Introduction
Oxidative stress occurs when redox homeostasis is
disrupted, which is usually accompanied by damaging effects to cell
survival. Additionally, oxidative stress has been implicated in
various pathologies, including liver diseases, neurodegenerative
diseases, cardiovascular diseases, cancer and diabetes (1–3).
Overproduction of reactive oxygen species (ROS) is considered to
serve a prominent role in oxidative stress; high concentrations of
ROS may result in cell death and damage to cellular structures
involving DNA, lipids and protein. Generally, the cellular
antioxidant defense system counterbalances ROS production to
maintain an appropriate balance between oxidants and antioxidants
(4). Therefore, antioxidant
therapy may be one strategy to prevent cells from excessive
exposure to oxidative stress and correct cellular redox homeostasis
(5).
Recent studies have demonstrated that the
transcription factor nuclear factor erythroid 2-related factor 2
(Nrf2) tightly regulates the cellular antioxidant system (6–8).
Nrf2 binds to and mediates the activation of antioxidant response
element (ARE)-dependent antioxidant target genes, including heme
oxygenase-1 (HO-1), NAD(P)H quinone oxidoreductase-1 (NQO1),
superoxide dismutase (SOD1 and 2), catalase, glutathione peroxidase
(GPx)1, GPx2, GPx4 and glutathione (6). The Nrf2-ARE signaling pathway is
known to be one of the important ROS-induced physiological
mechanisms in defense against oxidative damage (9). Therefore, the induction of Nrf2 and
further upregulation of antioxidant genes is considered an
important pathway to prevent diseases induced by oxidative stress,
including liver diseases, such as hepatitis, alcoholic and
non-alcoholic fatty liver diseases (10).
Previous studies have demonstrated that natural
products, including flavonoids, may be used as regulators of the
Nrf2-ARE signaling system in Nrf2 activation. Diosmetin
(3′,5,7-trihydroxy-4′-methoxyflavone) is a flavone initially found
in the legume Acacia farnesiana Wild and Olea
europaea L. leaves (11,12).
Diosmetin occurs naturally in various sources, including citrus
fruits, oregano and some specific medicinal herbs, including
Chrysanthemum morifolium, Origanum vulgare, Robiniapseudoacacia,
Rosa agrestis and Lespedeza davurica (13). Pharmacologically, diosmetin has
been reported to exhibit antioxidant (14,15),
antimicrobial (16),
anti-inflammatory (17),
anticancer (18) and estrogenic
(19) activities, and is used in
traditional Mongolian medicine to treat liver diseases (20). However, to date, very few studies
have focused on the hepatoprotective effects of diosmetin against
hydrogen peroxide (H2O2)-induced liver cell
damage, and the underlying molecular mechanism involved in the
expression of antioxidant genes remains to be elucidated.
The present study aimed to demonstrate the
protective effects of diosmetin against
H2O2-induced oxidative stress in the normal
human liver cell line L02 and to evaluate its role in activation of
the Nrf2-ARE signaling pathway for cytoprotection.
Materials and methods
Cell culture and treatment
Human normal hepatocytes (L02 cells) obtained from
Nanjing Key Gen Biotech Co., Ltd. (Nanjing, China) were cultured in
RPMI-1640 medium (HyClone; GE Healthcare, Chicago, IL, USA)
supplemented with 10% fetal bovine serum (HyClone; GE Healthcare)
and 100 U/ml penicillin-streptomycin at 37°C in a humidified
atmosphere containing 5% CO2. Diosmetin (Nanjing Zelang
Medical Technological Co., Ltd., Nanjing, China) stock solution was
prepared in dimethyl sulfoxide (DMSO) and diluted with RPMI-1640
medium (2.5, 5, 10, 20, 30 and 40 µM) prior to experimentation.
Cells in the negative control group were treated with DMSO alone at
a final concentration of <0.1% (v/v). The positive control was
treated with Trolox (40 µM, Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) or t-BHQ (30 µM, Sigma-Aldrich; Merck KGaA). In
vitro oxidative stress cell damage models were induced by 200
µM H2O2 (Sigma-Aldrich; Merck KGaA).
Cell viability, cell apoptosis and
lactate dehydrogenase (LDH) leakage assays
L02 cells were seeded in 96-well plates at a density
of 5×103 cells/well and cultured overnight.
Subsequently, cells were pretreated with various concentrations of
diosmetin (0–40 µM), Trolox (40 µM) or t-BHQ (30 µM) for 24 h at
37°C prior to exposure to 200 µM H2O2 for 6 h
at 37°C. Cell viability was estimated using the Cell Counting kit-8
colorimetric assay (Dojindo Molecular Technologies, Inc., Kumamoto,
Japan) according to the manufacturer's protocol. The release of LDH
was evaluated using an LDH assay kit (Nanjing Jiancheng
Bioengineering Institute, Nanjing, China) according to the
manufacturer's protocol. The proportions of apoptotic cells were
evaluated using an Annexin V/fluorescein isothiocyanate staining
kit (Beyotime Institute of Biotechnology, Haimen, China) according
to manufacturer's protocol. All cells were analyzed by flow
cytometry (BD Accuri™ C6 1.0.264.21, BD Biosciences, San Jose, CA,
USA).
