Introduction
Parkinson's disease (PD) is the most frequently
occurring neurodegenerative disease following Alzheimer's disease
(1,2). The primary pathogenesis is the loss
of dopamine (DA) neurons in the substantia nigra pars compacta and
the accumulation of ubiquitinated α-synuclein in the remaining
nigra DA neurons (1). At present,
the primary purpose of preclinical research is to identify a novel
therapeutic or therapeutic target, or develop a treatment strategy
to decrease the rate of and inhibit neurodegeneration (3). However, as of yet, no current drug
used in clinical practice has been able to inhibit the
neurodegeneration associated with the disease.
It has previously been demonstrated that oxidative
stress injury is the final pathological event in the progression of
PD (4). It has therefore been
hypothesized that if a particular medicine may inhibit the
generation of reactive oxygen species and nitric oxide and repair
the damaged mitochondrial complex, it may act as a neuroprotective
reagent (5).
Acetylcholinesterase (AChE) interferes via
hydrolysis of the acetylcholine neurotransmitter, to terminate
nerve impulses (6). It has
previously been demonstrated that AChE may promote cell apoptosis
and an AChE inhibitor in the treatment of Parkinson's disease may
prevent apoptosis of dopaminergic neurons in vitro and in
vivo by dopaminergic neurotoxicity (6).
Azadirachta indica is termed ‘Arishtha’ in
Sanskrit which means ‘the eliminator of pain’. It was traditionally
regarded as a therapeutic product in India (7). In Ayuveda medicine theory, the bark
is used in tonics, as an astringent, anthelmintic and anti-pyretic.
Furthermore, Azadirachta indica is an effective
lipid-lowering medicine, hypoglycemic agent, immunopotentiator,
hepatoprotective and is used in anti-inflammatory and
anti-fertility agents (8). The aim
of the present study was to evaluate the neuroprotective effects of
Azadirachta indica in the functional recovery of the
6-hydroxydopamine-induced rat Parkinson's model.
Materials and methods
Animals and experimental design
All protocols were approved by the Animal Care and
Welfare Committee of the Institute of Qilu Hospital of Shandong
University (Shandong, China). Male Wistar rats (300–350 g, 8–10
weeks, n=30) were purchased from Vital River Laboratories Co., Ltd.
(Beijing, China) and were maintained under temperature-controlled
conditions with a 12 h light/dark cycle and allowed food and water
ad libitum. Rats were divided into five groups (n=6 animals
per group): i) Sham group, ii) model group, iii) 500 mg/kg
azadirachta group, iv) 1,000 mg/kg azadirachta group; and v) 2,000
mg/kg azadirachta group. In the sham group, healthy rats were
injected intraperitoneally with normal saline. In the model group,
PD rats were injected intraperitoneally with normal saline. In the
three azadirachta treatment groups, PD rats were injected
intraperitoneally with 500, 1,000 and 2,000 mg/kg azadirachta
respectively, for 5 days.
6-Hydroxydopamine induced rat model of
PD
A total of 10 µg 6-hydroxydopamine, purity >98%
(Sigma-Aldrich, Merck KGaA, Darmstadt, Germany), at a final
concentration of 2 µg/µl, was dissolved in 0.1% ascorbic acid.
Wistar rats were anaesthetized with pentobarbital sodium (30 mg/kg,
intraperitoneally) and a burr hole was drilled and then a needle
inserted into the right substantia nigra pars compacta
(anterior-posterior: −5.2, lateral; +2.2, dorsal-ventral; −7.8
relative to bregma). Following infusion, the needle was kept in
place for 10 min. Then, the rotational behavior of the rats, which
was induced by administration of 5 µl of 6-hydroxydopamine (2
µg/µl), was tested 14 days following lesion formation.
Contralateral rotational turns were recorded over a period of 30
min.
Rotational testing
Following surgery, rats underwent rotational testing
and lesion measurement based on the severity of motor behavioral
disorder. Each rat was rotated 360°; the ipsilateral and to the
contralateral sides of the lesion of each rat was measured.
