Introduction
Knee osteoarthritis is a disease with the
degenerative changes in the articular hyaline cartilage (1). The incidence of knee osteoarthritis
is increasing due to prevalence in aging societies worldwide, which
is characterized by degenerative disease that mainly caused by
inflammation and dysfunction of synovial cells (2–4). A
systematic review has given the overview of factors (age, body mass
index, level of physical function and level of physical activity)
related to patients with hip or knee osteoarthritis (5). The pathological reasons of
osteoarthritis are complex in classification system manner and the
cellular pathogenesis in joints (6). Previous study has indicated that
inflammation is a variable feature of knee osteoarthritis, and is
associated with joint symptoms and progression of this disease
(7). A report has showed that
decreasing of tumor necrosis factor (TNF)-α, interleukin (IL)-1 and
IL-6 contents in joint fluid markedly improved symptoms in the
rabbit with knee osteoarthritis (8).
A previous study has investigated the association
between systemic and local inflammation and incident and
progressive radiographic secondary osteoarthritis and results found
that TNF-α inhibitor infliximab therapy is effective against hand
osteoarthritis (9). Qin et
al reported that TNF/TNFR signal transduction pathway-mediated
anti-apoptosis and anti-inflammatory effects of sodium ferulate on
IL-1b-induced rat osteoarthritis chondrocytes (10). Study also identified that
decreasing of circulating levels of TNF-α inhibited the development
of osteoarthritis (11). Systemic
blockade of STAT-3 can alleviate medial meniscus-induced
osteoarthritis in mice (12).
However, the associations between TNF-α and STAT3 pathway has not
reported.
Currently, toll-like receptor-3 (TLR-3) can regulate
the STAT3 pathway in rheumatoid arthritis fibroblast-like
synoviocytes (13). Evidences have
demonstrated that TLR-3 is overexpressed in synovial tissue in
patients with early rheumatoid, suggesting that TLR-3 signal
pathway may associate with the persistent inflammation and joint
destruction in this disease process (14). Zhu et al have found that
pristane-induced arthritis rats presented higher TLR3 expression
levels in the synovium, and increased the activity of the TLR3
signal pathway (15). Domagala
et al have reported that inhibition of IL-1β-induced
activation of extracellular signal-regulated kinase (ERK) signal
pathway showed benefits for the treatment of osteoarthritis
(16). Klosowska et al have
found that targeting of ERK/protein kinase B (AKT) signal pathway
reduced Fractalkine-induced osteoarthritis fibroblast migration via
alterations of cytoskeletal structure in the pathologic processes
of osteoarthritis (17). However,
the relationships between ERK/AKT signal pathway and osteoarthritis
have not well investigated, yet.
In the present study, we investigated the role of
TNF-α-mediated TLR-3/ERK/AKT pathways in synovial fibroblasts in
osteoarthritis model. We also analyzed the in vivo effects
of inhibition of TNF-α activity in monosodium iodoacetate-induced
osteoarthritis mice model.
Materials and methods
Ethics statement
This study was approved by Ethics Committee of the
309th Hospital of People's Liberation Army. All surgeries were
performed under intravenous injection of sodium pentobarbital
anesthesia (35 mg/kg).
Animals study
A total of 40 male C57BL/6 mice (25–32 g, 5–6 months
of age) were purchased from Shanghai SLAC Laboratory Animals Co.,
Ltd (Shanghai, China). All mice were housed under controlled
temperatures in a 12 h light/dark cycle with easy access to food
and water. All mice were identified by ear punching and used to
establish osteoarthritis mice model induced by monosodium
iodoacetate (0.2 mg per mice; Sigma-Aldrich Co., St. Louis, MO,
USA) as described previously (18). On day 10 after monosodium
iodoacetate administration, mice were randomly assigned to two
groups vehicle group (n=20) and TNF-α inhibitor group (n=20). Mice
were received subcutaneous injection of vehicle (0.2 mg/kg/day) or
TNF-α inhibitor (0.2 mg/kg/day; Lenalidomide, Sigma-Aldrich) for a
total of 32 days. All mice were sacrificed on day 40 for
histological analysis. Body weights of experimental mice were
measured prior and post treatments on day 40. The osteonecrosis
mice were anesthetized under IV pentobarbital anesthesia (35 mg/kg)
and sacrificed by cervical dislocation on day 40 for further
analysis.
Cells isolation and culture
Synovial fibroblasts were isolated from experimental
mice as described (19) and
cultured in DMEM medium with 10% fetal bovine serum (FBS; both
Sigma-Aldrich) at 37°C and 5% CO2 humidified atmosphere.
Synovial fibroblasts (1×106 cells/well) were seeded into
six-well plates and treated by TNF-α (2 mg/ml), TNF-α inhibitor (2
mg/ml) or PBS (all Sigma-Aldrich) for 24 h at 37°C for RT-qPCR and
western blot analysis.
Enzyme-linked immunosorbent assay
(ELISA)
Concentrations of TNF-α (MTA00B, Bio-Rad), IL-1β
(MLB00C), IL-4 (M4000B) and IL-6 (DY406; all Bio-Rad, Berkeley, CA,
USA) concentrations in serum from experimental mice were analyzed
using ELISA. TNF-α, IL-1β, IL-4 and IL-6 were measured by using
ELISA kits (Bio-Rad) according to the manufacturer's instructions.
The results were performed by ELISA reader system (1775×Mark™;
Bio-rad).
Knockdown of TLR-3
Small interference RNAs (siRNA) for TLR-3 (siRTLR-3)
were synthesized by Ribobio Co., Ltd. (Guangzhou, China). siRTLR-3
sense, 5′-CCUGAGCUGUCAAGCCACUACCUUU-3′ and antisense,
5′-AAAGGUAGUGGCUUGACAGCUCAGG-3′; siRcontrol sense,
5′-CCUGUCGAACUACCGCAUCCAGUUU-3′ and antisense,
5′-AAACUGGAUGCGGUAGUUCGACAGG-3′. Synovial fibroblasts
(1×106 cells/well) were seeded onto 6-well plates and
transiently transfected with 120 nmol siRTLR-3 with negative
control (NC) siRNA as control using RNAi MAX (Thermo Fisher
Scientific, Inc., Waltham, MA, USA) according to the manufacturers'
instructions. All experiments were performed in triplicate and
further analysis was performed after a 48-h transfection. After a
48-h transfection, cells were then treated with TNF-α inhibitor (2
mg/ml), or PBS for 24 h at 37°C for further analysis.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) assays
Total RNA was extracted from synovial fibroblasts
using TRIzol reagent (Thermo Fisher Scientific, Inc.) according to
the manufacturers' instructions. Extracted mRNA (1 µg) was
transcribed into cDNA at 42°C for 2 h using a reverse transcription
kit (Qiagen, Inc., Valencia, CA, USA) according to the
manufacturer's protocol. The cDNA (10 ng) was used for qPCR using
the SYBR-Green Master Mix system (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) according to the manufacturer's instrument. All
primers were synthesized by Invitrogen (Thermo Fisher Scientific,
Inc.) (Table I). After 120 sec
incubation at 95°C, PCR was performed using 40 cycles of
denaturation at 94°C for 30 sec, annealing at 56°C for 30 sec and
elongation at 72°C for 30 sec. Relative gene expression levels were
calculated using the 2−ΔΔCq method (20). The results were presented as the
n-fold change compared with β-actin using Quantiscan 2.1 (software
Demo of AB QuantStudio™ 12K Flex System; Thermo Fisher Scientific,
Inc.).
