Introduction
Osteoporosis is a metabolic bone disease that is
characterized by lower bone mass and bone microstructure and leads
to the increase of osteopsathyrosis and increased fractures
(1). National Institutes of Health
defines osteoporosis as a bone disease which the damages bone
strength, leads to degeneration of bone tissue microstructure and
an increased risk of fractures (2). Osteoporosis may be divided into two
types, primary and secondary: Primary osteoporosis occurs in aging
patients and is associated with the reduction of hormone secretion
and accounts for ~90% of cases (1), whereas secondary osteoporosis
accounts for 10% of cases (3).
Ovariectomy-induced osteoporosis (OIO) is the most common type of
osteoporosis. In postmenopausal osteoporosis, bone formation and
bone resorption is imbalanced, and occurs due to reduced estrogen
levels following menopause (3,4).
Clinical characteristics of postmenopausal osteoporosis include
chronic pain in lumbar spinal cord and limbs, humpback, shortened
height and fractures in skeleton, vertebra and forearm (5).
The common factors contributing to osteoporosis
pathogenesis may be divided as follows: i) Internal secretion; ii)
nutritional iii) physical; iv) immune; and v) genetic factors. The
reduction of estrogen levels in internal secretion is the primary
factor for the occurrence of osteoporosis following menopause
(6). Various drugs have various
disadvantages, reduced calcium absorption or they may lead to the
increase of osteopsathyrosis, particularly in estrogen replacement
therapy (7). Therefore, there is a
great potential to investigate the possible targets of anti-bone
absorption drugs. The osteoprotegerin/receptor activator of nuclear
factor κB (RANK)/RANK ligand (RANKL) system is the primary
determinant of bone mass and is a promising target (7,8).
Osteoporosis is a bone metabolism obstructive
disease of the whole body, which is characterized by damaging bone
tissue microstructure, thickening the bone cortex, reducing the
number of bone trabecula and increasing bone fragility. Osteoblasts
and osteoclasts regulate bone formation and bone resorption,
respectively (9). The dynamic
balance between them maintains normal bone mass (10). As the regulatory factors of bone
metabolism, phosphoinositide-3-kinase regulatory subunit 1 (PI3K)
and protein kinase B (AKT) may promote osteoblast precursors to
differentiate into mature osteoblasts and advocate bone formation
(11).
MicroRNAs (miRNAs) are a class of single-strand
non-coding RNAs with short sequence. They are extensively
distributed in animals and plants, where they have important roles
(12). miRNA is a small regulatory
molecule at the post-transcription level (13). It may specifically bind to the
3′-untranslated region of target mRNA; therefore, it may regulate
the expression of multiple genes. Therefore, it is involved in
multiple biological processes, including cell development,
differentiation, apoptosis and tumor genesis (13). Intracellular miRNAs expression may
be changed to some extent under disease conditions. Changes in
miRNA expression may lead to regulatory functional changes at the
post-transcription level (14).
Therefore, they may induce a series of biological functional
changes in cells. A recent study revealed that miRNAs may regulate
biological characteristics of multiple immune cells (13). They may have an important influence
on the regulation of the immune function of body. Therefore, to
investigate the effect and mechanism of microRNA-148a protection
against ovariectomy-induced osteoporosis the present study used
in vitro and in vivo models.
Materials and methods
Experimental design and diet
Female Sprague-Dawley rats (6-weeks old; weight,
180–230 g, n=20) were purchased from Animal Experiment Center of
Tianjin Medical University and were housed in polycarbonate cages
at (22±2°C) with 55±5% relative humidity, 12-h light/dark cycle
with free access to food and water for an adaptation period of 3
days. The experimental protocol was approved by the Animal Care and
Use Review Committee of Tianjin Hospital (Tianjin, China). Rats
were anesthetized with 2% of isoflurane (Sinopharm Chemical Reagent
Co., Ltd., Shanghai, China) and ovaries were removed bilaterally.
Rats were randomly divided into two treatment groups: i)
Sham-operated group (control); and ii) ovariectomized rats (model).
Rats were anesthetized with 2% of isoflurane (Sinopharm Chemical
Reagent Co., Ltd.), and peripheral blood was gathered from caudal
vein.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was prepared from blood samples using an
RNeasy Mini kit (Qiagen, Inc., Valencia, CA, USA). Total RNA (1 µg)
was reverse-transcribed at 37°C for 30 min and 85°C for 1 min to
synthesize complementary cDNA using the SuperScript III
First-Strand Synthesis System (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) using the following primers:
miR-148a RT-primer,
5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACAACAAAGTT-3′ and U6
RT-primer, 5′-CGCTTCACGAATTTGCGTGTCAT-3′. qPCR was performed using
the SYBR Premix Ex Tag kit (Takara Bio, Inc., Otsu, Japan) by an
ABI 7500 Sequencing Detection system (Applied Biosystems; Thermo
Fisher Scientific, Inc.). The reaction conditions were: 95°C for 10
min, followed by 40 cycles of 95°C for 30 sec and 60°C for 30 sec.