Measurements of oxidative stress and inflammation.
Blood samples were collected and centrifuged at 3,000 × g for 10
min at 4°C. Then, serum was collected and used to analyze catalase
(CAT; A007-1-1), glutathione-peroxidase (GSH)-PX (A005), tumor
necrosis factor (TNF)-α (H052), interleukin (IL)-1β (H002), IL-6
(H007) and nuclear factor (NF)-κB p65 (H202) levels, according to
the manufacturer's protocols (Nanjing Jiancheng Bioengineering
Institute, Nanjing, China). Absorbance values were measured by a
microplate reader (Molecular Devices, LLC, Sunnyvale, CA, USA) at
450 nm.
Measurements of inducible nitric oxide synthase
(iNOS) and AChE activity levels. Hippocampus tissue samples were
collected and homogenized in 1:3 (w/v) ice-cold
radioimmunprecipiatation assay buffer (Beyotime Institute of
Biotechnology, Nanjing, China) with a protease inhibitor mixture.
The concentration of protein was determined with a Bicinchoninic
acid (BCA) Protein Assay kit (Pierce; Thermo Fisher Scientific,
Inc., Waltham, MA, USA). The supernatant was collected and used to
analyze iNOS (A014-1-1) and AChE (A105-1) activity levels according
to the manufacturer's protocols (Nanjing Jiancheng Bioengineering
Institute). Absorbance values were measured with a microplate
Reader (Molecular Devices LLC) at 450 nm.
Western blotting
Hippocampus tissue samples were collected and
homogenized in 1:3 (w/v) ice-cold radiimmunoprecipitation assay
buffer with a protease inhibitor mixture. The concentration of
protein was determined with a BCA Protein Assay kit (Pierce, Thermo
Fisher Scientific, Inc.). Proteins (50 µg) were resolved by 10–12%
SDS-polyacrylamide gel electrophoresis and transferred to
polyvinylidene difluoride membranes. The membranes were incubated
overnight at 4°C with primary antibodies against: B cell lymphoma
(Bcl)-2 associated X protein (Bax, sc-6236, 1:500), cytochrome
c (sc-7159, 1:500) and β-actin (sc-7210, 1:4,000; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA). Following washing 4 times
with Tris buffered saline-Tween-20, the membranes were incubated
with horseradish peroxidase-conjugated anti-rabbit secondary
antibodies (sc-2004, 1:5,000; Santa Cruz Biotechnology, Inc.) for 1
h at room temperature. Immunoreactive bands were visualized using
an enhanced chemiluminescence detection kit (GE Healthcare Life
Sciences, Shanghai, China) and quantified using ImageJ software
v3.0 (National Institutes of Health, Bethesda, CA, USA) and an
Alliance LD system (Uvitec, Cambridge, UK).
Statistical analysis
All numerical data was reported as the mean ±
standard deviation using SPSS 20.0 (IBM Corp., Armonk, NY, USA,
n=3) and analyzed using one-way analysis of variance and a Tukey's
post hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Neuroprotective effects of Azadirachta
indica improve rotational behavior in 6-hydroxydopamine induced rat
Parkinson model
PD rats were used to investigate the neuroprotective
effects of Azadirachta indica and it was demonstrated that
it improved the rotational behavior. As presented in Fig. 1, there was a significant increase
in the turning values of the PD model group, when compared with the
sham group. In the 1,000 or 2,000 mg/kg Azadirachta indica
treated groups, turning values significantly decreased compared
with the PD model group.
