Introduction
Complete global brain ischemia and reperfusion
injury triggered by cardiac arrest (CA) are fundamental causes of
the reduced survival rates for patients with anabiosis following CA
(1). As brain tissues have a low
tolerance to hypoxic ischemia and its adverse reactions to ischemia
reperfusion, cerebral protection following cardiopulmonary
resuscitation (CPR) is difficult to treat (2). Complex mechanisms are responsible for
the cerebral lesions caused by CA/CPR, including free radical
formation, calcium overload, integrated enzyme reactions and the
activation of death signaling pathways (3). This subsequently leads to apoptosis
and necrosis of neurons. Previous clinical research and trials have
demonstrated that therapeutic hypothermia greatly increases
defibrillation success rates, ameliorates neural function and
survival, and improves overall prognosis (4). However, the optimal opportunity and
temperature range in which to conduct this therapy is difficult to
obtain; it is generally understood that therapeutic hypothermia
should be induced rapidly and at a low temperature during CPR
following sudden cardiac arrest (5).
A previous study have indicated that the expression
levels of transforming growth factor-β1 (TGF-β1) are increased
following cerebral ischemia, suggesting that TGF-β1 is associated
with the restoration and survival of neurons (6). However, certain exogenous intravenous
and local intracerebral injections of TGF-β1 may facilitate the
development of neurological impairment syndromes following cerebral
ischemia, and reduce infarction volumes and encephaledema. It is
understood that TGF-β1 is a multi-functional cytokine that is
involved in various pathological and physiological procedures,
stimulates secretion of the extra-cellular matrix and promotes
angiogenesis (7). Furthermore, it
exhibits anti-inflammatory, chemotaxis, movement-associated and
anti-proliferative properties, and serves an essential role in
repairing injured vessels (8).
A previous study indicated that, following
activation of the TGF-β1 receptor, signal transduction is primarily
mediated by phosphorylation of plasmosin; a member of the mothers
against decapentaplegic (Smad) protein family (9). Once combined with Smad4, activated
and phosphorylated Smad2 and 3 proteins facilitate the transport of
TGF-β1 to the cell nucleus, thus promoting its biological effects
(9).
As an alkaloid extracted from the kuh-seng compound,
oxymatrine demonstrates anti-inflammatory and anti-oxidative stress
properties, and has become an essential drug for the treatment of
tumors and hepatic fibrosis (10,11).
Studies involving diabetic rats have demonstrated that oxymatrine
improves cognitive impairment, reduces oxidative stress levels and
increases the expression levels of antioxidative factors, such as
superoxide dismutase (SOD) and glutathione (11). In addition, oxymatrine may
significantly reduce the expression levels of nuclear factor
(NF)-κB, tumor necrosis factor-α, interleukin (IL)-1β and caspase-3
(12). Following administration of
oxymatrine, the expression levels of melanoma
differentiation-associated protein, alanine transaminase, arginine
succinyltransferase, thyroglobulin, IL-6 and NF-κB were markedly
decreased, while the expression levels of SOD were increased
(13). The aim of the present