 | Table I.Sequences of primers for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Sequences of primers for reverse
transcription-quantitative polymerase chain reaction.
|
| Sequence (5′-3′) |
|---|
|
|
|
|---|
| Gene | Reverse | Forward |
|---|
| TNFα |
CCTATGTCTCAGCCTCTTCT |
CCTGGTATGAGATAGCAAAT |
| IL-1β |
GGCTGCTTCCAAACCTTTGA |
GAAGACACGGATTCCATGGT |
| IL-6 |
GTGAGGAACAAGCCAGAG |
TGACCAGAAGAAGGAATGC |
| IL-4 |
TACAGCCACCATGAGAAGGAC |
TGATCGTCTTTAGCCTTTCCA |
| MMP-3 |
GCCCTGGAACTCACACGACA |
TTGGAAACTCACACGCCAGAAG |
| RANKL |
AAGGCGAGAGATTCTTTCCCTG' |
ACTGGGGACAATTCACTAGAGC |
| MMP-9 |
CGGAGCACGGAGACGGGTAT |
TGAAGGGGAAGACGCACAGC |
| NF-κB |
CCGAAGAACCATCCGA |
CGGGAAGGACTTTATGTA |
| β-actin |
CAAGAGATGGCCACGGCTGCT |
TCCTTCTGCATCCTGTCGGCA |
Western blot analysis
Western blotting was used to evaluate protein levels
in this study as previously described (21). Briefly, a total of 1×107
synovial fibroblasts were lysed in a lysis buffer containing 1%
phenylmethanesulfonyl fluoride (Sigma-Aldrich) for 3 times of
freezing-thawing and centrifuged at 8,000 × g for 10 min at 4°C.
Concentration of proteins was measured by BCA kit (no. 23225;
Thermo Fisher Scientific, Inc.). Proteins (20 µg) were separated
using the 12% sodium dodecyl sulphate-polyacrylamide gels
(SDS-PAGE). Then proteins were transferred onto proteins to
polyvinylidene fluoride (PVDF) membrane (Millipore, Massachusetts,
MA, USA). Then the nitrocellulose membrane was blocked with 5%
(w/v) nonfat dry milk dissolved in tris-buffered saline plus
Tween-20 (TBST) solution for 2 h at 37°C and followed by incubation
with primary rabbit anti-mouse antibodies: TNF-α (1:1,000; ab6671),
IL-1β (1:1,000; ab200478), IL-4 (1:1,000; ab9728), IL-6 (1:500;
ab7737), PI3K (1:2,000; ab1678), ERK (1:2,000; ab196883), pERK
(1:2,000; ab214362), AKT (1:1,000; ab8805), pAKT (1:1,000;
ab133458), matrix metalloproteinase (MMP)-3 (1:1,000; ab53015) and
MMP-9 (1:1,000; ab38898), receptor activator of NF-κB ligand
(RANKL; 1:1,000; ab216484), NF-κBp65 (1:1,000; ab32536) and β-actin
(1:2,000, ab8226; all Abcam, Cambridge, UK) for 12 h at 4°C.
HRP-conjugated goat anti-rabbit IgG mAb (1:5,000; PV-6001;
ZSGB-BIO, Beijing, China) was added for 24 h at 4°C. A Ventana
Benchmark automated staining system was used for analyzing protein
expression (Olympus BX51; Olympus, Tokyo, Japan). Protein
expression signal was analyzed by scanning densitometry using a
Microtek ScanMaker 8700 (Zhongjing Technology Co. Ltd, Beijing,
China) with ScanWizard 5 software (Informer Technologies, Inc.,
Walnut, CA, USA).
Tissue preparation and histopathologic
analysis
The osteonecrosis mice were sacrificed on day 40 and
the joints and articular cartilages were separated and fixed in 10%
formalin. Paraffin-embedded joints and articular cartilages were
cut into at a 4-µm thickness sections. Tissue sections were stained
with 5% hematoxylin and eosin (H&E) for histological
evaluation. Safranin O-fast green and Toluidine blue staining was
used to evaluate proteoglycans in the cartilage matrix. Tissue
sections were also fixed in 10% formalin at 37°C for 24 h and
decalcificated using Gooding and Stewart's fluid (Wako Pure
Chemical Industries, Ltd., Osaka, Japan) and subsequently embedded
in paraffin. 4-µm thick sections were stained with 0.05% toluidine
blue (pH 4.1) and the severity of the degree of osteonecrosis was
evaluated by the modified Mankin scoring system (22). The Mankin scoring system was scored
as follows: 0, normal; 1, irregular surface; 2, pannus; 3, absence
of superficial cartilage layers; 4, slight disorganization; 5,
fissure into the calcified cartilage layer, and 6, disorganization.
Histopathological evaluation was performed by two independent
blinded observers.
Immunohistochemistry
The paraffinized joint tissue sections were heated
in an oven at 65°C for 24 h, dewaxed to water and rinsed with PBS
three times. The washed sections were placed in EDTA buffer (Beinuo
Bioscience Inc., Shanghai, China), and then boiled at a low heat
following an interval of 10 min at 65°C for a total of three
intervals. Following natural cooling, the sections were washed with
PBS three times, and were placed into 3% hydrogen peroxide solution
(Beina Bioscience Inc.), for incubation at 37°C for 10 min, to
block endogenous peroxidase. Free-floating sections were rinsed
with PBS and placed in a solution containing primary mouse
monoclonal antibodies directed against TNF-α (1:1,000; ab6671,
Abcam), IL-1β (1:1,000, ab200478), IL-4 (1:1,000, ab9728), IL-6 (1:
500; ab7737), PI3K (1:2,000; ab1678), ERK (1:2,000; ab196883), pERK
(1:2,000; ab214362), AKT (1:1,000; ab8805), pAKT (1:1,000;
ab133458; Abcam) at 4°C for 12 h. After rinsing, sections were
incubated for 1 h at 37°C with the avidin-biotin-peroxidase complex
(1:5,000 dilution; PV-6001; ZSGB-BIO). The sections were then
washed with PBS and observed by fluorescent video microscopy
(BZ-9000; Keyence Corp., Osaka, Japan).