Neuroprotective effects of Azadirachta
indica increase CAT and GSH-PX levels in 6-hydroxydopamine induced
rat Parkinson model
Furthermore, a significant decrease in CAT and
GSH-PX levels of the PD model group was observed compared with sham
group (Fig. 2). However, treatment
with 1,000 or 2,000 mg/kg Azadirachta indica significantly
increased CAT and GSH-PX levels in PD rats when compared with the
PD model group (Fig. 2).
 | Figure 2.CAT and GSH-PX levels in
6-hydroxydopamine induced rat Parkinson model. Neuroprotective
effects of Azadirachta indica improve (A) CAT and (B) GSH-PX
levels, in 6-hydroxydopamine induced rat Parkinson model. Sham,
sham group; Model, Parkinson model group; 500, 500 mg/kg
azadirachta group; 1,000, 1,000 azadirachta group; 2,000, 2,000
mg/kg azadirachta group. ##P<0.01 vs. sham group;
*P<0.01 vs. model group; **P<0.01 vs. model group. CAT,
catalase; GSH-PX, glutathione-peroxidase. |
Neuroprotective effects of Azadirachta
indica inhibit iNOS level in 6-hydroxydopamine induced rat
Parkinson model
When compared with sham group, iNOS activity level
was significantly enhanced in PD rats (Fig. 3). iNOS activity level was then
significantly inhibited by treatment with 1,000 or 2,000 mg/kg
Azadirachta indica compared with PD model group (Fig. 3).
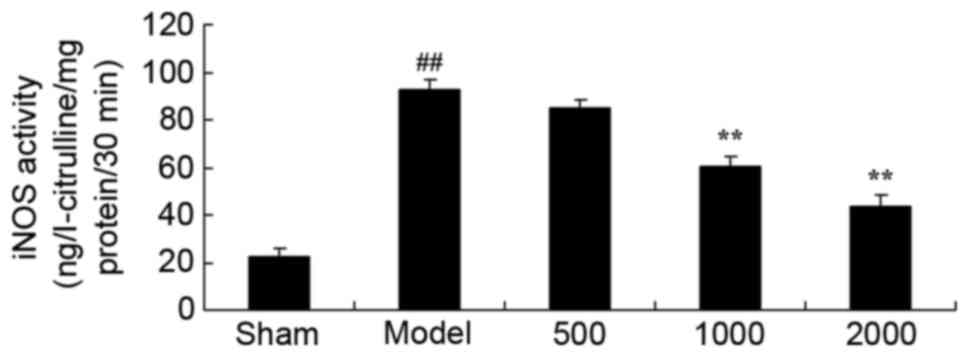 | Figure 3.iNOS level in 6-hydroxydopamine
induced rat Parkinson model. Treatment with Azadirachta
indica decreased iNOS level in 6-hydroxydopamine induced rat
Parkinson model. Sham, sham group; Model, Parkinson model group;
500, 500 mg/kg azadirachta group; 1,000, 1,000 azadirachta group;
2,000, 2,000 mg/kg azadirachta group. ##P<0.01 vs.
sham group; **P<0.01 vs. model group. iNOS, inducible nitric
oxide synthase. |
Neuroprotective effects of Azadirachta
indica inhibit inflammation in 6-hydroxydopamine induced rat
Parkinson model
Additionally, PD significantly enhanced TNF-α,
IL-1β, IL-6 and NF-κB of p65 levels in rats compared with sham
group (Fig. 4). However, treatment
with 1,000 or 2,000 mg/kg Azadirachta indica significantly
suppressed the PD-induced TNF-α, IL-1β, IL-6 and NF-κB of p65
levels in rats compared with PD model group (Fig. 4).
 | Figure 4.Inflammation in 6-hydroxydopamine
induced rat Parkinson model. Azadirachta indica decreases
(A) TNF-α, (B) IL-1β, (C) IL-6 and (D) NF-κB of p65 levels in
6-hydroxydopamine induced rat Parkinson model. Sham, sham group;
Model, Parkinson model group; 500, 500 mg/kg azadirachta group;
1,000, 1,000 azadirachta group; 2,000, 2,000 mg/kg azadirachta
group. ##P<0.01 vs. sham group; *P<0.01 vs. model
group; **P<0.01 vs. model group. TNF-α, tumor necrosis factor;
IL, interleukin; NF-κB; nuclear factor-κB p65 levels. |
Neuroprotective effects of Azadirachta
indica inhibit AChE activity in 6-hydroxydopamine induced rat
Parkinson model
As presented in Fig.
