Introduction
Irritable bowel syndrome (IBS) is a type of
functional gastrointestinal disorder (FGID) characterized by
symptoms such as abdominal pain or discomfort and stool
irregularities, unassociated with metabolic or organic
abnormalities (1,2). A number of factors are involved in
the pathophysiology of IBS, such as visceral sensitivity,
gastrointestinal (GI) motility, brain-gut interaction and
psychosocial stress (1,2). Interestingly, IBS occurs frequently
in patients recovering from infectious colitis, (3,4) and
we have reported that IBS-like symptoms are often observed in
patients with inflammatory bowel disease (IBD) even after the bowel
inflammation has been eliminated (5). In such patients, despite macroscopic
healing of the intestinal mucosa, IBS-like symptoms persist.
Although it has not been fully clarified how IBS symptoms are
manifested after infectious colitis or during remission after IBD,
it is tempting to speculate that a minimal degree of inflammation
and associated gut immune activation may play a pathophysiologic
role (3,4,6,7).
Among immune cells, mast cell is highlighted in the pathophysiology
of IBS since the number of mast cells is increased in the colonic
tissues in patients with IBS and moreover correlated with the
severity of their clinical symptoms (8,9). In
addition, recent evidences have revealed that macrophages play
pivotal roles in GI motility via acting on myenteric neural cells,
(10–13) and indeed, the infiltration of
macrophages in the colonic tissues may be enhanced in IBS patients
(9,14). Accordingly, in order to clarify the
pathophysiologic roles of immune cells, the investigation of mast
cells and macrophages in experimental IBS model appears to be
important.
Dextran sulfate sodium (DSS)-induced colitis is an
animal model used widely to investigate the pathophysiology of
various types of human colitis (15). It has been shown that mice treated
with DSS for 5–7 days develop severe acute colitis, and then
undergo healing of the damaged colonic tissue. However, the
pathophysiology and mucosal immune alteration after remission has
not been fully studied in this animal model. Then, to examine
whether the mice in remission after DSS-induced colitis are useful
as a model for IBS after acute colitis, we have observed those mice
in a time dependent manner. Subsequently, this animal model showed
significant alterations of GI motility and immune cell infiltration
in the GI tract, being possibly resemble to the subjects with
post-inflammatory IBS. In the present study, we therefore
investigated the involvement of immune cells, focusing mast cell
and macrophage that are highlighted in IBS studies, and analyzed
its relation to the alteration of GI motility in mice in remission
after acute colitis.
Materials and methods
Animal model
C57BL/6 mice (8-week-old females) were used in this
study. All the mice were maintained in cages on a 12 h light/dark
cycle under specific pathogen-free conditions and allowed free
access to food and water. The mice were administered 2% DSS
(molecular weight 36,000–50,000; ICN Biomedicals Inc, Aorano, OH,
USA) in drinking water for five days and sacrificed at various time
points thereafter. Their GI tissues were removed, cut open along
the longitudinal axis, rinsed with saline, and fixed in neutral
aqueous phosphate-buffered 10% formalin for histological
examinations. This animal experiment was carried out with the
approval of the Animal Use and Care Committee at Hyogo College of
Medicine.
Histological evaluation
The fixed tissues were embedded in paraffin, cut
perpendicularly to the surface at a thickness of 4 µm, and stained
with hematoxylin and eosin. The degree of inflammatory cell
infiltration in the small intestine and colon was scored on a scale
of 0 to 3 as follows: 0, normal; 1, inflammatory cell infiltration
into the mucosal layer; 2, up to the submucosal layer; 3, beyond
the submucosal layer. The scores were evaluated for all of the
slides from the small intestine and colon of each mouse, and the
results were averaged.
Immunohistochemistry
Immunohistochemical staining for the mannose
receptor (MR; a marker of M2-polarized macrophages) and tryptase (a
marker of mast cells) was performed using an Envision kit (Dako;
Agilent Technologies, Inc., Santa Clara, CA, USA) as described
previously (16). The primary
antibodies applied were: anti-MR (dilution 1:5,000; cat. no.
ab64693; Abcam, Cambridge, UK) and anti-tryptase antibody (dilution
1:4,000; cat. no. ab2378; Abcam). The sections were deparaffinized,
rehydrated and placed in PBS for MR staining or placed in 1X Dako
REAL Target Retrieval Solution (Dako; Agilent Technologies, Inc.,
Santa Clara, CA, USA) followed by microwave treatment for tryptase
staining. Then, to quench endogenous peroxidase activity, the
sections were pretreated with 0.3% H2O2 in
methanol for 25 min at room temperature. The sections were washed
in PBS and incubated with the primary antibodies at 4°C overnight.
