Introduction
Lung carcinoma is the leading cancer mortality
worldwide (1). The most common
lung cancer is non-small cell lung carcinoma (NSCLC), which differs
from small cell lung carcinoma (SCLC) in its histological and
cytological features (2). At
present, the treatment of NSCLC is very limited, including
chemotherapy, radiation therapy, surgical resection and targeted
therapy (3). The prognosis for
lung cancer is unfavorable, and novel therapeutic strategies to
improve the outcome of patients with NSCLC are required.
Traditional Chinese medicine is a source of novel compounds with
antineoplastic activity, including isocryptotanshinone, mangiferin
and xanthatin (4–6). Tetrahydrocurcumin (THC) is a
traditional Chinese medicine isolated from Curcuma wenyujin
(Chen & Ling, 1981). THC has a β-diketone structure and two
phenolic groups (Fig. 1) and has
anti-inflammatory, antioxidative and antitumor properties (7–12);
treatment of NSCLC via THC requires further investigation.
Autophagy is important for cellular functioning and
has a key role in various physiological and pathological processes
(13). In carcinoma, processes
with both promotional and suppression properties are considered a
double-edged sword (14). The
phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt)/mechanistic
target of rapamycin (mTOR) molecular signaling pathway is important
for autophagy. Activation of PI3K and mTOR leads to an increase in
the level of phosphorylated (p)-Akt, which can integrate upstream
signals active via the PI3K/Akt signaling pathway and
phosphorylation, suppressing autophagy (15,16).
In order to evaluate the clinical use of THC, the present study
investigated the antineoplastic mechanisms of THC in A549 cells.
The findings indicate that the PI3K/Akt/mTOR molecular signaling
pathway is involved in THC-induced autophagy.
Materials and methods
Chemicals and reagents
THC (Hangzhou Haoxin Biotech, Co., Ltd., Hangzhou,
China) was dissolved at a concentration of 200 µM in absolute
dimethyl sulfoxide (DMSO) as a stock solution, which was stored at
4°C and was diluted with DMSO for the subsequent experiments. DMSO,
tetramethylethylenediamine, ammonium persulfate, and acridine
orange were purchased from Sigma-Aldrich, Merck Millipore
(Darmstadt, Germany). RPMI-1640 medium, fetal bovine serum (FBS),
penicillin and streptomycin antibiotics, and phosphate-buffered
saline (PBS) were purchased from Gibco; Thermo Fisher Scientific,
Inc. (Waltham, MA, USA). Tris-HCl and 4xTris-HCl were purchased
from Shanghai Sangon Biotech Co., Ltd. (Shanghai, China).
Additionally, 30% acrylamide/sis solution (30%) and sodium dodecyl
sulfate (SDS) were purchased from Bio-Rad Laboratories, Inc.
(Shanghai, China). Tris and Glycine were purchased from Amresco,
LLC (Solon, OH, USA). Tween-20, methanol, and sodium chloride were
purchased from Sinopharm Chemical Reagent Co. Ltd. (Shanghai,
China). SDS-polyacrylamide gel electrophoresis (PAGE) protein
loading buffer (5X), cell lysis buffer for western blotting and
immunoprecipitation, phenylmethanesulfonyl fluoride, an ECL Plus
Luminescence kit, and a BCA Protein Assay kit were purchased from
Beyotime Institute of Biotechnology (Nantong, China). The Cell
Counting Kit-8 (CCK-8), glyceraldehyde 3-phosphate dehydrogenase
(GAPDH, MAB5465), goat anti-rabbit immunoglobulin G (IgG; GAR0072),
and goat anti-mouse IgG (GAM007) secondary antibodies were
purchased from MultiSciences Biotech Co., Ltd. (Shanghai, China). A
high-purity total RNA rapid extraction kit was purchased from
Generay Biotech Co., Ltd. (Shanghai, China). A PrimeScript™ RT
reagent kit was purchased from TaKaRa (Dalian, China). SuperReal
PreMix Color (SYBR® Green) was purchased from Tiangen
Biotech Co., Ltd. (Beijing, China). Akt (cat. no. 4691S), p-Akt
(cat. no. 4060S), mTOR (cat. no. 2983S), p-mTOR (cat. no. 5536S),
light chain (LC)3 I/II (cat. no. 4108S), p62 (cat. no. 13121S),
PI3K (cat. no. 4249P), and p-PI3K (cat. no. 4228S) antibodies were
purchased from Cell Signaling Technology, Inc. (Shanghai,
China).