Statistical analysis
Statistical analysis was completed using SPSS 19.0
statistical software (IBM SPSS, Armonk, NY, USA) with the
assistance of Microsoft Excel (Windows 2010; Microsoft Corporation,
Redmond, WA, USA). All data are expressed as the mean ± standard
deviation and experiments were performed three times. Statistical
analyses were performed using one-way ANOVA followed by Tukey's
multiple comparison post hoc tests using Graph Pad Prism 5
software. P<0.05 was considered to indicate a statistically
significant difference.
Results
Inflammatory cytokines expression in
iodoacetate-induced osteoarthritis mice
Inflammatory cytokines were investigated in
osteoarthritis mice model. As shown in Fig. 1A, serum levels of TNF-α, IL-1β,
IL-4 and IL-6 were significantly upregulated in osteoarthritis mice
compared to healthy mice (P<0.01). Results demonstrated that
TNF-α, IL-1β, IL-4 and IL-6 mRNA and protein levels were
significantly upregulated in synovial fibroblasts in osteoarthritis
mice compared to healthy mice (P<0.01, Fig. 1B and C). We observed that MMP-3,
MMP-9, RANKL and NF-κB mRNA and protein levels were significantly
upregulated in synovial fibroblasts in osteoarthritis mice compared
to healthy mice (P<0.01, Fig. 1D
and E). These results indicate that inflammatory cytokines are