Thereafter, the slides were incubated with horseradish
peroxidase-conjugated secondary antibodies (ready-to-use; cat. nos.
K4001 or K4003; Dako; Agilent Technologies, Inc.) at room
temperature for 30 min, visualized using 3,3′-diaminobenzidine
tetrahydrochloride with 0.05% H2O2 for 3 min,
and counterstained with Mayer's hematoxylin. Under a light
microscope (Olympus CX41; Olympus Corporation, Tokyo, Japan), MR-
and tryptase-positive inflammatory cells were counted in a 200-µm
stretch of the entire length of well-oriented tissue sections in at
least 4 randomly selected fields from the small intestine to colon
of each mouse, and the average was calculated.
GI transit time (GITT)
GITT was measured as described previously (17). In brief, the mice orally received
0.3 ml of 0.5% methylcellulose solution including 6% carmine red
(Wako Pure Chemical Industries, Ltd., Osaka, Japan). They were then
allowed access to food and water ad libitum until the first
red fecal pellet appeared. GITT was determined as the time period
between oral gavage and the appearance of the first red fecal
pellet.
Statistical analysis
All values were expressed as the mean ± SEM.
Significance of differences between two animal groups was analyzed
by Mann-Whitney U-test. Correlations among GITT, MR
expression and tryptase expression were assessed by linear
regression analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
Inflammatory cell infiltration in the
intestinal tract of mice after DSS-induced colitis
As shown in Fig. 1,
inflammatory cell infiltration in the intestinal tract of mice
after DSS-induced colitis was evaluated. Strong infiltration of
inflammatory cells was observed in not only the mucosal but also
the muscular layer in the colonic tissues of mice in the acute
phase of DSS-induced colitis (Fig.
1A). The severity of colonic inflammatory cell infiltration
peaked at 2–4 weeks after DSS induction (Fig. 1E). Thereafter, it gradually
declined but remained at a very weak level in the resolving phase
(Fig. 1E). Thus, although most
parts of the colonic mucosa appeared macoscopically normal, a
minimal degree of inflammatory cell infiltration was still evident
in some parts of colonic tissues in the resolving phase (Fig. 1B).
In the small intestine, weak infiltration of
inflammatory cells was microscopically evident in the acute phase
of DSS-induced colitis (Fig. 1C),
although the macroscopic appearance was normal. Moreover, it was
noteworthy that a minimal degree of inflammatory cell infiltration
was sustained in some parts of the small intestine in the
resolution phase (Fig. 1D and
F).
GITT in mice after DSS-induced
colitis
In normal mice, GITT was prolonged with increasing
age (from 8 to 32 weeks) (Fig. 2).
At two weeks after the start of the experiment, GITT was shorter in
mice with DSS-induced colitis than in normal controls, although the
difference was not significant. On the other hand, at four weeks
later, GITT was significantly longer in mice with DSS-induced
colitis than in normal controls. In contrast, however, GITT again
became significantly shorter in mice in the resolution phase (at 24
weeks) of DSS colitis.
Expression of MR and tryptase in mice
after DSS-induced colitis
We next examined the localization and population of
MR-positive macrophages and tryptase-positive mast cells in the
small intestine and colon of mice after DSS-induced colitis. In
normal mice, MR-positive macrophages were scattered in both the
mucosal and muscular layers of the colon (Fig. 3). In the distal colon, the number
of MR-positive macrophages in the mucosal layer increased with age,
whereas it remained very small in the muscular layer (Fig. 4). In mice with DSS-colitis, the
number of MR-positive macrophages was significantly increased in
the muscular layer throughout the small intestine and colon at 2
weeks after DSS induction (Fig.
4). Furthermore, it was significantly increased in the
small-intestinal muscular layer at 24 weeks after DSS induction,
and similar findings were observed in the muscular layer of the
colon (Figs. 3 and 4).
