Introduction
Osteoarthritis is a common arthritic disease in the
elderly, which is characterized by progressive destruction of
articular cartilage, synovial inflammation, subchondral bone
exposure and the formation of osteophytes (1). Although some drugs have been
demonstrated to reduce symptoms and slow the progression of
osteoarthritis, there are currently no effective pharmacological
treatments available to cure osteoarthritis.
Chondrocytes are the only cells that form the
articular cartilage; therefore, apoptosis of chondrocytes is a
critical step in the pathogenesis of osteoarthritis (2). Previous studies have revealed that
osteoarthritic chondrocytes exhibit a loss of mitochondrial
function, which precedes the classical signs of apoptosis (3–5).
Interleukin-1β (IL-1β) is a cytokine secreted by synovial cells and
macrophages, which serves an important role in amplifying
inflammation in osteoarthritis (6,7). In
response to IL-1β, chondrocytes secrete proinflammatory cytokines,
chemokines, neutral metalloproteinases and nitric oxide (NO)
(8). IL-1β has previously been
demonstrated to increase apoptosis in osteoarthritic chondrocytes
(9,10).
Tormentic acid (TA), which is a triterpene isolated
from the stem bark of the Vochysia divergens plant, has been
reported to exhibit anticancer, antioxidant, anti-inflammatory and
antiallodynic properties (11–13).
An et al (14) reported
that TA inhibited the expression of lipopolysaccharide-induced
tumor necrosis factor (TNF)-α, inducible NO synthase and
cyclooxygenase 2 in RAW264.7 cells. In addition, TA has exhibited
in vitro inhibitory activity against hepatocyte apoptosis by
decreasing the levels of cytochrome c, as well as
caspases-3, −8 and −9 (15).
However, the effects of TA on IL-1β-induced apoptosis of human
chondrocytes remain unclear. Therefore, the present study
investigated the in vitro effects of TA on the apoptosis of
IL-1β-treated human osteoarthritic chondrocytes.
Materials and methods
Culture of human articular cartilage
chondrocytes
Chondrocytes were isolated from cartilage as
previously described (16). The
cartilage was dissected and crushed into small pieces. The slices
were digested primarily with 1% pronase (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) for 2 h at 37°C and subsequently with 0.2%
(v/v) collagenase (Sigma-Aldrich; Merck KGaA) for 4 h at 37°C.
Primary chondrocytes were cultured in Dulbecco's Modified Eagle
Medium (DMEM; Invitrogen; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) supplemented with 10% fetal bovine serum (FBS; Invitrogen;
Thermo Fisher Scientific, Inc.), 100 U/ml penicillin and 100 mg/ml
streptomycin(Sigma-Aldrich; Merck KGaA) at 1×106
cells/ml in a 25-cm2 plastic culture flask at 37°C in an
atmosphere containing 5% CO2. Once cells reached 80%
confluence, they were passaged; chondrocytes from passage one were
used for subsequent experiments. Cartilage samples were derived
from eight patients (female; age, 64.3±5.2 years) with end-stage
osteoarthritisin Tianjin Hospital between May 2014 and October
2015. Informed written consent was obtained from patients and the
present study was approved by the Ethics Committee of Tianjin
Hospital (Tianjin, China).
Cell treatment
Human chondrocytes (1×104 cells/well) in
96-well plates were pretreated with various concentrations (2.5, 5
and 10 µM) of TA (Sigma-Aldrich; Merck KGaA) for 2 h and then
co-incubated in the absence or presence of IL-1β (10 ng/ml;
Sigma-Aldrich; Merck KGaA) for 24 h at 37°C. Chondrocytes exposed
to medium alone at room temperature served as the control
group.
Cell viability assay
A
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay was used to detect cell viability. Briefly, chondrocytes at a
density of 1×104 cells/well were plated in 96-well
culture plates. Following treatment, MTT was added to the cells at
a final concentration of 0.5 mg/ml and incubated for 4 h at 37°C.
Subsequently, the supernatant was removed, and the crystals were
dissolved in 100 µl dimethyl sulfoxide (Sigma-Aldrich; Merck KGaA).
The absorbance was measured at 490 nm using a Bio-Rad microplate
reader (Bio-Rad Laboratories Inc., Hercules, CA, USA).
Nucleosome ELISA assay for the detection of
apoptosis. The apoptosis of human chondrocytes was detected using a
Nucleosome ELISA kit (catalog no. KA1091; Abnova, Taipei, Taiwan)
according to the manufacturer's protocol. Following treatment,
chondrocytes were lysed in lysis buffer and the homogenates were
centrifuged at 10,000 × g at 4°C for 30 min to remove large genomic
DNA. The supernatant was added to ELISA plates coated with
anti-histone antibodies and incubated with a peroxidase-conjugated
anti-DNA antibody at 37°C for 1 h. Nucleosome detection was based
on a colorimetric change in substrate added to the wells following
conjugation.
