Introduction
Millions of people are affected by T cell-mediated
autoimmune diseases, including rheumatoid arthritis (RA), multiple
sclerosis (MS) and type 1 diabetes mellitus (1). Although autoimmune diseases occur
within different tissues, disease-associated autoimmune T cells are
frequently autoreactive and proliferate in the pathological state.
By contrast, the cells in the lesion tissue targeted by T cells are
injured by apoptosis or a reduced proliferative rate (2).
Previously, there have been reports about the role
of the Scutellariae radix compounds baicalein and baicalin
(3,4). For example, baicalein, baicalin and
wogonin have been demonstrated to serve roles in bladder cancer
cell lines including inhibition of cell proliferation in a
dose-dependent manner. Of the aforementioned compounds, baicalin
exhibited the greatest antiproliferative activity on human bladder
cancer cell lines (KU-1 and EJ-1) and a murine bladder cancer cell
line (MBT-2) (5,6). In addition, these compounds have been
reported to exhibit strong antioxidant and free radical-scavenging
activity (7). It was reported that
baicalein was able to significantly attenuate the clinical severity
of experimental autoimmune encephalomyelitis (EAE), an animal model
of MS (8). The inhibited migration
of autoimmune T cells into the central nervous system (CNS) caused
by treatment with baicalein may be attributed to the reduced
activation of microglia, which was indicated previously by
suppressed phagocytosis, and decreased production of
proinflammatory cytokines and chemokines in the CNS (9,10).
Baicalein additionally selectively induces apoptosis in activated
lymphocytes and ameliorates concanavalin A-induced hepatitis in
mice (11). Although baicalein and
baicalin have similar chemical structure, baicalin exhibited
slightly different effects in autoimmune diseases via various
mechanisms. For instance, baicalin exhibited protective effects on
myelin in rats with EAE, although the anti-inflammatory activity of
baicalin is due to the binding of chemokines (12,13).
The present study tested the effect of baicalein and baicalin in
other autoimmune diseases, including an RA model and a colitis
model, and compared the different mechanisms of the autoimmune
disorders. The results of the present study may provide a basis for
the treatment of autoimmune diseases using these natural
compounds.
Materials and methods
Chemicals
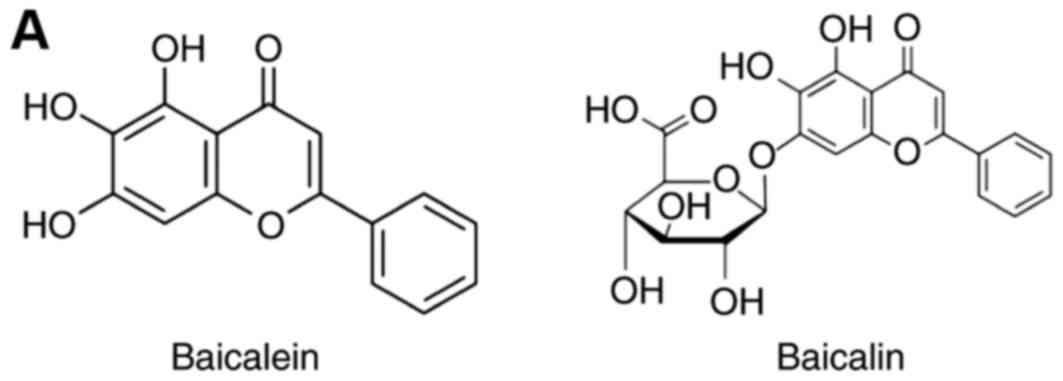
Baicalein and baicalin (Fig. 1A) were purchased from Sigma-Aldrich
(Merck KGaA, Darmstadt, Germany). For the in vitro study,
the drugs were used at a concentration of 100 µM baicalein and 200
µM baicalin. The compounds were added 1 h prior to cell activation.
For the in vivo study, baicalein and baicalin were both used
at a dose of 20 mg/kg.