Cell lines and cell culture
The A549 human lung adenocarcinoma epithelial cell
line was purchased from the Cell Bank of Type Culture Collection of
Chinese Academy of Sciences (Shanghai, China). The cells were
cultured in RPMI-1640 supplemented with 10% FBS, penicillin G (100
U) and streptomycin (100 µg/ml). Cells were incubated in a
humidified atmosphere containing 5% CO2 at 37°C.
Cell viability assay
The CCK-8 assay was used to quantify the effect of
THC on the cell viability of A549 cells. Cells were seeded in
96-well plates (5×103 cells/well) in 90 µl medium for 24
h. The cells were subsequently treated cells with 10 µl of 0, 30,
70, 100 or 130 µM THC for 12, 24, 48 and 72 h. Following the THC
treatment, 10 µl CCK-8 was added to each well and incubated for an
additional 2 h at 37°C. The present study quantified cell viability
at 490 nm using a plate reader (Molecular Devices, LLC, Sunnyvale,
CA, USA).
Flow cytometry
To verify the effect of THC on autophagy in A549
cells, the present study quantified cellular autophagy using flow
cytometry. A549 cells were seeded in 6-well plates
(3×105 cells/well) for 24 h and treated with 0, 10, 30,
70, 100 or 130 µM THC for 12, 24, 48, and 72 h. Cells were
harvested, washed twice with cold PBS, and stained for 15 min at
4°C with acridine orange. The fluorescence intensity of the cells
was detected using channels FL1 and FL3 of the flow cytometer (BD
Biosciences, Franklin Lakes, NJ, USA). The analyses were repeated
three times. The autophagy rate (%) was calculated as follows:
Autophagy rate (%)=(FL3/FL1) ×100.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Using TRIzol reagent, the present study treated
total mRNA from the A549 cells with 0, 30, 70, 100 and 130 µmol/l
THC for 24 h at 37°C. Following the THC treatment, the mRNA
expression levels in A549 cells were determined using qPCR assay on
a CFX Connect Real-Time PCR system (Bio-Rad Laboratories, Inc.).
The present study extracted total RNA using a high-purity total RNA
rapid extraction kit and synthesized the cDNA using a PrimeScript™
RT reagent kit; in an ice bath the RT reaction was mixed and then
reacted at 37°C for 15 min and then at 85°C for 5 sec. The reaction
was then stopped and stored at 4°C until use. Each sample was run
in triplicate in a final volume of 20.0 µl containing 10 µl 2X
SuperReal Color PreMix, 1 µl forward primer (10 µM), 1 µl reverse
primer (10 µM) and 8 µl cDNA (β-actin forward,
TGACGTGGACATCCGCAAAG, reverse, CTGGAAGGTGGACAGCGAGG; Beclin-1
forward, AAACCAGATGCGTTATGCCC and reverse, GCGACC CAGCCTGAAGTTAT).
Cycling conditions for the qPCR were as follows: 1 cycle at 95°C
for 15 min followed by 40 cycles at 95°C for 10 sec, 57°C for 15
sec, and 72°C for 15 sec. At the end of each reaction, a melting
curve analysis was performed. β-actin was used as the reference
gene, and the 2−ΔΔCq method was used to determine the
relative expression of each gene (17).
Western blot analysis
A549 cells (2×105 cells/plate) were
treated with 0, 30, 70, 100, and 130 µmol/l THC for 24 h at 37°C
and lysed in cell lysis buffer for western blotting and
immunoprecipitation. Proteins were quantified using the BCA Protein
Assay kit. Protein (30 µg/lane) were subjected to 12% SDS-PAGE and
transferred to a polyvinylidene fluoride membranes. The membranes
were blocked with 5% non-fat milk for 1 h, followed by overnight
incubation at 4°C with the following primary antibodies: Akt,
p-Akt, LC3 I/II, mTOR, p-mTOR, p62, p-PI3K, and PI3K (all 1:1,000).
Subsequently, the membranes were incubated with the corresponding
horseradish-peroxidase secondary antibody, goat anti-rabbit IgG,
and goat anti-mouse IgG (all 1:5,000) for 1 h at room temperature.
The blots were developed and visualized using the ECL Plus
Luminescence kit and ChemiDoc XRS+ System (Bio-Rad
Laboratories).