Measurement of caspase activity
Caspase-3 activity was detected usingacaspase-3
colorimetric assay kit (catalog no. E13183; Invitrogen; Thermo
Fisher Scientific, Inc.). Briefly, following treatment,
chondrocytes were lysed in lysis buffer and centrifuged at 10,000 ×
g for 1 min, and the supernatants were collected. Subsequently,
equal amounts of protein were reacted with the aminomethylcoumarin
(AMC)-derived substrate Z-DEVD-AMC at 37°C for 90 min. The activity
of caspase-3 was determined using a plate reader set at 405 nm.
Western blot analysis
Chondrocytes were lysed in 100 µl lysis buffer (1%
Triton X-100, 1% deoxycholic acid, 20 mM NaCl and 25 mM Tris; pH
7.4). The cell lysate supernatants were harvested by centrifugation
at 10,000 × g for 10 min at 4°C. Protein concentrations of the cell
supernatants were measured using a bicinchoninic acid protein assay
kit. Equal amounts of protein (30 µg) were separated by 12%
SDS-PAGE and transferred onto a nitrocellulose membrane (Bio-Rad
Laboratories, Inc.). Following blocking with 5% non-fat milk in
Tris-buffered saline (TBS) with 0.1% Tween-20 (TBST) at room
temperature for 1 h, the blots were incubated with primary
antibodies overnight at 4°C. The following antibodies were used:
Mouse anti-B-cell lymphoma (Bcl)-2 (dilution, 1:1,000; catalog no.