The following primers used for qPCR were: MicroRNA-148a forward,
5′-GCTAGTGTTCTGAGACACTCCG-3′ and reverse, 5′-GTGCAGGGTCCGAGGT-3′;
and U6 forward, 5′-GCTTCGGCAGCACATATACTAAAAT-3′ and reverse,
5′-CGCTTCACGAATTTGCGTGTCAT-3′. The relative expression was analyzed
by the 2−ΔΔCq method (15).
H&E staining
The caput femoris (n=6/group) was collected and
fixed with 10% paraformaldehyde for 72 h at room temperature.
Formalin-fixed paraffin-embedded sections of caput femoris were
sectioned at 10 µM and stained with H&E for 30 min at room
temperature. Tissue samples were observed using an Olympus
fluorescent photomicroscope (Olympus Corporation, Tokyo,
Japan).
Cell culture and transfection
MC3T3-E1 cells were obtained from The Cell Bank of
Type Culture Collection of Chinese Academy of Sciences (Shanghai,
China) and maintained in Dulbecco's modified Eagle's medium (Gibco;
Thermo Fisher Scientific, Inc.) supplemented with 10%
heat-inactivated fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.) and at 37°C with 5% CO2. MC3T3-E1
cells treated with RANKL (50 ng/ml; R&D Systems, Inc.,
Minneapolis, MN, USA) for 4 days. MC3T3-E1 cells (1×105
cell/well) were transfected with 100 nM microRNA-148a
(5′-GAGGCAAAGTTCTGAGA-3′ and 5′-AGAACTTTGTCTC-3′), 100 nM
anti-microRNA-148a (5′-CTCCGTTTCAAGACTCTGGC-3′ and
5′-TCTTGAAACAGAGGAGA-3′), and negative mimics (5′-CCCCCCCCC-3′)
using Lipofectamine® 3000 (Thermo Fisher Scientific,
Inc.). MC3T3-E1 cells were treated with RANKL (50 ng/ml; R&D
Systems, Inc.) for 3 days, 6 h after transfection. ERα inhibitor,
AZD9496 (0.1 nM; MedChemExpress, Shanghai, China) was added to
transfected cells for 24 h at 37°C.
Cytotoxicity assay
Following transfection at 24, 48 and 72 h the cells
were seeded onto 96-well plates at a density of 3×104
cells/well. The cells were stained with MTT solution for 4 h and
dimethyl sulfoxide was added into the wells for 20 min. The
absorbance was detected using a FlexStation 3 Multi-Mode microplate
reader (Molecular Devices, LLC, Sunnyvale, CA, USA) at 492 nm.
Apoptosis assay
Cells were seeded onto 6-well plates at a density of
1×106 cells/well 48 h after transfection. The cells were
then stained with Annexin-V and propidium iodide assay kit (BD
Biosciences, Franklin Lakes, NJ, USA) for 15 min. The apoptotic
rate was quantified using C6 flow cytometer (BD Biosciences) and
analyzed using FlowJo 7.6.1 (FlowJo; Tree Star, Inc., Ashland, OR,
USA).
Western blotting and caspase-3/9
activity levels
Protein was extracted with RIPA lysis buffer
(Beyotime Institute of Biotechnology, Jiangsu, China). Total
protein was quantified using Enhanced BCA Protein Assay kit
(Beyotime Institute of Biotechnology). Equal quantity (50 µg) of
total protein was resolved by 10% SDS-PAGE and transferred onto a
polyvinylidene difluoride membrane (EMD Millipore, Bedford, MA,
USA). The membranes were blocked with 5% non-fat milk in
tris-buffered saline with 0.1% Tween-20 for 1 h at 37°C and
incubated with various primary antibodies: Bcl2-associated X (Bax;
1:500; sc-6236; Santa Cruz Biotechnology, Inc., Dallas, TX, USA),
PI3K (1:500; sc-7174; Santa Cruz Biotechnology, Inc.), estrogen
receptor a (ERα; sc-544, 1:500; Santa Cruz Biotechnology, Inc.),
phosphorylated (p)-AKT (cat. nos. sc-293125 and sc-7985-R, 1:500;
Santa Cruz Biotechnology, Inc.) and GAPDH (cat. no. sc-25778;
1:2,000; Santa Cruz Biotechnology, Inc.) at 4°C overnight and
subsequently incubated with an anti-mouse immunoglobulin G (IgG)
antibody conjugated to horseradish peroxidase (cat. no. AAT-16440;
1:5,000; Amyjet Scientific Inc.,) for 1 h at 37°C. The membranes
were visualized using enhanced chemiluminescence kits (GE
Healthcare, Chicago, IL, USA) and assessed by densitometry using
MacBiophotonics ImageJ version 1.41a (https://imagej.nih.gov/ij/).
Equal quantity (50 µg) of total protein was used to
quantify caspase-3/9 activity levels using commercial kits (C1116
and C1158; Beyotime Institute of Biotechnology). The absorbance was
detected using a FlexStation 3 Multi-Mode microplate reader at 405
nm.