study was to examine the protective effects of oxymatrine against
CPR, as well as the potential underlying mechanisms of oxymatrine
in cardiac fibrosis.
Materials and methods
Animal experiments
Animal experiments were approved by the University
Laboratory Animal Research Committee of The First Hospital of Jilin
University (Changchun, China). A total of 32 adult Sprague-Dawley
(SD) rats (weight, 250±30 g) were purchased from the Qinghai
Experimental Animal Center (Zhejiang, China) and maintained in a
specific pathogen-free environment (22–24°C, 55–60% humidity, 12 h
light/dark cycle and free access to food and water). Rats were
anaesthetized by intraperitoneal injection of chloral hydrate (300
mg/kg, intraperitoneal, Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) and a CPR model was established by asphyxiation. To do
this, the tracheal intubation was closed at the end of expiration
for 5 min. SD rats were randomly divided into sham-operated (n=6),
model (n=10), 25 mg/kg oxymatrine-treated (n=8) and 50 mg/kg
oxymatrine-treated (n=8) groups. In the 25 and 50 mg/kg oxymatrine
groups, SD rats were administered with 25 and 50 mg/kg oxymatrine
(Intragastrically) once a day for 4 weeks, respectively. In
sham-operated and model groups, rats were anaesthetized by
intraperitoneal injection of chloral hydrate without the CPR model,
and administered with normal saline once a day for 4 weeks
(Intragastrically). Oxymatrine (purity, >95%) was purchased from
Shanghai Leiyunshang Green Valley Pharmaceutical Co., Ltd. (Xi'an,
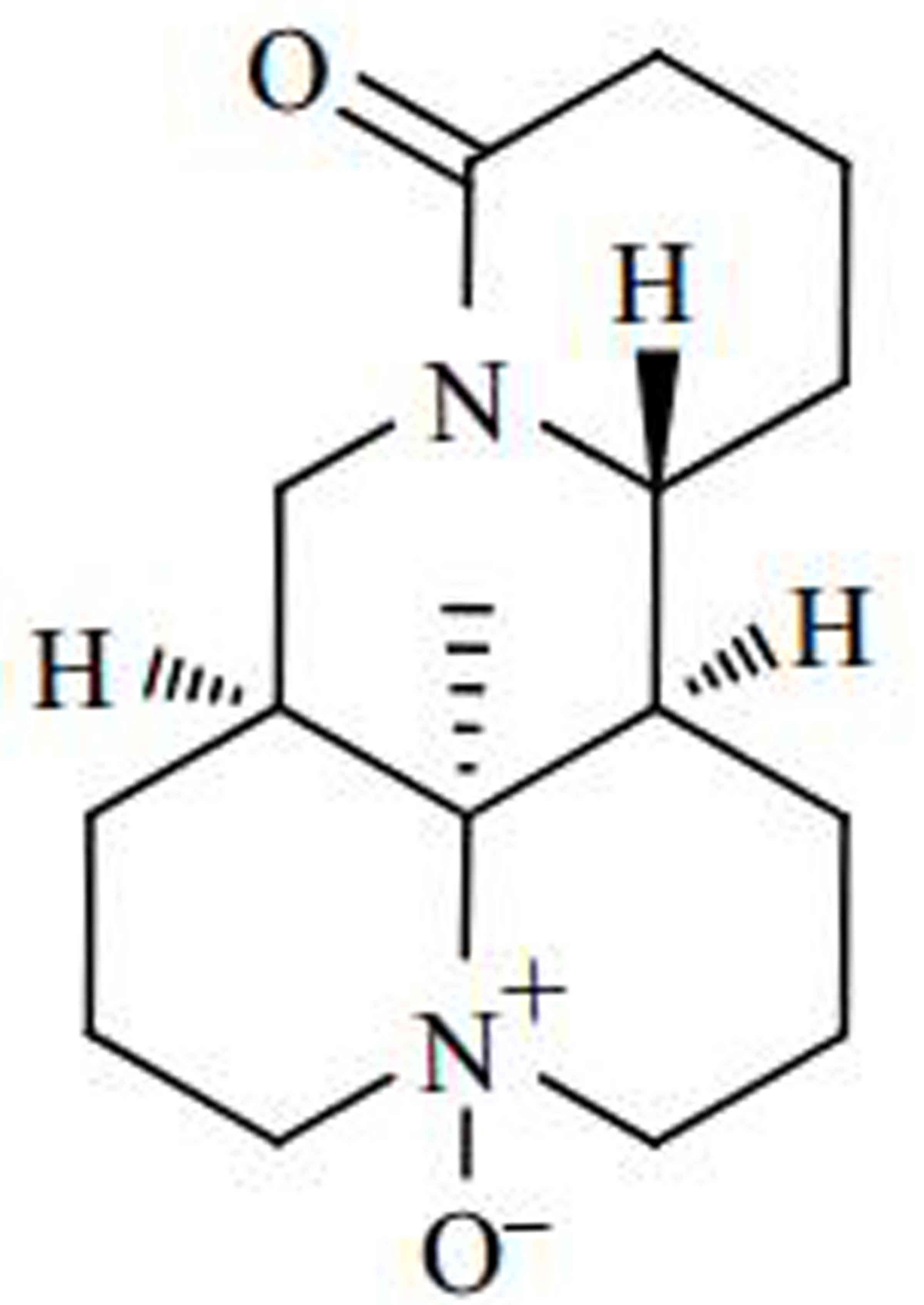
China). Its structural formula is presented in Fig. 1.
Enzyme-linked immunosorbent assay
(ELISA) analysis of troponin I concentration and ejection
fraction
After treatment with oxymatrine, rat was
anaesthetized and peripheral blood was acquired from caudal vein.
Serum was separated after centrifugation at 1,000 × g for 10 min at
4°C to measure troponin I concentrations using an ELISA kit (cat.
no. 2010–2-HSP; Life Diagnostics, Inc., West Chester, PA, USA)
according to the manufacturer's protocol. The absorbance value was
detected at a wavelength of 450 nm.
The percentage ejection fraction (EF) was calculated
using the same area-length method described previously (14). Lactate levels (cat no. M002;
Nanjing Jiancheng Bioengineering Institute, Nanjing, China) were
determined from 0.3-ml samples at baseline. A partial pressure of
oxygen (PaO2) value of <5 mmHg was considered to
indicate hypoxia, and a PaO2 value of between 100 and
300 mmHg was considered to indicate mild hyperoxia. The myocardial
performance index (MPI) was calculated as (a-b)/b, where a=mitral
valve closure-to-opening interval and b=left ventricular ejection
time. Oxygen extraction was calculated as the difference between
the arterial (Ca) and venous concentrations (Cv) of oxygen
(CaO2-CvO2). Therefore, CaO2 =
1.38 × hemoglobin (HB) × arterial oxygen saturation +
PaO2 × 0.0031; CvO2 = 1.38 × HB × mixed
venous oxygen saturation + mixed venous oxygen tension
(PvO2) × 0.0031.
Hydroxyproline content assessment
The hydroxyproline content of heart tissues was
assessed following a previously described method (13) using ELISA kits (cat no. A030-2;
Nanjing Jiancheng Bioengineering Institute) according to the
manufacturer's protocol. The absorbance value was detected at a
wavelength of 450 nm.
