Introduction
Prostate cancer (PCa), a highly common malignant
tumour in the urinary and reproductive systems, is the second most
common cancer and the sixth leading cause of cancer-related deaths
worldwide (1,2). In recent years, the incidence and
mortality of PCa in China have gradually increased with progressing
economy (3). Despite the unclear
aetiology of this malignancy, both hereditary and environmental
components have been demonstrated to participate in prostate
carcinogenesis, where age, race and family history are the only
well-established risk factors (4,5).
Over the past decades, considerable advances have been achieved in
the diagnosis and treatment of PCa. However, the long-term survival
of patients with PCa remains poor (6). The 5-year relative survival rate of
patients with PCa at the advanced metastatic stage is only 28%
(7). The local or distant
metastasis, recurrence and development of hormone refractory
disease remain the major causes of death (8,9).
Therefore, the molecular mechanisms underlying the formation and
progression of PCa must be elucidated to develop novel and
effective therapeutic methods for treatment of patients with this
malignancy.
MicroRNAs (miRNAs) are a subset of non-coding,
single-stranded, short RNA molecules that are 19–24 nucleotides in
length (10). miRNAs regulate gene
expression primarily by binding to the 3′-untranslated regions
(3′-UTRs) of their target genes in a sequence-dependent recognition
manner, thereby inducing mRNA degradation or blocking the
translation of the encoded protein (11). At least one-third of human genes
are estimated to be regulated by miRNAs, which serve key roles in
numerous biological processes, such as differentiation, organ
development, immune responses, proliferation, apoptosis and signal
transduction (12). Several lines
of evidence suggest that miRNAs are aberrantly expressed in almost
all types of human cancer, including PCa (13,14).
miRNAs act as either oncogene or tumour suppressor due to the
different cellular contexts of malignancies (15,16).
miRNAs also participate in tumourigenesis and tumour development by
regulating cell proliferation, cell cycle, cell survival,
apoptosis, angiogenesis, cell migration, cell invasion, and
metastasis (17). Therefore,
investigating cancer-related miRNAs is important to provide novel
therapeutic targets for PCa treatment.
miR-136 is significantly decreased in multiple human
cancers (18–20). However, limited information is
available regarding the expression pattern and biological functions
of miR-136 in PCa. Hence, the present study aims to elucidate the
expression pattern, biological functions and underlying molecular
mechanisms of miR-136 in PCa.
Materials and methods
Clinical specimens
This study was approved by the Ethical Committee of
Ningbo No. 2 Hospital. Written informed consent was provided by all
patients prior to enrollment in this study. A total of 27 pairs of
PCa tissues and adjacent noncancerous tissues (NCTs) were obtained
from patients who underwent surgery at Ningbo No. 2 Hospital from
June 2014 to February 2016. None patients had been treated with any
treatment including chemotherapy, radiotherapy and
androgen-deprivation treatment before surgery. Following surgical
resection, all tissues were immediately frozen in liquid nitrogen
and stored at −80°C until further use.
Cell culture and transfection
Human PCa cells (DU145, PC3, LNCaP and 22RV1) and
normal prostatic epithelial cell line (RWPE-1) were acquired from
Institute of Biochemistry and Cell Biology at the Chinese Academy
of Sciences (Shanghai, China). All PCa cell lines were cultured in
RPMI-1640 medium with 10% fetal bovine serum (FBS), 100 U/ml
penicillin and 100 µg/ml streptomycin (all from Gibco, Grand
Island, NY, USA). RWPE-1 cells were grown in
keratinocyte-serum-free medium containing 50 mg/ml bovine pituitary
extract and 5 ng/ml recombinant human epidermal growth factor (all
from Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA).
All cell lines were maintained at 37°C in a humidified atmosphere
of 5% CO2 and 95% air.
miR-136 mimics and negative control miRNA mimics
(miR-NC) were purchased from GenePharma Co., Ltd. (Shanghai,
China). MAP2K4 overexpression plasmid (pCMV-MAP2K4) and blank
plasmid pCMV were chemically synthesized by Guangzhou RiboBio Co.,
Ltd. (Guangzhou, China). For transfection, cells were plated in
6-well plates 24 h prior to transfection. Cell transfection was
carried out using Lipofectamine 2000 (Invitrogen Life Technologies,
Carlsbad, CA, USA) according to the manufacturer's
instructions.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from tissues or cells using
TRIzol reagent (Thermo Fisher Scientific, Inc.) in accordance with
the manufacturer's instructions. A NanoDrop 2000 (Thermo Fisher
Scientific, Inc., Wilmington, DE, USA) was used to determine the
RNA purity and concentration. To quantify miR-136 expression, the
complementary DNA (cDNA) was synthesized using the TaqMan MicroRNA
Reverse Transcription kit and the quantitative PCR was performed
using the TaqMan MicroRNA PCR kit (both from Applied Biosystems,
Foster City, CA, USA). U6 snRNA used as an control for miR-136
expression. For detection of MAP2K4 mRNA, a PrimeScript RT Reagent
kit (Takara Biotechnology, Co., Ltd., Dalian, China) was utilized
to synthesize the cDNA. The PCR amplification for the
quantification of MAP2K4 mRNA was carried out using the SYBR Premix
Ex Taq™ kit (Takara Biotechnology, Co., Ltd.). The
relative expression of MAP2K4 mRNA was illustrated as fold
difference relative to GAPDH. Relative expression was analyzed
using the 2−ΔΔCq method (21).
Cell Counting Kit-8 (CCK-8) assay
PCa cell proliferation was detected by the CCK-8
assay in accordance with the manufacturer's instructions.
Transfected cells were collected 24 h post-transfection. For the
CCK-8 assay, cells were seeded in 96-well plates at a density of
3×103 cells/well. The CCK-8 assay was performed at 0,
24, 48, 72 and 96 h after incubation. In brief, 10 µl of the CCK-8
reagent (Dojindo Laboratories, Kumamoto, Japan) was added, and the
plate was incubated at 37°C with 5% CO2 for 2 h.