In normal mice, tryptase expression was detected in
the immune cells in the lamina propria but was hardly evident in
the muscular layer throughout the small intestine and colon
(Fig. 5). In those mice, the
number of tryptase-positive cells in the mucosal layer increased
with age (Fig. 6). In mice with
DSS-colitis, the number of tryptase-positive cells was
significantly greater in not only the mucosal but also the muscular
layer in the small intestine or colon between 4 and 12 weeks after
DSS induction (Figs. 5 and
6).
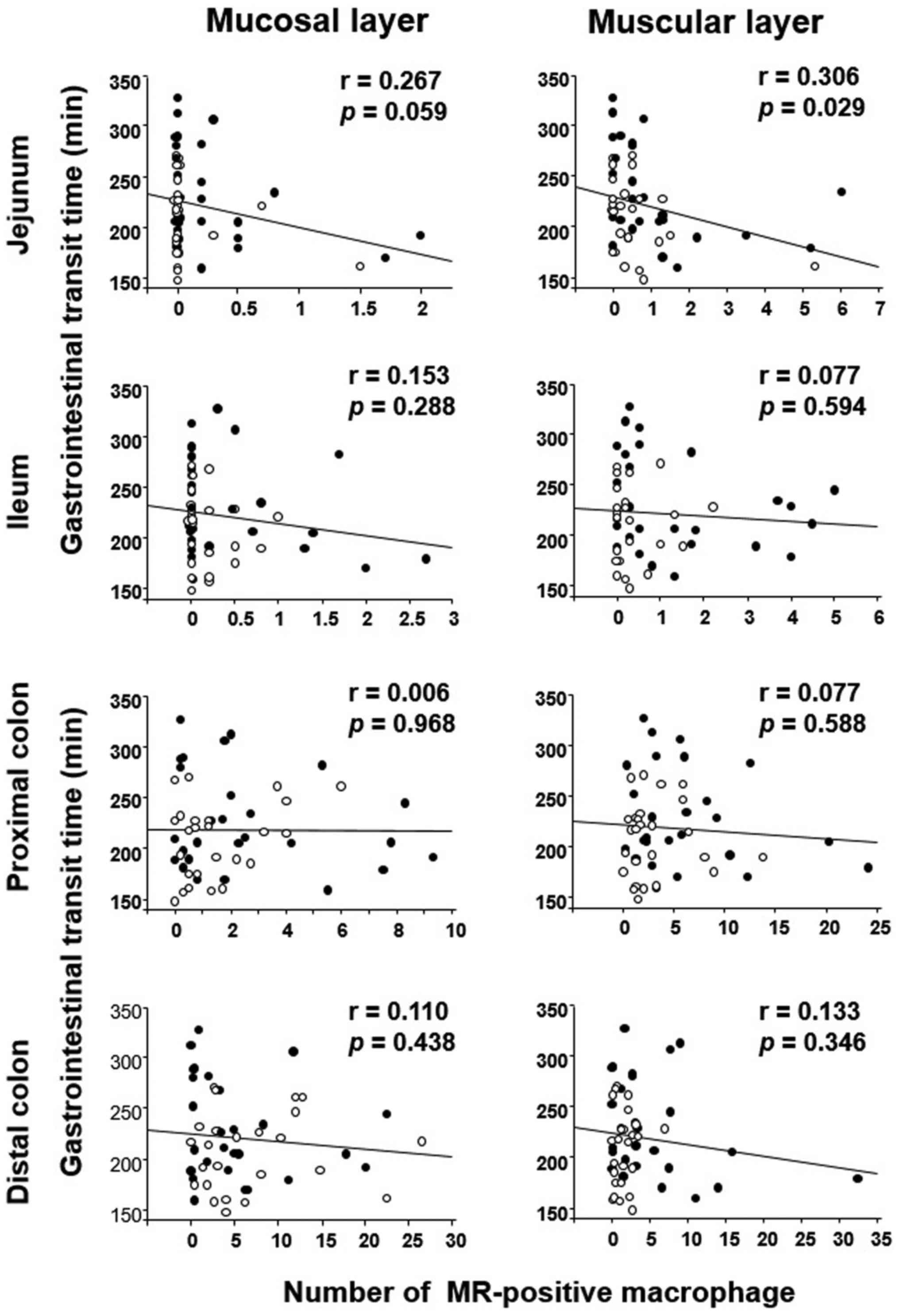
GITT and its association with MR or
tryptase expression
The correlation between GITT and MR or tryptase
expression was evaluated in the experimental mice (DSS-treated and
untreated) by linear regression analysis. GITT was negatively
correlated with the number of MR-positive cells in the muscular
layer of the jejunum (Fig. 7). In
terms of tryptase expression, GITT was positively correlated with
the number of tryptase-positive cells in the muscular layer of the
jejunum and colon (Fig. 8).