Luciferase reporter assay
mRNA of ERα 3′UTR was inserted downstream of the
luciferase reporter gene in a pMIR-REPORT vector (Thermo Fisher
Scientific, Inc.). HEK293 cells (1×105 cell) were
co-transfected with miR-148a mimic and ERα-3′UTR (Promega
Corporation, Madison, WI, USA) using Lipofectamine® 2000
(Thermo Fisher Scientific, Inc.). The luciferase activities were
measured using the Dual-Luciferase Reporter Assay System (Promega
Corporation) after 48 h of incubation at 37°C with 5%
CO2. The ratio of Renilla luciferase of firefly
luciferase was calculated for each well.
Statistical analysis
Data are expressed as the mean ± standard deviation.
Statistical significance was determined using Student's t-test or
one-way analysis of variance which was followed by Tukey's Honest
Significant Difference as a post hoc test. P<0.05 was considered
to indicate a statistically significant difference.
Results
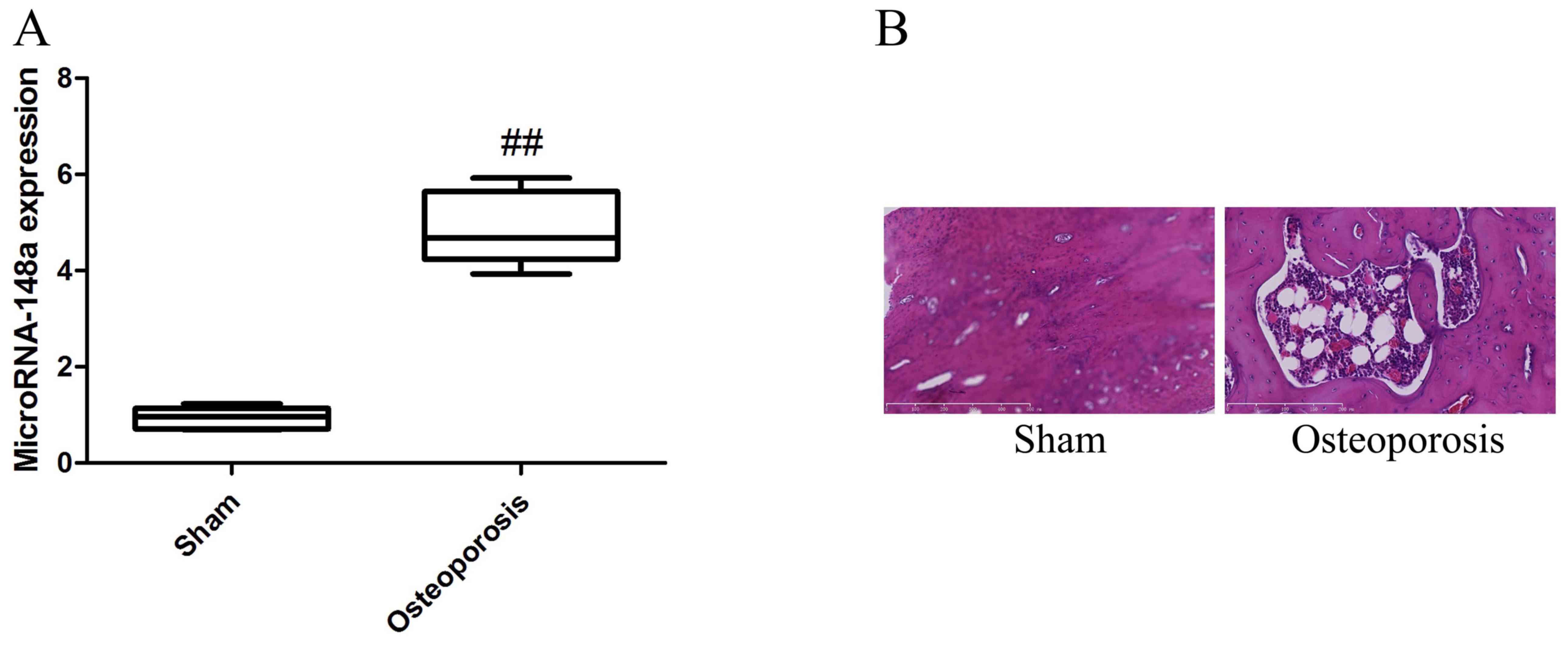
Expression of microRNA-148a in
osteoporosis rats following ovariectomy
RT-qPCR was used to analyze the changes of microRNAs
in osteoporosis rats following ovariectomy and it was determined
that microRNA-148a expression level was significantly upregulated
in the osteoporosis rats following ovariectomy when compared the
sham group (Fig. 1).