Measurement of intracellular ROS and
mitochondrial membrane potential (MMP)
Intracellular ROS production was detected using an
intracellular ROS assay kit (Beyotime Institute of Biotechnology)
and MMP was measured using rhodamine 123 (Rh123; Sigma-Aldrich;
Merck KGaA). Cells (5×105 cells/well) were pretreated
with various concentrations of diosmetin and t-BHQ (30 µM) for 24 h
at 37°C, and were then incubated with 200 µM
H2O2 for 6 h at 37°C. Following staining with
2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA, in the ROS
assay kit; 10 µM) for 20 min or Rh123 (1 µM) for 30 min at 37°C,
cells were analyzed by flow cytometry (BD Accuri™ C6 1.0.264.21, BD
Biosciences, San Jose, CA, USA), images of the stained cells were
observed under an inverted fluorescence microscope (IX71;Olympus
Corporation, Tokyo, Japan).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from L02 cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) and was reverse transcribed into cDNA using
a PrimeScript™ RT reagent kit (Takara Bio, Inc., Otsu, Japan)
according to manufacturer's protocol. RT-qPCR was conducted using
SYBR® Premix Ex Taq™ II kit (Takara Bio, Inc.) on an
Applied Biosystems Quant Studio™ 6 Flex thermocycler (Thermo Fisher
Scientific, Inc.). The RT-qPCR conditions were as follows: 95°C for
30 sec, 40 cycles of amplification (95°C for 5 sec, 60°C for 30
sec, and 72°C for 30 sec), and 95°C for 15 sec, 60°C for 1 min,
95°C for 15 sec. The PCR primers used were as follows: Nrf2
forward, 5′-GCGACGGAAAGAGTATGAGC-3′, and reverse,
5′-ACCTGGGAGTAGTTGGCAGA-3′;HO-1 forward,
5′-CTGACCCATGACACCAAGGAC-3′, and reverse,
5′-AAAGCCCTACAGCAACTGTCG-3′; NQO1 forward,
5′-GGCAGAAGAGCACTGATCGTA-3′, and reverse,
5′-TGATGGGATTGAAGTTCATGGC-3′; and GAPDH forward,
5′-ACGGATTTGGTCGTATTGGG-3′ and reverse, 5′-TGATTTTGGAGGGATCTCGC-3′.
The 2−ΔΔCq method was used for quantitative calculation
(21).
Western blot analysis
Following treatments, cells were lysed using
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology). The lysates were centrifuged at 12,000 × g for 10
min at 4°C, and the supernatants were collected and stored at
−80°C. Protein concentrations were determined using a Bicinchoninic
Acid assay kit (Beyotime Institute of Biotechnology). Equivalent
amounts of lysate protein (50 µg) were separated by 10% SDS-PAGE
and were then transferred to polyvinylidene fluoride membranes (EMD
Millipore, Billerica, MA, USA). The membranes were blocked with 5%
skimmed milk powder diluted in TBS with Tween 20 at room
temperature for 1 h. Then membranes were probed with monoclonal
anti-Nrf2 (1:2,000; cat. no. ab62352), HO-1 (1:20,000; cat. no.