Discussion
FGIDs frequently occur in patients after infectious
colitis (3,4) although endoscopic examinations reveal
no apparent abnormality in the enteric lumen, suggesting that some
form of cryptic molecular alteration plays a pathophysiologic role.
In this context, it is tempting to speculate that
post-inflammation-associated factors are central to the mechanism
of FGID development after inflammation (3,4,6,7).
Although DSS-induced colitis is a well-established animal model,
the associated GI motility has not been examined intensively. In
the present study, we investigated GITT in mice with DSS-induced
colitis at various time intervals. As shown in Fig. 1, GITT was shortened in the acute
phase of DSS-induced colitis, reflecting the fact that diarrhea
occurs during this period in this model (15). On the other hand, GITT was
conversely prolonged during the healing process from 4 to 12 weeks
after DSS induction. Histopathologic examination using microscopy
demonstrated mild infiltration of inflammatory cells, implying that
alteration of the immune system may affect GI motility.
Furthermore, we found that a minimal degree of inflammatory cell
infiltration remained in the intestine in the resolution phase
after 24 weeks and that GITT became significantly shortened again
at this time point. These findings suggest that alteration of the
immune system certainly affects GI motility, although further
studies of the infiltrating immune cells and the mediators they
produce would be warranted.
In the present study, we investigated mice after
induction of DSS-colitis focusing on macrophages and mast cells as
these have received attention as key players in FGIDs after
inflammation (8,10). MR-positive macrophages and
tryptase-positive mast cells were observed in not only the mucosal
but also the muscular layer of the intestinal tract. Interestingly,
at 2 and 24 weeks after DSS-colitis induction, the number of
macrophages was increased in the muscular layer of the intestinal
tract, and GITT was simultaneously shortened. Moreover, from 4 to
12 weeks after DSS-colitis induction, the number of mast cells was
increased, and GITT was prolonged. These finding strongly suggest
that macrophages are involved in the acceleration of GI motility
whereas mast cells are associated with the suppression of GI
motility. Indeed, we showed that GITT was negatively correlated
with the number of muscule-associated macrophages and positively
correlated with that of mast cells. Although it is difficult to
explain how GI motility is affected by these infiltrating immune
cells, some alteration in their profile may be associated with a
change in GI motility during the healing process after acute
colitis.
With regard to the involvement of macrophages and
mast cells in post-inflammation GI dysmotility, interaction between
the enteric nerve system (ENS) and smooth muscle (18,19)
is greatly affected by immune cell-producing mediators such as
cytokines, chemokines, neuropeptides or proteases (11,20,21).
Indeed, mast cells are able to release histamine, serotonin,
tryptase and prostaglandins, and those mediators are possible to
act on their specific receptors of myenteric neural cells, leading
to altered motor function (8).
Similar to mast cells, macrophages are likely to affect ENS and
smooth muscles with various mediators (10–13).
Interestingly, macrophages have been recently classified into the
M1 and M2 type that mainly produce Th1 and Th2 cytokines,
respectively (22,23). In detail, M1 macrophages release
proinflammatory cytokines such as TNFα, IL-1β and IL-6 and their
stimulation acts on ENS and smooth muscle, resulting in the
suppression of GI motility (10,12).