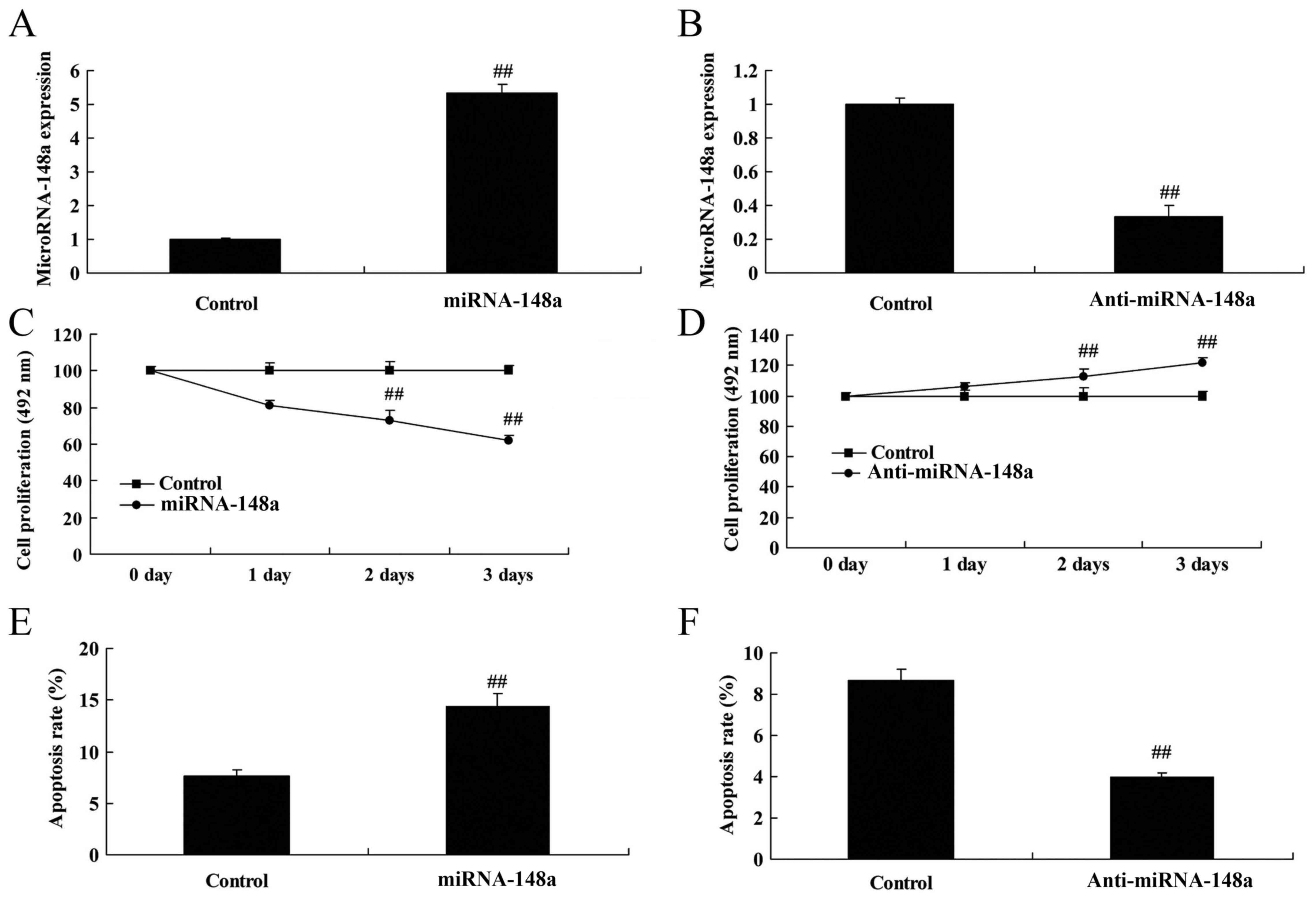
Effects of microRNA-148a on bone cell
growth in vitro
The function of microRNA-148a on bone cell growth in
osteoblasts in vitro was investigated using microRNA-148a
and anti-microRNA-148a mimics to upregulate and downregulate
microRNA-148a expression in osteoblasts in vitro (Fig. 2). Overexpression of microRNA-148a
inhibited bone cell growth and induced apoptosis in osteoblasts
in vitro (Fig. 2C and E).