Ethics statement
All animal procedures were performed in strict
accordance with the recommendations in the Guide for the Care and
Use of the Animal Biosafety Level 3 Laboratory (of Wuhan University
(Wuhan, China). The protocol was approved by the Committee on the
Ethics of Animal Experiments of Wuhan University (permit no. SCXK
2008-0004).
Collagen-induced arthritis (CIA)
model
A total of 50 C57BL/6J male mice (6–8 weeks of age;
weight, 25 g) were obtained from the Animal Biosafety Level 3
Laboratory of Wuhan University. The mice were housed under standard
laboratory conditions (a 12-h light/dark cycle with an average room
temperature of 23°C and a relative humidity of 55%, with food and
water available ad libitum). Mice received a subcutaneous
injection of 0.3 ml collagen (Sigma-Aldrich; Merck KGaA) at the
base of the tail. The vehicle group was treated with PBS. Affected
joints were counted daily for 35 days, mice were sacrificed at the
end of the treatment with baicalein/baicalin and the synovial
fluids (SF) were processed for ELISA analysis.
DSS induced colitis
Colitis was induced in mice with 2.5% w/v DSS (MP
Biomedicals, LLC, Santa Ana, CA, USA) provided ad libitum
for 9 days. DSS induces mucosal damage in the colon, resulting in
an influx of commensal flora to the submucosal layers; this induces
an immune response that closely parallels human colitis. Control
groups received tap water only, DSS group received 2.5% DSS, DSS +
baicalein group received 2.5% DSS and baicalein treatment, and DSS
+ baicalin group was treated with 2.5% DSS and baicalin.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Cells The cells are colon epithelial cells isolated
from DSS mice and the lymphocytes isolated from the synovial fluid
of CIA mice. For isolation of colon epithelial cells, the entire
colon was dissected out and cut longitudinally. The feces were
removed and the lumen was cleaned with PBS. The colon was
subsequently cut into 1-cm-long pieces, transferred into a 50 ml
conical flask containing PBS and stirred vigorously at 37°C for 30
min. The tissue was subsequently strained through a tea strainer
and the debris was washed in PBS to isolate any residual epithelial
cells. The collected PBS solution was centrifuged at 1,000 × g for
5 min at room temperature. The cells were then suspended in 1 ml
PBS, added to a Percoll gradient (40 and 25% Percoll solutions, GE
Healthcare, Chicago, IL, USA), and subjected to density gradient
centrifugation for 25–30 min at 1,000 × g at room temperature.
Subsequently, the cells at the interface (colon epithelial cells)
were collected, washed twice with PBS and used for isolation of
RNA. CD4+ T cells were sorted by fluorescence-activated
cell sorting from the SF of CIA mice. FACS for CD4+ T
cells was performed using the BD FACS Aria flow cytometer (BD
Biosciences, San Jose, CA, USA) by gating on CD4+ cells
after Fc block. Cells were washed once with staining buffer
[Pharmingen Stain Buffer containing bovine serum albumin (BSA)
proteins; BD Biosciences; cat. no. 554657]. The supernatant was
discarded and cells were resuspend in the staining buffer at a
density up to 50×106 cells/ml to maximize the efficiency
of staining. For lymphocytes expressing high levels of Fc receptor
(FcR), a monoclonal antibody that binds to FcgR (Purified Rat
Anti-Mouse CD16/CD32; Mouse BD Fc Block™; cat. no. 553142; BD
Biosciences) was used to bind the receptor on ice for 10–15 min.