markedly upregulated in serum and synovial fibroblasts in
osteoarthritis mice model.
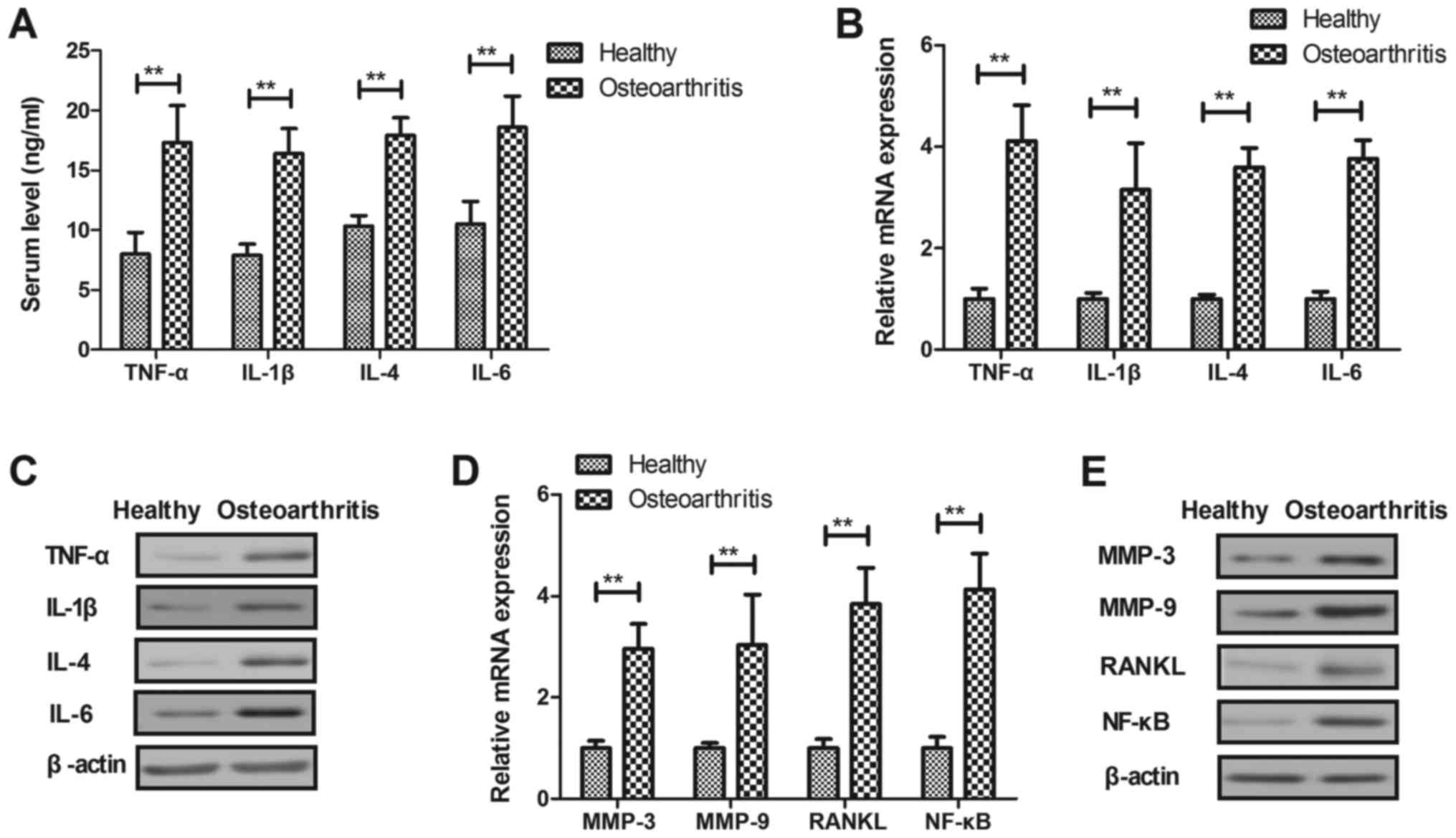 | Figure 1.Inflammatory cytokines expression in
osteoarthritis and healthy mice. (A) Serum levels of TNF-α, IL-1β,
IL-4 and IL-6 in osteoarthritic and healthy mice. (B) mRNA and (C)
protein levels of TNF-α, IL-1β, IL-4 and IL-6 in synovial
fibroblasts in osteoarthritic and healthy mice. (D) mRNA and (E)
protein levels of MMP-3, MMP-9, RANKL and NF-κB in synovial
fibroblasts in osteoarthritic and healthy mice. **P<0.01. TNF,
tumor necrosis factor; IL, interleukin; MMP, matrix
metalloproteinase; NF, nuclear factor; RANKL, receptor activator of
NF-κB ligand. |
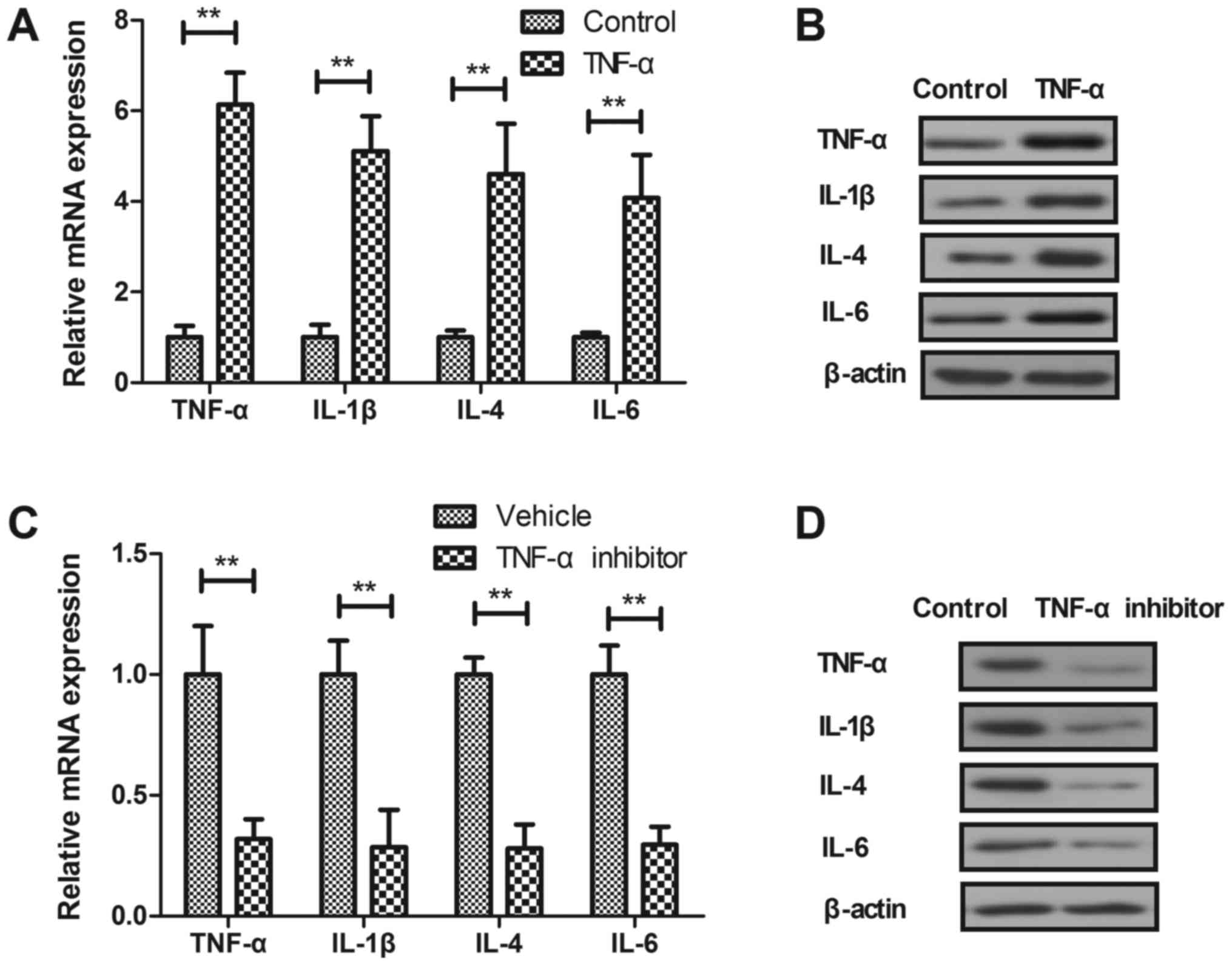
Effect of TNF-α on inflammatory
cytokines expression levels in synovial fibroblasts
In vitro assay showed that TNF-α increased
TNF-α, IL-1β, IL-4 and IL-6 mRNA and protein levels were
significantly upregulated in synovial fibroblasts (Fig. 2A and B). TNF-α inhibitor treatment
downregulated TNF-α, IL-1, IL-4 and IL-6 mRNA and protein levels in
synovial fibroblasts (Fig. 2C and
D). These results indicate that TNF-α inhibitor can lead to
downregulation of inflammatory cytokines expression levels in
synovial fibroblasts in vitro.
