective components are
considered to be safe, reliable and inexpensive. The mechanism of
emodin has been extensively researched, and osthole, as an emerging
Chinese medicine, has been widely investigated in recent years. Hao
et al (25) demonstrated
that osthole exhibited a therapeutic effect on pulmonary fibrosis,
while Chen et al (26)
reported beneficial effects of osthole on cognitive impairment in
animals, which may occur via the Nrf2 pathway. These results
indicate that osthole may act on the nervous system. Other studies
have demonstrated that osthole may also exhibit an active role in
non-nervous system diseases. Osthole was previously reported to
exhibit anti-inflammatory effects through NF-κB and
mitogen-activated protein kinase signaling pathways (27). Furthermore, Liu et al
(1) demonstrated the effect of
osthole in mitochondrial disorders, Wang et al (28) reported that osthole inhibited
inflammatory cytokine release in adipocytes, Huang and Dong
(29) indicated that osthole
exhibited anti-inflammatory effects in chronic kidney failure
though the NF-κB pathway, and Hao and Liu (30) concluded that osthole reduced
inflammatory responses in rats with pulmonary fibrosis. Although
previous reports have investigated the role of osthole in the
process of inflammation, the present study focused on inflammation
in neurodegenerative diseases by using BV2 microglial cells. In
addition to investigating conventional inflammatory factors, the
present study also investigated the activation of the NF-κB and
Nrf2 signaling pathways following osthole treatment. According to
the results of western blotting and ELISA, osthole reduced the
expression of proteins associated with NF-κB and Nrf2 pathways.
These results highlight the potential medicinal value of Fructus
cnidii, which is another term for the traditional Chinese herb She
Chuang Zi. With the discovery of the mechanism of action of this
traditional Chinese medicine, osthole may have potential as an
anti-inflammatory drug option.
Inflammation is a common pathological phenomenon
that occurs in various disease states (31). BV2 cells are a type of mouse
microglial cell, which are present in the brain and spinal cord
(32). They are considered to be
the first and most important line of defense in the central nervous
system. A number of studies have demonstrated that macrophages
stimulated by external antigens, such as LPS, release IL-1β, TNF-α
and other important inflammatory factors (33–37).
These inflammatory cytokines promote an inflammatory reaction and
lead to tissue damage. The results of the present study also
demonstrated that stimulation of BV2 cells with LPS also led to the
production of inflammatory factors, including TNF-α, IL-6 and
IL-1β. It was also observed in the present study that the
traditional Chinese medicine osthole affected this process and
reduced the inflammatory response of BV2 cells following LPS
stimulation.
In conclusion, the results of the present study
demonstrated that osthole may protect inflammatory BV2 cells
against inflammation induced by LPS stimulation, and this may occur
through inhibition of the NF-κB and Nrf2 signaling pathways.
However, a previous report by Chen et al (38) indicated that osthole was not able
to inhibit the Nrf2 pathway. As a result, it may be concluded that,
in the present study, the alterations in the expression of proteins
associated with the Nrf2 pathway may occur as a result of the
anti-inflammatory effects of osthole; it was previously reported
that LPS induced Nrf2 and HO-1 activation (39). In the present study, osthole also
reduced the release of inflammatory factors such as IL-1β and
TNF-α. These results may provide the scientific foundation for the
effect of osthole in the prevention of nerve inflammation.
Therefore, further investigation of this traditional Chinese is
required to determine whether it may be employed for the treatment
of various types of inflammation in the future.
References
|
1
|
Liu WB, Zhou J, Qu Y, Li X, Lu CT, Xie KL,
Sun XL and Fei Z: Neuroprotective effect of osthole on
MPP+-induced cytotoxicity in PC12 cells via inhibition
of mitochondrial dysfunction and ROS production. Neurochem Int.
57:206–215. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ogawa H, Sasai N, Kamisako T and Baba K:
Effects of osthol on blood pressure and lipid metabolism in
stroke-prone spontaneously hypertensive rats. J Ethnopharmacol.
112:26–31. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhou F, Zhong W, Xue J, Gu ZL and Xie ML:
Reduction of rat cardiac hypertrophy by osthol is related to
regulation of cardiac oxidative stress and lipid metabolism.
Lipids. 47:987–994. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu J, Xu R and Zhao X: Mechanisms for
effect of osthole on inhibiting the growth and invasion of bladder
cancer cells. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 41:345–352.
2016.(In Chinese). PubMed/NCBI
|
|
5
|
Pan Z, Fang Z, Lu W, Liu X and Zhang Y:
Osthole, a coumadin analog from Cnidium monnieri (L.) Cusson,
stimulates corticosterone secretion by increasing steroidogenic
enzyme expression in mouse Y1 adrenocortical tumor cells. J
Ethnopharmacol. 175:456–462. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chun J, Tosun A and Kim YS:
Anti-inflammatory effect of corymbocoumarin from Seseli gummiferum
subsp. corymbosum through suppression of NF-κB signaling pathway
and induction of HO-1 expression in LPS-stimulated RAW 264.7 cells.