Absorbance at 450 nm wavelength was recorded on a multilabel plate
reader (BioTek Instruments, Inc., Winooski, VT, USA). Each assay
was performed in triplicate and repeated three times.
Matrigel invasion assay
The invasion ability of PCa cells was examined using
Transwell chambers (EMD Millipore, Billerica, MA, USA) coated with
Matrigel (BD Biosciences, San Jose, CA, USA). The transfected cells
were harvested 48 h post-transfection by using 0.2% trypsin/EDTA
solution (Gibco) and washed with fetal bovine serum (FBS)-free
RPMI-1640 medium. A total of 5×104 transfected cells in
FBS-free RPMI-1640 medium were added to the upper chamber.
RPMI-1640 medium supplemented with 10% FBS was then added to the
lower chamber to serve as chemoattractant. After incubation at 37°C
with 5% CO2 for 24 h, the cells attached to the upper
surface of the chamber were removed by a cotton swab. The invasive
cells were fixed with 100% methanol and stained with 0.5% crystal
violet solution (Beyotime Institute of Biotechnology, Haimen,
China). The invasive cells were then photographed and counted in
five randomly selected fields under an inverted microscope (×200;
Olympus, Tokyo, Japan).
Bioinformatics analysis
Potential target genes for miR-136 were predicted
using (http://www.targetscan.org/index.html) and miRanda
(http://www.microrna.org/microrna/).
Luciferase reporter assay
For luciferase reporter assay, the luciferase
plasmids, pmirGLO-MAP2K4-3′-UTR wild-type (Wt1 and 2) and
pmirGLO-MAP2K4-3′-UTR mutant (Mut1 and 2), were chemically
synthesized and confirmed by GenePharma Co., Ltd. Cells were seeded
into 24-well plates at a density of 1.5×105 cells each
well and cultured at 37°C with 5% CO2 for 24 h. miR-136 mimics or
miR-NC was transfected into cells, together with
pmirGLO-MAP2K4-3′-UTR Wt (1 and 2) or pmirGLO-MAP2K4-3′-UTR Mut (1
and 2), using Lipofectamine 2000 according to the manufacturer's
instructions. At 24 h post-transfection, luciferase activities were
measured by using a Dual-Luciferase Reporter Assay system (Promega,
Madison, WI, USA), according to the manufacturer's instructions.
Renilla luciferase activity was used as an internal
control.
Western blot analysis
Total protein was extracted from tissues or cells
using RIPA lysis buffer (Beyotime Institute of Biotechnology,
Jiangsu, China). After centrifugation at 12,000 × g for 15 min at
4°C, a BCA Protein Assay kit (Beyotime Institute of Biotechnology)
was adopted to detect the protein concentration. Equal amounts of
protein was loaded on a 10% sodium dodecyl sulfate-polyacrylamide
gel and transferred to polyvinylidene difluoride membranes (EMD