Subsequently, CD4 mAb (FITC Rat Anti-Mouse CD4; cat. no. 553046; BD
Biosciences) was used to detect the desired cell population by
incubation for 20–30 min on ice in the dark, followed by two washes
with Pharmingen Stain Buffer containing BSA proteins. Finally, the
cell sorting analysis was performed at 4°C. The data were analyzed
with the FlowJo software version 6.3.2 (Tree Star, Inc., Ashland,
OR, USA). Cells were treated with baicalein/baicalin in
vitro and the culture period was 8 h. Total RNA was extracted
from the cells using the RNeasy mini kit (Qiagen China Co., Ltd.,
Shanghai, China), followed by cDNA synthesis using the Superscript
III first strand synthesis kit (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) at 25°C for 10 min, 50°C for 30
min and 85°C for 5 min. qPCR was performed on a Bio-Rad amplifier
using the Bio-Rad real time PCR mix, including SYBR Green dye (both
Bio-Rad Laboratories, Inc., Hercules, CA, USA). The following
thermocycling conditions were used for the PCR: 50°C for 2 min, 10
min at 95°C; 40 cycles of 95°C for 15 sec and 60°C for 1 min. Data
were analyzed using the Cq value normalized to the endogenous
reference gene GAPDH (14). The
following primers were used for the PCR: Ki-67 (forward:
5′-ACCGTGGAGTAGTTTATCTGGG-3′; reverse:
5′-TGTTTCCAGTCCGCTTACTTCT-3′); STAT3 (forward:
5′-CAATACCATTGACCTGCCGAT-3′; reverse: 5′-GAGCGACTCAAACTGCCCT-3′);
STAT4 (5′-forward: TGGCAACAATTCTGCTTCAAAAC-3′; reverse:
5′-GAGGTCCCTGGATAGGCATGT-3′); STAT6 (5′-forward:
CTCTGTGGGGCCTAATTTCCA-3′; reverse: 5′-CATCTGAACCGACCAGGAACT-3′);
GAPDH (5′-forward: AGGTCGGTGTGAACGGATTTG-3′; forward:
5′-TGTAGACCATGTAGTTGAGGTCA-3′).
ELISA analysis
Cytokine production was measured using commercial
kit for interleukin (IL)-2 (cat. no. DY402), interferon (IFN)-γ
(cat. no. DY485), tumor necrosis factor (TNF)-α (cat. no. DY410;
all R&D Systems, Inc., Minneapolis, MN, USA), IL-6 (cat. no.
550950) and IL-17 (cat. no. 555067; both BD Pharmingen; BD
Biosciences), according to the manufacturers' protocols.
MTT assay
Cells were incubated with MTT reagent (5 µg/ml final
concentration) at 37°C for 4 h. Formazan was solubilized by adding
100 µl DMSO into each well. The extent of formazan production was
determined by an ELISA reader at a wavelength of 550 nm, while 630
nm served as the reference wavelength. The results were calculated
according to the manufacturer's instructions (Vybrant™ MTT Cell
Proliferation Assay kit; Thermo Fisher Scientific, Inc.).
Histology
The colon was washed with cold PBS, measured and
weighed. It was Swiss-rolled and sections were stained with
hematoxylin and eosin as described previously (15). A histopathological score was
generated in a blinded fashion using a widely used grading tool
(16) examining crypts, epithelia,
goblet cells, cellular infiltration and edema. Scores of 0–1
reflect normal morphology, and 2–4, 5–7 and 8–10 represent mild,
moderate, and severe colitis, respectively.
Statistical analysis
The data were analyzed using SPSS 17.0 software
(SPSS, Inc., Chicago, IL, USA) to evaluate the differences between
the groups. The data are reported as the mean ± standard deviation.
P<0.05 was considered to indicate a statistically significant
difference. One-way analysis of variance with the
Student-Newman-Keuls post hoc test was used to calculate the
differences between the multiple comparison groups. The
Mann-Whitney U test was used to analyze the differences between two
groups.
Results
Baicalein attenuates mouse CIA
Baicalein and baicalin administration exhibited
protective activity in two animal models in vivo. Firstly,
the effect on the mouse CIA model was investigated. Mice developed
stable arthritis symptoms on day 10 and the severity of the disease
in the vehicle-treated group worsened continuously with time. The
baicalein-treated group had a lower arthritis score and fewer
affected joints compared with the vehicle group during the entire
course of treatment, while baicalin exerted no significant effect
on the CIA model (Fig. 1B and C).