Int Immunopharmacol. 31:207–215. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Menden HL, Xia S, Mabry SM, Navarro A, Nyp
MF and Sampath V: Nicotinamide adenine dinucleotide phosphate
oxidase 2 regulates LPS-induced inflammation and alveolar
remodeling in the developing lung. Am J Respir Cell Mol Biol.
55:767–778. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bozic I, Savic S, Laketa D, Bjelobaba I,
Milenkovic I, Pekovic S, Nedeljkovic N and Lavrnja I: Benfotiamine
attenuates inflammatory response in LPS stimulated BV-2 microglia.
PLoS One. 10:e01183722015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang L, Guo H and Jin X, Meng X, Yan L,
Dan Z, Wu S, Zhou H, Peng L, Xie Q and Jin X: Oleoylethanolamide
exerts anti-inflammatory effects on LPS-induced THP-1 cells by
enhancing PPARα signaling and inhibiting the NF-κB and
ERK1/2/AP-1/STAT3 pathways. Sci Rep. 6:346112016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shi J, Shan S, Li H, Song G and Li Z:
Anti-inflammatory effects of millet bran derived-bound polyphenols
in LPS-induced HT-29 cell via ROS/miR-149/Akt/NF-κB signaling
pathway. Oncotarget. 8:74582–74594. 2017.PubMed/NCBI
|
|
11
|
Fan C, Wu L, Zhang G, Xu F, Zhang S, Zhang
X, Sun L, Yu Y, Zhang Y and Ye RD: 4′-Hydroxywogonin suppresses
lipopolysaccharide-induced inflammatory responses in RAW 264.7
macrophages and acute lung injury mice. PLoS One. 12:e01811912017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Minagar A, Shapshak P, Fujimura R, Ownby
R, Heyes M and Eisdorfer C: The role of macrophage/microglia and
astrocytes in the pathogenesis of three neurologic disorders:
HIV-associated dementia, Alzheimer disease, and multiple sclerosis.
J Neurol Sci. 202:13–23. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gasparini C, Celeghini C, Monasta L and
Zauli G: NF-kappaB pathways in hematological malignancies. Cell Mol
Life Sci. 71:2083–2102. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shih RH, Wang CY and Yang CM: NF-kappaB
signaling pathways in neurological inflammation: A mini review.
Front Mol Neurosci. 8:772015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Brambilla R, Bracchi-Ricard V, Hu WH,
Frydel B, Bramwell A, Karmally S, Green EJ and Bethea JR:
Inhibition of astroglial nuclear factor kappaB reduces inflammation
and improves functional recovery after spinal cord injury. J Exp
Med. 202:145–156. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Noort AR, Tak PP and Tas SW: Non-canonical
NF-κB signaling in rheumatoid arthritis: Dr Jekyll and Mr Hyde?
Arthritis Res Ther. 17:152015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Duh EJ, Maury WJ, Folks TM, Fauci AS and
Rabson AB: Tumor necrosis factor alpha activates human
immunodeficiency virus type 1 through induction of nuclear factor
binding to the NF-kappa B sites in the long terminal repeat. Proc
Natl Acad Sci USA. 86:pp. 5974–5978. 1989; View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Khodir AE, Atef H, Said E, ElKashef HA and
Salem HA: Implication of Nrf2/HO-1 pathway in the coloprotective
effect of coenzyme Q10 against experimentally induced ulcerative
colitis. Inflammopharmacology. 25:119–135. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cao S, Du J and Hei Q: Lycium barbarum
polysaccharide protects against neurotoxicity via the Nrf2-HO-1
pathway. Exp Ther Med. 14:4919–4927. 2017.PubMed/NCBI
|
|
20
|
Kwon YW, Cheon SY, Park SY, Song J and Lee
JH: Tryptanthrin suppresses the activation of the LPS-treated BV2
microglial cell line via Nrf2/HO-1 antioxidant signaling. Front
Cell Neurosci. 11:182017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bachewal P, Gundu C, Yerra VG, Kalvala AK,
Areti A and Kumar A: Morin exerts neuroprotection via attenuation
of ROS induced oxidative damage and neuroinflammation in
experimental diabetic neuropathy. Biofactors. Nov 28–2017.(Epub
ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Truong VL, Jun M and Jeong WS: Role of
resveratrol in regulation of cellular defense systems against
oxidative stress. Biofactors. Nov 28–2017.(Epub ahead of print).
PubMed/NCBI
|
|
23
|
Chen QM and Maltagliati AJ: Nrf2 at the
heart of oxidative stress and cardiac protection. Physiol Genomics.