Millipore). After blocking with 5% non-fat dry milk in
Tris-buffered saline containing 0.05% Tween-20 (TBST) at room
temperature for 2 h, the membranes were incubated at 4°C overnight
with primary antibodies: Mouse anti-human MAP2K4 monoclonal
antibody (sc-136314, 1:1,000 dilution) or mouse anti-human GAPDH
monoclonal antibody (sc-47724, 1:1,000 dilution) (both from Santa
Cruz Biotechnology, Inc., Santa Cruz, CA, USA). Afterwards, the
membranes were washed with TBST for three times and probed with
goat-anti mouse horseradish peroxidase-conjugated secondary
antibody (sc-2005, 1:5,000 dilution; Santa Cruz Biotechnology,
Inc.) at room temperature for 1 h. Finally, the proteins were
visualized using ECL Immunoblot Detection system (Pierce, Rockford,
IL, USA). Densitometry of protein bands was quantified with
Quantity One software (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). GAPDH served as a loading control.
Statistical analysis
Data are presented as the median ± standard
deviation, and analyzed using SPSS software (version 20.0; SPSS
Inc., Chicago, IL, USA). Differences between groups were analyzed
using student's t-test or one-way ANOVA with a Student-Newman-Keuls
post hoc test. The association between miR-136 and MAP2K4
expression levels was determined using Spearman's correlation
analysis. P<0.05 were considered to indicate a statistically
significant difference.
Results
miR-136 is downregulated in PCa
tissues and cell lines
To investigate the expression pattern of miR-136 in
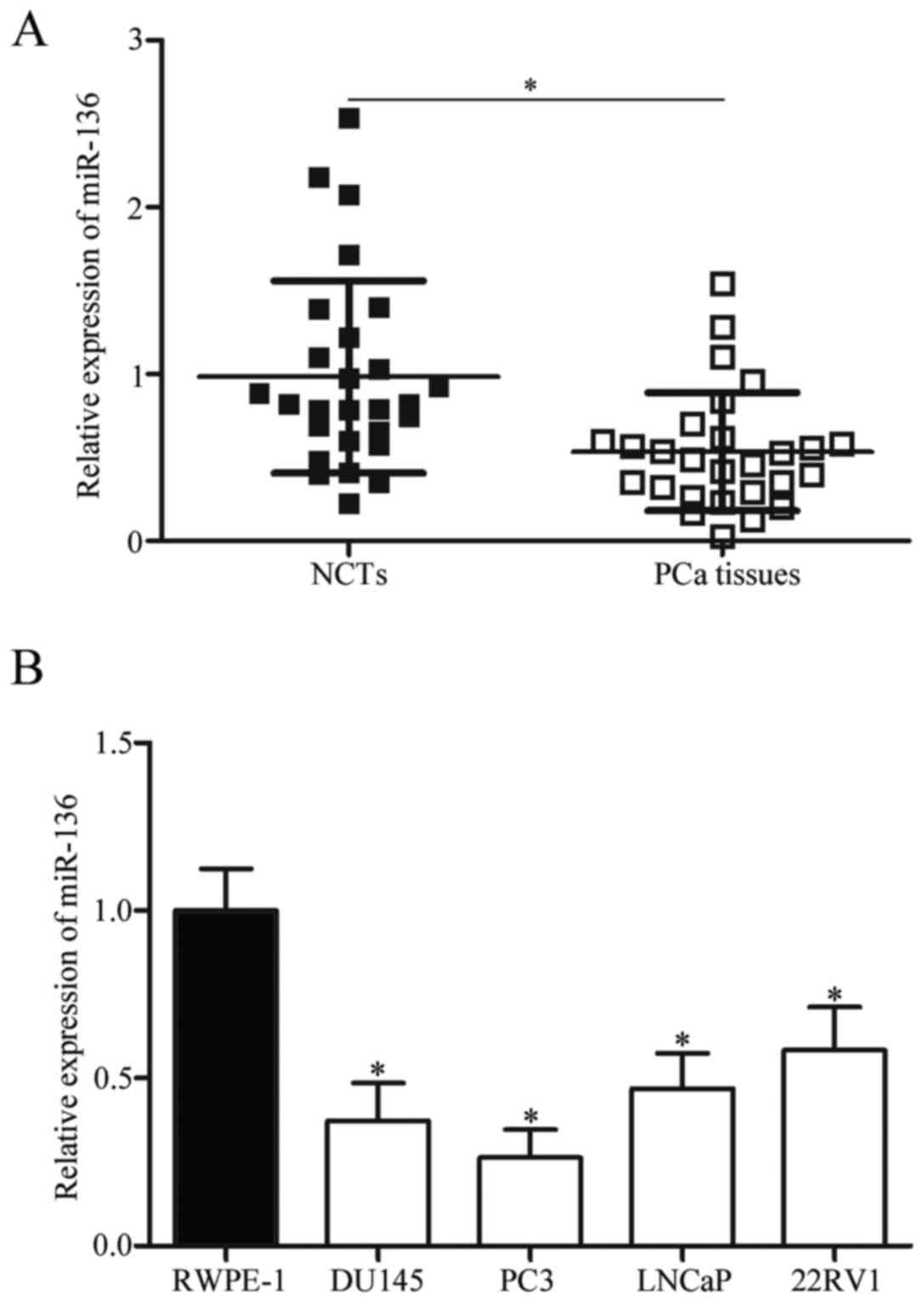
PCa, we firstly measured the expression level of miR-136 in 27
pairs of PCa tissues and NCTs. Results showed that miR-136
expression was downregulated in PCa tissues than in NCTs (Fig. 1A; P<0.05). In addition, miR-136
expression was detected in four PCa cell lines (DU145, PC3, LNCaP
and 22RV1) and a normal prostatic epithelial cell line (RWPE-1).
The expression level of miR-136 was lower in all examined PCa cell
lines than in RWPE-1 (Fig. 1B;
P<0.05). These results suggested that the decreased expression
of miR-136 was likely associated with PCa progression.
miR-136 overexpression inhibits cell
proliferation and invasion in PCa
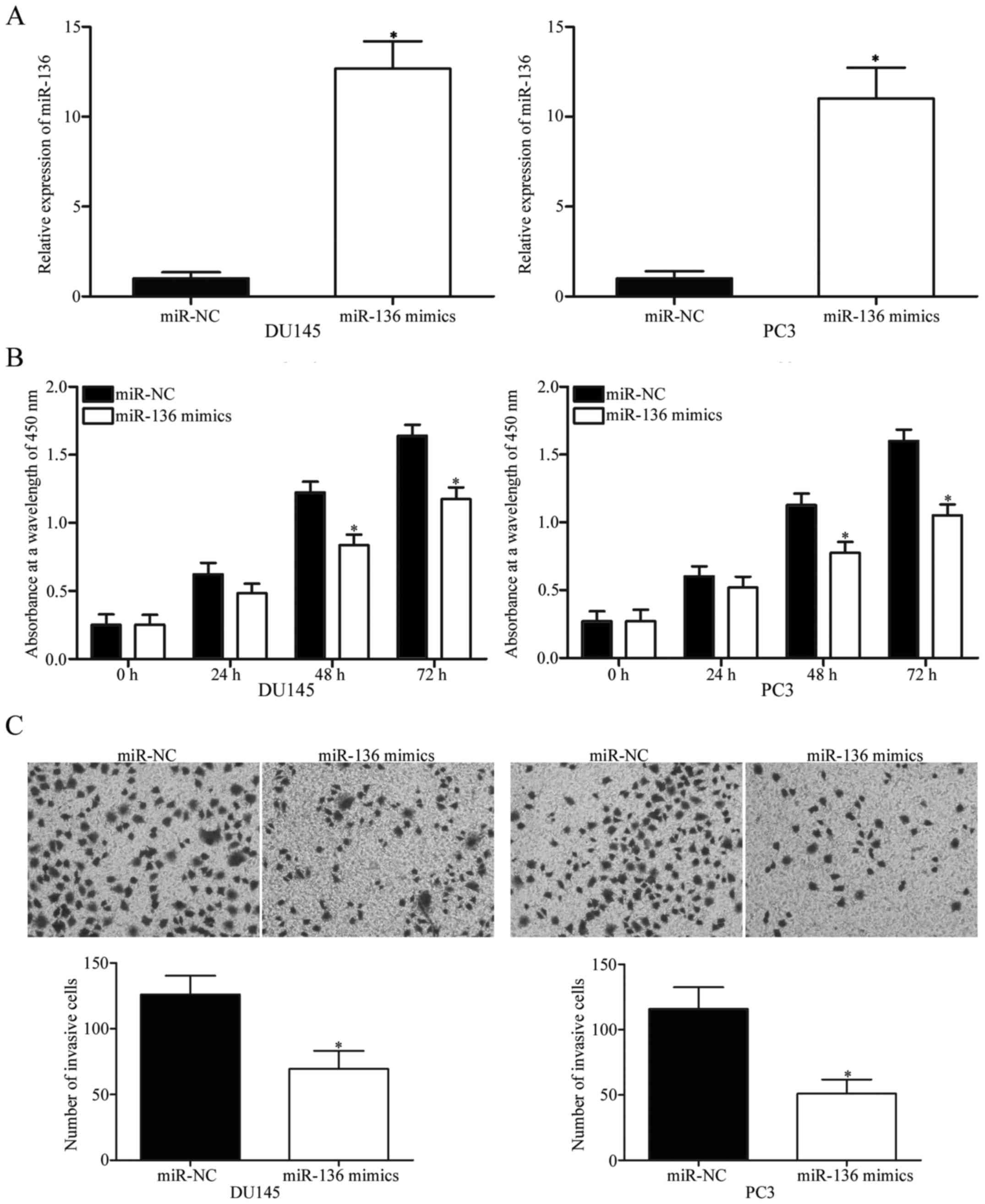
To evaluate the biological roles of miR-136 in PCa,
we transfected DU145 and PC3 cells, which were expressed relatively
lower miR-136 levels, with miR-136 mimics to increase miR-136
expression levels. After transfection, RT-qPCR confirmed that
transfection with miR-136 mimics significantly increases the
miR-136 levels relative to those of the cells transfected with
miR-NC (Fig. 2A; P<0.05).