This result was confirmed by measuring cytokine production in the
SF. Baicalein was able to suppress Th1-type cytokine production,
including IFN-γ, TNF-α and IL-2, in addition to the Th17-type
cytokine IL-17, suggesting that it may inhibit T cell function
in vivo (Fig. 1D).
Baicalein regulates CIA mouse T cell
proliferation via signal transducer and activator of transcription
(STAT) expression in tyrosine-protein kinase JAK (JAK)/STAT
signaling
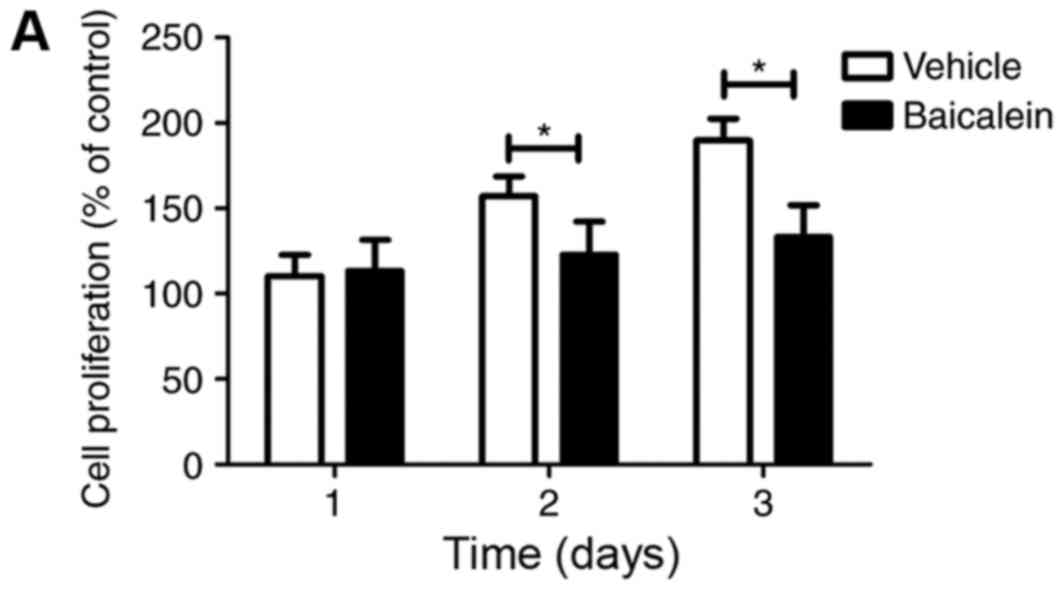
Furthermore, the role of baicalein in T cell
proliferation was investigated. Treatment with 100 nM baicalein
in vitro significantly inhibited the proliferation of
CD4+ T cells sorted from the SF of CIA mice upon
anti-CD3 Ab activation, demonstrated via the MTT assay, and
supported by the Ki67 expression analysis using qPCR (Fig. 2A and B). To elucidate the primary
mechanism underlying the role of baicalein in proliferation,
JAK/STAT pathway gene expression was assessed and the cells were
treated with baicalein in vitro. As presented in Fig. 2C, baicalein significantly reduced
the expression of STAT3 and STAT4, although it did not exert the
same effect on STAT6 expression in infiltrated SF T cells (Fig. 2C), suggesting that baicalein
regulated joint inflammatory signaling by suppressing STAT3/STAT4
expression in the JAK/STAT pathway.