sc-7382), mouse anti-Bcl-2-associated X protein (Bax; dilution,
1:1,000; catalog no. sc-7480), rabbit anti-phosphorylated
(p)-phosphoinositide 3-kinase (PI3K; dilution, 1:1,500; catalog no.
sc-293115), mouse anti-PI3K (dilution, 1:1,500; catalog no.
sc-71892), mouse anti-p-protein kinase B(p-Akt; dilution, 1:1,000;
catalog no. sc-52940), mouse anti-Akt (dilution, 1:1,000; catalog
no. sc-5298) and mouse anti-GAPDH (dilution, 1:3,000; catalog no.
sc-365062). All primary antibodies were purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). Subsequently, the membranes
were incubated with rabbit anti-mouse (dilution, 1:3,000; catalog
no. 61–6520) or goat anti-rabbit (dilution, 1:3,000; catalog no.
65–6120) immunoglobulin G-horseradish peroxidase-labeled secondary
antibodies(Invitrogen; Thermo Fisher Scientific, Inc.) at room
temperature for 1 h. Finally, protein bands were visualized using
an enhanced chemiluminescence kit (Amersham; GE Healthcare Life
Sciences, Little Chalfont, UK). Semi-quantitative analysis was
performed using Gel-Pro Analyzer version 4.0 software (Media
Cybernetics, Inc., Rockville, MD, USA).
Statistical analysis
Data was analyzed using SPSS software version 13.0
(SPSS, Inc., Chicago, IL, USA). Results from at least three