Subsequently, CCK-8 assay was performed to investigate the effect
of miR-136 overexpression on PCa cell proliferation. Results
revealed that upregulating miR-136 significantly decreases the
growth rate relative to that in the miR-NC-transfected DU145 and
PC3 cells at 48 and 72 h after plating (Fig. 2B; P<0.05). We also examined the
effect of miR-136 on cell invasion ability by using a Matrigel
invasion assay. The ectopic expression of miR-136 decreased the
invasion capacities of both DU145 and PC3 cells relative to those
in the miR-NC group (Fig. 2C;
P<0.05). These results suggest that miR-136 is involved in
regulating PCa progression and may serve as a tumour
suppressor.
MAP2K4 is a direct target of miR-136
in PCa
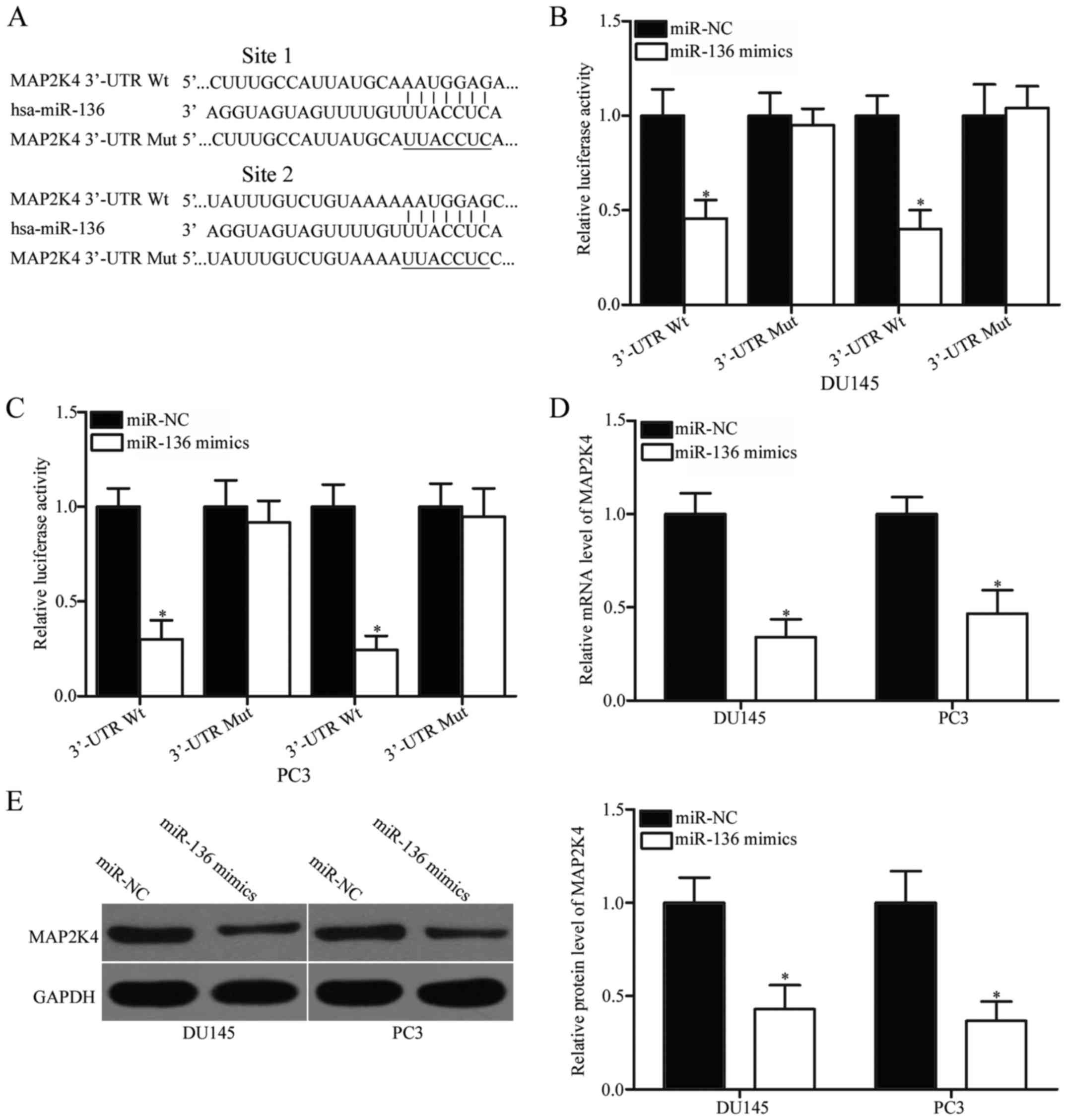
To study the mechanism underlying the
tumour-suppressive roles of miR-136 in PCa, we conducted
bioinformatics analysis to predict the potential targets of the
miRNA. MAP2K4, which is highly expressed in PCa and contributes to
PCa occurrence and development (22–24),
has been predicted as a major target of miR-136 and selected for
further confirmation. The 3′-UTR of MAP2K4 contains two predicted
binding sites for miR-136 (Fig.