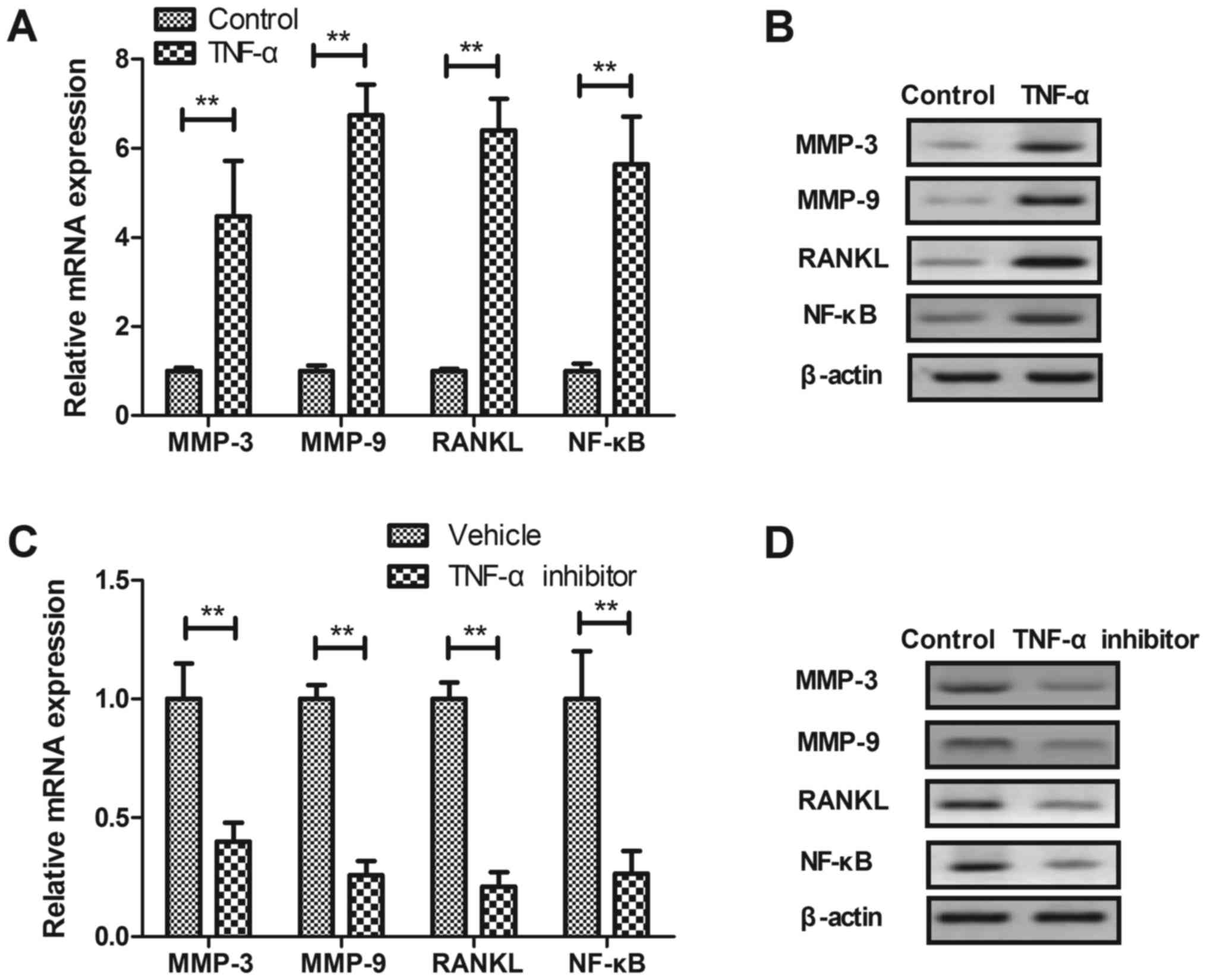
Effect of TNF-α on expression of
pro-inflammation factors in synovial fibroblasts
We investigated the effects of TNF-α on
pro-inflammation factors in synovial fibroblasts. We showed that
TNF-α treatment stimulated mRNA and protein expression levels of
MMP-3, MMP-9, RANKL and NF-κB in synovial fibroblasts (P<0.01,
Fig. 3A and B). TNF-α inhibitor
treatment significantly decreased mRNA and protein expression
levels of MMP-3, MMP-9, RANKL and NF-KB in synovial fibroblasts
(P<0.01, Fig. 3C and D). These
results suggest that TNF-α is a target for inhibition of
pro-inflammation factors in synovial fibroblasts.
Effect of TNF-α on TLR-3-medicated
ERK/AKT signal pathway
We investigated the regulatory effects of TNF-α on
TLR-3-medicated ERK/AKT signal pathway in synovial fibroblasts.
Results demonstrated that TLR-3, ERK and AKT expression levels were
significantly upregulated in synovial fibroblasts in osteoarthritis
mice compared to healthy mice (P<0.01, Fig. 4A). TNF-α treatment increased TLR-3,
ERK and AKT expression levels in synovial fibroblasts compared to
control (P<0.01, Fig. 4B).
Reversely, TNF-α inhibitor significantly decreased TLR-3, ERK and
AKT expression levels in synovial fibroblasts compared to control
(P<0.01, Fig. 4C). These
results suggest that TNF-α can regulate TLR-3-medicated ERK/AKT
signal pathway in synovial fibroblasts.
TNF-α regulates inflammatory factors
expression via TLR-3-meidated ERK/AKT signal pathway
We further analyzed the possible mechanism medicated
by TNF-α in synovial fibroblasts. Results revealed that knockdown
of TLR-3 (siRTLR-3) canceled TNF-α inhibitor-decreased expression
and phosphorylation levels of ERK and AKT in synovial fibroblasts
(Fig. 5A). TNF-α
inhibitor-decreased TNF-α, IL-1β, IL-4 and IL-6 expression levels
were also abolished by siRTLR-3 in synovial fibroblasts (Fig. 5B). Also, TNF-α inhibitor-decreased
MMP-3, MMP-9, RANKL and NF-κB expression levels were canceled by
TLR-3 knockdown in synovial fibroblasts (Fig. 5C). These results indicate that
TNF-α regulates inflammatory factors expression via TLR-3-meidated
ERK/AKT signal pathway in synovial fibroblasts.
 | Figure 5.TNF-α regulates inflammatory factor
expression via the TLR-3-meidated ERK/AKT signaling pathway. (A)
Knockdown of TLR-3 reverses TNF-α inhibitor-decreased protein
expression and phosphorylation of ERK and AKT in synovial
fibroblasts. (B) Knockdown of TLR-3 abolishes TNF-α
inhibitor-decreased TNF-α, IL-1β, IL-4 and IL-6 protein expression
in synovial fibroblasts. (C) Knockdown of TLR-3 abolishes TNF-α
inhibitor-decreased protein expression of MMP-3, MMP-9, RANKL and
NF-κB in synovial fibroblasts. siRTLR-3, TLR-3 knockdown; TNF,
tumor necrosis factor; MMP, matrix metalloproteinase; NF, nuclear
factor; RANKL, receptor activator of NF-κB ligand; TLR, toll-like
receptor; ERK, extracellular signal-regulated kinase; AKT, protein
kinase B; p, phosphorylated; IL, interleukin. |
TNF-α inhibitor improves bone
destruction in iodoacetate-induced osteoarthritis mice model
Finally, we further investigated the in vivo
role of TNF-α inhibitor in the iodoacetate-induced osteoarthritis
mice model. As shown in Fig. 6A,
TNF-α inhibitor improved the osteoarthritis determined by
osteoarthritis score. Results demonstrated that TNF-α inhibitor
markedly downregulated bone destruction compared to control
(Fig. 6B). TNF-α inhibitor also
improved body weight for osteoarthritis mice compared to control
(Fig. 6C). Inflammatory factors of
TNF-α, IL-1, IL-4 and IL-6 expression levels were downregulated in
cartilago articularis (Fig. 6D).
TNF-α inhibitor decreased TLR-3 and increased expression and
phosphorylation levels of ERK and AKT in cartilago articularis
(Fig. 6E). These results suggest
that TNF-α inhibitor presents many benefits for the treatment of
osteoarthritis mice model.