Western blot analysis
Heart tissue samples were homogenized using 10 µg/ml
radioimmunoprecipitation buffer and protease inhibitors (EMD
Millipore, Billerica, MA, USA). The supernatant was collected and
total protein was quantified using a bicinchoninic acid assay
(Beyotime Institute of Biotechnology, Shanghai, China). Total
protein (50 µg) was separated by 12% SDS-PAGE and transferred onto
nitrocellulose membranes. Following blocking with 5% nonfat milk in
Tris-buffered saline with 0.1% Tween-20, membranes were incubated
with the following primary antibodies at 4°C overnight: Anti-TGF-β1
(cat no. sc-7892, dilution, 1:4,000; Santa Cruz Biotechnology,
Inc), anti-TGF-β receptor type 1 (TβR1; cat no. 3712, dilution,
1:3,000; Cell Signaling Technology, Inc.), anti-Smad3 (cat no.
sc-8332, dilution, 1:4,000; Santa Cruz Biotechnology, Inc) and
anti-β-actin (cat no. sc-7210, dilution, 1:2,000; Santa Cruz
Biotechnology, Inc). This was followed by incubation with secondary
antibodies (dilution, 1:5,000, cat no. sc-2030; Santa Cruz
Biotechnology, Inc.) for 1 h at room temperature. Densitometry was
detected using Beyo enhanced chemiluminescence Plus (Beyotime
Institute of Biotechnology) and performed using ImageJ software
version 1.47 (National Institutes of Health, Bethesda, MD,
USA).
Statistical analysis
Data are expressed as the mean ± standard error and
SPSS software version 17.0 (SPSS, Inc., Chicago, IL, USA) was used.
Student's t-test were used to analyze the differences between
groups. P<0.05 was considered to indicate a statistically
significance difference.
Results
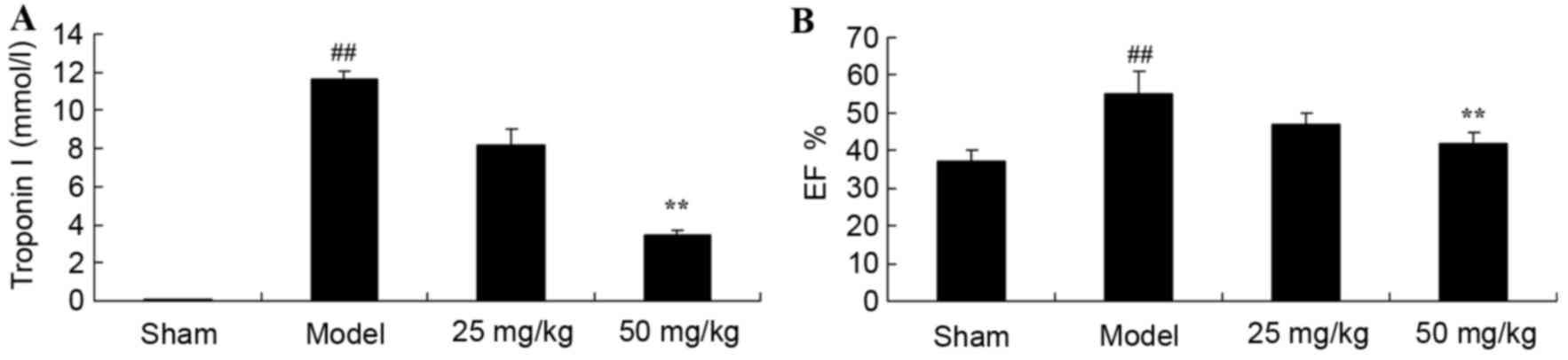
Oxymatrine treatment reduces troponin
I levels and EF in a rat model of CPR
The present study investigated whether oxymatrine
may protect against an increase in troponin I levels and EF in a
rat model of CPR. The levels of troponin I and the percentage EF of
rats in the model group were significantly increased when compared
with the sham-operated group (Fig.
2). However, treatment with 50 mg/kg oxymatrine significantly
reduced troponin I levels and the EF percentage when compared with
the model group (Fig. 2).
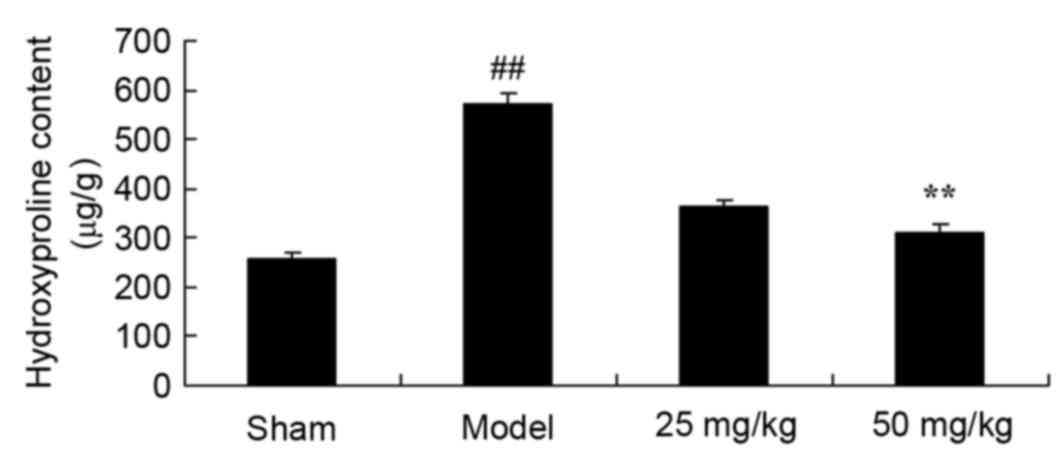
Oxymatrine treatment protects against
increases in heart hydroxyproline content in a rat model of
CPR
At 4 weeks following induction of the rat CPR model,
hydroxyproline content was significantly increased in the model
group when compared with the sham group (Fig. 3). Following administration of 50
mg/kg oxymatrine, hydroxyproline content was significantly
ameliorated when compared with the model group (Fig. 3).