Baicalin ameliorates DSS-induced
colitis
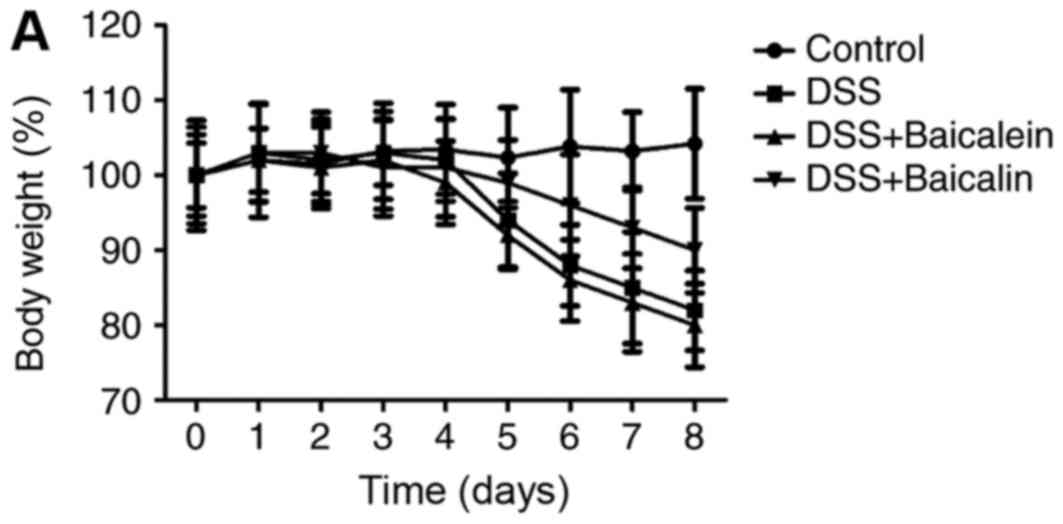
The efficacy of baicalein and baicalin was
investigated in another type of autoimmune disease. A DSS-induced
colitis model in mice was produced via treatment with 3% DSS. The
body weight of the baicalin-treated group was increased compared
with the vehicle-treated group at the peak of the disease, which
was consistent with the colon length and histology scores, while
baicalein exerted no effect on the DSS model (Fig. 3A-C). Similarly, the serum IL-2,
IFN-γ and IL-17 levels were reduced by baicalin (Fig. 3D).
Baicalin regulated epithelial cell
proliferation in DSS-induced colitis via STAT expression in
JAK/STAT signaling
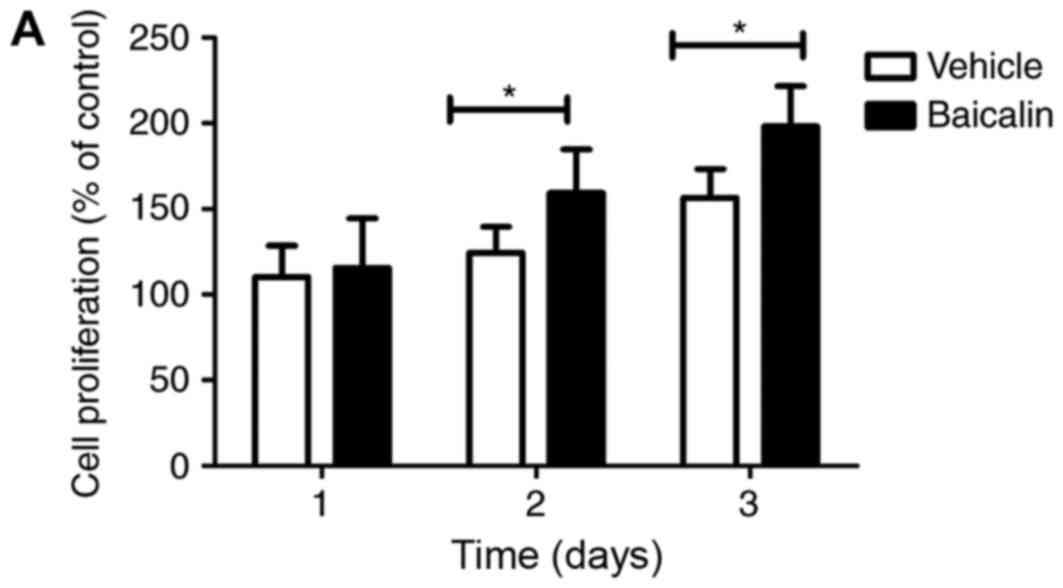
To determine the effect of baicalin on epithelial
proliferation, colon epithelial cells (CECs) were isolated and
treated with baicalin in vitro. Baicalin was able to promote
the proliferation of CECs as measured by CCK8 staining, suggesting
that it was able to repair the epithelial barrier and promote
mucosal wound healing in the disease state (Fig. 4A). This was consistent with the
proliferation marker Ki67 analysis using qPCR (Fig. 4B). In order to elucidate the
possible mechanism underlying the effect of baicalin on CEC
proliferation, the JAK-STAT signaling pathway was selected for
analysis and the mRNA expression of STAT subtype genes was assessed
by qPCR. It was demonstrated that baicalin may reduce the
expression of the STAT4 gene locus, while having no significant
effect on STAT3/STAT6 (Fig. 4C),
suggesting that baicalin mediated CEC proliferation through the
JAK-STAT signaling pathway.