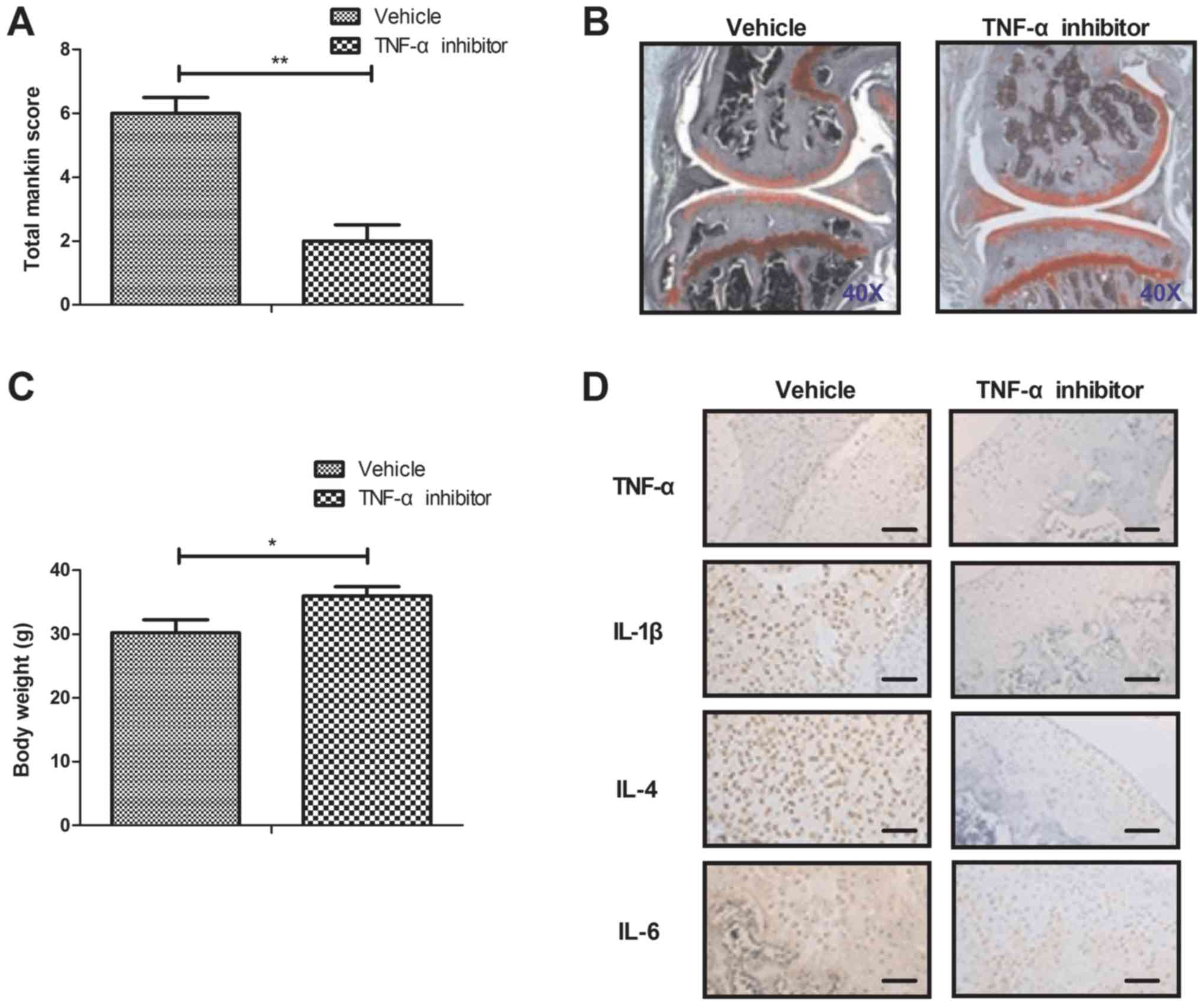 | Figure 6.TNF-α inhibitor improves bone
destruction in a mouse model of iodoacetate-induced osteoarthritis.
(A) TNF-α inhibitor improves osteoarthritis as determined by the
osteoarthritis score. (B) Representative articular tissue in
vehicle and TNF-α inhibitor treated mice. TNF-α inhibitor
downregulates bone destruction compared with the control.
Magnification, ×40. (C) TNF-α inhibitor improves body weight of
osteoarthritis mice compared with the control. (D) TNF-α inhibitor
decreases TNF-α, IL-1, IL-4 and IL-6 expression in articular
cartilage. Scale bars, 20 µm. (E) TNF-α inhibitor decreases TLR-3
and increases the expression and phosphorylation of ERK and AKT in
articular cartilage. Scale bars, 20 µm. *P<005 and **P<0.01.
TNF, tumor necrosis factor; TLR, toll-like receptor; ERK,
extracellular signal-regulated kinase; AKT, protein kinase B; p,
phosphorylated; IL, interleukin. |
Discussion
In recent years, many reports have found that
anti-TNF-α therapy has achieved the therapeutic effects for
patients with osteoarthritis by targeting TNF-α in synovial fluid
(9,23–25).
In this study, we analyzed the relationships between TNF-α and
TLR-3-mediatred ERK/AKT signal pathway in synovial fibroblasts in
mice with osteoarthritis. Güler-Yüksel et al have indicated
that treatment with TNF-α inhibitor infliximab could reduce hand
osteoarthritis (9). Findings in
this study indicate that TNF-α upregulates the inflammatory
cytokines and decreases ERK/AKT signal pathway in synovial
fibroblasts in mice with osteoarthritis. Notably, we found that
TNF-α increased TLR-3 expression in synovial fibroblasts in mice
with osteoarthritis. Here, we reported that TNF-α can increase
regulate inflammation in synovial fibroblasts via regulation of
TLR-3-mediated ERK/AKT signal pathway in mice with osteoarthritis
both in vitro and in vivo.
Study has observed that osteoarthritis Marker YKL-39
is stimulated by IL-4 in differentiating macrophages (26). We reported that TNF-α increased
Il-4 expression in synovial fibroblasts. Treatment with TNF-α
inhibitor infliximab treatment significantly reduced hand
osteoarthritis in patients with rheumatoid arthritis (9). In addition, suppressing the release
of synovia IL-1β and TNF-α in knee osteoarthritis of rabbits may
contribute to the treatment of osteoarthritis (27). Our results showed that TNF-α
treatment upregulated IL-1 and IL-6 expression levels in synovial
fibroblasts and in cartilago articularis in osteoarthritis mice
model. Study has showed that TNF-α and MMP-3 correlated
significantly with the swollen joint count (28). MMP-3 and MMP-9 expression levels
were upregulated in osteoarthritis mice, which can be downregulated
by TNF-α inhibitor (29).