Statistical analysis
Data are presented as the mean ± standard deviation.
Student's t-test and one-way analysis of variance followed by a
Tukey's post hoc test were used to analyze the data. Differences
between experimental groups were assessed using SPSS version 19.0
(IBM Corporation, Armonk, NY, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
THC promotes cell proliferation
inhibition
To evaluate the effects of THC on cell
proliferation, A549 cells were treated with 0, 30, 70, 100, and 130
µM THC for 12, 24, 48 and 72 h. Cell viability was determined using
a CCK-8 assay. A representative micrograph is presented in Fig. 2A (magnification, ×40). The
inhibitory effect increased with higher THC concentration at 12 and
24 h. THC inhibited cell growth in a dose-dependent manner at each
time point (Fig. 2B).
THC induces autophagy in A549
cells
The aforementioned findings suggested that THC
induced an autophagic response in A549 cells. Flow cytometry
determined that THC-induced autophagy in A549 cells (Fig. 3).
THC increases Beclin-1 expression
Beclin-1 is associated with genes involved in the
formation of autophagosomes and may influence the rate of
autophagy. Following THC treatment in A549 cells, a dose-dependent
increase in Beclin-1 expression was observed (P<0.05; Table I, Fig.
4).
 | Table I.Expression data for Beclin-1 at 12 and
24 h (n=5, mean ± standard deviation). |
Table I.
Expression data for Beclin-1 at 12 and
24 h (n=5, mean ± standard deviation).
|
| THC treatment |
|---|
|
|
|
|---|
| Time | CK | 30 µM | 70 µM | 100 µM | 130 µM |
|---|
| 12 h | 0.98±0.04 | 1.06±0.03 | 1.45±0.04 |
2.28±0.03a |
2.92±0.08a |
| 24 h | 0.98±0.03 | 1.04±0.08 | 1.34±0.11 |
2.17±0.13a |
2.74±0.05a |
THC-induced autophagy involves
inhibition of the PI3K/Akt/mTOR signaling pathway
The PI3K/Akt/mTOR molecular signaling pathway is
involved in autophagy regulation, and suppression of the
PI3K/Akt/mTOR pathway promotes autophagy. To examine the role of
this pathway in THC-induced autophagy, the present study quantified
the expression of mTOR, p-mTOR, Akt, p-Akt LC3-II/LC3-I, p62, PI3K,
and p-PI3K using western blotting. Treatment with THC for 24 h led