5, a significant increase in AChE activity was observed in the
PD model group, compared with the sham group. However,
Azadirachta indica at 1,000 or 2,000 mg/kg resulted in a
significant decrease in AChE activity compared with PD model group
(Fig. 5).
 | Figure 5.AChE activity in 6-hydroxydopamine
induced rat Parkinson model. Azadirachta indica decreases
AChE activity in the 6-hydroxydopamine induced rat Parkinson model.
Sham, sham group; Model, Parkinson model group; 500, 500 mg/kg
azadirachta group; 1,000, 1,000 azadirachta group; 2,000, 2,000
mg/kg azadirachta group. ##P<0.01 vs. sham group;
*P<0.01 vs. model group; **P<0.01 vs. model group. AChE,
acetylcholinesterase. |
Neuroprotective effects of Azadirachta
indica inhibit cyclo-oxygenase (COX)-2 protein expression in
6-hydroxydopamine induced rat Parkinson model
As presented in Fig.
6, there was a significant increase in COX-2 protein expression
in the PD model group, compared with sham group. Treatment with
1,000 or 2,000 mg/kg of Azadirachta indica significantly
suppressed COX-2 protein expression in 6-hydroxydopamine induced
rats (Fig. 6).
 | Figure 6.COX-2 protein expression in
6-hydroxydopamine induced rat Parkinson model. (A) Representative
image and (B) quantitative analysis of COX-2 protein expression in
6-hydroxydopamine induced rat Parkinson model samples treated with
differing concentrations of Azadirachta indica, detected via
western blotting. Sham, sham group; Model, Parkinson model group;
500, 500 mg/kg azadirachta group; 1,000, 1,000 azadirachta group;
2,000, 2,000 mg/kg azadirachta group. ##P<0.01 vs.
sham group; *P<0.01 vs. model group; **P<0.01 vs. model
group. COX-2, cyclo-oxygenase-2. |
Neuroprotective effects of Azadirachta
indica inhibit caspase-3 and caspase-9 activity in
6-hydroxydopamine induced rat Parkinson model
Statistical analysis indicated that caspase-3 and
caspase-9 activities in PD model rats significantly increased, when
compared with sham group (Fig. 7).
In addition, 1,000 or 2,000 mg/kg treatment with Azadirachta
indica significantly reduced the caspase-3 and caspase-9
activities in PD rats compared with PD model group (Fig. 7).
Neuroprotective effects of Azadirachta
indica inhibits Bax protein expression in 6-hydroxydopamine induced
rat Parkinson model
PD significantly induced Bax protein expression in
rats compared with the sham group (Fig. 8). Correspondingly, treatment with
Azadirachta indica significantly suppressed the protein
expression of Bax in PD rats compared with PD model group (Fig. 8).
 | Figure 8.Bax protein expression in
6-hydroxydopamine induced rat Parkinson model. (A) Representative
image and (B) quantitative analysis of Bax protein expression in
6-hydroxydopamine induced rat Parkinson model samples treated with
differing concentrations of Azadirachta indica, detected via
western blotting. Sham, sham group; Model, Parkinson model group;
500, 500 mg/kg azadirachta group; 1,000, 1,000 azadirachta group;
2,000, 2,000 mg/kg azadirachta group. ##P<0.01 vs.
sham group; *P<0.01 vs. model group; **P<0.01 vs. model
group. Bax, B cell lymphoma-2 associated X protein. |
Neuroprotective effects of Azadirachta
indica inhibit cytochrome c in 6-hydroxydopamine induced rat
Parkinson model
Statistical analysis revealed that PD significantly
activated cytochrome c protein expression in PD rats
compared with the sham group (Fig.