Evidences have showed that RANKL and NF-KB expression levels were
increased in synovial tissue from patients with rheumatoid
arthritis, spondyloarthropathy and osteoarthritis (30). We reported that TNF-α inhibitor
significantly downregulated RANKL and NF-KB in synovial fibroblasts
and in synovial tissue in osteoarthritis mice. These results
indicate that TNF-α is a mediator for inflammation in the processes
of osteoarthritis mice. We will evaluate the effects TNF-α on P-38,
P-65 and IRF-3 expression in synovial fibroblasts in our future
work.
Currently, TLR-3 SNP is associated with knee
osteoarthritis and increasing of TLR-3 levels has been observed in
a Chinese Han population (31). We
found that TLR-3 was upregulated and TNF-α inhibitor decreased
TLR-3 expression levels in synovial fibroblasts. Domagala et
al have suggested that inhibition of IL-1β-induced activation
of MEK/ERK pathway provided a potential mechanism for the treatment
of osteoarthritis (16). Fu et
al have showed that regulation of the PI3K/AKT signaling
pathway could inhibit inflammation and chondrocyte apoptosis in a
rat model of osteoarthritis (32).
In this study, we found that TNF-α inhibitor downregulated TLR-3,
ERK and AKT expression levels in synovial fibroblasts in mice model
of osteoarthritis. Ballak et al have found that TLR-3
appears to play a redundant role in obesity-induced inflammation
and insulin resistance (33).
Additionally, TLR-3 mediated the synovial inflammation in
rheumatoid arthritis (34). We
reported that TNF-α regulates inflammation via TLR-3-mediated
ERK/AKT signal pathway in synovial fibroblasts.
In conclusion, this study found that TNF-α inhibitor
treatment not only decreased inflammatory factors, but also
associated with osteoarthritis score in mice model. The
pro-inflammatory cytokines in osteoarthritis, such as MMP-3, MMP-9,
RANKL and NF-KB, which participate in the pathogenesis of
osteoarthritis, are downregulated by treatment of TNF-α inhibitor.
Importantly, findings have indicated that TNF-α can regulate
inflammation expression via TLR-3-mediated ERK/AKT signal pathway
in synovial fibroblasts both in vitro and in vivo.
Therefore, ERK/AKT may provide a novel potential target for
osteoarthritis therapy. However, further reports need to elucidate
the possible mechanisms mediated by TNF-α in the pathogenesis of
osteoarthritis.
Acknowledgements
Not applicable.
Funding
The present study was supported by National Science
Foundation (grant no. 30801159), the 12th five-year plan of the
military (grant no. 39770714) and Beijing municipal starting
special (grant no. 2016-3-5071).
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
FYY, CQX, CLJ and JTS performed the experiments and
analyzed the experimental data. XWH designed the study.
Ethics approval and consent to
participate
The present study was approved by Ethics Committee
of the 309th Hospital of People's Liberation Army.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Xing F, Lu B, Kuang MJ, Wang Y, Zhao YL,
Zhao J, Sun L, Wang Y, Ma JX and Ma XL: A systematic review and
meta-analysis into the effect of lateral wedge arch support insoles
for reducing knee joint load in patients with medial knee
osteoarthritis. Medicine (Baltimore). 96:e71682017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Aujla RS and Esler CN: Total knee
arthroplasty for osteoarthritis in patients less than fifty-five
years of age: A systematic review. J Arthroplasty. 32:2598–2603.e1.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Alrushud AS, Rushton AB, Kanavaki AM and
Greig CA: Effect of physical activity and dietary restriction
interventions on weight loss and the musculoskeletal function of
overweight and obese older adults with knee osteoarthritis: A
systematic review and mixed method data synthesis. BMJ Open.
7:e0145372017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Alentorn-Geli E, Samuelsson K, Musahl V,
Green CL, Bhandari M and Karlsson J: The association of
recreational and competitive running with hip and knee
osteoarthritis: A systematic review and meta-analysis. J Orthop
Sports Phys Ther. 47:373–390. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Veenhof C, Huisman PA, Barten JA, Takken T
and Pisters MF: Factors associated with physical activity in
patients with osteoarthritis of the hip or knee: A systematic
review. Osteoarthritis Cartilage. 20:6–12. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Davis AM: Osteoarthritis year in review:
Rehabilitation and outcomes. Osteoarthritis Cartilage. 20:201–206.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stannus O, Jones G, Cicuttini F,
Parameswaran V, Quinn S, Burgess J and Ding C: Circulating levels
of IL-6 and TNF-α are associated with knee radiographic
osteoarthritis and knee cartilage loss in older adults.
Osteoarthritis Cartilage. 18:1441–1447. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yuan PW, Liu DY, Chu XD, Hao YQ, Zhu C and
Qu Q: Effects of preventive administration of juanbi capsules on
TNF-alpha, IL-1 and IL-6 contents of joint fluid in the rabbit with
knee osteoarthritis. J Tradit Chin Med. 30:254–258. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Güler-Yüksel M, Allaart CF, Watt I,
Goekoop-Ruiterman YP, de Vries-Bouwstra JK, van Schaardenburg D,
van Krugten MV, Dijkmans BA, Huizinga TW, Lems WF and Kloppenburg
M: Treatment with TNF-α inhibitor infliximab might reduce hand
osteoarthritis in patients with rheumatoid arthritis.
Osteoarthritis Cartilage. 18:1256–1262. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Qin J, Shang L, Ping AS, Li J, Li XJ, Yu
H, Magdalou J, Chen LB and Wang H: TNF/TNFR signal transduction
pathway-mediated anti-apoptosis and anti-inflammatory effects of
sodium ferulate on IL-1β-induced rat osteoarthritis chondrocytes in
vitro. Arthritis Res Ther. 14:R2422012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li ZC, Han N, Li X, Li G, Liu YZ, Sun GX,
Wang Y, Chen GT and Li GF: Decreased expression of microRNA-130a
correlates with TNF-α in the development of osteoarthritis. Int J
Clin Exp Pathol. 8:2555–2564. 2015.PubMed/NCBI
|
|
12
|
Latourte A, Cherifi C, Maillet J, Ea HK,
Bouaziz W, Funck-Brentano T, Cohen-Solal M, Hay E and Richette P:
Systemic inhibition of IL-6/Stat3 signalling protects against
experimental osteoarthritis. Ann Rheum Dis. 76:748–755. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee SY, Yoon BY, Kim JI, Heo YM, Woo YJ,
Park SH, Kim HY, Kim SI and Cho ML: Interleukin-17 increases the
expression of Toll-like receptor 3 via the STAT3 pathway in
rheumatoid arthritis fibroblast-like synoviocytes. Immunology.