to a significant reduction in levels of p-mTOR, p-Akt and p62
compared to treatment for 12 h (Fig.
5). These findings indicate that the PI3K/Akt/mTOR pathway is
involved in THC-induced autophagic cell proliferation inhibition
via inhibition of Akt and mTOR phosphorylation.
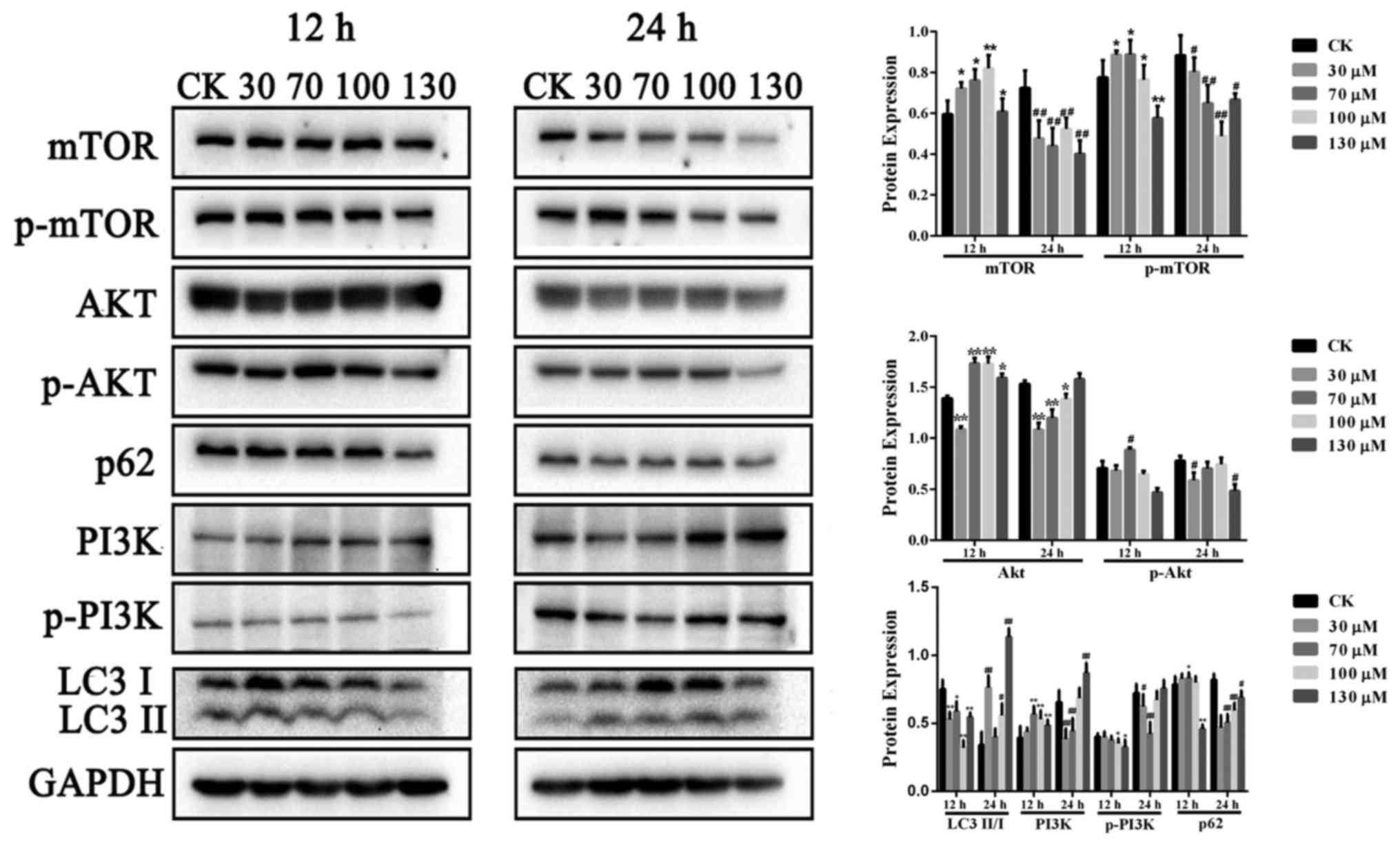 | Figure 5.Expression levels of p-mTOR, p-Akt,
p-PI3K, LC3I/II and p62 in the THC 24 h treatment group were
markedly reduced when compared with the 12 h. Treatment with THC
for 24 h led to a significant reduction in levels of p-mTOR, p-Akt,
and p62 compared to treatment for 12 h. *P<0.05 vs. 24 h CK
group, **P<0.01vs. 24 h CK group, #P<0.05 vs. 24 h
CK group and ##P<0.01 vs. 24 h CK group; THC,
tetrahydrocurcumin; p, phosphorylated; mTOR, mechanistic target of
rapamycin; Akt, protein kinase B; PI3K, phosphoinositide 3-kinase;
LC3, light chain 3I/II. |
Discussion
THC has anti-inflammatory, antioxidative and
antitumor effects (7–12). Previous studies have revealed that
THC inhibits the invasion of breast cancer (10,18).
The present study confirmed the suppressive effect of THC on A549
lung cancer cells. THC inhibits the proliferation of A549 cells by
inducing autophagy via the PI3K/Akt/mTOR signaling pathway
(Fig. 6).
The present study revealed a potential novel
molecular mechanism for the antitumor effects of THC in A549 cells.
Treatment of A549 cells with THC led to increased autophagy.
Concurrently, activation of the PI3K/Akt/mTOR molecular pathway was
inhibited by THC, which indicates that activation of the
PI3K/Akt/mTOR pathway may be associated with autophagy. This is
consistent with the previously reported mechanism of action of THC
in the literature (19). Wu et
al determined that THC induced autophagic cell death via the
PI3K/Akt/mTOR and MAPK molecular signaling pathways in HL-60 human
leukemia cells (19). Therefore,
it may be concluded that THC-induced autophagic cell proliferation
inhibition is the predominant mechanism underlying inhibition of
A549 cell proliferation. This conclusion may contribute to the
future development and clinical application of curcumin antitumor
drugs.