independent experiments were expressed as the mean ± standard
deviation. Differences were analyzed using a Student's t-test or
one-way analysis of variance followed by Tukey's posthoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effects of TA on human chondrocyte
viability
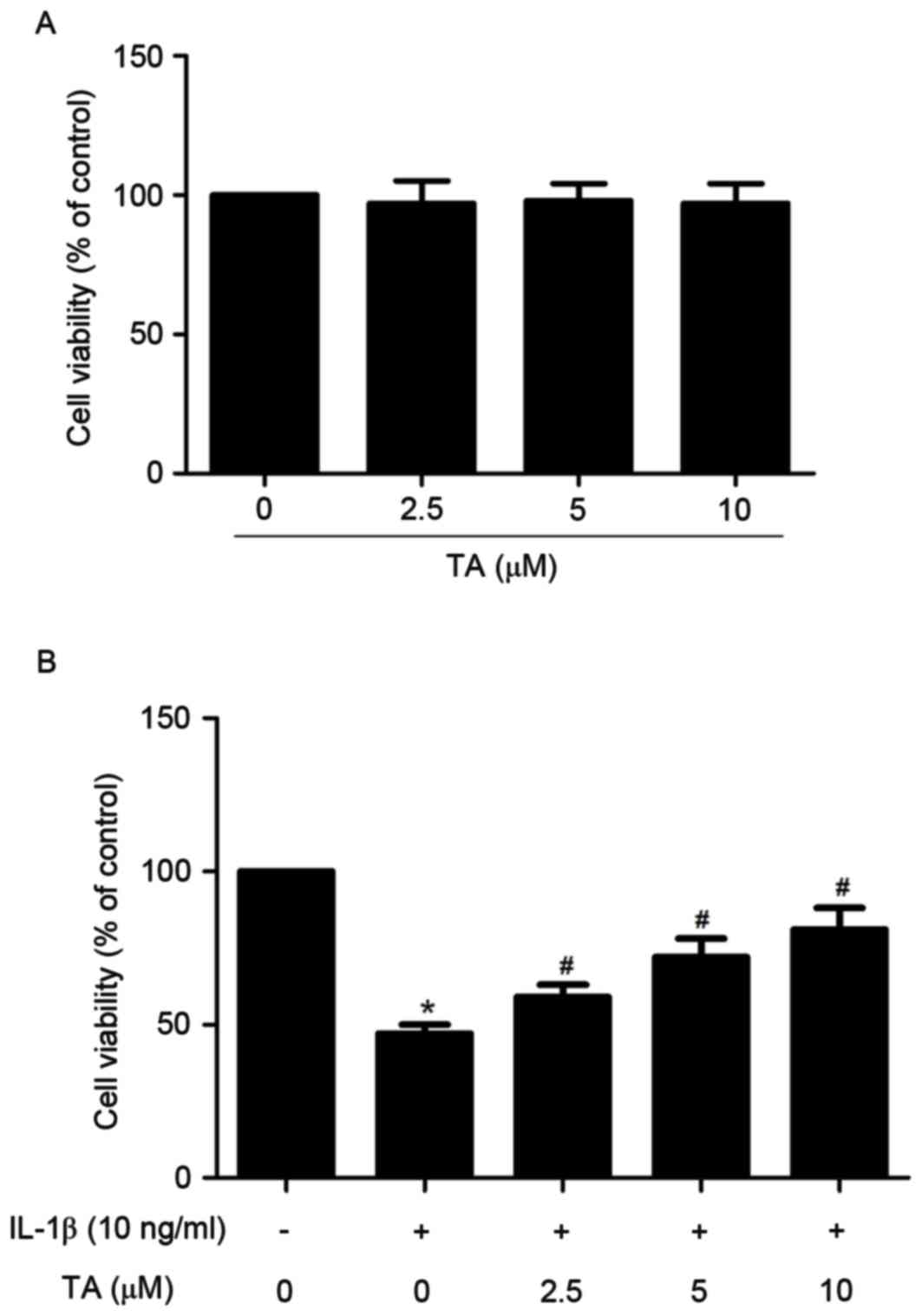
The effects of TA on IL-1β-stimulated human
chondrocytes were evaluated using an MTT assay. As shown in
Fig. 1A, human chondrocyte cell
viability was unaffected by TA. Following incubation with IL-1β (10
ng/ml), cell viability was significantly decreased in human
chondrocytes. However, when IL-1β-stimulated chondrocytes were
pretreated with various doses of TA for 2 h, cell viability
increased significantly (Fig.
1B).
TA suppresses IL-1β-induced apoptosis
of chondrocytes
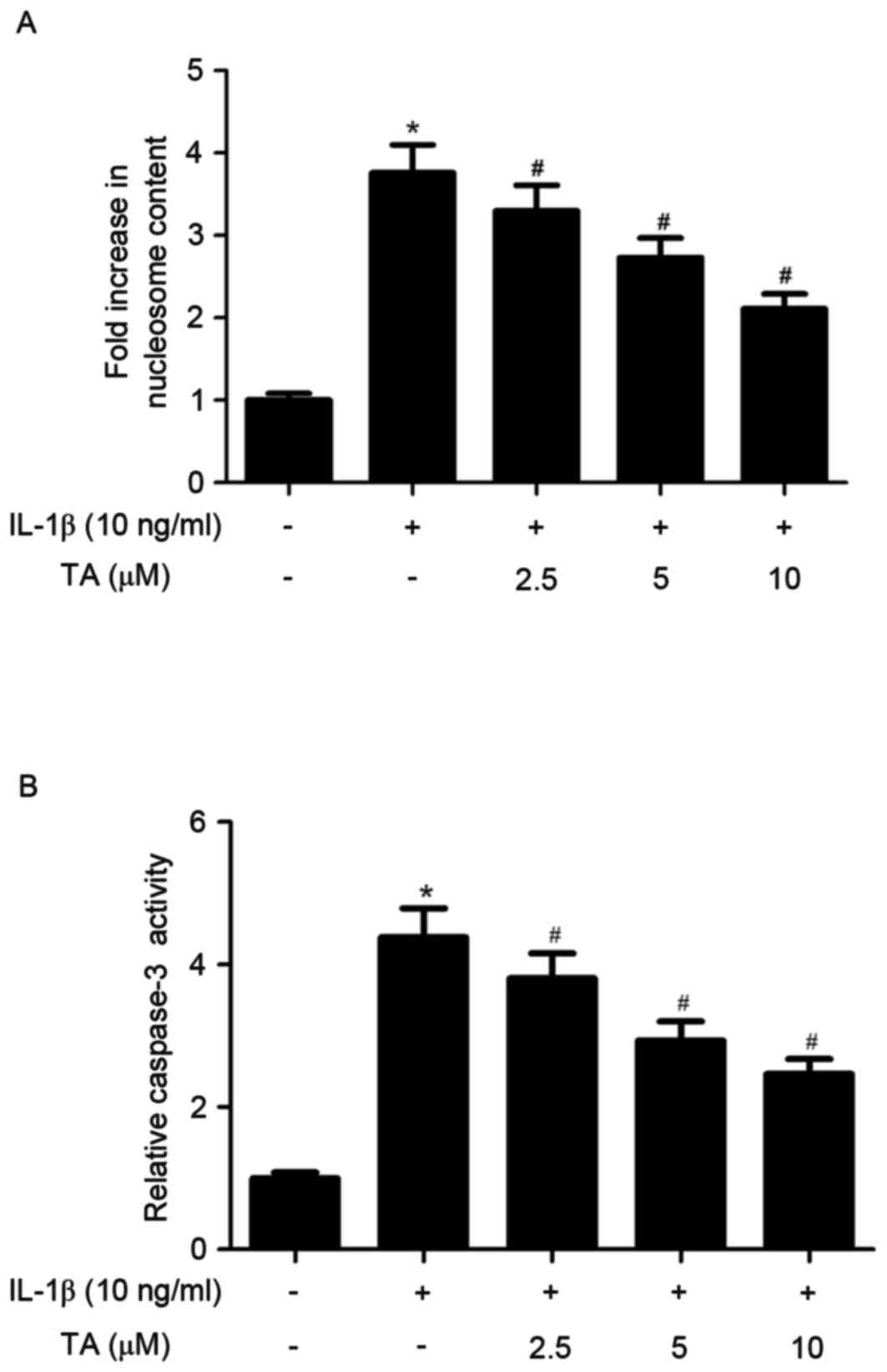
A Nucleosome ELISA assay was performed to measure
the effects of TA on apoptosis of IL-1β-stimulated human
chondrocytes. The results revealed that IL-1β treatment
significantly induced chondrocyte apoptosis. However, TA
pretreatment significantly inhibited apoptosis induced by IL-1β
(Fig. 2A). Caspase-3 activity was
also determined in the various groups using the caspase-3
colorimetric assay kit. As shown in Fig. 2B, TA significantly suppressed