ab68477), NQO1 (1:20,000; cat. no. ab80588; Abcam, Cambridge, UK)
and β-actin primary antibodies (1:5,000; cat. no. T0022; Affinity
Biosciences, Cincinnati, OH, USA) overnight at 4°C, and were then
incubated with goat anti-rabbit (1:6,000; cat. no. 33101ES60) or
anti-mouse (1:6,000; cat. no. 33201ES60) horseradish
peroxidase-conjugated secondary antibodies (YEASEN Biosciences,
Shanghai, China; ww.yeasen.com) for 1 h at room
temperature. Blots were visualized using an enhanced
chemiluminescent method (EMD Millipore) and were analyzed using a
gel image analysis system (Bio-Rad ChemiDoc XRS; Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Small interfering RNA (siRNA)
assays
Nrf2 siRNAs (cat. no. siB140820100848) and a
negative control (cat. no. siP01001) were purchased from Guangzhou
RiboBio, Co., Ltd. (Guangzhou, China). siRNAs (100 nM) were
transfected into L02 cells for 24 h at 37°C using
Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) prior to H2O2/diosmetin
treatment. Subsequently, the expression levels of Nrf2, HO-1 and
NQO1 were detected by western blotting; β-actin was used as an
internal control.
Statistical analysis
Data are expressed as the mean ± standard deviation,
and the differences in mean values were analyzed by one-way
analysis of variance followed by the least significant difference
test. P<0.05 was considered to indicate a statistically
significant difference. Data were analyzed using SPSS version 16.0
(SPSS, Inc., Chicago, IL, USA).
Results
Diosmetin attenuates
H2O2-induced L02 cell cytotoxicity
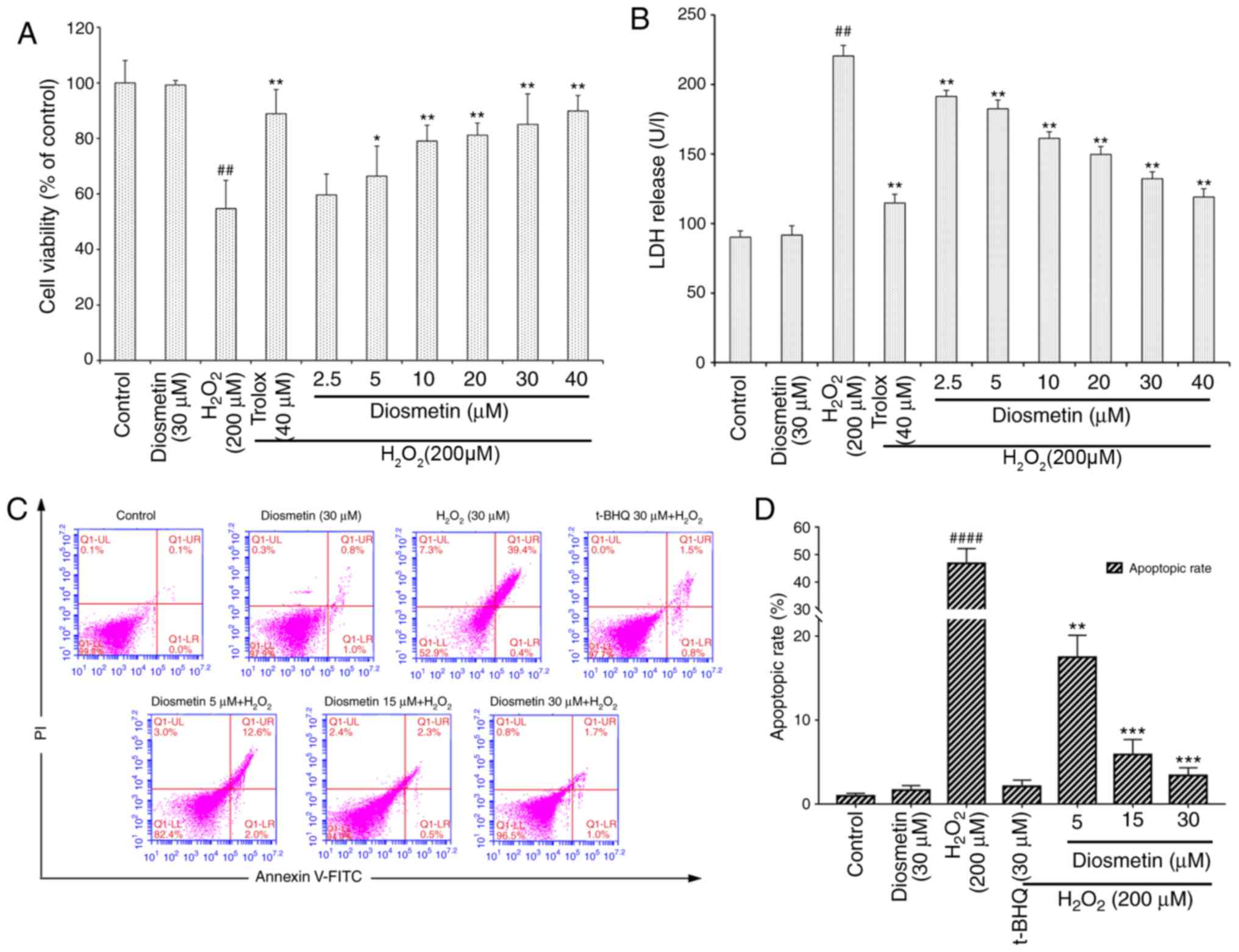
The viability of cells treated with diosmetin alone
was similar to that of the control group. However, compared with in
the control group, cells exposed to 200 µM
H2O2 for 6 h revealed a significant decrease
in cell viability (54.7±6.9%; P<0.01). Conversely, the viability
of cells pretreated with various concentrations of diosmetin (2.5,
5, 10, 20, 30 and 40 µM) was restored in a dose-dependent manner;
with the exception of the 2.5 µM-treated group, the cell viability
of the other diosmetin-treated groups were significantly increased
compared with in the H2O2-treated group
(P<0.05 and P<0.01). The cytoprotective effects of 20, 30 and
40 µM diosmetin were similar to those exerted by the positive
control 40 µM Trolox, and there were no significant differences in
cell viability among these groups (P>0.05; Fig. 1A).
As presented in Fig.
1B, the cellular LDH release assay demonstrated that the LDH
levels in the culture medium of H2O2-treated
cells were significantly increased compared with in the control
group (P<0.01). The LDH levels were not markedly different