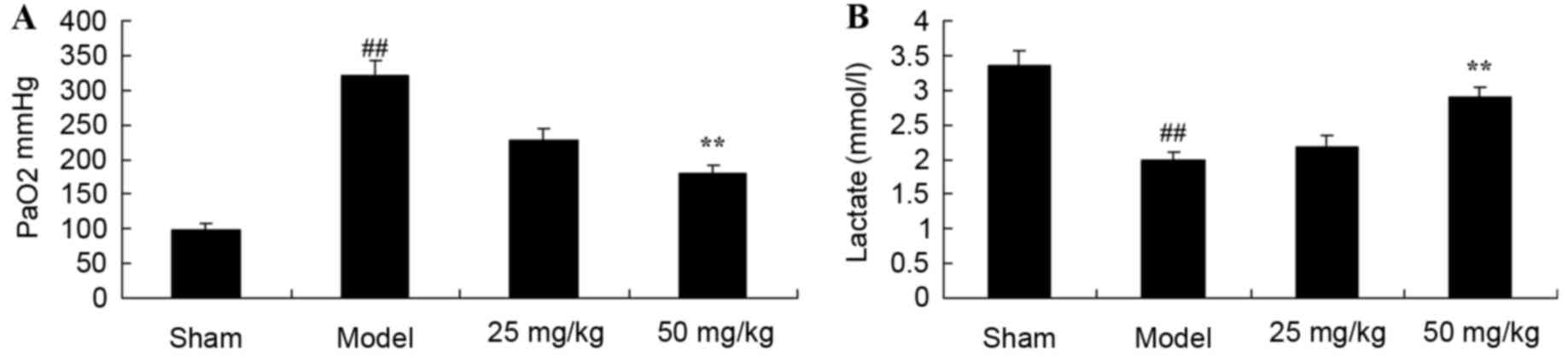
Oxymatrine treatment protects against
increased PaO2 levels and decreased lactate levels in a
rat model of CPR
Following induction of the CPR model,
PaO2 levels were significantly decreased, while lactate
levels were significantly decreased in the CPR model group when
compared with the sham group (Fig.
4). Following treatment with 50 mg/kg oxymatrine,
PaO2 levels decreased and lactate levels increased when
compared with the model group (Fig.
4).
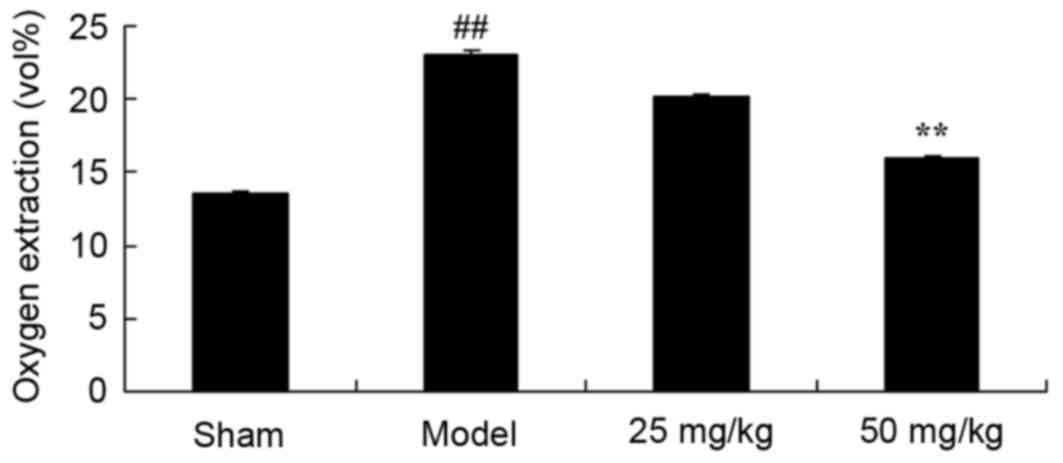
Oxymatrine treatment protects against
increased oxygen extraction in a rat model of CPR
In order to evaluate whether oxymatrine protects
against increased oxygen extraction in a rat model of CPR, the
level of oxygen extraction was compared among all experimental
groups. A significant increase in oxygen extraction was observed in
the model group when compared with the sham group (Fig. 5). By contrast, 50 mg/kg oxymatrine
administration significantly reduced oxygen extraction when
compared with the model group (Fig.