9). Notably, treatment with Azadirachta indica
significantly suppressed cytochrome c in PD rats (Fig. 9).
 | Figure 9.Cytochrome c in
6-hydroxydopamine induced rat Parkinson model. (A) Representative
image and (B) quantitative analysis of cytochrome c protein
expression in 6-hydroxydopamine induced rat Parkinson model samples
treated with differing concentrations of Azadirachta indica,
detected via western blotting. Sham, sham group; Model, Parkinson
model group; 500, 500 mg/kg azadirachta group; 1,000, 1,000
azadirachta group; 2,000, 2,000 mg/kg azadirachta group.
##P<0.01 vs. sham group; *P<0.01 vs. model group;
**P<0.01 vs. model group. |
Neuroprotective effects of Azadirachta
indica inhibit p53 protein expression in 6-hydroxydopamine induced
rat Parkinson model
As presented in Fig.
10, it was observed that there was a significant increase in
p53 protein expression in PD rats compared with the sham group.
However, Azadirachta indica significantly suppressed p53
protein expression in PD rats (Fig.
10).
 | Figure 10.p53 protein expression in
6-hydroxydopamine induced rat Parkinson model. (A) Representative
image and (B) quantitative analysis of p53 protein expression in
6-hydroxydopamine induced rat Parkinson model samples treated with
differing concentrations of Azadirachta indica, detected via
western blotting. Sham, sham group; Model, Parkinson model group;
500, 500 mg/kg azadirachta group; 1,000, 1,000 azadirachta group;
2,000, 2,000 mg/kg azadirachta group. ##P<0.01 vs.
sham group; *P<0.01 vs. model group; **P<0.01 vs. model
group. |
Discussion
PD is additionally termed paralysis agitans, the
incidence rate of PD is as high as 10% in the population
>65-years-old, and China has a similar PD incidence rate,
according to an epidemiological survey (9,10).
PD is a chronic progressive disease of the nervous system that
results from dysfunction of the extrapyramidal system and is a
neurodegenerative disease, similar to Alzheimer's and lateral
sclerosis of muscular atrophy (9,10).
The neuroprotective effect of Azadirachta indica improved
rotational behavior in the 6-hydroxydopamine induced rat Parkinson
model.
Oxidative stress is the imbalance between oxidation
and anti-oxidation in cells, and results from excess active oxygen
and loss of antioxidants (11,12).
Oxidative stress is necessary at the optimum physiological range,
to stimulate proliferation or remove the aging cell components.
However, a large amount of oxidative stress may damage the normal
structure of tissues or the function of cells (12). The DA metabolism level is greater
than the basal level, therefore DA neurons in the substantia nigra
pars compacta are particularly vulnerable to damage resulting from
oxidative stress (4). The present
study demonstrated that Azadirachta indica significantly
increased CAT and GSH-PX levels in PD rats. Omobowale et al
(7) suggested that Azadirachta
indica significantly attenuates oxidative stress and
inflammation in dogs.
AChE has previously been demonstrated to act as a
promoting factor of apoptosis. Therefore, the targeting of AChE as
a site of anti-apoptosis is a novel idea in the neuroprotective
therapy of PD (13). The
disequilibrium of dopamine and acetylcholine in the corpus striatum
is key in the pathogenesis of PD (14). The present study demonstrated that
Azadirachta indica at 1,000 or 2,000 mg/kg resulted in a
significant decrease in AChE activity in PD model rats. Soares
et al (15) suggested that
azadirachtin results in anti-inflammatory and anti-nociceptive
activities in mice via inhibition of AChE.
In the brain of PD patients, the increase of
inflammation and damage of the blood brain barrier may enhance the
interaction between the central nervous system and the peripheral
immune system. As a result, an increased number of leukocytes enter
into the brain parenchyma (16).