between the control group and the group treated with diosmetin
alone (P>0.05). Pretreatment with the lowest concentration of
diosmetin (2.5 µM) for 24 h prior to H2O2
exposure significantly reduced LDH release (P<0.01), and
diosmetin reduced LDH release in a dose-dependent manner (2.5–40
µM). The highest concentration of diosmetin (40 µM) exerted a
similar effect to the positive control (40 µM Trolox). These
findings indicated that diosmetin exerted protective effects
against H2O2-induced cytotoxicity, as
demonstrated by LDH release and cell viability assays.
There were also significant differences in the rates
of cell apoptosis and death among the various groups. In cells
treated with 200 µM H2O2, the cell apoptotic
rate (47.1±5.5%) was much greater than in the control group
(0.7±0.2%, P<0.0001). However, pretreatment with increasing
concentrations of diosmetin (5, 15 and 30 µM) significantly reduced
H2O2-induced cell apoptosis in a
concentration-dependent manner (Fig.
1C and D).
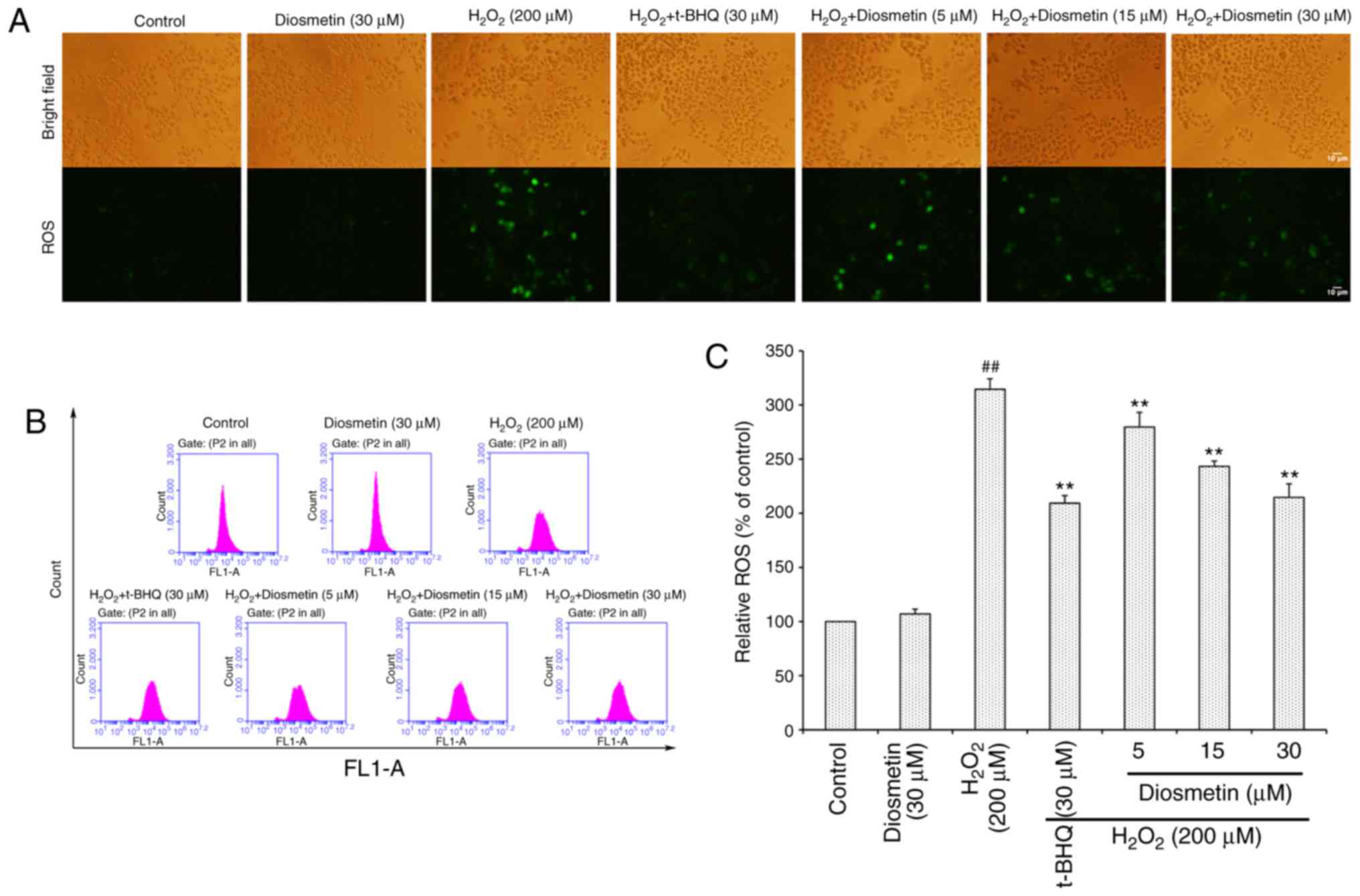
Diosmetin inhibits
H2O2-induced intracellular ROS accumulation
and MMP loss
To directly determine the production of
intracellular ROS, DCFH-DA-labeled cells were measured using an
inverted fluorescence microscope (Fig.
2A). The results demonstrated that the control group of cells
exhibited very weak green fluorescence; however, the fluorescence
intensity of H2O2-exposed cells was markedly
enhanced. Conversely, diosmetin pretreatment reduced the effects of
H2O2 on fluorescence intensity.
As illustrated in Fig.
2B and C, when cells were treated with 200 µM
H2O2 alone, the intracellular ROS level was
more than three times that of the control group. However,
pretreatment with increasing concentrations of diosmetin (5, 15 and
30 µM) significantly attenuated H2O2-induced
ROS accumulation in a concentration-dependent manner (P<0.01).
In addition, 30 µM diosmetin inhibited ROS accumulation to a
similar level as that in the positive control group, which was
treated with tertiary butylhydroquinone (t-BHQ, 30 µM).
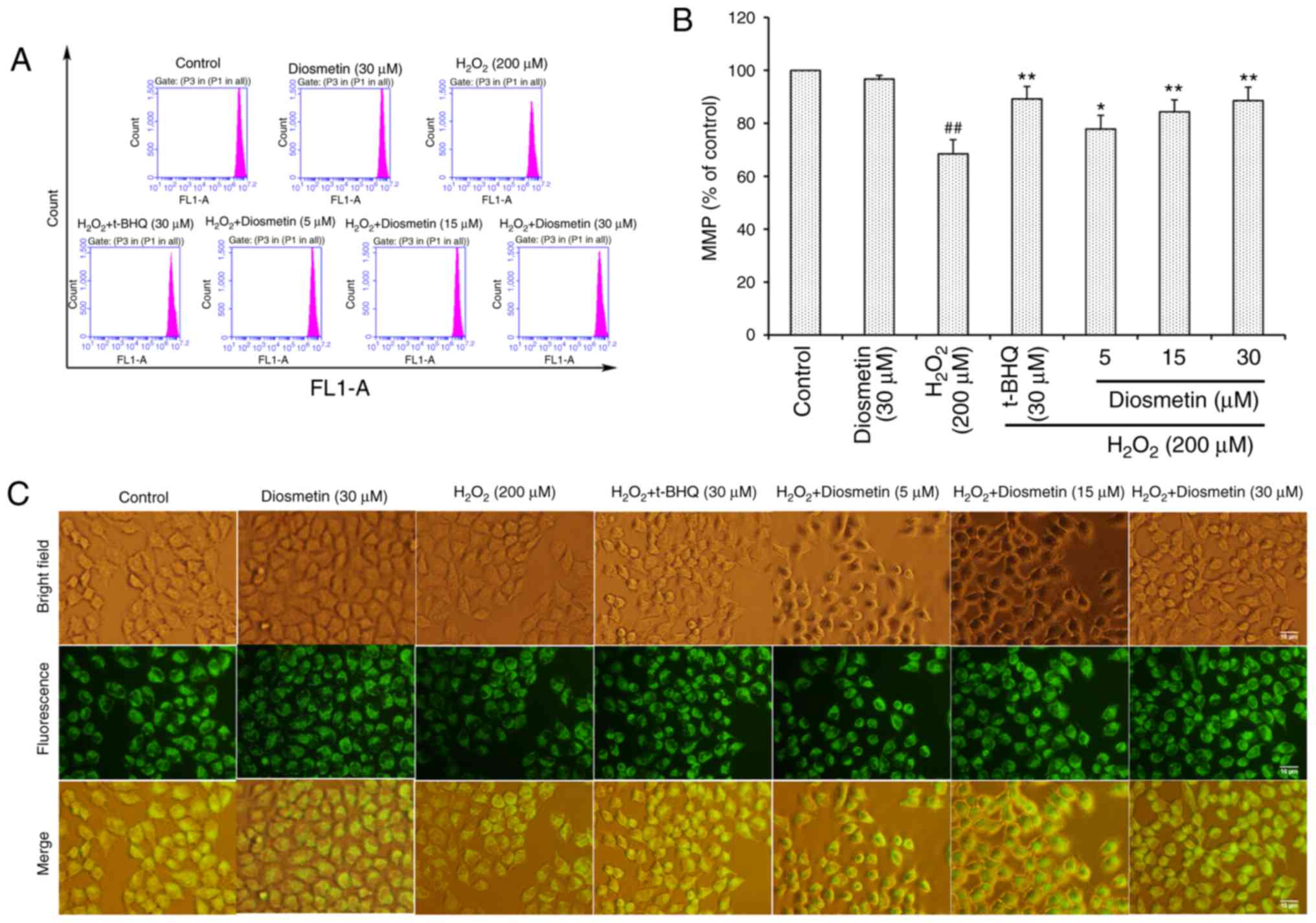
As presented in Fig. 3A
and B, the MMP of L02 cells treated with diosmetin alone was
similar to that of the control group. However, in L02 cells treated
with 200 µM H2O2, MMP was significantly
decreased (68.5±5.3%) compared with in the control group
(P<0.01). Conversely, pretreatment with diosmetin significantly
prevented the loss of MMP in a dose-dependent manner (P<0.05 or
P<0.01). Furthermore, 30 µM diosmetin exhibited a similar
inhibitory effect to 30 µM t-BHQ. These results further supported
the conclusion reached by observations made under fluorescence
microscopy (Fig. 3C).