5).
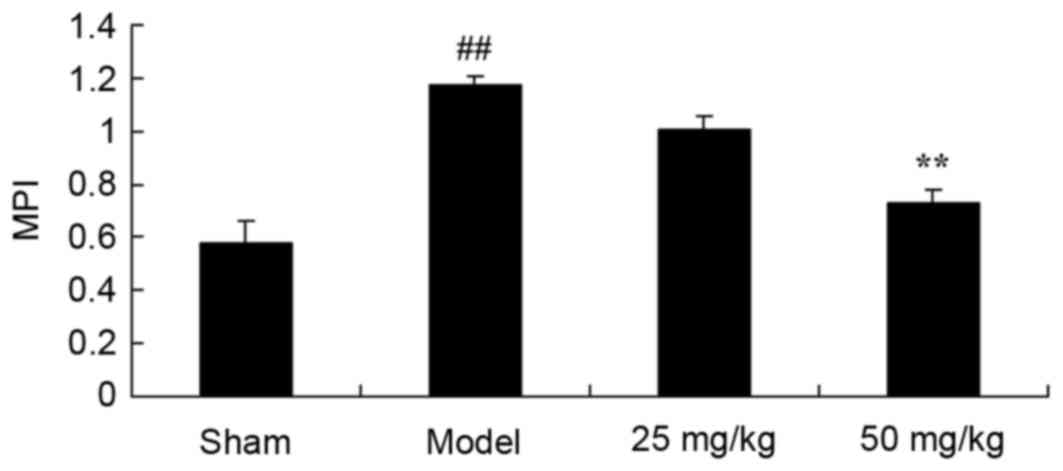
Oxymatrine treatment protects against
increased MPI in a rat model of CPR
The present study investigated whether oxymatrine
may protect against increases in MPI in the rat model of CPR. The
MPI of model rats was significantly increased when compared with
the sham group (Fig. 6). By
contrast, continuous administration of 50 mg/kg oxymatrine for 4
weeks, was associated with a significant reduction in MPI when
compared with the model group (Fig.
6).
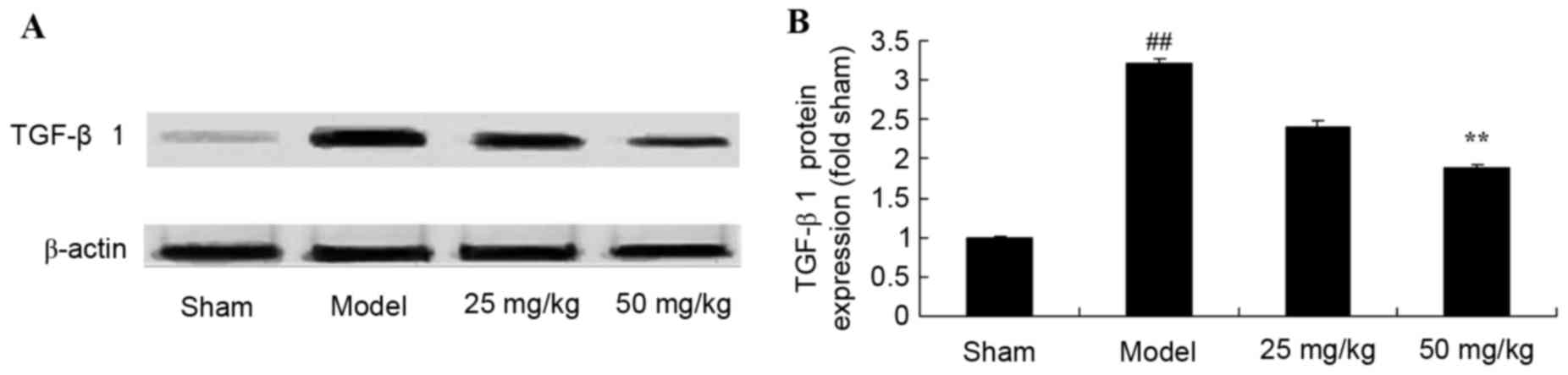
Oxymatrine treatment reduces the
protein expression levels of TGF-β1 in a rat model of CPR
A total of 4 weeks following CPR model induction,
TGF-β1 protein expression levels were increased significantly in
the model group when compared with the sham group (Fig. 7). By contrast, following 4 weeks of
treatment with 50 mg/kg oxymatrine, TGF-β1 protein expression
levels were significantly suppressed when compared with the model
group (Fig. 7).