Under inflammatory conditions, peripheral immune cells enter the
central nervous system and may result in nerve inflammation and
neurodegeneration via paracrine and endocrine signaling (17). In the present study, it was
demonstrated that Azadirachta indica significantly
suppressed the PD-induced levels of TNF-α, IL-1β, IL-6, NF-κB of
p65 and COX-2 in rats. In addition, Dkhil et al (8) reported the suppressive inflammatory
effects of the Azadirachta indica extract against Eimeria
papillata-induced coccidiosis.
In addition, a pathological study on the brain of PD
patients suggested that NO is important in the progression of PD
(18). Under physiological
conditions, an appropriate level of NO in cells is very important
to maintain redox balance and cell proliferation (19). However, the excess accumulation of
NO may result in an imbalance of redox reactions, and NO is toxic
to DA neurons. A previous study indicated that NO is involved in
DNA damage, poly (ADP-ribose) polymerase 1 activation and cell
death induced by 1-methyl-4-phenylpyridinium (19). In the present study, iNOS activity
level was significantly inhibited by 1,000 or 2,000 mg/kg
Azadirachta indica compared with PD rats. Kim et al
(20) suggested that
Azadirachta indica protects against lethal endotoxemia and
sepsis in mice via suppression of iNOS and inflammatory
diseases.
It has previously been demonstrated that the
increase of apoptosis is the primary cause of the loss of DA
neurons in the substantia nigra corpus striatum in PD patients.
Bcl-2, Bax, caspase-3, cytochrome c and p53 are associated
with apoptosis of DA neurons in the substantia nigra in the
midbrain (21). The apoptosis of
nerve cells in PD patients is regulated by a series of genes
associated with apoptosis, of which the Bcl-2 gene family is
considered one of the most important (22). The known members of the Bcl-2 gene
family include Bcl-2, Bcl-xl, Bcl-w, p53 and other cell apoptosis
inhibiting genes and Bcl-xs, Bax, Bcl-2 associated agonist of cell
death, Bcl-2 antagonist/killer 1, p53 and other cell apoptosis
promoting genes (23). In
addition, the present study revealed that treatment with
Azadirachta indica significantly suppressed the protein
expression of Bax and p53 in PD rats. Manosroi et al
(24) indicated that
Azadirachta indica extracts exhibit cytotoxic and
melanogenesis-inhibitory activities via suppression of caspases-3,
−8, and −9 and inhibition of Bax.
The release of mitochondrial cytochrome c is
additionally important in cell apoptosis (25). Cytochrome c release in the
cytoplasm may trigger a cascade reaction of activation, which leads
to cell apoptosis (26). The
release of cytochrome c results from the increased
permeability of the mitochondrial outer membrane, which
subsequently mediates activity of the cell apoptotic cascade
(27). As the inducing factor of
apoptosis, cytochrome c activates the apoptosome with apoptosis
protease activating factor, caspase-9 precursor and ATP/dATP, and
then activates caspase-3, which triggers the cascade reaction of
caspases and leads to cell apoptosis (25). The present study demonstrated that
Azadirachta indica significantly suppressed cytochrome
c levels in PD rats. Manikandan et al (28) suggested that Azadirachta
indica may exhibit anti-oxidant, anti-angiogenic,
anti-proliferative and anti-apoptotic effects via regulation of
Bax, cytochrome c and caspase-3.
In conclusion, the present study suggested that
neuroprotective effects of Azadirachta indica resulted in
functional recovery in the 6-hydroxydopamine induced rat Parkinson
model and the mechanism involved suppression of oxidative stress
and inflammation, and inhibition of AChE activity and apoptosis.
These findings suggest that Azadirachta indica may act as a
novel therapeutic in the future for the treatment of PD.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
XX designed the experiment; LW, LM and YL performed
the experiments. XX and LW conducted data analysis; XX wrote the
manuscript.
Ethics approval and consent to
participate
All protocols were approved by the Animal Care and
Welfare Committee of the Institute of Qilu Hospital of Shandong
University (Shandong, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
González-Burgos E, Fernandez-Moriano C and
Gómez-Serranillos MP: Potential neuroprotective activity of Ginseng
in Parkinson's disease: A review. J Neuroimmune Pharmacol.