Diosmetin upregulates Nrf2, NQO1 and
HO-1 expression in H2O2-stressed L02
cells
The Nrf2-ARE signaling pathway is known to serve a
pivotal role in cellular defense against oxidative stress. Since
diosmetin may attenuate H2O2-induced
oxidative stress in L02 cells, it was hypothesized that treatment
with diosmetin may activate expression of the transcription factor
Nrf2 and ARE-dependent antioxidant target genes, including NQO1 and
HO-1. Therefore, activation of Nrf2, NQO1 and HO-1 were
investigated in diosmetin-treated L02 cells using western blot
analysis and RT-qPCR. As expected, pretreatment with diosmetin
dose-dependently increased the protein expression levels of Nrf2,
NQO1 and HO-1. Notably, 30 µM diosmetin induced significant protein
accumulation of Nrf2, NQO1 and HO-1 compared with in the
H2O2-treated group (P<0.01; Fig. 4A and B).
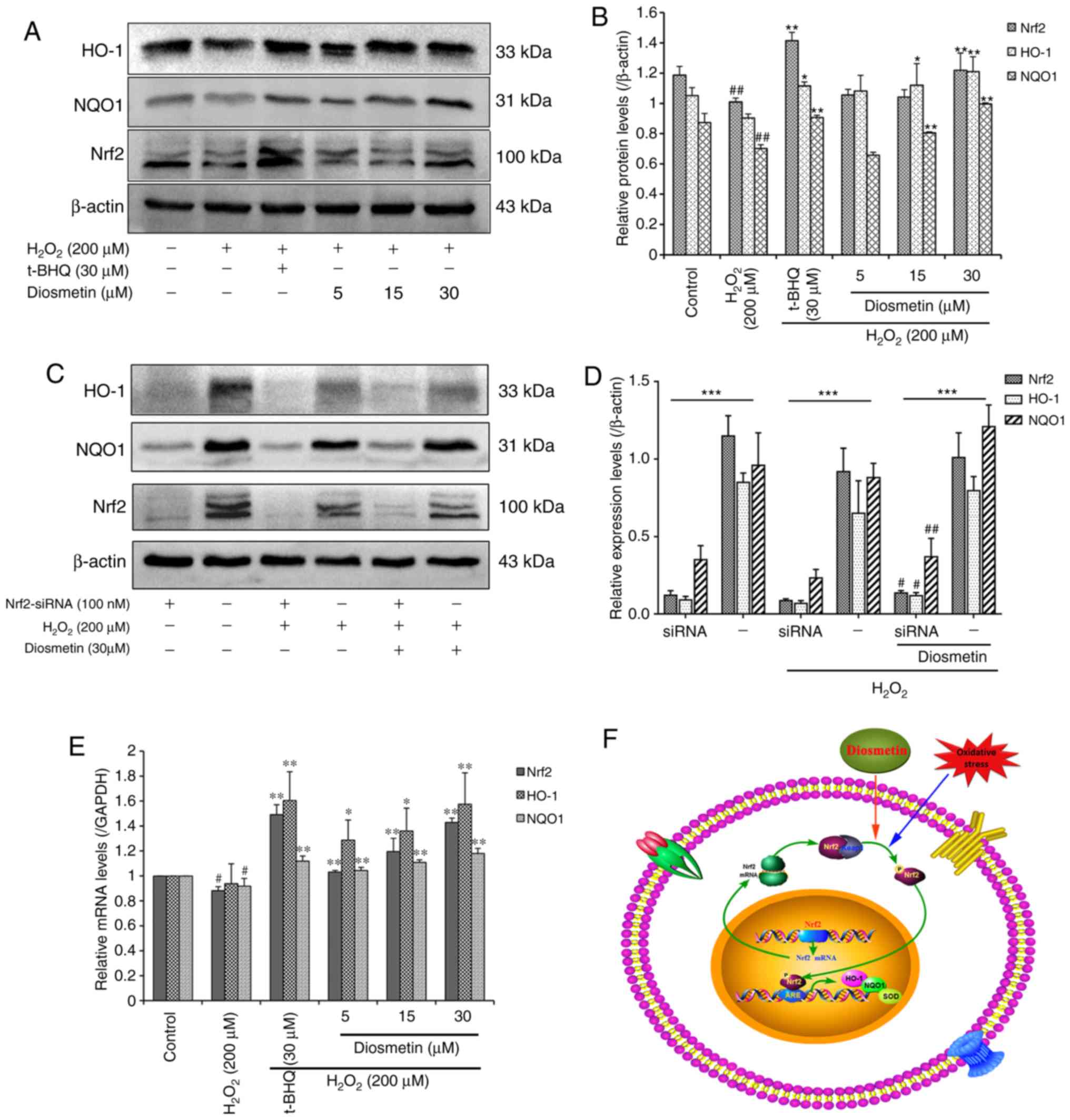 | Figure 4.Effects of diosmetin on the
expression levels of HO-1, NQO1 and Nrf2 in
H2O2-induced L02 cells. (A) Relative protein
expression levels of HO-1, NQO1 and Nrf2 were detected by western
blotting. (B) Scanning densitometry was used for semi-quantitative
analysis of western blotting. Data are presented as the mean ±
standard deviation of three independent experiments.