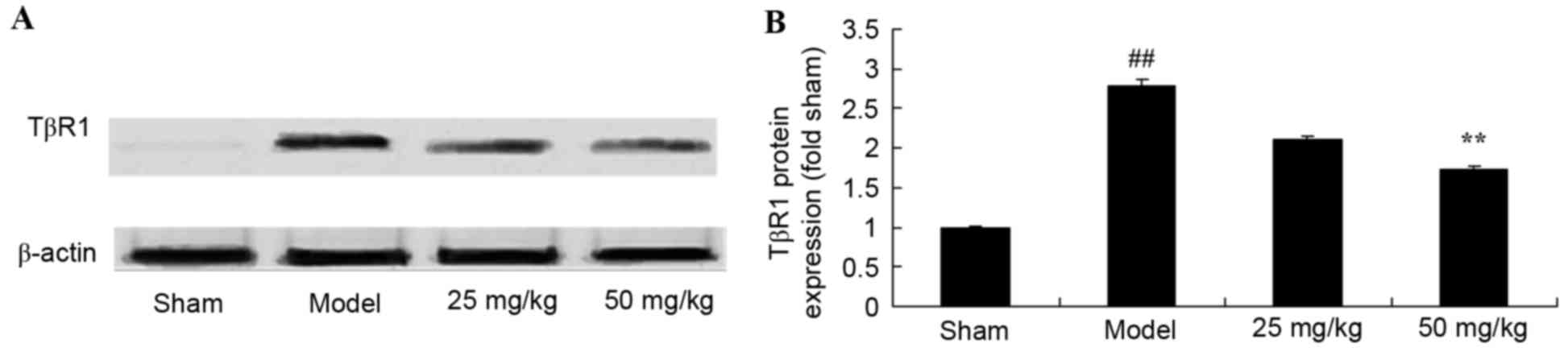
Oxymatrine treatment reduces the
protein expression levels of TβR1 in a rat model of CPR
Representative western blot images are presented in
Fig. 8A. Compared with the sham
group, CPR significantly induced TβR1 protein expression levels
(Fig. 8). By contrast, treatment
with 50 mg/kg oxymatrine significantly reduced TβR1 protein
expression levels when compared with the model group (Fig. 8).
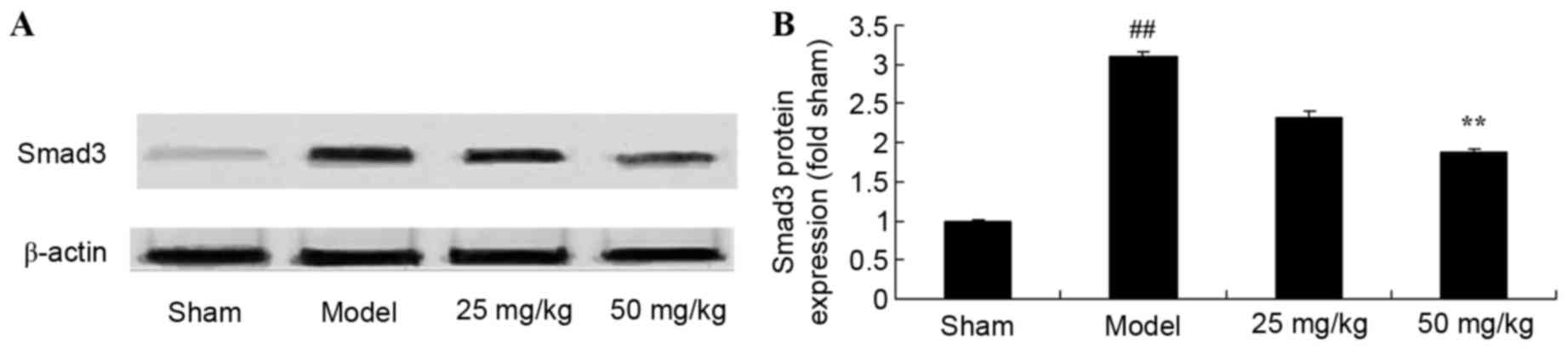
Oxymatrine treatment reduces the
protein expression levels of Smad3 in a rat model of CPR
Representative western blot images are presented in
Fig. 9A. Compared with the sham
group, CPR significantly induced Smad3 protein expression levels
(Fig. 9). However, treatment with
50 mg/kg oxymatrine significantly reduced Smad3 protein levels when
compared with the model group (Fig.
9).
Discussion
CA is a primary cause of mortality and severe
disability worldwide. Recovery of cerebral function is a complex
issue during CPR (15).
Reperfusion following cerebral ischemia may trigger serious
cerebral anoxia, resulting in dysneuria (5). The present study demonstrated that
oxymatrine significantly inhibited troponin I levels, EF and
hydroxyproline content in a rat model of CPR.
CPR may lead to severe hypoxic-ischemic states. Once
circulation has halted for 4–6 min, irreversible nerve injury may
occur (16). Even if resuscitation
is successful, numerous oxygen radicals may induce oxidative
stress, thus provoking ischemia reperfusion injuries (16). A previous study revealed that
oxidative stress injury is a key underlying mechanism of cerebral
injury following CPR, and oxygen extraction assessment demonstrates
the oxygen-intake capability of brain tissues and is a key
indicator of hypoxic-ischemic encephalopathy (17). The present study demonstrated that
oxymatrine significantly reduced PaO2, increased lactate
levels, decreased oxygen extraction and reduced the MPI of CPR
model rats. Shen et al (13) indicated that oxymatrine protects
against myocardial fibrosis via the TGF-β1-Smad signaling
pathway.