10:14–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rektor I, Goldemund D, Bednarik P,
Bednařík P, Sheardová K, Michálková Z, Telecká S, Dufek M and
Rektorová I: Impairment of brain vessels may contribute to
mortality in patients with Parkinson's disease. Mov Disord.
27:1169–1172. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fuxe K, Marcellino D, Genedani S and
Agnati L: Adenosine A(2A) receptors, dopamine D(2) receptors and
their interactions in Parkinson's disease. Mov Disord.
22:1990–2017. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tapias V, Cannon JR and Greenamyre JT:
Pomegranate juice exacerbates oxidative stress and nigrostriatal
degeneration in Parkinson's disease. Neurobiol Aging. 35:1162–1176.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Prigione A, Isaias IU, Galbussera A,
Brighina L, Begni B, Andreoni S, Pezzoli G, Antonini A and
Ferrarese C: Increased oxidative stress in lymphocytes from
untreated Parkinson's disease patients. Parkinsonism Relat Disord.
15:327–328. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tassorelli C, Furnari A, Buscone S,
Alfonsi E, Pacchetti C, Zangaglia R, Pichiecchio A, Bastianello S,
Lozza A, Allena M, et al: Pisa syndrome in Parkinson's disease:
Clinical, electromyographic, and radiological characterization. Mov
Disord. 27:227–235. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Omobowale TO, Oyagbemi AA, Oyewunmi OA and
Adejumobi OA: Chemopreventive effect of methanolic extract of
Azadirachta indica on experimental Trypanosoma brucei
induced oxidative stress in dogs. Pharmacognosy Res. 7:249–258.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dkhil MA, Al-Quraishy S, Moneim Abdel AE
and Delic D: Protective effect of Azadirachta indica extract
against Eimeria papillata-induced coccidiosis. Parasitol Res.
112:101–106. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kohl Z, Ben Abdallah N, Vogelgsang J,
Tischer L, Deusser J, Amato D, Anderson S, Müller CP, Riess O,
Masliah E, et al: Severely impaired hippocampal neurogenesis
associates with an early serotonergic deficit in a BAC α-synuclein
transgenic rat model of Parkinson's disease. Neurobiol Dis.
85:206–217. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kumar R, Hauser RA, Mostillo J, Dronamraju
N, Graf A, Merschhemke M and Kenney C: Mavoglurant (AFQ056) in
combination with increased levodopa dosages in Parkinson's disease
patients. Int J Neurosci. 126:20–24. 2013. View Article : Google Scholar
|
|
11
|
Abdenour B and Charles R: Innovative
anthocyanins formulation protects neuronal-like cells against
oxidative stress-induced damage: Pharmacotherapeutic application
for Alzheimer's disease. Free Radic Biol Med. 75 Suppl 1:S452014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lu J, Wu L, Jiang T, Wang Y, Zhao H, Gao
Q, Pan Y, Tian Y and Zhang Y: Angiotensin AT2 receptor stimulation
inhibits activation of NADPH oxidase and ameliorates oxidative
stress in rotenone model of Parkinson's disease in CATHa cells.
Neurotoxicol Teratol. 47:16–24. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Steultjens MP, Stolwijk-Swüste J, Roorda
LD, Dallmeijer AJ, van Dijk GM, Post B and Dekker J: CARPA Study
Group: WOMAC-pf as a measure of physical function in patients with
Parkinson's disease and late-onset sequels of poliomyelitis:
Unidimensionality and item behaviour. Disabil Rehabil.