##P<0.01 vs. the control group. *P<0.05 and
**P<0.01 vs. the H2O2 model group. (C)
Expression levels of HO-1, NQO1 and Nrf2 following treatment with
100 nM Nrf2 siRNA and 30 µM diosmetin. (D) Scanning densitometry
was used to semi-quantify the results of western blotting. Data are
presented as the mean ± standard deviation of three independent
experiments. ***P<0.01 vs. Nrf2 siRNAs group with negative
control. #P<0.05 and ##P<0.01 vs. the
H2O2+ Nrf2 siRNA group. (E) Relative mRNA
expression levels of HO-1, NQO1 and Nrf2 were analyzed by reverse
transcription-quantitative polymerase chain reaction.
#P<0.05 vs. the control group. *P<0.05 and
**P<0.01 vs. the H2O2 model group. (F)
Schematic representation of Nrf2-ARE signaling pathway activation
by oxidative stress and diosmetin. In the cytoplasm, Keap1 inhibits
the Nrf2 signaling pathway by promoting Nrf2 ubiquitination. When
oxidative stress occurs in L02 cells, diosmetin facilitates the
dissociation of Nrf2-Keap1, phosphorylation of Nrf2 and nuclear
translocation. In the nucleus, Nrf2 promotes the expression of
HO-1, NQO1 and SOD antioxidants by binding to the ARE regions. ARE,
antioxidant response element; H2O2, hydrogen
peroxide; HO-1, heme oxygenase-1; Keap1, Kelch-like ECH-associated
protein 1; NQO1, NAD(P)H quinone oxidoreductase-1; Nrf2, nuclear
factor erythroid 2-related factor 2; siRNA, small interfering RNA;
SOD, superoxide dismutase; t-BHQ, tertiary butylhydroquinone. |
To assess the functional role of diosmetin and Nrf2
in H2O2-induced oxidative stress and damage,
the present study investigated whether diosmetin may rescue the
expression of Nrf2 inhibited by siRNA. The results revealed that
transient inhibition of Nrf2 by siRNA resulted in significant
downregulation of HO-1 and NQO1 in three groups (control,
H2O2 and diosmetin; Fig. 4C and D). However, treatment with 30
µM diosmetin rescued the inhibitory effects of Nrf2 siRNA to a
certain extent compared with in the
H2O2-induced group, and increased the
expression of Nrf2, HO-1 and NQO1 (P<0.01 or P<0.05).
Furthermore, pretreatment with diosmetin and t-BHQ also led to a
significant increase in the mRNA expression levels of Nrf2, NQO1
and HO-1 in a dose-dependent manner (Fig. 4E).
Collectively, these data suggested that treatment
with certain concentrations of diosmetin may activate the
expression of Nrf2, which may regulate transcription of the
antioxidant enzymes HO-1 and NQO1. Furthermore, increased
expression of HO-1 and NQO1 may protect L02 cells from
H2O2-induced oxidative stress and damage
(Fig. 4F).
Discussion
Oxidative stress has been reported to be involved in
the pathogenesis of numerous human diseases, including hepatitis,
alcoholic and non-alcoholic fatty liver diseases (2,22).
It is widely believed that natural antioxidant products have broad
protective effects against oxidative stress. Therefore, searching
for natural antioxidant compounds with effective cytoprotective
potential may provide novel therapeutic strategies for liver
diseases. H2O2-induced cell injury is a
broadly accepted cell model for evaluating the hepatoprotective
effects of natural antioxidant compounds (23). The present study demonstrated that
diosmetin may attenuate H2O2-induced L02 cell
injury by increasing cell viability, decreasing LDH release and
blocking the loss of MMP. The protective effects of diosmetin
against H2O2-induced L02 cell damage were
associated with reduced ROS levels, activation of Nrf2 and
upregulation of downstream phase II detoxifying enzymes, including
HO-1 and NQO1. To the best of our knowledge, the present study is
the first to demonstrate that diosmetin possessed potent
hepatoprotective effects and suppressed numerous molecular events,
which are implicated in oxidative stress, via activation of the
ROS/Nrf2/NQO1-HO-1 signaling axis in human hepatocytes.
Consequently, the findings of the present study indicated that
diosmetin, as a natural antioxidant, may be used as a
pharmacologically effective drug against oxidative liver
disorders.
Two previous studies revealed that diosmetin
exhibited antioxidant effects in other cell types. Ge et al
(24) demonstrated that diosmetin
may inhibit transforming growth factor-β1-induced intracellular ROS
generation in human bronchial epithelial cells. Liao et al
(14) reported that diosmetin may
effectively attenuate 2,2-azobis(2-amidinopropane)
dihydrochloride-induced erythrocyte hemolysis and
CuCl2-induced plasma oxidation via the prevention of
intracellular ROS generation. In addition, the antioxidant activity
of diosmetin was revealed in a 1,1-diphenyl-2-picrylhydrazyl model
system in vitro (15).
However, to the best of our knowledge, there are no studies
available to date regarding the effects of diosmetin on
H2O2-induced oxidative stress in human liver
cells. The results of the present study revealed that cells
pretreated with diosmetin exhibited significantly increased cell
viability and reduced LDH release compared with in cells exposed to
H2O2 alone, and the effects were similar to
those of the positive control Trolox. Another property positive
control t-BHQ was also used to demonstrate the protective effects
of diosmetin. These results indicated that diosmetin exhibits an
excellent antioxidant capacity to attenuate
H2O2-induced oxidative stress in human
hepatocytes.