However, downregulation of microRNA-148a promoted bone cell growth
and reduced apoptosis in osteoblasts in vitro (Fig. 2D and F). Additionally,
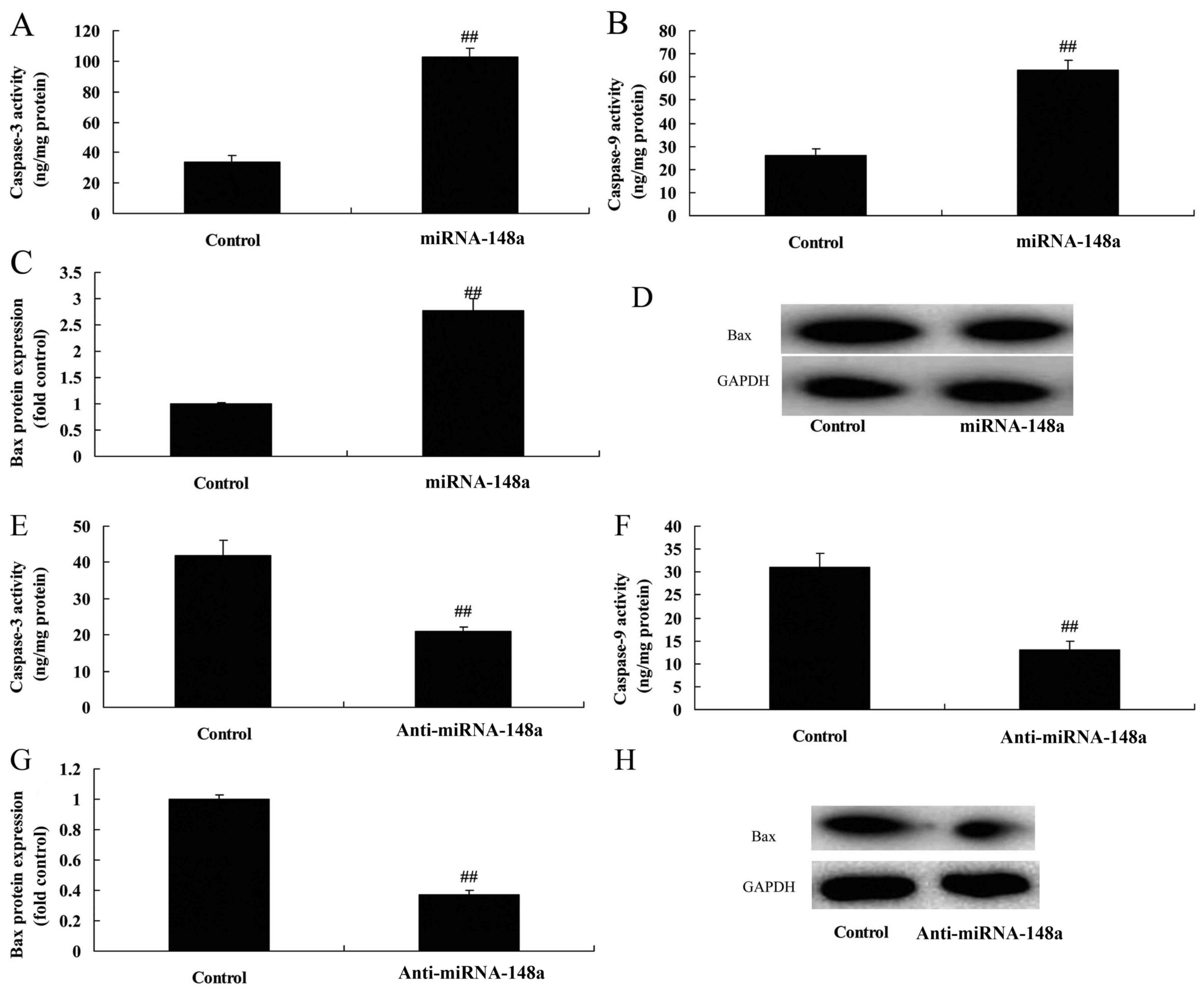
overexpression of microRNA-148a induced Bax protein expression and
caspase-3/9 activity in osteoblasts in vitro (Fig. 3A-D). Downregulation of
microRNA-148a reduced Bax protein expression and caspase-3/9
activity in osteoblasts in vitro (Fig. 3E-H).
Effects of microRNA-148a on bone cell
growth in vitro through PI3K/AKT signaling by ERa
The present study investigated the effect of
microRNA-148a on bone cell growth in vitro, and the effect
of ovariectomy-induced osteoporosis association with ERa.
Bioinformatics and luciferase reporter assays revealed that ERα was
a target gene of microRNA-148a (Fig.
4A) and overexpression of microRNA-148a reduced and reduced
ERa, PI3K and p-Akt protein expression levels in osteoblasts in
vitro (Fig. 4B-E). However,
the downregulation of microRNA-148a upregulated ERα, PI3K and p-Akt
protein expression levels in osteoblasts in vitro (Fig. 4F-I).
 | Figure 4.Effect of miRNA-148a expression on
osteoblast cell growth in vitro PI3K/AKT signaling. (A)
Bioinformatics and luciferase reporter assays revealed that ERα may
be a target gene of miRNA-148a. (B) Western blotting was used to
quantify ERα, PI3K and p-AKT protein expression in the miRNA-148a
group. Quantification of (C) PI3K, (D) ERα and (E) p-Akt protein
expression levels in the miRNA-148 a group. (F) Western blotting
was used to quantify ERα, PI3K and p-AKT protein expression in the
miRNA-148a group. Quantification of (G) ERα, (H) PI3K and (I) p-Akt
protein expression levels in the anti-miRNA-148 a group.