3A). To determine whether miR-136 can directly interact with
the 3′-UTR of MAP2K4, we conducted a luciferase reporter assay.
Results showed that miR-136 overexpression significantly repressed
the activity of the MAP2K4-3′-UTR luciferase plasmid containing a
wild-type gene (1 and 2; Fig. 3B and
C; P<0.05). By contrast, no change in luciferase activity
was observed when the miR-136 binding site was mutated (1 and 2).
This observation indicates that miR-136 directly interacted with
the two target regions in the 3′-UTR of MAP2K4. RT-qPCR and western
blotting were performed to illustrate that miR-136 can regulate the
endogenous expression of MAP2K4. Our data showed that the enforced
expression of miR-136 reduced the MAP2K4 expression in DU145 and
PC3 cells at the mRNA (Fig. 3D;
P<0.05) and protein (Fig. 3E;
P<0.05) levels. These findings suggest that MAP2K4 is a direct
target of miR-136 in PCa.
Increased MAP2K4 levels are inversely
associated with miR-136 expression in PCa tissues
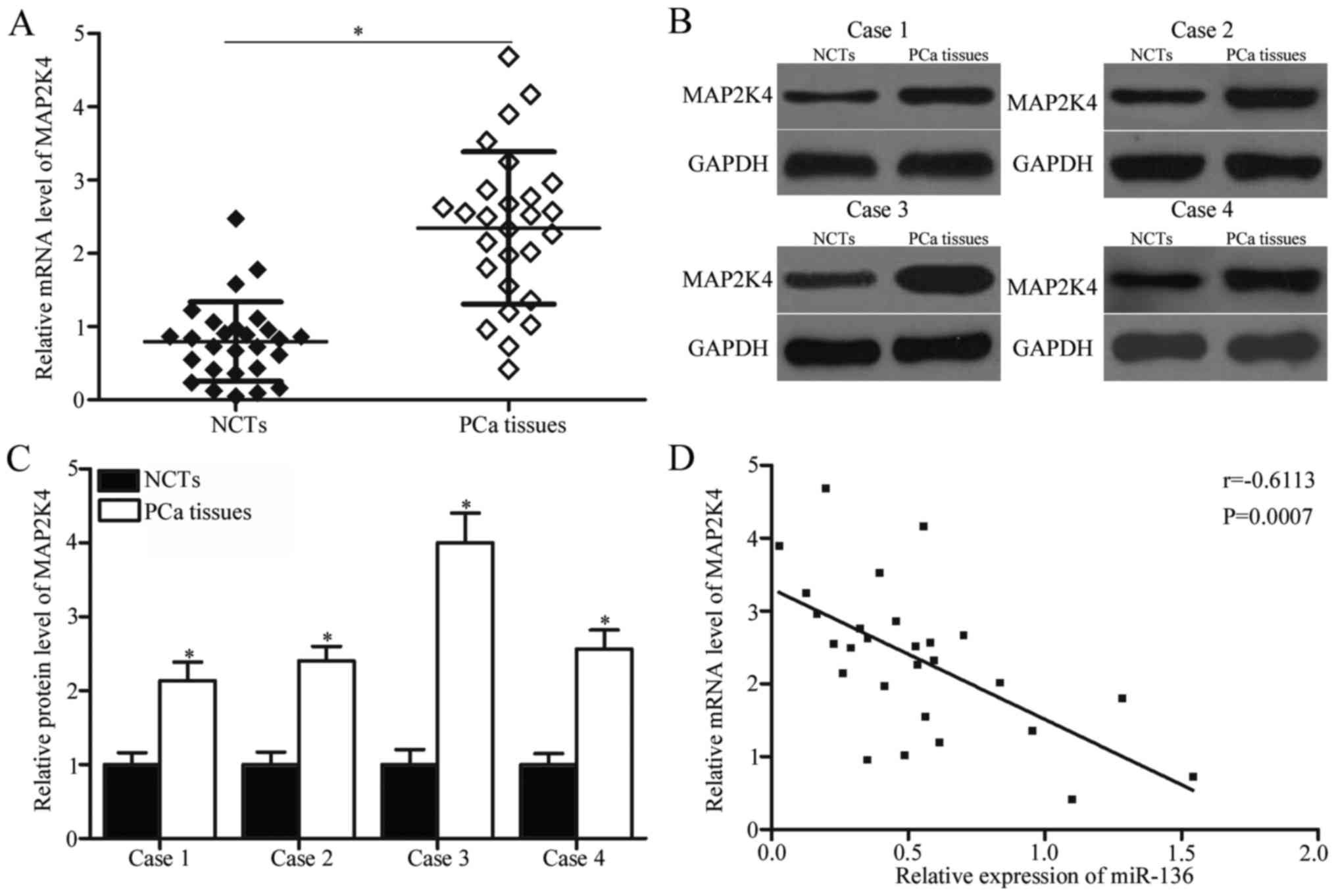
To further explore the relationship between miR-136
and MAP2K4 in PCa, we detected MAP2K4 expression levels in PCa
tissues and NCTs. RT-qPCR results indicated that MAP2K4 mRNA
expression was significantly increased in the PCa tissues than in
the NCTs (Fig. 4A; P<0.05). In
addition, the protein expression level of MAP2K4 was examined in
four randomly selected paired PCa tissues and NCTs. The data of
western blotting revealed that protein level of MAP2K4 was higher
in the PCa tissues than in the NCTs (Fig. 4B and C; P<0.05). A negative
correlation was observed between miR-136 and MAP2K4 mRNA expression
in PCa tissues (Fig. 4D;
r=−0.6113, P=0.0007). This negative correlation suggests that the
upregulation of MAP2K4 in PCa may partially be due to the
downregulation of miR-136.