Oxidative stress is considered to serve a marked
role in the development of mitochondrial dysfunction, thus
contributing to increased mitochondrial membrane permeability and
resulting in depolarization of the MMP (25). Furthermore, a reduction in
mitochondrial integrity may increase ROS production and decrease
adenosine triphosphate production (26). In addition, high levels of ROS may
in turn damage mitochondrial function, resulting in irreversible
membrane damage and eventually cell death (27). The present study reported that MMP
was significantly decreased in H2O2-treated
L02 cells, whereas diosmetin pretreatment reduced the loss of MMP
in a dose-dependent manner, and the inhibitory effects of 30 µM
diosmetin were similar to those of the positive control (30 µM
t-BHQ). Therefore, the ability of diosmetin to maintain
mitochondrial membrane integrity may be due to its ROS scavenging
activity.
As a well-characterized oxidative stress inducer,
H2O2 may trigger intracellular ROS generation
in various human cell lines (28).
Additionally, H2O2 is able to easily pass
through the cell membrane via aquaporins or by simple diffusion,
and evoke lipid peroxidation, and DNA and protein damage, which
result in significant oxidative damage (29). The present study confirmed that
cells exposed to H2O2 generated a large
amount of ROS in L02 cells; however, when L02 cells were pretreated
with diosmetin, the H2O2-induced
intracellular ROS accumulation was significantly attenuated.
Therefore, the protective effects of diosmetin against
H2O2-induced cytotoxicity may be mainly
attributed to its ROS scavenging capacity.
It has previously been indicated that antioxidants
may exhibit their antioxidant activity not by directly scavenging
intracellular oxidants, but by inducing the endogenous antioxidant
defense system (30). Activation
of the antioxidant system is known to serve a significant role in
cellular defense against oxidative impacts; detoxifying enzymes,
including HO-1 and NQO1, which are regulated by Nrf2, are important
parts of the system (4). Nrf2,
which is a member of the cap ‘n’ collar family, is a basic leucine
zipper transcription factor that serves as a critical regulator of
antioxidants and detoxifying enzymes, in order to protect against
oxidative stress-induced cell damage and apoptosis (31). When stimulated by inducers, Nrf2 is
released from its cytosolic inhibitor, Kelch-like ECH-associated
protein 1, after which translocases into the nucleus and binds to
the ARE to promote the expression of numerous phase II enzymes,
including NQO1 and HO-1 (8,32).
Since the Nrf2/ARE signaling pathway has been reported to offer
protection against oxidative damage, the induction of NQO1 and HO-1
regulated by the Nrf2/ARE signaling pathway may provide a
therapeutic strategy for liver diseases in cases of oxidative
stress (33). However, the
regulatory mechanisms involved in mediating Nrf2 activation are not
yet fully understood. The present study hypothesized that increased
expression of NQO1 and HO-1 may be dependent upon activation of the
Nrf2/ARE signaling pathway. As expected, the mRNA and protein
expression levels of Nrf2 were increased in a dose-dependent manner
in diosmetin-pretreated L02 cells, and the mRNA and protein
expression levels of NQO1 and HO-1 were also dose-dependently
increased. Collectively, the results of the present study indicated
that diosmetin-mediated protection against
H2O2-induced L02 cell injury may be
attributed to upregulation of HO-1 andNQO1 via the Nrf2/ARE
signaling pathway; to the best of our knowledge, the present study
is the first to reveal activation of the Nrf2/ARE signaling pathway
by diosmetin.
In conclusion, the present study demonstrated that
diosmetin may exert hepatoprotective effects against
H2O2-induced L02 cell damage by upregulating
the expression of NQO1 and HO-1 via Nrf2 activation, which may
contribute to the suppression of ROS generation and increased MP.
Therefore, the findings of the present study provided a scientific
basis for the hepatoprotective effects of diosmetin and suggested
that it may be used as a promising natural protective agent for the
treatment of various liver diseases associated with oxidative
stress.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural
Science Foundation of the Higher Education Institutions of Anhui
Province (grant nos. KJ2016A473, KJ2017A215 and KJ2015A263), and
National Natural Science Foundation of China (grant no.
81771381).
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
CW, YL and CL drafted the paper and participated in
the data analysis. SW and DW performed the RT-qPCR and western blot
analysis. NW and QX performed the cell viability, cell apoptosis
and LDH leakage assays. WJ measured the intracellular ROS. MQ
measured the MMP. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhang H, Davies KJA and Forman HJ:
Oxidative stress response and Nrf2 signaling in aging. Free Radic
Biol Med. 88:314–336. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Webb C and Twedt D: Oxidative stress and
liver disease. Vet Clin North Am Small Anim Pract. 38(125–135):
v2008. View Article : Google Scholar
|
|
3
|
Zhang B, Dong JL, Chen YL, Liu Y, Huang
SS, Zhong XL, Cheng YH and Wang ZG: Nrf2 mediates the protective
effects of homocysteine by increasing the levels of GSH content in
HepG2 cells. Mol Med Rep. 16:597–602. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Espinosa-Diez C, Miguel V, Mennerich D,
Kietzmann T, Sánchez-Pérez P, Cadenas S and Lamas S: Antioxidant
responses and cellular adjustments to oxidative stress. Redox Biol.
6:183–197. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hu Y, Wang S, Wang A, Lin L, Chen M and
Wang Y: Antioxidant and hepatoprotective effect of Penthorum
chinense Pursh extract against t-BHP-induced liver damage in L02
cells. Molecules. 20:6443–6453. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Leiser SF and Miller RA: Nrf2 signaling, a
mechanism for cellular stress resistance in long-lived mice. Mol
Cell Biol. 30:871–884. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yan B, Ma Z, Shi S, Hu Y, Ma T, Rong G and
Yang J: Sulforaphane prevents bleomycin-induced pulmonary fibrosis
in mice by inhibiting oxidative stress via nuclear factor erythroid
2-related factor-2 activation. Mol Med Rep. 15:4005–4014. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Han MH, Park C, Lee DS, Hong SH, Choi IW,
Kim GY, Choi SH, Shim JH, Chae JI, Yoo YH and Choi YH:
Cytoprotective effects of esculetin against oxidative stress are
associated with the upregulation of Nrf2-mediated NQO1 expression
via the activation of the ERK pathway. Int J Mol Med. 39:380–386.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nguyen T, Nioi P and Pickett CB: The
Nrf2-antioxidant response element signaling pathway and its
activation by oxidative stress. J Biol Chem. 284:13291–13295. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tang W, Jiang YF, Ponnusamy M and Diallo
M: Role of Nrf2 in chronic liver disease. World J Gastroenterol.