LC3 has been previously used as a specific marker to
monitor autophagy and LC3-II (the conjugated form of LC3) is highly
correlated with autophagosome number (20,21).
p62 is a selective autophagy substrate (22). The present study determined that
THC induction activated the levels of the autophagy-associated
protein LC3II/I, reduction in autophagy for the decreasing p62
expression. This suggested that THC inhibition of A549 lung cancer
cells may be achieved through the autophagy pathway.
The PI3K/AKT/mTOR molecular signaling cascade
regulates cell autophagy. As an important member of the
PI3K-associated kinase family, mTOR is associated with cell
proliferation and metabolism. Previous studied suggested that
suppression of Akt and downstream target protein mTOR activation
may induce autophagy (23–25). The PI3K/Akt/mTOR pathway has a key
role in the pathogenesis of NSCLC. Therefore, inhibition of
PI3K/Akt/mTOR pathway activation may be a suitable therapeutic
target for future lung cancer treatments (26,27).
The findings of the present study revealed that THC may induce the
antitumor effects of autophagy in A549 cells by reducing activation
of the PI3K/Akt/mTOR pathway, which suggests that THC is a
potential treatment for lung cancer.
In conclusion, THC induced autophagic cell
proliferation inhibition in NSCLC cells via suppression of the
PI3K/Akt/mTOR molecular signaling pathway. To the best of our
knowledge the current findings present a novel anticancer mechanism
of THC.
Acknowledgements
Dr. Sun Wei (Department of Pharmacy, The Second
Affiliated Hospital and Yuying Children's Hospital of Wenzhou
Medical University, Wenzhou, China) revised and analyzed the
manuscript.
Funding
The current study was supported by the Zhejiang
Science and Technology Department Public Service Technology
Application Project (grant no. 2016C33SA510004), Huzhou Science and
Technology Project (grant no. 2016GY52) and Zhejiang Medical
Association Foundation (grant no. 2016ZYC-A70).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GS, HL and BL conceived and designed the experiments
and acquired reagents, materials, and/or analysis tools. HL, FC,
YW, WF and WS performed the experiments. HL and BL analyzed the
data. GS and BL produced the manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Absenger G, Terzic J and Bezan A: ASCO
update: Lung cancer. Memo. 10:224–227. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Guo S, Luo W, Liu L, Pang X, Zhu H, Liu A,
Lu J, Ma DL, Leung CH, Wang Y and Chen X: Isocryptotanshinone, a
STAT3 inhibitor, induces apoptosis and pro-death autophagy in A549
lung cancer cells. J Drug Target. 24:934–942. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shi W, Deng J, Tong R, Yang Y, He X, Lv J,
Wang H, Deng S, Qi P, Zhang D and Wang Y: Molecular mechanisms
underlying mangiferin-induced apoptosis and cell cycle arrest in
A549 human lung carcinoma cells. Mol Med Rep. 13:3423–3432. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang L, Ruan J, Yan L, Li W, Wu Y, Tao L,
Zhang F, Zheng S, Wang A and Lu Y: Xanthatin induces cell cycle
arrest at G2/M checkpoint and apoptosis via disrupting NF-κB
pathway in A549 non-small-cell lung cancer cells. Molecules.
17:3736–3750. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chong L, Zhang W, Nie Y, Yu G, Liu L, Lin
L, Wen S, Zhu L and Li C: Protective effect of curcumin on acute
airway inflammation of allergic asthma in mice through Notch1-GATA3
signaling pathway. Inflammation. 37:1476–1485. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kukongviriyapan U, Apaijit K and
Kukongviriyapan V: Oxidative stress and cardiovascular dysfunction
associated with cadmium exposure: Beneficial effects of curcumin
and tetrahydrocurcumin. Tohoku J Exp Med. 239:25–38. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ji JL, Huang XF and Zhu HL: Curcumin and
its formulations: Potential anti-cancer agents. Anticancer Agents
Med Chem. 12:210–218. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Han X, Deng S, Wang N, Liu Y and Yang X:
Inhibitory effects and molecular mechanisms of tetrahydrocurcumin
against human breast cancer MCF-7 cells. Food Nutr Res.
60:306162016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yoysungnoen B, Bhattarakosol P, Changtam C
and Patumraj S: Effects of tetrahydrocurcumin on tumor growth and
cellular signaling in cervical cancer xenografts in nude mice.