On the other hand, M2 macrophages may suppress the expression of
proinflammatory cytokines in M1 macrophage by release
anti-inflammatory cytokines including IL-10, (10,24)
possibly resulting in the acceleration of GI motility. In this
context, we showed in the present study that M2 macrophage were
increased in the muscular layer of the intestinal tract after
remission of colitis, supporting the possibility that these may be
involved in the acceleration of GI motility observed during the
period of colitis remission. On the other hand, not only M2
macrophages but also mast cells are known to infiltrate into the
muscle layer in the intestine and may accelerate GI motility
through stimulation with Th2 cytokines, histamine or serotonin
(8). Therefore, we expected that
the increase of muscle-associated mast cells would result in
acceleration of GI motility. Conversely, however, the data we
obtained indicated the opposite situation, i.e., that
muscle-associated mast cells might be involved in delayed GI
motility. In humans, mast cells in the colonic mucosa are increased
in patients with not only diarrhea but also constipation
predominant IBS, (25,26) suggesting that these mast cells may
not be a factor in the modification of intestinal movements.
Although this study did not obtain any evidence for a mechanical
role of muscle-associated mast cells in intestinal motility, the
present animal model may be useful for investigating the role of
mast cells in post-inflammatory FGIDs.
In summary, we have shown that GI dysmotility occurs
in mice that are in remission after colitis, and that
histopathologically, there is a significant increase of macrophages
and mast cells in the muscular layer of the intestinal tract.
Moreover, we have demonstrated that muscle-associated macrophages
may be involved in the acceleration of GI motility, whereas
muscle-associated mast cells may be associated with its delay.
Together, our findings suggest that the increased number of
macrophages and/or mast cells in the intestinal muscular layer
plays a pathophysiologic role in post-colitis GI dysmotility.
Acknowledgements
The authors would like to thank Chiyomi Itoh and
Mayumi Yamada of Hyogo College of Medicine for their technical
assistance.
Funding
This work was supported in part by Grants-in-aid for
Scientific Research (grant no.17K09363) from the Ministry of
Education, Culture, Sports, Science and Technology, Japan.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
HF conceived a designed research and interpreted
results of experiments. TT, TO, JW and HM also contributed to the
design of the study and the interpretation of experimental results.
MK and HF performed experiments, analyzed data, prepared figures
and drafted manuscript. MK, HF, TT, TO, JW and HM approved final
version of manuscript. HF, TT, TO, JW and HM edited and revised
manuscript.
Ethics approval and consent to
participate
This study involved no human data or tissues. The
animal experiments were carried out with the approval of the Animal
Use and Care Committee at Hyogo College of Medicine.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
GI
|
gastrointestinal
|
|
IBS
|
irritable bowel syndrome
|
|
DSS
|
dextran sulfate sodium
|
|
MR
|
mannose receptor
|
|
GITT
|
gastrointestinal transit time
|
|
FGID
|
functional gastrointestinal
disorder
|
|
IBD
|
inflammatory bowel disease
|
|
ENS
|
enteric nerve system
|
References
|
1
|
Longstreth GF, Thompson WG, Chey WD,
Houghton LA, Mearin F and Spiller RC: Functional bowel disorders.
Gastroenterology. 130:1480–1491. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Enck P, Aziz Q, Barbara G, Farmer AD,
Fukudo S, Mayer EA, Niesler B, Quigley EM, Rajilić-Stojanović M,
Schemann M, et al: Irritable bowel syndrome. Nat Rev Dis Primers.
2:160142016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
DuPont AW: Post-infectious irritable bowel
syndrome. Curr Gastroenterol Rep. 9:378–384. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Beatty JK, Bhargava A and Buret AG:
Post-infectious irritable bowel syndrome: Mechanistic insights into
chronic disturbances following enteric infection. World J
Gastroenterol. 20:3976–3985. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tomita T, Kato Y, Takimoto M, Yamasaki T,
Kondo T, Kono T, Tozawa K, Yokoyama Y, Ikehara H, Ohda Y, et al:
Prevalence of irritable bowel syndrome-like symptoms in Japanese
patients with inactive inflammatory bowel disease. J
Neurogastroenterol Motil. 22:661–669. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Törnblom H, Lindberg G, Nyberg B and
Veress B: Full-thickness biopsy of the jejunum reveals inflammation
and enteric neuropathy in irritable bowel syndrome.