miRNA-148a, overexpressing group; Anti-miRNA-148a, downregulating
group. ##P<0.01 vs. control group. miRNA, microRNA;
p, phosphorylated; AKT, protein kinase B; PI3K, phosphoinositide
3-kinase; ERα, estrogen receptor α. |
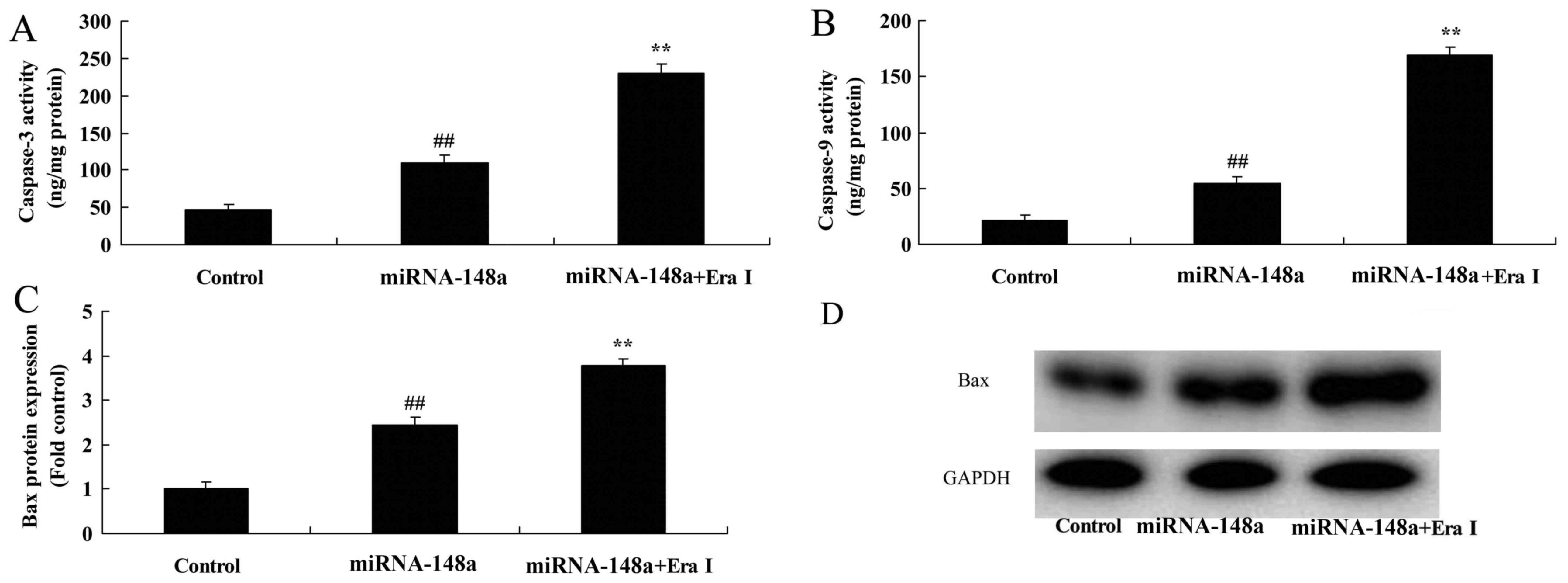
Inhibition of ERα increases the effect
of microRNA-148a on apoptosis in osteoblasts in vitro
In order to investigate the underlying effect of
microRNA-148a on osteoporosis, the present study used ERα
inhibitor, 0.1 nM of AZD9496, to reduce reduced ERα expression in
osteoblasts in vitro. As presented in Fig. 5A-D, the inhibition of ERα (BHPI,
inhibitor used) reduced ERα, PI3K and p-Akt protein expression in
osteoblasts in vitro following microRNA-148a transfection
when compared with the microRNA-148a only group. The inhibition of
ERα increased the effect of the microRNA-148a the inhibition of
osteoblast growth and activation of apoptosis in osteoblasts in
vitro when compared with the microRNA-148a only group (Fig. 5E and F). Additionally, the
inhibition of ERα increased Bax protein expression and caspase-3/9
activity in osteoblasts in vitro (Fig. 6).
Inhibition of PI3K reduces the effect
of microRNA-148a on osteoblast apoptosis in vitro
In order to investigate whether the effect of
microRNA-148a on osteoblast apoptosis in vitro by PI3K/AKT
signaling the present study used an PI3K inhibitor to reduce
PI3K/AKT signaling in osteoblasts in vitro. As presented in
Fig. 7A-C, the PI3K inhibitor
reduced PI3K/AKT signaling in osteoblasts in vitro following
microRNA-148a transfection when compared with microRNA-148a only
group. Subsequently, the inhibition of PI3K reduced the effect of
microRNA-148a on the inhibition of osteoblast proliferation and
increased the apoptotic rate in osteoblasts in vitro when
compared with the microRNA-148a only group (Fig. 7D and E). The inhibition of PI3K
also increased Bax protein expression levels and caspase-3,
caspase-9 activity in osteoblasts in vitro (Fig. 7F-I).
Discussion
Patients with osteoporosis worldwide have exceeded
200 million, including 80% of patients with postmenopausal
osteoporosis and fractures in 50% of postmenopausal women are
associated with osteoporosis (7).
In addition, a previous study revealed the that annual bone loss
rate of women after 5–10 years of menopause is 2–4% and which is
evidently higher than 1% estimated in men (6). OIO is a common disease which
frequently occurs in the elderly. The commonest one is
postmenopausal osteoporosis (16).