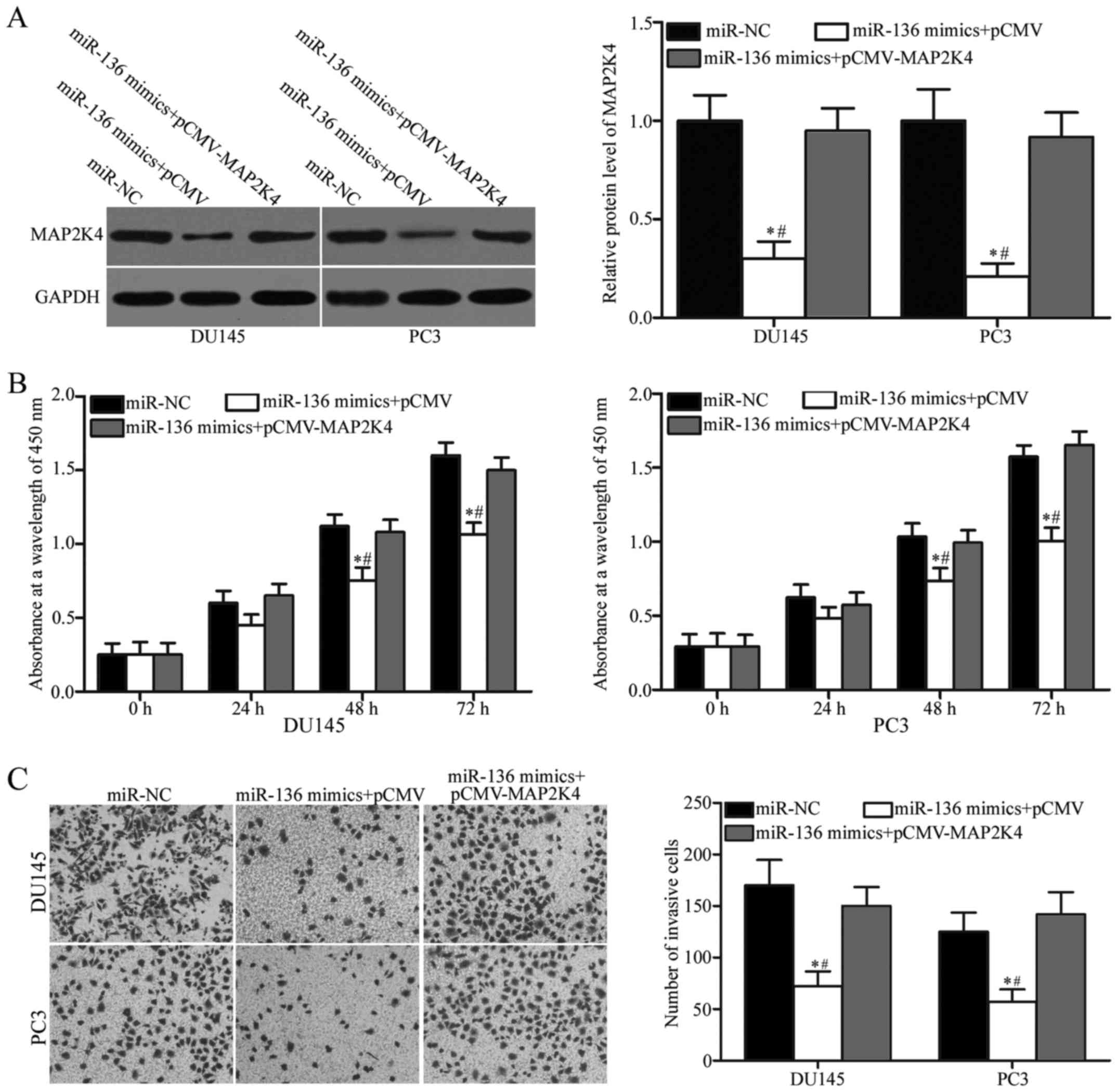
MAP2K4 overexpression reverses miR-136
inhibitory effects on the cell proliferation and invasion of
PCa
To investigate whether miR-136 exerts its tumour
suppressive roles in PCa by regulating MAP2K4, we carried out a
rescue experiment involving DU145 and PC3 cells cotransfected with
miR-136 mimics and pCMV or pCMV-MAP2K4. After transfection, Western
blotting analysis revealed that the reduced expression of MAP2K4
induced by miR-136 mimics can be significantly recovered by
cotransfection with pCMV-MAP2K4 in DU145 and PC3 cells (Fig. 5A; P<0.05). Subsequently,
functional assays showed that restored MAP2K4 expression can
reverse the inhibitory effects of miR-136 overexpression on the
proliferation (Fig. 5B; P<0.05)
and invasion (Fig. 5C; P<0.05)
of DU145 and PC3 cells. These results clearly demonstrated that
miR-136 can regulate the malignant biological behaviour of PCa
cells by inhibiting MAP2K4.
Discussion
Accumulated evidence shows that PCa initiation and
progression are controlled by miRNAs (25–27).
Therefore, investigating PCa-related miRNAs may provide novel
biomarkers for diagnosing and treating the patients with this
disease. In the present study, we found that miR-136 was obviously
downregulated in PCa tissues and cell lines. Upregulation of
miR-136 suppressed the cell proliferation and invasion of PCa.
Additionally, MAP2K4 was validated as a direct target of miR-136 in
PCa. MAP2K4 was upregulated in PCa tissues, and its expression
levels were inversely correlated with those of miR-136. Moreover,
restored MAP2K4 expression rescued the miR-136 inhibitory effects
on the cell proliferation and invasion of PCa. Therefore, our
present data suggest that miR-136 is a brake for the rapid growth
and invasion of PCa.
miR-136 dysregulation has been reported in multiple
human cancer types. For example, miR-136 was downregulated in
gastric cancer tissues and cell lines. Meanwhile, decreased miR-136
expression was significantly associated with increased peritoneal
metastasis and worsened prognosis in patients with gastric cancer
(18). In triple-negative breast
cancer, the expression level of miR-136 is lower in tumour tissues
than in normal tissues and negatively correlated with increasing
World Health Organisation grades (19). In glioma, miR-136 was mildly
expressed and significantly associated with malignancy grade.