Nov 29–2017.(Epub ahead of print).
|
|
24
|
Aldaba-Muruato LR, Muñoz-Ortega MH,
Macías-Pérez JR, Pulido-Ortega J, Martínez-Hernández SL and
Ventura-Juárez J: Adrenergic regulation during acute hepatic
infection with Entamoeba histolytica in the hamster: Involvement of
oxidative stress, Nrf2 and NF-KappaB. Parasite. 24:462017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hao Y and Liu Y: Osthole alleviates
bleomycin-induced pulmonary fibrosis via modulating
angiotensin-converting enzyme 2/angiotensin-(1–7) axis and
decreasing inflammation responses in rats. Biol Pharm Bull.
39:457–465. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen Z, Mao X, Liu A, Gao X, Chen X, Ye M,
Ye J, Liu P, Xu S, Liu J, et al: Osthole, a natural coumarin
improves cognitive impairments and BBB dysfunction after transient
global brain ischemia in C57 BL/6J mice: Involvement of Nrf2
pathway. Neurochem Res. 40:186–194. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wu SJ: Osthole attenuates inflammatory
responses and regulates the expression of inflammatory mediators in
HepG2 cells grown in differentiated medium from 3T3-L1
preadipocytes. J Med Food. 18:972–979. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang XL, Shang X, Cui Y, Zhao X, Zhang Y
and Xie ML: Osthole inhibits inflammatory cytokine release through
PPARα/γ-mediated mechanisms in LPS-stimulated 3T3-L1 adipocytes.
Immunopharmacol Immunotoxicol. 37:185–192. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Huang T and Dong Z: Osthole protects
against inflammation in a rat model of chronic kidney failure via
suppression of nuclear factor-κB, transforming growth factor-β1 and
activation of phosphoinositide 3-kinase/protein kinase B/nuclear
factor (erythroid-derived 2)-like 2 signaling. Mol Med Rep.
16:4915–4921. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hao Y and Liu Y: Osthole alleviates
bleomycin-induced pulmonary fibrosis via modulating
angiotensin-converting enzyme 2/angiotensin-(1–7) axis and
decreasing inflammation responses in rats. Biol Pharm Bull.
39:457–465. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee HH, Shin JS, Lee WS, Ryu B, Jang DS
and Lee KT: Biflorin, isolated from the flower buds of syzygium
aromaticum L., suppresses LPS-induced inflammatory mediators via
STAT1 inactivation in macrophages and protects mice from endotoxin
shock. J Nat Prod. 79:711–720. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cho N, Moon EH, Kim HW, Hong J, Beutler JA
and Sung SH: Inhibition of nitric oxide production in BV2
microglial cells by triterpenes from tetrapanax papyriferus.
Molecules. 21:4592016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sun Y and Shang D: Inhibitory effects of
antimicrobial peptides on lipopolysaccharide-induced inflammation.
Mediators Inflamm. 2015:1675722015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tai Y, Qiu Y and Bao Z: Magnesium
lithospermate B suppresses lipopolysaccharide-induced
neuroinflammation in BV2 microglial cells and attenuates
neurodegeneration in lipopolysaccharide-injected mice. J Mol
Neurosci. Dec 1–2017.(Epub ahead of print). PubMed/NCBI
|
|
35
|
He Y, She H, Zhang T, Xu H, Cheng L, Yepes
M, Zhao Y and Mao Z: p38 MAPK inhibits autophagy and promotes
microglial inflammatory responses by phosphorylating ULK1. J Cell
Biol. Dec 1–2017.(Epub ahead of print).
|
|
36
|
Xu L, Zheng X, Wang Y, Fan Q, Zhang M, Li
R, Ye J, Wu X, Zhao W and Zhang Y: Berberine protects acute liver
failure in mice through inhibiting inflammation and
mitochondria-dependent apoptosis. Eur J Pharmacol. 819:161–168.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang S, Zhang Z, Wang Y, Gadahi JA, Xie Q,
Xu L, Yan R, Song X and Li X: Toxoplasma gondii excretory/secretory
antigens (TgESAs) suppress pro-inflammatory cytokine secretion by
inhibiting TLR-induced NF-κB activation in LPS-stimulated murine
macrophages. Oncotarget. 8:88351–88359. 2017.PubMed/NCBI
|
|
38
|
Chen XJ, Zhang B, Hou SJ, Shi Y, Xu DQ,
Wang YX, Liu ML, Dong HY, Sun RH, Bao ND, Jin FG and Li ZC: Osthole
improves acute lung injury in mice by up-regulating
Nrf-2/thioredoxin 1. Respir Physiol Neurobiol. 188:214–222. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tursun X, Zhao Y, Alat Z, Xin X, Tursun A,
Abdulla R and AkberAisa H: Anti-inflammatory effect of rosa rugosa
flower extract in lipopolysaccharide-stimulated RAW264.7
macrophages. Biomol Ther (Seoul). 24:184–190. 2016. View Article : Google Scholar : PubMed/NCBI
|