IL-1β-induced caspase-3 activation in human chondrocytes.
TA promotes anti-apoptotic protein and
inhibits proapoptotic protein expression in chondrocytes
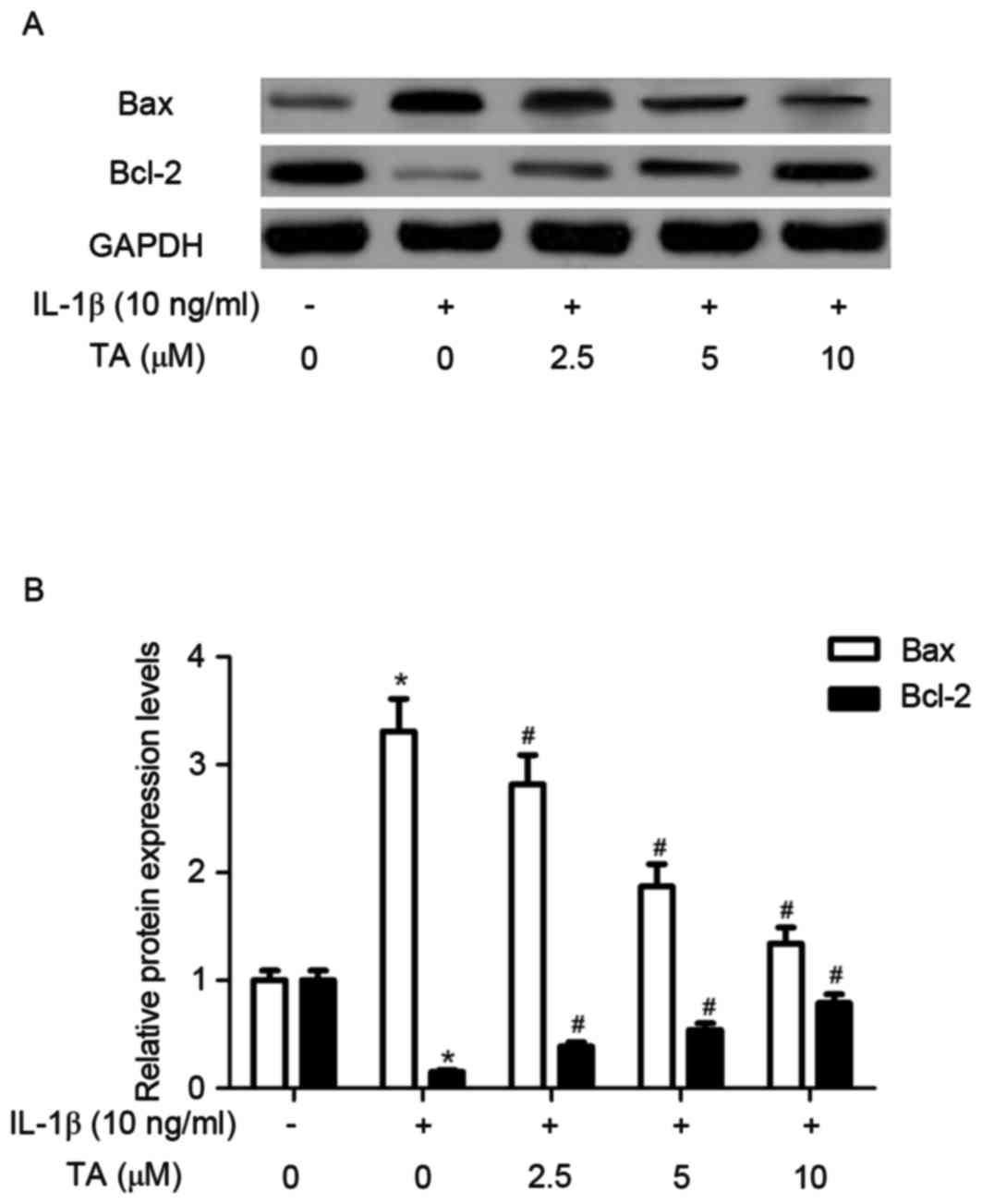
To evaluate whether TA modulates the expression of
apoptosis-associated proteins, the effects of TA on Bax and Bcl-2
expression in IL-1β-stimulated human chondrocytes were investigated
by western blot analysis. The results demonstrated that IL-1β
significantly increased the expression of Bax protein and inhibited
the expression of Bcl-2 protein. Conversely, in the TA-pretreated
group, Bax expression was downregulated and the expression of Bcl-2
was upregulated in human chondrocytes (Fig. 3).
TA reduces IL-1β-induced apoptosis of
human chondrocytes via the PI3K/Akt pathway
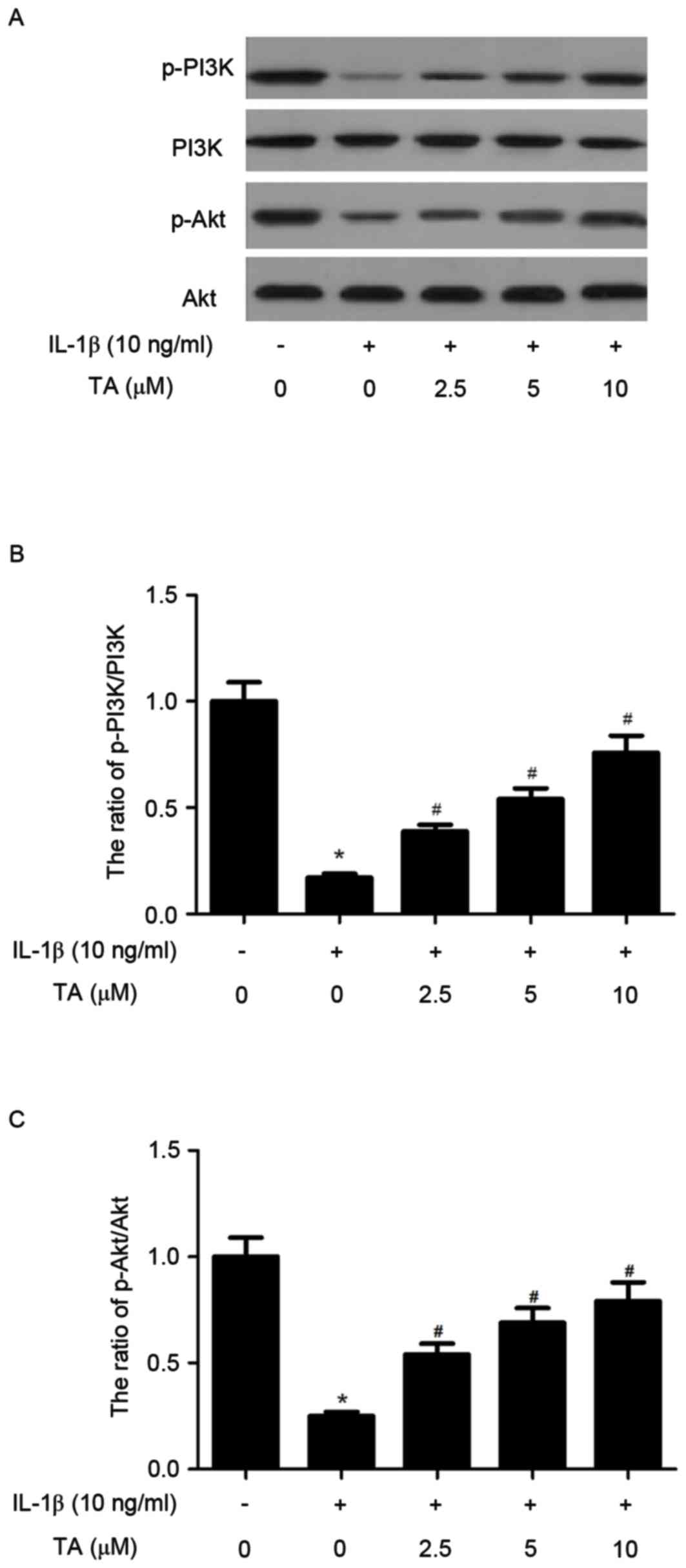
The present study also determined whether the
PI3K/Akt pathway is associated with the protective effects of TA on
IL-1β-induced apoptosis of human chondrocytes. As shown in Fig. 4, the expression levels of p-PI3K
and p-Akt were significantly decreased following 24 h of IL-1β
stimulation compared with the control group. However, pretreatment
with TA for 2 h significantly increased p-PI3K and p-Akt expression
in IL-1β-induced chondrocytes.