Gastroenterology. 123:1972–1979. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ford AC and Talley NJ: Mucosal
inflammation as a potential etiological factor in irritable bowel
syndrome: A systematic review. J Gastroenterol. 46:421–431. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wouters MM, Vicario M and Santos J: The
role of mast cells in functional GI disorders. Gut. 65:155–168.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Barbara G, Cremon C, Carini G, Bellacosa
L, Zecchi L, De Giorgio R, Corinaldesi R and Stanghellini V: The
immune system in irritable bowel syndrome. J Neurogastroenterol
Motil. 17:349–359. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cipriani G, Gibbons SJ, Kashyap PC and
Farrugia G: Intrinsic gastrointestinal macrophages: Their phenotype
and role in gastrointestinal motility. Cell Mol Gastroenterol
Hepatol. 2:120–130.e1. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shea-Donohue T, Notari L, Sun R and Zhao
A: Mechanisms of smooth muscle responses to inflammation.
Neurogastroenterol Motil. 24:802–811. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Türler A, Schwarz NT, Türler E, Kalff JC
and Bauer AJ: MCP-1 causes leukocyte recruitment and subsequently
endotoxemic ileus in rat. Am J Physiol Gastrointest Liver Physiol.
282:G145–G155. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao A, Urban JF Jr, Anthony RM, Sun R,
Stiltz J, van Rooijen N, Wynn TA, Gause WC and Shea-Donohue T: Th2
cytokine-induced alterations in intestinal smooth muscle function
depend on alternatively activated macrophages. Gastroenterology.
135:217–225.e1. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Boyer J, Saint-Paul MC, Dadone B,
Patouraux S, Vivinus MH, Ouvrier D, Michiels JF, Piche T and Tulic
MK: Inflammatory cell distribution in colon mucosa as a new tool
for diagnosis of irritable bowel syndrome: A promising pilot study.
Neurogastroenterol Motil. 30:2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chassaing B, Aitken JD, Malleshappa M and
Vijay-Kumar M: Dextran sulfate sodium (DSS)-induced colitis in
mice. Curr Protoc Immunol. 104:252014.PubMed/NCBI
|
|
16
|
Kitayama Y, Fukui H, Hara K, Eda H, Kodani
M, Yang M, Sun C, Yamagishi H, Tomita T, Oshima T, et al: Role of
regenerating gene i in claudin expression and barrier function in
the small intestine. Transl Res. 173:92–100. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Eda H, Fukui H, Uchiyama R, Kitayama Y,
Hara K, Yang M, Kodani M, Tomita T, Oshima T, Watari J, et al:
Effect of Helicobacter pylori infection on the link between GLP-1
expression and motility of the gastrointestinal tract. PLoS One.
12:e01772322017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mayer EA, Tillisch K and Gupta A:
Gut/brain axis and the microbiota. J Clin Invest. 125:926–938.
2015. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wood JD: Neuropathophysiology of
functional gastrointestinal disorders. World J Gastroenterol.
13:1313–1332. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lakhan SE and Kirchgessner A:
Neuroinflammation in inflammatory bowel disease. J
Neuroinflammation. 7:372010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang M, Fukui H, Eda H, Xu X, Kitayama Y,
Hara K, Kodani M, Tomita T, Oshima T, Watari J, et al: Involvement
of gut microbiota in association between GLP-1/GLP-1 receptor
expression and gastrointestinal motility. Am J Physiol Gastrointest
Liver Physiol. 312:G367–G373. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Canton J, Neculai D and Grinstein S:
Scavenger receptors in homeostasis and immunity. Nat Rev Immunol.
13:621–634. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Novoselov VV, Sazonova MA, Ivanova EA and
Orekhov AN: Study of the activated macrophage transcriptome. Exp
Mol Pathol. 99:575–580. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Deng B, Wehling-Henricks M, Villalta SA,
Wang Y and Tidball JG: IL-10 triggers changes in macrophage
phenotype that promote muscle growth and regeneration. J Immunol.
189:3669–3680. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chadwick VS, Chen W, Shu D, Paulus B,
Bethwaite P, Tie A and Wilson I: Activation of the mucosal immune
system in irritable bowel syndrome. Gastroenterology.
122:1778–1783. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Barbara G, Stanghellini V, De Giorgio R,
Cremon C, Cottrell GS, Santini D, Pasquinelli G, Morselli-Labate
AM, Grady EF, Bunnett NW, et al: Activated mast cells in proximity
to colonic nerves correlate with abdominal pain in irritable bowel
syndrome. Gastroenterology. 126:693–702. 2004. View Article : Google Scholar : PubMed/NCBI
|