Discussion
The results of the present study demonstrated that
the components of Scutellariae radix (termed Huang-Qin in
Chinese), baicalein and baicalin, regulate T cell and epithelial
cell function to exert a therapeutic effect on autoimmune disease.
The distinct biological activities of baicalein and baicalin were
assessed in diverse autoimmune diseases. Baicalein ameliorated the
severity of disease in the CIA model, while baicalin attenuated
DSS-induced colitis; the in vitro study demonstrated that
the two compounds mediated cell activation via the JAK-STAT pathway
and cell proliferation. It has been reported that baicalein and
baicalin exert multiple physiological activities. Baicalin
ameliorates camptothecin-induced intestinal toxicity in rats
(17). In vitro, baicalin
exhibited a protective role in renal cell injury (18). It has additionally been reported
that baicalein and baicalin are potent inhibitors of reverse
transcriptase, and that they suppress the human T-cell leukemia
virus and promote the apoptosis of human immunodeficiency
virus-infected CEM cells (19).
Furthermore, the antitumor effects of baicalein and baicalin on
human hepatoma cell lines have been reported (20).
Animal models of autoimmune arthritis and colitis
have proven to be valuable research tools for the study of the
pathogenic mechanisms of these autoimmune diseases, in addition to
the testing of novel therapeutics. Firstly, the present study
identified that baicalein was effective in the murine RA model
induced by type II collagen. CIA has been the most widely used
model of RA; it shares several pathological features with RA, and
collagen is an important protein in cartilage, the target tissue in
RA (21,22). Additionally, of the antigen-defined
models that are based on cartilage proteins, CIA has the shortest
duration between immunization and disease manifestation. In the
present study, treatment with baicalein in vivo reduced the
arthritis score and attenuated the expression of inflammatory
cytokines in the serum. This was due to the in vitro
regulation of T cell proliferation via the JAK-STAT signaling
pathway; in particular, it suppressed the expression of STAT3 and
STAT4. In another experiment, it was demonstrated that the
Scutellariae radix component baicalin alleviated disease
severity in the mouse colitis model induced by DSS. Inflammatory
bowel disease (IBD) is an autoimmune disease characterized by
chronic, uncontrolled inflammation in the intestinal mucosa,
affecting millions of people worldwide. The DSS-challenged mice
exhibited stable symptoms of IBD, with weight loss, shorter colon
length, rectal bleeding and loose stools (23). However, the control mice exhibited
more severe disease compared with baicalin-treated animals, which
presented with reduced weight loss and increased colon length.
Furthermore, proinflammatory responses were suppressed in the colon
in baicalin-treated mice. In contrast to the inhibition of T cell
proliferation in the RA model induced by baicalein, the other
Scutellariae radix component baicalin was able to promote
epithelial proliferation by amplifying STAT4 gene expression and
the downstream pathway. The JAK-STAT pathway is a direct signal
transduction pathway in the nucleus: The extracellular signaling
proteins, including cytokines (for example, IFN-γ and interleukins)
and growth factors, interact with transmembrane receptors at the
cell surface in order to monitor the extracellular environment, JAK
family gene expression is activated and the substrate STAT proteins
are phosphorylated (24,25). Different STAT subtypes are involved
in cell proliferation, differentiation and transformation (26–28).