A previous study indicated that expression levels of
Smad3 increase during the early stages of cerebral ischemia
(18). Smad3 is involved in a
series of pathophysiological alterations, including the increase of
cerebral microvessel permeability, aseptic inflammation,
blood-brain barrier damage and encephaledema (19). Synthetic Smad3 inhibitors may
inhibit these alterations to some extent (20). The present study demonstrated that
oxymatrine significantly reduced Smad3 protein levels when compared
with the model group.
The TGF subfamily includes TGF-β1, activin and bone
morphogenetic protein, which possess various functions associated
with cell growth and differentiation (6). TGF-β1 is a multi-functional cytokine
(6). Almost all cell types,
including epithelial, endothelial and nerve cells, as well as
connective tissues, may interact with TGF-β1 and express the
corresponding receptors (21).
TGF-β1 is involved in cell proliferation and differentiation,
embryonic development, extracellular matrix synthesis and
angiogenesis (8). It serves an
essential role in pathological processes involved in fibrosis, DNA
damage repair and tumor growth. TGF-β1 possesses neuroprotective
functions in various cerebral injuries, including hypoxia-ischemia,
glutamate excitotoxicity, amyloid-β protein accumulation, oxidative
stress and human immunodeficiency virus infection (22). Accordingly, the results of the
present study indicated that oxymatrine significantly suppressed
Smad3 protein expression levels in a rat model of CPR. Fan et
al (23) reported that
oxymatrine inhibits collagen synthesis via Smad3-mediated induction
of the TGF-β1 signaling pathway.
The TGF-β1/TβR1 signaling pathway is pleiotropic and
serves fundamental roles in tissue repair, the accumulation of the
extracellular matrix, excessive fibrosis and the proliferation and
metastasis of tumors (24). TGF-β1
transduces signals via the transmembrane receptor complex together
with serine and threonine enzymes (25). As a signaling molecule in
cytoplasm, TβR1 directly transduces TGF-β1 signals into the cell
nucleus via the cytomembrane. A previous study suggested that
TGF-β1/TβR1 may be closely associated with the regulation of
inflammation, and activation of the TGF-β1/Smad3 signaling pathway
may be pro- or anti-inflammatory (26). In the present study, oxymatrine
significantly suppressed TGF-β1/TβR1 protein expression levels in a
rat model of CPR. Guo et al (27) indicated that oxymatrine protects
against diabetic nephropathy via TGF-β1, and Fan et al
(23) reported that oxymatrine
inhibits collagen synthesis via the Smad3-induced TGF-β1 signaling
pathway.
In conclusion, the results of the present study
demonstrated that oxymatrine markedly reduced troponin I levels,
EF, hydroxyproline content, PaO2, oxygen extraction and
MPI, and increased lactate levels in a rat model of CPR, which may
be associated with suppression of reduction the TGF-β1/TβR1/Smad3
signaling pathway. In further study, more in depth signaling
pathway analysis and the underlying mechanism of oxymatrine will be
explored. The results of this research suggested that oxymatrine
treatment may protect against the effects of CPR via regulation of
the TGF-β1/Smad3 signaling pathway and may be a novel drug for CPR
in a clinical setting.
Acknowledgements
The present study was supported by the Natural
Science Foundation of China (grant no. 81471830).
References
|
1
|
Takahashi K, Sasanuma N, Itani Y, Tanaka
T, Domen K, Masuyama T, Ohyanagi M and Suzuki K: Impact of early
interventions by a cardiac rehabilitation team on the social
rehabilitation of patients resuscitated from cardiogenic
out-of-hospital cardiopulmonary arrest. Intern Med. 54:133–139.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Blomberg H, Gedeborg R, Berglund L,
Karlsten R and Johansson J: Poor chest compression quality with
mechanical compressions in simulated CPR: A randomized, cross-over
manikin study. Resuscitation. 82:1332–1337. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chung TN, Kim SW, You JS, Cho YS, Chung
SP, Park I and Kim SH: The specific effect of metronome guidance on
the quality of one-person CPR and rescuer fatigue. J Emerg Med.
43:1049–1054. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ducros L, Vicaut E, Soleil C, Le Guen M,
Gueye P, Poussant T, Mebazaa A, Payen D and Plaisance P: Effect of
the addition of vasopressin or vasopressin plus nitroglycerin to
epinephrine on arterial blood pressure during CPR in humans. J
Emerg Med. 41:453–459. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jones CM, Thorne CJ, Colter PS, Macrae A,
Brown GA and Hulme J: Rescuers may vary their side of approach to a
casualty without impact on cardiopulmonary resuscitation
performance. Emerg Med J. 30:74–75. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Maitra SR, Bhaduri S, El-Maghrabi MR and
Shapiro MJ: Inhibition of matrix metalloproteinase on hepatic
transforming growth factor beta1 and caspase-3 activation in
hemorrhage. Acad Emerg Med. 12:797–803. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang X, Qu X, Zhong Q, Li Q and Wang D:
Expression of transforming growth factor beta 1 in lung tissue
during cardiopulmonary bypass-induced lung injury in dogs. Thorac
Cardiovasc Surg. 61:747–753. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hutcheson JD, Ryzhova LM, Setola V and
Merryman WD: 5-HT(2B) antagonism arrests non-canonical
TGF-β1-induced valvular myofibroblast differentiation. J Mol Cell
Cardiol. 53:707–714. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu XN, Wang S, Yang Q, Wang YJ, Chen DX
and Zhu XX: ESC reverses epithelial mesenchymal transition induced
by transforming growth factor-β via inhibition of Smad signal
pathway in HepG2 liver cancer cells. Cancer Cell Int. 15:1142015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
He M, Wu Y, Wang M, Chen W, Yuan W and
Jiang J: The clinical value of oxymatrine in preventing lamivudine
induced YMDD mutation: A Meta-analysis. Evid Based Complement
Alternat Med. 2015:9716162015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jiang G, Liu X, Wang M, Chen H, Chen Z and
Qiu T: Oxymatrine ameliorates renal ischemia-reperfusion injury
from oxidative stress through Nrf2/HO-1 pathway. Acta Cir Bras.