Discussion
It has previously been reported that there are
significant associations between increasing chondrocyte apoptosis
and the severity of osteoarthritis in in vitro and in
vivo studies (17–19). Therefore, it is essential to
elucidate the molecular mechanisms underlying chondrocyte apoptosis
in osteoarthritis. The present study investigated the effects of TA
on IL-1β-induced chondrocyte apoptosis, and revealed that TA
promotes cell viability and inhibits cell apoptosis in
IL-1β-stimulated human chondrocytes.
Proinflammatory cytokines, such as TNF-α and IL-1β,
serve important roles in degenerative joint diseases including OA.
Previous studies have demonstrated that IL-1β promotes the
imbalance between excessive cartilage destruction and cartilage
repair (20,21). IL-1β has been widely used to mimic
the osteoarthritic microenvironment in in vitro studies
(22). In the present study,
IL-1β-induced human chondrocytes were chosen as a model to study
the protective effects of TA on chondrocytes. A significant
decrease in cell viability was observed in IL-1β-stimulated
chondrocytes. However, when TA was added to IL-1β-treated
chondrocytes, there was a significant restoration in chondrocyte
viability.
Chondrocyte apoptosis is responsible for the
development and progression of osteoarthritis. During the
progression of osteoarthritis, apoptotic cells are the main source
of numerous catabolic factors, including proteases, proinflammatory
mediators, NO and oxygen radicals (23,24).
The present study demonstrated that IL-1β treatment significantly
induced chondrocyte apoptosis. These in vitro observations
are consistent with previous reports, which revealed that IL-1β
suppresses viability and induces apoptotic signaling in human
chondrocytes harvested from articular cartilage and osteoarthritic
cartilage (4,25). However, TA pretreatment markedly
inhibited apoptosis induced by IL-1β.
Caspases area family of cysteine proteases, which
act as common death effector molecules in several apoptotic
pathways (26). A previous study
reported that caspase-3 activity is markedly enhanced in
chondrocyte apoptosis induced by IL-1β (27). In addition, the Bax/Bcl-2 family
consists of many important regulators of apoptosis; therefore,
alterations in the levels of anti- and proapoptotic proteins
mayaffect the apoptotic process (28,29).
The present study revealed that TA markedly suppressed caspase-3
activity induced by IL-1β in human chondrocytes, and downregulated
Bax expression and upregulated Bcl-2 expression in IL-1β-stimulated
human chondrocytes. These results indicated that TA exhibited
protective effects on human chondrocytes in the presence of IL-1β
by inhibiting chondrocyte apoptosis.
Previous studies have suggested that the PI3K/Akt
signaling pathway may be involved in the regulation of chondrocyte
apoptosis (30–32). p-Akt activates the Bcl-2-associated
death promoter protein, a downstream substrate of Akt, leading to
the release of Bcl-2 and subsequently, the inhibition of apoptosis
(33). Conversely, suppression of
PI3K/Akt signaling blocks proteoglycan synthesis in chondrocytes
and reduces chondrocyte survival (34,35).
The present study demonstrated that pretreatment with TA for 2 h
markedly increased p-PI3K and p-Akt expression in chondrocytes
treated with IL-1β. These results indicated that TA may inhibit
IL-1β-induced chondrocyte apoptosis via activation of the PI3K/Akt
signaling pathway.
In conclusion, the results of the present study
suggested that TA attenuated IL-1β-induced apoptosis via activation
of the PI3K/Akt signaling pathway in human chondrocytes. Therefore,
TA may be a potential therapeutic target for the treatment of
osteoarthritis.