Log-rank testing indicated that glioma patients with low miR-136
expression exhibited poorer prognosis than those with high miR-136
levels (20). In ovarian cancer,
miR-136 expression was decreased in tumour tissues than in the
normal control tissues. Low miR-136 expression was associated with
poor overall survival in patients with ovarian cancer (28). miR-136 downregulation was observed
in colon cancer (29) and melanoma
(30). However, in nonsmall-cell
lung cancer, miR-136 expression was significantly upregulated in
primary tumours and cell lines (31). These conflicting findings suggest
that the expression pattern of miR-136 is tissue specific, and this
miRNA may be developed as a prognostic marker in cancer.
miR-136 plays tumour-suppressive roles in several
tumour types. For instance, Zheng et al found that
upregulating miR-136 expression inhibits the peritoneal metastasis
of gastric cancer in vitro and in vivo (18). Meanwhile, Yan et al reported
that the resumption of expression of miR-136 decreases cell
migration and invasion in triple-negative breast cancer (19). Multiple studies showed that miR-136
overexpression promotes cell apoptosis (32), increases chemosensitivity (33) to temozolomide and reverses
cisplatin resistance (20) in
glioma. Yuan et al revealed that restoring miR-136
expression attenuates cell proliferation and invasion in colon
cancer (29). Wang et al
indicated that miR-136 overexpression suppresses melanoma cell
growth, metastasis, and epithelial-mesenchymal transition as well
as induces apoptosis in vitro (30). Jeong et al (28) and Zhao et al (34) demonstrated that enforced miR-136
expression represses cell viability, proliferation, cancer stem
cell spheroid formation and angiogenesis; promotes apoptosis; and
reduces the chemoresistance to cisplatin in ovarian cancer.
Nevertheless, miR-136 plays an oncogenic role in nonsmall-cell lung
cancer by regulating anchorage-dependent and -independent
proliferation (31). These
contradicting findings indicated that miR-136 acts as a tumour
suppressor in certain cancers and an oncogene in others.
Several targets of miR-136 have been identified,
including HOXC10 in gastric cancer; RASAL2 (19) in breast cancer; E2F1 (20), AEG-1 (32,33)
and Bcl-2 in glioma; PPP2R2A (31)
in nonsmall-cell lung cancer; LRH-1 (29) in colon cancer; PMEL (30) in melanoma; and Notch3 (28) in ovarian cancer. In the present
study, MAP2K4 was demonstrated to be a novel target of miR-136 in
PCa. MAP2K4, located on chromosome 17, is a member of the MAPK
signalling pathway (35). A large
body of evidence has indicated that MAP2K4 is aberrantly expressed
in numerous tumours, such as osteosarcoma (36), tongue squamous cell carcinoma
(37), breast cancer (38) and pancreatic cancer (39). MAP2K4 is involved in various
pathophysiological processes of tumourigenesis and tumour
development, including cell proliferation, cycle, apoptosis,
invasion and metastasis (36,39–41).
In PCa, MAP2K4 is overexpressed in tumour tissues. Increased MAP2K4
expression is correlated with raised pathological stage at
prostatectomy (24). Upregulating
MAP2K4 promoted cell proliferation and metastasis while inhibiting
G1-S phase arrest and cell apoptosis in PCa (22,23).
With the emerging correlation of MAP2K4 with aggressive PCa
progression, MAP2K4 may be a promising therapeutic target for
treating patients with PCa.
In conclusion, the present study proved that miR-136
is downregulated in PCa tissues and cell lines. miR-136
overexpression inhibited PCa cell proliferation and invasion by
directly targeting MAP2K4. Our current results demonstrated that
using miR-136/MAP2K4-based reagents may be a novel therapeutic
approach for patients with PCa.
References
|
1
|
Peyromaure EM, Mao K, Sun Y, Xia S, Jiang
N, Zhang S, Wang G, Liu Z and Debré B: A comparative study of
prostate cancer detection and management in China and in France.
Can J Urol. 16:4472–4477. 2009.PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu D, Zhou Y, Pan H, Qu P and Zhou J:
MicroRNA99a inhibits cell proliferation, colony formation ability,
migration and invasion by targeting fibroblast growth factor
receptor 3 in prostate cancer. Mol Med Rep. 11:1469–1475. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rubin MA, Maher CA and Chinnaiyan AM:
Common gene rearrangements in prostate cancer. J Clin Oncol.
29:3659–3668. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Deng X, He G, Liu J, Luo F, Peng X, Tang
S, Gao Z, Lin Q, Keller JM, Yang T and Keller ET: Recent advances
in bone-targeted therapies of metastatic prostate cancer. Cancer
Treat Rev. 40:730–738. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Souza AG, Bastos VAF, Silva IBB, Marangoni
K and Goulart VA: Different gene therapy strategies: A overview for
prostate cancer. Curr Gene Ther. 16:287–291. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Koupparis A and Gleave ME: Multimodal
approaches to high-risk prostate cancer. Curr Oncol. 17 Suppl
2:S33–S37. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee YS and Dutta A: MicroRNAs: Small but
potent oncogenes or tumor suppressors. Curr Opin Investig Drugs.
7:560–564. 2006.PubMed/NCBI
|
|
11
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Krol J, Loedige I and Filipowicz W: The
widespread regulation of microRNA biogenesis, function and decay.
Nat Rev Genet. 11:597–610. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fazi F and Blandino G: MicroRNAs: Non
coding pleiotropic factors in development, cancer prevention and
treatment. Microrna. 2:812013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Massillo C, Dalton GN, Farré PL, De Luca P
and De Siervi A: Implications of microRNA dysregulation in the
development of prostate cancer. Reproduction. 154:R81–R97. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Volinia S, Calin GA, Liu CG, Ambs S,
Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, et
al: A microRNA expression signature of human solid tumors defines
cancer gene targets. Proc Natl Acad Sci USA. 103:pp. 2257–2261.
2006; View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Spizzo R, Nicoloso MS, Croce CM and Calin
GA: SnapShot: MicroRNAs in cancer. Cell. 137:586. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zheng J, Ge P, Liu X, Wei J, Wu G and Li
X: miR-136 inhibits gastric cancer-specific peritoneal metastasis
by targeting HOXC10. Tumour Biol. 39:10104283177062072017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yan M, Li X, Tong D, Han C, Zhao R, He Y
and Jin X: miR-136 suppresses tumor invasion and metastasis by
targeting RASAL2 in triple-negative breast cancer. Oncol Rep.