As the global population is aging the morbidity of osteoporosis is
growing at an alarming rate. Therefore, prevention of osteoporosis
may become an important research topic in the future (17). To the best of our knowledge, the
present study is the first to demonstrate that microRNA-148a
expression was upregulated in osteoporosis rats following
ovariectomy.
Thousands of miRNAs have been previously discovered
in various organisms with increasingly intensive research on miRNA
(12). miRNAs regulate various
activities in organisms, such as growth, development and aging
(18). It has been estimated that
~50% human genes are regulated by miRNAs. This is currently
verified by using bioinformatics and experiments where one miRNA
may specifically bind to multiple genes (18). Additionally, one gene may also be
regulated by multiple miRNAs, thus exerting the regulatory function
(19). The presents study
determined that overexpression of microRNA-148a inhibited
osteoblast proliferation and induced apoptosis of osteoblasts in
vitro.
Although instruments and methods used for detecting
the degree of osteoporosis quantitatively domestically and overseas
are constantly improving constantly, there is a lack of effective
measures in terms of prevention, additionally drug treatments are
very limited (20). Currently, ERα
remains to be the primary target for the prevention of bone loss in
postmenopausal women (21). A
previous study revealed that ERa may prevent the occurrence of
postmenopausal osteoporosis (22).
The present in vitro study revealed that overexpression of
microRNA-148a reduced ERα, PI3K and p-AKT protein expression levels
in osteoblasts in vitro. Ma et al previously reported
that microRNA-148a suppresses estrogen induced viability and
migration through ERα expression in breast cancer cells (23). Zhang et al suggested that
microRNA-148a promotes cancer cell growth by targeting PI3K/Akt
protein expression in osteosarcoma (24).
PI3K in the signaling pathway required for
osteoblast differentiation. PI3K stimulates mesenchymal stem cells
(MSCs) to differentiate into the osteoblasts and promote bone
formation (25). In MSCs,
activating PI3K/AKT signal pathway may reduce bone morphogenetic
protein-induced alkaline phosphatase (ALP) and osteopontin
expression, in order to impact osteoprogenitor cells and reduce
osteoblasts (10). PI3K
kinase-specific inhibitor prevents the PI3K signaling pathway from
excitation. A previous study used ALP staining to observe changes
of preosteoblastic cell MC3T3E1 differentiation and revealed that
blocking the excitation of the signal pathway may limit ALP
activity. Based on the findings of the current study it is possible
that the inhibition of ERα or PI3K may significantly increase the
effect of microRNA-148a on apoptosis of osteoblasts in
vitro.
In conclusion, the present study demonstrated that
microRNA-148a significantly increased apoptosis in ovariectomized
rats via PI3K/AKT signaling. Therefore, microRNA-148a/PI3K/AKT
signaling pathway is a promising candidate for the development of
future therapeutic agents against osteoporosis in postmenopausal
women.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
YX designed the experiment, analyzed the data and
wrote the manuscript. YX, BL and JL performed the experiment.
Ethics approval and consent to
participate
The experimental protocol was approved by the Animal
Care and Use Review Committee of Tianjin Hospital (Tianjin,
China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Krantz E, Trimpou P and Landin-Wilhelmsen
K: Effect of growth hormone treatment on fractures and quality of
life in postmenopausal osteoporosis: A 10-year follow-up study. J
Clin Endocrinol Metab. 100:3251–3259. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hansen KE, Nabak AC, Johnson RE,
Marvdashti S, Keuler NS, Shafer MM and Abrams SA: Isotope
concentrations from 24-h urine and 3-h serum samples can be used to
measure intestinal magnesium absorption in postmenopausal women. J
Nutr. 144:533–537. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sugimoto T, Nakamura T, Nakamura Y, Isogai
Y and Shiraki M: Profile of changes in bone turnover markers during
once-weekly teriparatide administration for 24 weeks in
postmenopausal women with osteoporosis. Osteoporos Int.
25:1173–1180. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bonnick S, De Villiers T, Odio A, Palacios
S, Chapurlat R, DaSilva C, Scott BB, Le Bailly De Tilleghem C,
Leung AT and Gurner D: Effects of odanacatib on BMD and safety in
the treatment of osteoporosis in postmenopausal women previously
treated with alendronate: A randomized placebo-controlled trial. J
Clin Endocrinol Metab. 98:4727–4735. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu HF, He HC, Yang L, Yang ZY, Yao K, Wu
YC, Yang XB and He CQ: Pulsed electromagnetic fields for
postmenopausal osteoporosis and concomitant lumbar osteoarthritis
in southwest China using proximal femur bone mineral density as the
primary endpoint: Study protocol for a randomized controlled trial.
Trials. 16:2652015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ruixo Pérez JJ, Zheng J and Mandema JW:
Similar relationship between the time course of bone mineral
density improvement and vertebral fracture risk reduction with
denosumab treatment in postmenopausal osteoporosis and prostate
cancer patients on androgen deprivation therapy. J Clin Pharmacol.