Acknowledgements
The present study was funded by the Tianjin Hospital
Science Foundation (grant no. TJYY1601) and by the National Natural
Science Foundation of China (grant no. 81702208 and 81501919).
References
|
1
|
Martel-Pelletier J: Pathophysiology of
osteoarthritis. Osteoarthr Cartilage. 12 Suppl A:S31–S33. 2014.
View Article : Google Scholar
|
|
2
|
Johnson E, Charchandi A, Babis G and
Soucacos P: Apoptosis in osteoarthritis: Morphology, mechanisms,
and potential means for therapeutic intervention. J Surg Orthop
Adv. 17:147–152. 2007.
|
|
3
|
Grishko V, Xu M, Ho R, Mates A, Watson S,
Kim JT, Wilson GL and Pearsall AW IV: Effects of hyaluronic acid on
mitochondrial function and mitochondria-driven apoptosis following
oxidative stress in human chondrocytes. J Biol Chem. 284:9132–9139.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dave M, Attur M, Palmer G, Al-Mussawir HE,
Kennish L, Patel J and Abramson SB: The antioxidant resveratrol
protects against chondrocyte apoptosis via effects on mitochondrial
polarization and ATP production. Arthritis Rheum. 58:2786–2797.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Blanco FJ, Rego I and Ruiz-Romero C: The
role of mitochondria in osteoarthritis. Nat Rev Rheumatol.
7:161–169. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Goldring MB: Osteoarthritis and cartilage:
The role of cytokines. Curr Rheumatol Rep. 2:459–465. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bassleer CT, Combal JP, Bougaret S and
Malaise M: Effects of chondroitin sulfate and interleukin-1 beta on
human articular chondrocytes cultivated in clusters. Osteoarthr
Cartilage. 6:196–204. 1998. View Article : Google Scholar
|
|
8
|
Daheshia M and Yao JQ: The interleukin 1
beta pathway in the pathogenesis of osteoarthritis. J Rheumatol.
35:2306–2312. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yasuhara R, Miyamoto Y, Akaike T, Akuta T,
Nakamura M, Takami M, Morimura N, Yasu K and Kamijo R:
Interleukin-1beta induces death in chondrocyte-like ATDC5 cells
through mitochondrial dysfunction and energy depletion in a
reactive nitrogen and oxygen species-dependent manner. Biochem J.
389:315–323. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sanchez C, Deberg MA, Piccardi N, Msika P,
Reginster JY and Henrotin YE: Osteoblasts from the sclerotic
subchondral bone downregulate aggrecan but upregulate
metalloproteinases expression by chondrocytes. This effect is
mimicked by interleukin-6,-1beta and oncostatin M pre-treated
non-sclerotic osteoblasts. Osteoarthr Cartilage. 13:979–987. 2005.
View Article : Google Scholar
|
|
11
|
Banno N, Akihisa T, Tokuda H, Yasukawa K,
Higashihara H, Ukiya M, Watanabe K, Kimura Y, Hasegawa J and
Nishino H: Triterpene acids from the leaves of Perilla frutescens
and their anti-inflammatory and antitumor-promoting effects. Biosci
Biotech Biochem. 68:85–90. 2004. View Article : Google Scholar
|
|
12
|
Song YL, Zhang L, Gao JM, Du GH and Cheng
YX: Speciosaperoxide, a new triterpene acid, and other terpenoids
from Chaenomeles speciosa. J Asian Nat Prod Res. 10:217–222. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bortalanza LB, Ferreira J, Hess SC, Delle
Monache F, Yunes RA and Calixto JB: Anti-allodynic action of the
tormentic acid, a triterpene isolated from plant, against
neuropathic and inflammatory persistent pain in mice. Eur J
Pharmacol. 453:203–208. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
An HJ, Kim IT, Park HJ, Kim HM, Choi JH
and Lee KT: Tormentic acid, a triterpenoid saponin, isolated from
Rosa rugosa, inhibited LPS-induced iNOS, COX-2, and TNF-α
expression through inactivation of the nuclear factor-κb pathway in
RAW 264.7 macrophages. Int Immunopharmacol. 11:504–510. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lin X, Zhang S, Huang R, Tan S, Liang S,
Wu X, Zhuo L and Huang Q: Protective effect of tormentic acid from
Potentilla chinensis against lipopolysaccharide/D-galactosamine
induced fulminant hepatic failure in mice. Int Immunopharmacol.
19:365–372. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shakibaei M and Merker HJ: beta1-Integrins
in the cartilage matrix. Cell Tissue Res. 296:565–573. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Aigner T and Kim HA: Apoptosis and
cellular vitality: Issues in osteoarthritic cartilage degeneration.
Arthritis Rheum. 46:1986–1996. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Goldring MB: Update on the biology of the
chondrocyte and new approaches to treating cartilage diseases. Best
Pract Res Clin Rheumatol. 20:1003–1025. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mistry D, Oue Y, Chambers MG, Kayser MV
and Mason RM: Chondrocyte death during murine osteoarthritis.