Biomed Res Int 2016. 2016.doi: 10.1155/2016/1781208. View Article : Google Scholar
|
|
12
|
Lin B, Yu H, Lin Y, Cai C, Lu H and Zhu X:
Suppression of GRASP65 phosphorylation by tetrahydrocurcumin
protects against cerebral ischemia/reperfusion injury via ERK
signaling. Mol Med Rep. 14:4775–4780. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang Y and Qin Z: Coordination of
autophagy with other cellular activities. Acta Pharmacol Sin.
34:585–594. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Janku F, McConkey DJ, Hong DS and Kurzrock
R: Autophagy as a target for anticancer therapy. Nat Rev Clin
Oncol. 8:528–539. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Levine B and Kroemer G: Autophagy in the
pathogenesis of disease. Cell. 132:27–42. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zi D, Zhou ZW, Yang YJ, Huang L, Zhou ZL,
He SM, He ZX and Zhou SF: Danusertib induces apoptosis, cell cycle
arrest, and autophagy but inhibits epithelial to mesenchymal
transition involving PI3K/Akt/mTOR signaling pathway in human
ovarian cancer cells. Int J Mol Sci. 16:27228–27251. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kang N, Wang MM, Wang YH, Zhang ZN, Cao
HR, Lv YH, Yang Y, Fan PH, Qiu F and Gao XM: Tetrahydrocurcumin
induces G2/M cell cycle arrest and apoptosis involving p38 MAPK
activation in human breast cancer cells. Food Chem Toxicol.
67:193–200. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu JC, Lai CS, Badmaev V, Nagabhushanam K,
Ho CT and Pan MH: Tetrahydrocurcumin, a major metabolite of
curcumin, induced autophagic cell death through coordinative
modulation of PI3K/Akt-mTOR and MAPK signaling pathways in human
leukemia HL-60 cells. Mol Nutr Food Res. 55:1646–1654. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang XY, Zhang TT, Song DD, Zhou J, Han
R, Qin ZH and Sheng R: Endoplasmic reticulum chaperone GRP78 is
involved in autophagy activation induced by ischemic
preconditioning in neural cells. Mol Brain. 8:202015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kuma A, Matsui M and Mizushima N: LC3, an
autophagosome marker, can be incorporated into protein aggregates
independent of autophagy: Caution in the interpretation of LC3
localization. Autophagy. 3:323–328. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bjørkøy G, Lamark T, Brech A, Outzen H,
Perander M, Overvatn A, Stenmark H and Johansen T: p62/SQSTM1 forms
protein aggregates degraded by autophagy and has a protective
effect on huntingtin-induced cell death. J Cell Biol. 171:603–614.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Paglin S, Lee NY, Nakar C, Fitzgerald M,
Plotkin J, Deuel B, Hackett N, McMahill M, Sphicas E, Lampen N and
Yahalom J: Rapamycin-sensitive pathway regulates mitochondrial
membrane potential, autophagy, and survival in irradiated MCF-7
cells. Cancer Res. 65:11061–11070. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim SH, Son KM, Kim KY, Yu SN, Park SG,
Kim YW, Nam HW, Suh JT, Ji JH and Ahn SC: Deoxypodophyllotoxin
induces cytoprotective autophagy against apoptosis via inhibition
of PI3K/AKT/mTOR pathway in osteosarcoma U2OS cells. Pharmacol Rep.
69:878–884. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pant K, Saraya A and Venugopal SK:
Oxidative stress plays a key role in butyrate-mediated autophagy
via Akt/mTOR pathway in hepatoma cells. Chem Biol Interact.
273:99–106. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shi H, Pu J, Zhou XL, Ning YY and Bai C:
Silencing long non-coding RNA ROR improves sensitivity of
non-small-cell lung cancer to cisplatin resistance by inhibiting
PI3K/Akt/mTOR signaling pathway. Tumour Biol. 39:2017.doi:
10.1177/1010428317697568. View Article : Google Scholar :
|
|
27
|
Jiang W, Zhang W, Wu L, Liu L, Men Y, Wang
J, Liang J, Hui Z, Zhou Z, Bi N and Wang L: MicroRNA-related
polymorphisms in PI3K/Akt/mTOR pathway genes are predictive of
limited-disease small cell lung cancer treatment outcomes. Biomed
Res Int 2017. 2017.doi: 10.1155/2017/6501385. View Article : Google Scholar
|




