54:503–512. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stuss M, Rieske P, Cegłowska A,
Stêpień-Kłos W, Liberski PP, Brzeziańska E and Sewerynek E:
Assessment of OPG/RANK/RANKL gene expression levels in peripheral
blood mononuclear cells (PBMC) after treatment with strontium
ranelate and ibandronate in patients with postmenopausal
osteoporosis. J Clin Endocrinol Metab. 98:E1007–E1011. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bai P, Sun Y, Jin J, Hou J, Li R, Zhang Q
and Wang Y: Disturbance of the OPG/RANK/RANKL pathway and systemic
inflammation in COPD patients with emphysema and osteoporosis.
Respir Res. 12:1572011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Baek JM, Kim JY, Yoon KH, Oh J and Lee MS:
Ebselen is a potential anti-osteoporosis agent by suppressing
receptor activator of nuclear factor kappa-B ligand-induced
osteoclast differentiation in vitro and lipopolysaccharide-induced
inflammatory bone destruction in vivo. Int J Biol Sci. 12:478–488.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li H, Li T, Fan J, Li T, Fan L, Wang S,
Weng X, Han Q and Zhao RC: miR-216a rescues dexamethasone
suppression of osteogenesis, promotes osteoblast differentiation
and enhances bone formation, by regulating c-Cbl-mediated PI3K/AKT
pathway. Cell Death Differ. 22:1935–1945. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yuan FL, Xu RS, Jiang DL, He XL, Su Q, Jin
C and Li X: Leonurine hydrochloride inhibits osteoclastogenesis and
prevents osteoporosis associated with estrogen deficiency by
inhibiting the NF-κB and PI3K/Akt signaling pathways. Bone.
75:128–137. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hao L, Fu J, Tian Y and Wu J: Systematic
analysis of lncRNAs, miRNAs and mRNAs for the identification of
biomarkers for osteoporosis in the mandible of ovariectomized mice.
Int J Mol Med. 40:689–702. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen J, Qiu M, Dou C, Cao Z and Dong S:
MicroRNAs in bone balance and osteoporosis. Drug Dev Res.
76:235–245. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li H, Wang Z, Fu Q and Zhang J: Plasma
miRNA levels correlate with sensitivity to bone mineral density in
postmenopausal osteoporosis patients. Biomarkers. 19:553–556. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brixen K, Chapurlat R, Cheung AM, Keaveny
TM, Fuerst T, Engelke K, Recker R, Dardzinski B, Verbruggen N,
Ather S, et al: Bone density, turnover, and estimated strength in
postmenopausal women treated with odanacatib: A randomized trial. J
Clin Endocrinol Metab. 98:571–580. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Binkley N, Bolognese M,
Sidorowicz-Bialynicka A, Vally T, Trout R, Miller C, Buben CE,
Gilligan JP and Krause DS: Oral Calcitonin in Postmenopausal
Osteoporosis (ORACAL) Investigators: A phase 3 trial of the
efficacy and safety of oral recombinant calcitonin: The Oral
Calcitonin in Postmenopausal Osteoporosis (ORACAL) trial. J Bone
Miner Res. 27:1821–1829. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ma Y, Shan Z, Ma J, Wang Q, Chu J, Xu P,
Qin A and Fan S: Validation of downregulated microRNAs during
osteoclast formation and osteoporosis progression. Mol Med Rep.
13:2273–2280. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jiang ZS and Hao ZH: An insertion/deletion
polymorphism within the 3′-untranslated region of COL1A2 confers
susceptibility to osteoporosis. Mol Med Rep. 14:4415–4421. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang D, Maalouf NM, Adams-Huet B, Moe OW
and Sakhaee K: Effects of sex and postmenopausal estrogen use on
serum phosphorus levels: A cross-sectional study of the National
Health and Nutrition Examination Survey (NHANES) 2003–2006. Am J
Kidney Dis. 63:198–205. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Martynetz FA, Pessole Biondo-Simões Mde L,
Martynetz JR, Martynetz TD, Zimerman E and Neto HM: Tomografic and
tensiometric assessment on femurs from oophorectomized rats
subjected to hormone replacement therapy. Rev Bras Ortop. 45:40–45.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sanders S and Geraci SA: Osteoporosis in
postmenopausal women: Considerations in prevention and treatment:
(women's health series). South Med J. 106:698–706. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ma F, Feng Y, Li W, Li Z, Liu T and Li L:
miR-148a suppresses estrogen-induced viability and migration of
breast cancer cells via inhibition of estrogen receptor α
expression. Exp Ther Med. 13:2515–2522. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang H, Wang Y, Xu T, Li C, Wu J, He Q,
Wang G, Ding C, Liu K, Tang H and Ji F: Increased expression of
microRNA-148a in osteosarcoma promotes cancer cell growth by
targeting PTEN. Oncol Lett. 12:3208–3214. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ge P, Cui Y, Liu F, Luan J, Zhou X and Han
J: L-carnitine affects osteoblast differentiation in NIH3T3
fibroblasts by the IGF-1/PI3K/Akt signalling pathway. Biosci
Trends. 9:42–48. 2015. View Article : Google Scholar : PubMed/NCBI
|