Osteoarthr Cartilage. 12:131–141. 2004. View Article : Google Scholar
|
|
20
|
Caron JP, Fernandes JC, Martel-Pelletier
J, Tardif G, Mineau F, Geng C and Pelletier JP: Chondroprotective
effect of intraarticular injections of interleukin-1 receptor
antagonist in experimental osteoarthritis. Suppression of
collagenase-1 expression. Arthritis Rheum. 39:1535–1544. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Joosten LA, Helsen MM, Saxne T, van De Loo
FA, Heinegard D and van Den Berg WB: IL-1alpha beta blockade
prevents cartilage and bone destruction in murine type II
collagen-induced arthritis, whereas TNF-alpha blockade only
ameliorates joint inflammation. J Immunol. 163:5049–5055.
1999.PubMed/NCBI
|
|
22
|
Largo R, Alvarez-Soria M, Dıez-Ortego I,
Calvo E, Sanchez-Pernaute O, Egido J and Herrero-Beaumont G:
Glucosamine inhibits IL-1beta-induced NFkappaB activation in human
osteoarthritic chondrocytes. Osteoarthritis Cartilage. 11:290–298.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mazzetti I, Grigolo B, Pulsatelli L,
Dolzani P, Silvestri T, Roseti L, Meliconi R and Facchini A:
Differential roles of nitric oxide and oxygen radicals in
chondrocytes affected by osteoarthritis and rheumatoid arthritis.
Clin Sci. 101:593–599. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Venkatesan N, Barre L, Benani A, Netter P,
Magdalou J, Fournel-Gigleux S and Ouzzine M: Stimulation of
proteoglycan synthesis by glucuronosyltransferase-I gene delivery:
A strategy to promote cartilage repair. Proc Natl Acad Sci USA.
101:pp. 18087–18092. 2004; View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang L, Gai P, Xu R, Zheng Y, Lv S, Li Y
and Liu S: Shikonin protects chondrocytes from
interleukin-1beta-induced apoptosis by regulating PI3K/Akt
signaling pathway. Int J Clin Exp Pathol. 8:2982015.PubMed/NCBI
|
|
26
|
Samali A, Zhivotovsky B, Jones D, Nagata S
and Orrenius S: Apoptosis: Cell death defined by caspase
activation. Cell Death Differ. 6:495–496. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shakibaei M, Schulze-Tanzil G, John T and
Mobasheri A: Curcumin protects human chondrocytes from
IL-1beta-induced inhibition of collagen type II and beta1-integrin
expression and activation of caspase-3: An immunomorphological
study. Anna Anat. 187:487–497. 2005. View Article : Google Scholar
|
|
28
|
Chen J, Chu Y, Cao J, Yang Z, Guo X and
Wang Z: T-2 toxin induces apoptosis, and selenium partly blocks,
T-2 toxin induced apoptosis in chondrocytes through modulation of
the Bax/Bcl-2 ratio. Food Chem Toxicol. 44:567–573. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Malemud CJ and Gillespie HJ: The role of
apoptosis in arthritis. Curr Rheumatol Rev. 1:131–142. 2005.
View Article : Google Scholar
|
|
30
|
Sugimori K, Matsui K, Motomura H, Tokoro
T, Wang J, Higa S, Kimura T and Kitajima I: BMP-2 prevents
apoptosis of the N1511 chondrocytic cell line through
PI3K/Akt-mediated NF-kappaB activation. J Bone Miner Metab.
23:411–419. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Oh CD and Chun JS: Signaling mechanisms
leading to the regulation of differentiation and apoptosis of
articular chondrocytes by insulin-like growth factor-1. J Biol
Chem. 278:36563–36571. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ikegami D, Akiyama H, Suzuki A, Nakamura
T, Nakano T, Yoshikawa H and Tsumaki N: Sox9 sustains chondrocyte
survival and hypertrophy in part through Pik3ca-Akt pathways.
Development. 138:1507–1519. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Stiles BL: PI-3-K and AKT: Onto the
mitochondria. Adv Drug Deliver Rev. 61:1276–1282. 2009. View Article : Google Scholar
|
|
34
|
Yin W, Park JI and Loeser RF: Oxidative
stress inhibits insulin-like growth factor-I induction of
chondrocyte proteoglycan synthesis through differential regulation
of phosphatidylinositol 3-Kinase-Akt and MEK-ERK MAPK signaling
pathways. J Biol Chem. 284:31972–31981. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Starkman BG, Cravero JD, Delcarlo M and
Loeser RF: IGF-I stimulation of proteoglycan synthesis by
chondrocytes requires activation of the PI 3-kinase pathway but not
ERK MAPK. Biochem J. 389:723–729. 2005. View Article : Google Scholar : PubMed/NCBI
|