141:353–361. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ospelt C, Brentano F, Rengel Y, Stanczyk
J, Kolling C, Tak PP, Gay RE, Gay S and Kyburz D: Overexpression of
toll-like receptors 3 and 4 in synovial tissue from patients with
early rheumatoid arthritis: Toll-like receptor expression in early
and longstanding arthritis. Arthritis Rheum. 58:3684–3692. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhu W, Meng L, Jiang C, He X, Hou W, Xu P,
Du H, Holmdahl R and Lu S: Arthritis is associated with
T-cell-induced upregulation of Toll-like receptor 3 on synovial
fibroblasts. Arthritis Res Ther. 13:R1032011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Domagala F, Martin G, Bogdanowicz P,
Ficheux H and Pujol JP: Inhibition of interleukin-1beta-induced
activation of MEK/ERK pathway and DNA binding of NF-kappaB and
AP-1: Potential mechanism for diacerein effects in osteoarthritis.
Biorheology. 43:577–587. 2006.PubMed/NCBI
|
|
17
|
Klosowska K, Volin MV, Huynh N, Chong KK,
Halloran MM and Woods JM: Fractalkine functions as a
chemoattractant for osteoarthritis synovial fibroblasts and
stimulates phosphorylation of mitogen-activated protein kinases and
Akt. Clin Exp Immunol. 156:312–319. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mohan G, Perilli E, Kuliwaba JS, Humphries
JM, Parkinson IH and Fazzalari NL: Application of in vivo
micro-computed tomography in the temporal characterisation of
subchondral bone architecture in a rat model of low-dose monosodium
iodoacetate-induced osteoarthritis. Arthritis Res Ther.
13:R2102011. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zimmermann T, Kunisch E, Pfeiffer R, Hirth
A, Stahl HD, Sack U, Laube A, Liesaus E, Roth A, Palombo-Kinne E,
et al: Isolation and characterization of rheumatoid arthritis
synovial fibroblasts from primary culture-primary culture cells
markedly differ from fourth-passage cells. Arthritis Res. 3:72–76.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kurien BT and Scofield RH: Western
blotting. Methods. 38:283–293. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bar-Yehuda S, Rath-Wolfson L, Del Valle L,
Ochaion A, Cohen S, Patoka R, Zozulya G, Barer F, Atar E,
Piña-Oviedo S, et al: Induction of an antiinflammatory effect and
prevention of cartilage damage in rat knee osteoarthritis by CF101
treatment. Arthritis Rheum. 60:3061–3071. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ma CH, Lv Q, Yu YX, Zhang Y, Kong D, Niu
KR and Yi CQ: Protective effects of tumor necrosis factor-α
blockade by adalimumab on articular cartilage and subchondral bone
in a rat model of osteoarthritis. Braz J Med Biol Res. 48:863–870.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chevalier X, Ravaud P, Maheu E, Baron G,
Rialland A, Vergnaud P, Roux C, Maugars Y, Mulleman D, Lukas C, et
al: Adalimumab in patients with hand osteoarthritis refractory to
analgesics and NSAIDs: A randomised, multicentre, double-blind,
placebo-controlled trial. Ann Rheum Dis. 74:1697–1705. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fioravanti A, Fabbroni M, Cerase A and
Galeazzi M: Treatment of erosive osteoarthritis of the hands by
intra-articular infliximab injections: A pilot study. Rheumatol
Int. 29:961–965. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gratchev A, Schmuttermaier C, Mamidi S,
Gooi L, Goerdt S and Kzhyshkowska J: Expression of osteoarthritis
marker YKL-39 is stimulated by transforming growth factor beta
(TGF-beta) and IL-4 in differentiating macrophages. Biomark
Insights. 3:39–44. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang J, Zhuo LS, Wang YY, Peng ZL, Huang
YR, Wang Y and Yang L: Effects of electroacupuncture on synovia
IL-1beta and TNF-alpha contents in the rabbit with knee
osteoarthritis. Zhen Ci Yan Jiu. 32:115–118. 2007.(In Chinese).
PubMed/NCBI
|
|
28
|
Mahmoud RK, El-Ansary AK, El-Eishi HH,
Kamal HM and El-Saeed NH: Matrix metalloproteinases MMP-3 and MMP-1
levels in sera and synovial fluids in patients with rheumatoid
arthritis and osteoarthritis. Ital J Biochem. 54:248–257.
2005.PubMed/NCBI
|
|
29
|
Sun R, Huang Y, Zhang H and Liu R: MMP-2,
TNF-α and NLRP1 polymorphisms in Chinese patients with ankylosing
spondylitis and rheumatoid arthritis. Mol Biol Rep. 40:6303–6308.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Crotti TN, Smith MD, Weedon H, Ahern MJ,
Findlay DM, Kraan M, Tak PP and Haynes DR: Receptor activator
NF-kappaB ligand (RANKL) expression in synovial tissue from
patients with rheumatoid arthritis, spondyloarthropathy,
osteoarthritis, and from normal patients: Semiquantitative and
quantitative analysis. Ann Rheum Dis. 61:1047–1054. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang HY, Lee HS, Lee CH, Fang WH, Chen HC,
Salter DM and Su SL: Association of a functional polymorphism in
the promoter region of TLR-3 with osteoarthritis: A two-stage
case-control study. J Orthop Res. 31:680–685. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fu D, Shang X, Ni Z and Shi G: Shikonin
inhibits inflammation and chondrocyte apoptosis by regulation of
the PI3K/Akt signaling pathway in a rat model of osteoarthritis.
Exp Ther Med. 12:2735–2740. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ballak DB, van Asseldonk EJ, van Diepen
JA, Jansen H, Hijmans A, Joosten LA, Tack CJ, Netea MG and
Stienstra R: TLR-3 is present in human adipocytes, but its
signalling is not required for obesity-induced inflammation in
adipose tissue in vivo. PLoS One. 10:e01231522015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Roelofs MF, Wenink MH, Brentano F,
Abdollahi-Roodsaz S, Oppers-Walgreen B, Barrera P, van Riel PL,
Joosten LA, Kyburz D, van den Berg WB and Radstake TR: Type I
interferons might form the link between Toll-like receptor (TLR)
3/7 and TLR4-mediated synovial inflammation in rheumatoid arthritis
(RA). Ann Rheum Dis. 68:1486–1493. 2009. View Article : Google Scholar : PubMed/NCBI
|