The results of the present study demonstrated that the natural
compounds of Scutellariae radix were able to directly
regulate the expression of STAT genes in the JAK-STAT pathway. In
conclusion, the two components of Scutellariae radix,
baicalein and baicalin, exhibited therapeutic effects in two types
of autoimmune disease. The two compounds were able to modulate the
proliferation of the target cells in arthritis and colitis through
various STAT subtypes in the JAK-STAT pathway (baicalein, STAT3/4;
baicalin, STAT4).
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81401230).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JX and ZG designed and performed the experiments,
analyzed the data, and wrote the manuscript. JLiu, GY, MS, JLi and
XX performed experiments and data analysis.
Ethics approval and consent to
participate
All animal procedures were performed in strict
accordance with the recommendations in the Guide for the Care and
Use of the Animal Biosafety Level 3 Laboratory of Wuhan University
(Wuhan, China). The protocol was approved by the Committee on the
Ethics of Animal Experiments of Wuhan University (permit no. SCXK
2008-0004).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tobón GJ, Youinou P and Saraux A: The
environment, geo-epidemiology, and autoimmune disease: Rheumatoid
arthritis. J Autoimmun. 35:10–14. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kragstrup TW, Jalilian B, Keller KK, Zhang
X, Laustsen JK, Stengaard-Pedersen K, Hetland ML, Hørslev-Petersen
K, Junker P, Østergaard M, et al: Changes in soluble CD18 in murine
autoimmune arthritis and rheumatoid arthritis reflect disease
establishment and treatment response. PLoS One. 11:e01484862016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pan TL, Wang PW, Huang CH, Leu YL, Wu TH,
Wu YR and You J: Herbal formula, Scutellariae radix and Rhei
rhizoma attenuate dimethylnitrosamine-induced liver fibrosis in a
rat model. Sci Rep. 5:117342015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen CY, Chen JX, Li W, Li H and Yang B:
Comparative chemical and statistical analysis of cultivated and
wild Radix Scutellariae. Am J Chin Med. 39:1029–1041. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ikemoto S, Sugimura K, Yoshida N, Yasumoto
R, Wada S, Yamamoto K and Kishimoto T: Antitumor effects of
Scutellariae radix and its components baicalein, baicalin, and
wogonin on bladder cancer cell lines. Urology. 55:951–955. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li HL, Zhang S, Wang Y, Liang RR, Li J, An
P, Wang ZM, Yang J and Li ZF: Baicalein induces apoptosis via a
mitochondrial-dependent caspase activation pathway in T24 bladder
cancer cells. Mol Med Rep. 7:266–270. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shieh DE, Liu LT and Lin CC: Antioxidant
and free radical scavenging effects of baicalein, baicalin and
wogonin. Anticancer Res. 20:2861–2865. 2000.PubMed/NCBI
|
|
8
|
Xu J and Zhang Y, Xiao Y, Ma S, Liu Q,
Dang S, Jin M, Shi Y, Wan B and Zhang Y: Inhibition of
12/15-lipoxygenase by baicalein induces microglia PPARβ/δ: A
potential therapeutic role for CNS autoimmune disease. Cell Death
Dis. 4:e5692013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Suk K, Lee H, Kang SS, Cho GJ and Choi WS:
Flavonoid baicalein attenuates activation-induced cell death of
brain microglia. J Pharmacol Exp Ther. 305:638–645. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen CJ, Raung SL, Liao SL and Chen SY:
Inhibition of inducible nitric oxide synthase expression by
baicalein in endotoxin/cytokine-stimulated microglia. Biochem
Pharmacol. 67:957–965. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang Y, Shan L, Hua Y, Wang D, Zeng H,
Liu R, Zhang W and Hu Z: Baicalein selectively induces apoptosis in
activated lymphocytes and ameliorates concanavalin a-induced
hepatitis in mice. PLoS One. 8:e695922013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Krakauer T, Li BQ and Young HA: The
flavonoid baicalin inhibits superantigen-induced inflammatory
cytokines and chemokines. FEBS Lett. 500:52–55. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li BQ, Fu T, Gong WH, Dunlop N, Kung H,
Yan Y, Kang J and Wang JM: The flavonoid baicalin exhibits
anti-inflammatory activity by binding to chemokines.