30:422–429. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang Y, Yan R and Hu Y: Oxymatrine
inhibits lipopolysaccharide-induced inflammation by down-regulating
Toll-like receptor 4/nuclear factor-kappa B in macrophages. Can J
Physiol Pharmacol. 93:253–260. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shen XC, Yang YP, Xiao TT, Peng J and Liu
XD: Protective effect of oxymatrine on myocardial fibrosis induced
by acute myocardial infarction in rats involved in
TGF-β1-Smads signal pathway. J Asian Nat Prod Res.
13:215–224. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ye S, Weng Y, Sun S, Chen W, Wu X, Li Z,
Weil MH and Tang W: Comparison of the durations of mild therapeutic
hypothermia on outcome after CPR in the rat. Circulation.
125:123–129. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Edelson DP, Call SL, Yuen TC and Vanden
Hoek TL: The impact of a step stool on CPR: A cross-over mannequin
study. Resuscitation. 83:874–878. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao S, Qian J, Wang J, Tang G, Zhengfei
Y, Jena C, Xiaobo WU, Neil D, Caijing L and Wanchun T: Effects of
oxygen concentrations on postresuscitation myocardial oxidative
stress and myocardial function in a rat model of Cardiopulmonary
Resuscitation. Crit Care Med. 43:e560–e566. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kruzliak P, Pechanova O and Kara T: New
perspectives of nitric oxide donors in cardiac arrest and CPR
treatment. Heart Fail Rev. 19:383–390. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xue M, McKelvey K, Shen K, Minhas N, March
L, Park SY and Jackson CJ: Endogenous MMP-9 and not MMP-2 promotes
rheumatoid synovial fibroblast survival, inflammation and cartilage
degradation. Rheumatology (Oxford). 53:2270–2279. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim BJ, Hur JW, Park JS, Kim JH, Kwon TH,
Park YK and Moon HJ: Expression of matrix metalloproteinase-2 and
−9 in human ligamentum flavum cells treated with tumor necrosis
factor-α and interleukin-1β. J Neurosurg Spine. 24:428–435. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Stan MS, Sima C, Cinteza LO and
Dinischiotu A: Silicon-based quantum dots induce inflammation in
human lung cells and disrupt extracellular matrix homeostasis. FEBS
J. 282:2914–2929. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tuuminen R, Nykänen AI, Krebs R, Soronen
J, Pajusola K, Keränen MA, Koskinen PK, Alitalo K and Lemström KB:
PDGF-A, -C, and -D but not PDGF-B increase TGF-beta1 and chronic
rejection in rat cardiac allografts. Arterioscler Thromb Vasc Biol.
29:691–698. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang J, Yu J, Ding CP, Han SP, Zeng XY and
Wang JY: Transforming growth factor-beta in the red nucleus plays
antinociceptive effect under physiological and pathological pain
conditions. Neuroscience. 291:37–45. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fan DL, Zhao WJ, Wang YX, Han SY and Guo
S: Oxymatrine inhibits collagen synthesis in keloid fibroblasts via
inhibition of transforming growth factor-β1/Smad signaling pathway.
Int J Dermatol. 51:463–472. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yan X, Liao H, Cheng M, Shi X, Lin X, Feng
XH and Chen YG: Smad7 Protein Interacts with Receptor-regulated
Smads (R-Smads) to inhibit transforming growth factor-β
(TGF-β)/Smad signaling. J Biol Chem. 291:382–392. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Du S, Li H, Cui Y, Yang L, Wu J, Huang H,
Chen Y, Huang W, Zhang R, Yang J, et al: Houttuynia cordata
inhibits lipopolysaccharide-induced rapid pulmonary fibrosis by
up-regulating IFN-γ and inhibiting the TGF-β1/Smad pathway. Int
Immunopharmacol. 13:331–340. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xu Z, Ramachandran S, Gunasekaran M, Zhou
F, Trulock E, Kreisel D, Hachem R and Mohanakumar T: MicroRNA-144
dysregulates the transforming growth factor-β signaling cascade and
contributes to the development of bronchiolitis obliterans syndrome
after human lung transplantation. J Heart Lung Transplant.
34:1154–1162. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Guo C, Han F, Zhang C, Xiao W and Yang Z:
Protective effects of oxymatrine on experimental diabetic
nephropathy. Planta Med. 80:269–276. 2014. View Article : Google Scholar : PubMed/NCBI
|