20:13079–13087. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Garavito G, Rincón J, Arteaga L, Hata Y,
Bourdy G, Gimenez A, Pinzón R and Deharo E: Antimalarial activity
of some Colombian medicinal plants. J Ethnopharmacol. 107:460–462.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Meirinhos J, Silva BM, Valentão P, Seabra
RM, Pereira JA, Dias A, Andrade PB and Ferreres F: Analysis and
quantification of flavonoidic compounds from Portuguese olive (Olea
europaea L.) leaf cultivars. Nat Prod Res. 19:189–195. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Patel K, Gadewar M, Tahilyani V and Patel
DK: A review on pharmacological and analytical aspects of
diosmetin: A concise report. Chin J Integr Med. 19:792–800. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liao W, Ning Z, Chen L, Wei Q, Yuan E,
Yang J and Ren J: Intracellular antioxidant detoxifying effects of
diosmetin on 2,2-azobis(2-amidinopropane) dihydrochloride
(AAPH)-induced oxidative stress through inhibition of reactive
oxygen species generation. J Agric Food Chem. 62:8648–8654. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bai N, Zhou Z, Zhu N, Zhang L, Quan Z, He
K, Zhang QY and Ho CH: Antioxidative flavonoids from the flower of
Inula Britannica. J Food Lipid. 12:141–149. 2005. View Article : Google Scholar
|
|
16
|
Meng JC, Zhu QX and Tan RX: New
antimicrobial mono- and sesquiterpenes from Soroseris hookeriana
subsp. erysimoides. Planta Med. 66:541–544. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Domínguez M, Avila JG, Nieto A and
Céspedes CL: Anti-inflammatory activity of Penstemon gentianoides
and Penstemon campanulatus. Pharm Biol. 49:118–124. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu B, Shi Y, Peng W, Zhang Q, Liu J, Chen
N and Zhu R: Diosmetin induces apoptosis by upregulating p53 via
the TGF-β signal pathway in HepG2 hepatoma cells. Mol Med Rep.
14:159–164. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Androutsopoulos V, Wilsher N, Arroo RR and
Potter GA: Bioactivation of the phytoestrogen diosmetin by CYP1
cytochromes P450. Cancer Lett. 274:54–60. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Obmann A, Werner I, Presser A, Zehl M,
Swoboda Z, Purevsuren S, Narantuya S, Kletter C and Glasl S:
Flavonoid C- and O-glycosides from the Mongolian medicinal plant
Dianthus versicolor Fisch. Carbohydr Res. 346:1868–1875. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Marí M, Colell A, Morales A, von Montfort
C, Garcia-Ruiz C and Fernández-Checa JC: Redox control of liver
function in health and disease. Antioxid Redox Signal.
12:1295–1331. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Senthil Kumar KJ, Liao JW, Xiao JH, Gokila
Vani M and Wang SY: Hepatoprotective effect of lucidone against
alcohol-induced oxidative stress in human hepatic HepG2 cells
through the up-regulation of HO-1/Nrf-2 antioxidant genes. Toxicol
In Vitro. 26:700–708. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ge A, Ma Y, Liu YN, Li YS, Guo H, Zhang
JX, Wang QX, Zeng XN and Huang M: Diosmetin prevents
TGF-beta1-induced epithelial-mesenchymal transition via ROS/MAPK
signaling pathways. Life Sci. 153:1–8. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Beal MF: Mitochondria take center stage in
aging and neurodegeneration. Ann Neurol. 58:495–505. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Somayajulu M, Mccarthy S, Hung M, Sikorska
M, Borowy-Borowski H and Pandey S: Role of mitochondria in neuronal
cell death induced by oxidative stress; neuroprotection by Coenzyme
Q10. Neurobiol Dis. 18:618–627. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dumont M and Beal MF: Neuroprotective
strategies involving ROS in Alzheimer disease. Free Radic Biol Med.
51:1014–1026. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zorov DB, Filburn CR, Klotz LO, Zweier JL
and Sollott SJ: Reactive oxygen species (ROS)-induced ROS release:
A new phenomenon accompanying induction of the mitochondrial
permeability transition in cardiac myocytes. J Exp Med.
192:1001–1014. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sies H: Role of metabolic H2O2 generation:
Redox signaling and oxidative stress. J Biol Chem. 289:8735–8741.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li T, Chen B, Du M, Song J, Cheng X, Wang
X and Mao X: Casein glycomacropeptide hydrolysates exert
cytoprotective effect against cellular oxidative stress by
up-regulating HO-1 expression in HepG2 cells. Nutrients. 9:pii:
E31. 2017. View Article : Google Scholar
|
|
31
|
Huang Y, Li W, Su Z and Kong AN: The
complexity of the Nrf2 pathway: Beyond the antioxidant response. J
Nutr Biochem. 26:1401–1413. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Suzuki T and Yamamoto M: Molecular basis
of the Keap1-Nrf2 system. Free Radic Biol Med. 88:93–100. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ma Z, Li C, Qiao Y, Lu C, Li J, Song W,
Sun J, Zhai X, Niu J, Ren Q and Wen A: Safflower yellow B
suppresses HepG2 cell injury induced by oxidative stress through
the AKT/Nrf2 pathway. Int J Mol Med. 37:603–612. 2016. View Article : Google Scholar : PubMed/NCBI
|