36:65–71. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen W, Yang Y, Chen B, Lu P, Zhan L, Yu
Q, Cao K and Li Q: miR-136 targets E2F1 to reverse cisplatin
chemosensitivity in glioma cells. J Neurooncol. 120:43–53. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C (T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pavese JM, Ogden IM, Voll EA, Huang X, Xu
L, Jovanovic B and Bergan RC: Mitogen-activated protein kinase
kinase 4 (MAP2K4) promotes human prostate cancer metastasis. PLoS
One. 9:e1022892014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wan X, Huang W, Yang S, Zhang Y, Zhang P,
Kong Z, Li T, Wu H, Jing F and Li Y: Androgen-induced miR-27A acted
as a tumor suppressor by targeting MAP2K4 and mediated prostate
cancer progression. Int J Biochem Cell Biol. 79:249–260. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lotan TL, Lyon M, Huo D, Taxy JB, Brendler
C, Foster BA, Stadler W and Rinker-Schaeffer CW: Up-regulation of
MKK4, MKK6 and MKK7 during prostate cancer progression: An
important role for SAPK signalling in prostatic neoplasia. J
Pathol. 212:386–394. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sun Y, Jia X, Hou L and Liu X: Screening
of differently expressed miRNA and mRNA in prostate cancer by
integrated analysis of transcription data. Urology. 94:313.e1–e6.
2016. View Article : Google Scholar
|
|
26
|
Wang Z, Xu L, Hu Y, Huang Y, Zhang Y,
Zheng X, Wang S, Wang Y, Yu Y, Zhang M, et al: miRNA let-7b
modulates macrophage polarization and enhances tumor-associated
macrophages to promote angiogenesis and mobility in prostate
cancer. Sci Rep. 6:256022016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li JZ, Li J, Wang HQ, Li X, Wen B and Wang
YJ: miR-141-3p promotes prostate cancer cell proliferation through
inhibiting kruppel-like factor-9 expression. Biochem Biophys Res
Commun. 482:1381–1386. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jeong JY, Kang H, Kim TH, Kim G, Heo JH,
Kwon AY, Kim S, Jung SG and An HJ: MicroRNA-136 inhibits cancer
stem cell activity and enhances the anti-tumor effect of paclitaxel
against chemoresistant ovarian cancer cells by targeting Notch3.
Cancer Lett. 386:168–178. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yuan Q, Cao G, Li J, Zhang Y and Yang W:
MicroRNA-136 inhibits colon cancer cell proliferation and invasion
through targeting liver receptor homolog-1/Wnt signaling. Gene.
628:48–55. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang JJ, Li ZF, Li XJ, Han Z, Zhang L and
Liu ZJ: Effects of microRNA-136 on melanoma cell proliferation,
apoptosis, and epithelial-mesenchymal transition by targetting PMEL
through the Wnt signaling pathway. Biosci Rep. 37:BSR201707432017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shen S, Yue H, Li Y, Qin J, Li K, Liu Y
and Wang J: Upregulation of miR-136 in human non-small cell lung
cancer cells promotes Erk1/2 activation by targeting PPP2R2A.
Tumour Biol. 35:631–640. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang Y, Wu J, Guan H, Cai J, Fang L, Li J
and Li M: MiR-136 promotes apoptosis of glioma cells by targeting
AEG-1 and Bcl-2. FEBS Lett. 586:3608–3612. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wu H, Liu Q, Cai T, Chen YD, Liao F and
Wang ZF: MiR-136 modulates glioma cell sensitivity to temozolomide
by targeting astrocyte elevated gene-1. Diagn Pathol. 9:1732014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhao H, Liu S, Wang G, Wu X, Ding Y, Guo
G, Jiang J and Cui S: Expression of miR-136 is associated with the
primary cisplatin resistance of human epithelial ovarian cancer.
Oncol Rep. 33:591–598. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yoshida BA, Dubauskas Z, Chekmareva MA,
Christiano TR, Stadler WM and Rinker-Schaeffer CW:
Mitogen-activated protein kinase kinase 4/stress-activated
protein/Erk kinase 1 (MKK4/SEK1), a prostate cancer metastasis
suppressor gene encoded by human chromosome 17. Cancer Res.
59:5483–5487. 1999.PubMed/NCBI
|
|
36
|
Tesser-Gamba F, Petrilli AS, de Seixas
Alves MT, Filho RJ, Juliano Y and Toledo SR: MAPK7 and MAP2K4 as
prognostic markers in osteosarcoma. Hum Pathol. 43:994–1002. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wu X, Gong Z, Sun L, Ma L and Wang Q:
MicroRNA-802 plays a tumour suppressive role in tongue squamous
cell carcinoma through directly targeting MAP2K4. Cell Prolif.
50:2017. View Article : Google Scholar
|
|
38
|
Liu S, Liu YY and Li R: Expressions of
MAP2K4 and estrogen receptor and their clinical significance in
invasive breast cancer. Nan Fang Yi Ke Da Xue Xue Bao. 37:488–493.
2016.(In Chinese). PubMed/NCBI
|
|
39
|
Wang L, Pan Y and Dai JL: Evidence of MKK4
pro-oncogenic activity in breast and pancreatic tumors. Oncogene.
23:5978–5985. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yeasmin S, Nakayama K, Rahman MT, Rahman
M, Ishikawa M, Katagiri A, Iida K, Nakayama N and Miyazaki K: MKK4
acts as a potential tumor suppressor in ovarian cancer. Tumour
Biol. 32:661–670. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ishikawa M, Nakayama K, Rahman MT, Rahman
M, Katagiri A, Iida K and Miyazaki K: Functional and
clinicopathological analysis of loss of MKK4–– expression in
endometrial cancer. Oncology. 79:238–246. 2010. View Article : Google Scholar : PubMed/NCBI
|