34:1423–1430. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Antonini A and Tinazzi M: Targeting pain
in Parkinson's disease. Lancet Neurol. 14:1144–1145. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Soares DG, Godin AM, Menezes RR, Nogueira
RD, Brito AM, Melo IS, Coura GM, Souza DG, Amaral FA, Paulino TP,
et al: Anti-inflammatory and antinociceptive activities of
azadirachtin in mice. Planta Med. 80:630–636. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu XL, Wang P, Liu YH and Xue YX: Effects
of poly (ADP-ribose) polymerase inhibitor 3-aminobenzamide on
blood-brain barrier and dopaminergic neurons of rats with
lipopolysaccharide-induced Parkinson's disease. J Mol Neurosci.
53:1–9. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rohn TT and Catlin LW: Immunolocalization
of influenza A virus and markers of inflammation in the human
Parkinson's disease brain. PLoS One. 6:e204952011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huerta C, Sánchez-Ferrero E, Coto E,
Blázquez M, Ribacoba R, Guisasola LM, Salvador C and Alvarez V: No
association between Parkinson's disease and three polymorphisms in
the eNOS, nNOS, and iNOS genes. Neurosci Lett. 413:202–205. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pontone GM, Palanci J, Williams JR and
Bassett SS: Screening for DSM-IV-TR cognitive disorder NOS in
Parkinson's disease using the Mattis Dementia Rating Scale. Int J
Geriatr Psychiatry. 28:364–371. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim WH, Song HO, Jin CM, Hur JM, Lee HS,
Jin HY, Kim SY and Park H: The methanol extract of Azadirachta
indica A. juss leaf protects mice against lethal endotoxemia
and sepsis. Biomol Ther (Seoul). 20:96–103. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhu L, Zhu B, Yang L, Zhao X, Jiang H and
Ma F: RelB regulates Bcl-xl expression and the irradiation-induced
apoptosis of murine prostate cancer cells. Biomed Rep. 2:354–358.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shrivastava P, Vaibhav K, Tabassum R, Khan
A, Ishrat T, Khan MM, Ahmad A and Islam F, Safhi MM and Islam F:
Anti-apoptotic and anti-inflammatory effect of Piperine on 6-OHDA
induced Parkinson's rat model. J Nutr Biochem. 24:680–687. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yasuda T, Hayakawa H, Nihira T, Ren YR,
Nakata Y, Nagai M, Hattori N, Miyake K, Takada M, Shimada T, et al:
Parkin-mediated protection of dopaminergic neurons in a chronic
MPTP-minipump mouse model of Parkinson disease. J Neuropathol Exp
Neurol. 70:686–697. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Manosroi A, Kitdamrongtham W, Ishii K,
Shinozaki T, Tachi Y, Takagi M, Ebina K, Zhang J, Manosroi J,
Akihisa R and Akihisa T: Limonoids from Azadirachta indica
var. siamensis extracts and their cytotoxic and
melanogenesis-inhibitory activities. Chem Biodivers. 11:505–531.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Witt SN and Flower TR: Alpha-Synuclein,
oxidative stress and apoptosis from the perspective of a yeast
model of Parkinson's disease. FEMS Yeast Res. 6:1107–1116. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bayir H, Kapralov AA, Jiang J, Huang Z,
Tyurina YY, Tyurin VA, Zhao Q, Belikova NA, Vlasova II, Maeda A, et
al: Peroxidase mechanism of lipid-dependent cross-linking of
synuclein with cytochrome C: Protection against apoptosis versus
delayed oxidative stress in Parkinson disease. J Biol Chem.
284:15951–15969. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Itoh K, Weis S, Mehraein P and
Müller-Höcker J: Defects of cytochrome c oxidase in the substantia
nigra of Parkinson's disease: And immunohistochemical and
morphometric study. Mov Disord. 12:9–16. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Manikandan P, Letchoumy PV, Gopalakrishnan
M and Nagini S: Evaluation of Azadirachta indica leaf
fractions for in vitro antioxidant potential and in vivo modulation
of biomarkers of chemoprevention in the hamster buccal pouch
carcinogenesis model. Food Chem Toxicol. 46:2332–2343. 2008.
View Article : Google Scholar : PubMed/NCBI
|
