Immunopharmacology. 49:295–306. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Park CM, Reid PE, Walker DC and MacPherson
BR: A simple, practical ‘swiss roll’ method of preparing tissues
for paraffin or methacrylate embedding. J Microsc. 145:115–120.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu Y, Hunt NH and Bao S: The role of
granulocyte macrophage-colony-stimulating factor in acute
intestinal inflammation. Cell Res. 18:1220–1229. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Takasuna K, Kasai Y, Kitano Y, Mori K,
Kobayashi R, Hagiwara T, Kakihata K, Hirohashi M, Nomura M, Nagai
E, et al: Protective effects of kampo medicines and baicalin
against intestinal toxicity of a new anticancer camptothecin
derivative, irinotecan hydrochloride (CPT-11), in rats. Jpn J
Cancer Res. 86:978–984. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lin M, Li L, Li L, Pokhrel G, Qi G, Rong R
and Zhu T: The protective effect of baicalin against renal
ischemia-reperfusion injury through inhibition of inflammation and
apoptosis. BMC Complement Altern Med. 14:192014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Baylor NW, Fu T, Yan YD and Ruscetti FW:
Inhibition of human T cell leukemia virus by the plant flavonoid
baicalin (7-glucuronic acid, 5,6-dihydroxyflavone). J Infect Dis.
165:433–437. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chang WH, Chen CH and Lu FJ: Different
effects of baicalein, baicalin and wogonin on mitochondrial
function, glutathione content and cell cycle progression in human
hepatoma cell lines. Planta Med. 68:128–132. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Di Sante G, Tolusso B, Fedele AL, Gremese
E, Alivernini S, Nicolò C, Ria F and Ferraccioli G: Collagen
specific T-cell repertoire and HLA-DR alleles: Biomarkers of active
refractory rheumatoid arthritis. EBioMedicine. 2:2037–2045. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schultz HS, Guo L, Keller P, Fleetwood AJ,
Sun M, Guo W, Ma C, Hamilton JA, Bjørkdahl O, Berchtold MW and
Panina S: OSCAR-collagen signaling in monocytes plays a
proinflammatory role and may contribute to the pathogenesis of
rheumatoid arthritis. Eur J Immunol. 46:952–963. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Palone F, Vitali R, Cucchiara S,
Pierdomenico M, Negroni A, Aloi M, Nuti F, Felice C, Armuzzi A and
Stronati L: Role of HMGB1 as a suitable biomarker of subclinical
intestinal inflammation and mucosal healing in patients with
inflammatory bowel disease. Inflamm Bowel Dis. 20:1448–1457. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liongue C, O'Sullivan LA, Trengove MC and
Ward AC: Evolution of JAK-STAT pathway components: Mechanisms and
role in immune system development. PLoS One. 7:e327772012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Abell K and Watson CJ: The Jak/Stat
pathway: A novel way to regulate PI3K activity. Cell Cycle.
4:897–900. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zoranovic T, Grmai L and Bach EA:
Regulation of proliferation, cell competition, and cellular growth
by the Drosophila JAK-STAT pathway. JAKSTAT.
2:e254082013.PubMed/NCBI
|
|
27
|
Kowshik J, Baba AB, Giri H, Reddy Deepak
G, Dixit M and Nagini S: Astaxanthin inhibits JAK/STAT-3 signaling
to abrogate cell proliferation, invasion and angiogenesis in a
hamster model of oral cancer. PLoS One. 9:e1091142014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Katsuyama T, Comoglio F, Seimiya M, Cabuy
E and Paro R: During Drosophila disc regeneration, JAK/STAT
coordinates cell proliferation with Dilp8-mediated developmental
delay. Proc Natl Acad Sci USA. 112:E2327–E2336. 2015. View Article : Google Scholar : PubMed/NCBI
|