Introduction
Chimeric antigen receptor (CAR) T cells contain an
antibody-derived target region and a single chain fragment
variable, which is an antibody-derived target region infused to the
membrane of T cells to control signaling. They are divided into
four types according to their structure and activity. CAR-T cells
are cellular immunotherapies that have been studied internationally
for nearly 20 years, although the use of these engineered cells
only began to be widespread in recently. At the 2017 ASH Meeting on
Hematologic Malignancies (1), the
exceptional performance of CD19-CAR-T cells in the treatment of
hematologic malignancies was recognized. However, as suitable
targets for CAR-T solid tumors are often expressed in healthy
tissues leading to CAR-T cells attacking normal cells their use is
limited and the prospects for CAR-T therapy for solid tumors are
not good. The use of the cluster of differentiation (CD)19 antigen
has led to the emergence of several associated complications, such
as low gamma globulin hematic disease, cytokine storm and
off-target effects (2). Hence,
there is a need to explore more effective regimens of existing
treatments to reduce such complications. Certain studies have
focused on the incorporation of suicide genes into fourth
generation CAR-T cells to stimulate apoptosis of target cells;
however, complications have still been experienced (3,4).
Chemotherapeutic agents have many useful clinical applications,
although the exact effects of these on CAR-T cells remain unknown.
Therefore, the aims of the present study were to determine the
effect of chemotherapeutic agents on CAR-T cells using the in
vitro Cell Counting kit-8 (CCK-8) assay and to evaluate the
abilities of fludarabine (FDR) and mafosfamide (MFA) to induce
apoptosis of CD19-CAR-T cells through the use of Annexin
V/propidium iodide double staining, a JC-1 fluorescent probe for
detection of alterations in cell membrane potential and flow
cytometric analysis to assess concentrations of caspase-3/7 to
identify the apoptotic signaling pathways of CD19-CAR-T cells.
Since CD19-CAR-T cells have demonstrated excellent response rates
in patients with acute lymphoblastic leukemia, a common
hematological disease (5–8), CD19-CAR-T cells were used in the
present study.
Materials and methods
Treatment of CD19-CAR-T cells with
chemotherapeutic agents
CD19-CAR-T cells were donated by Biothera
Pharmaceuticals, Inc. (Eagan, MN, USA) and cultured in serum-free
primary cell culture medium (Hangzhou Union Biotechnology Co.,
Ltd., Guangzhou, China) at 37°C and 5% CO2. CD19-CAR-T
cells were cultured at a concentration of 2×105 cells in
90 µl immune cell serum-free medium (Youkang serum free medium;
Union Biotechnology Co., Ltd., Hangzhou, China) supplemented with
FDR (Genzyme Europe B.V., Naarden, Netherlands) at concentrations
of 6.25, 12.5, 25, 50 or 100 µg/ml, or MFA (Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) at concentrations of 1.25,
2.5, 5, 10 or 20 µg/ml for 24, 48, 72 or 96 h at room temperature.
Each sample was prepared in triplicate. Serum-free medium (10 µl)
and 90 µl 2×105 CD19-CAR-T cells in immune cell
serum-free medium served as a negative control.
Inhibition of CD19-CAR-T cell
viability by the CCK-8 assay
Inhibition of CD19-CAR-T cells incubated with FDR
and MFA for 24, 48, 72 and 96 h were tested using a CCK-8 assay
(Biyuntian Biological Engineering Co., Ltd., Shanghai, China),
according to the manufacturer's protocol. At each time point, each
concentration was distributed among 3 wells; normal, control and
blank control wells. The normal well received cells, culture medium
and chemotherapeutic agents (A dosing group). The control well
received cells and culture medium (A0 dosing group). The blank
control well received culture medium (A blank group). After culture
for 24, 48, 72 and 96 h, 10 µl CCK-8 solution was removed from each
well and incubated at 37°C and 5% CO2 for 2 h, and the
optical density (OD) was measured using a SpectraMax M Series
Multi-Mode Microplate reader (Molecular Devices, LLC, Sunnyvale,
CA, USA) at 450 nm wavelength. The % cell viability was calculated
as (the OD value of the A0 dosing group - the OD value of the A
dosing group)/(the OD value of the A0 dosing group - the OD value
of the A blank dosing group) ×100%.
Annexin V/propidium iodide,
caspase-3/7 and mitochondrial membrane potential analysis of
CD19-CAR-T cells by flow cytometry
CD19-CAR-T cells were cultured in serum-free medium
(Youkang serum free medium; Union Biotechnology Co., Ltd.,
Hangzhou, China) and stimulated with 2% interleukin-2 (Novoprotein
Biotechnology Co., Ltd., Shanghai, China) every 2–3 days until the
cell concentration reached 2×105 cells per 90 µl. Then,
FDR (12.5 µg/ml) and MFA (10 µg/ml) were added to the cultures, for
12, 24 or 48 h and divided into normal and control groups.
CD19-CAR-T cells were cultured in serum-free medium (normal group).
Annexin V positive and propidium iodide positive using a
fluorescein isothiocyanate (FITC) Annexin V/Dead Cell Apoptosis kit
(Thermo Fisher Scientific, Inc., Waltham, MA, USA) were added to
CD19-CAR-T cells (control group). Apoptotic cells were induced by
cytokine depletion by washing and suspending cells in serum-free
medium three times. Following incubation for 24 h, apoptotic cell
death was detected using a CKX41 inverted microscope (Olympus
Corporation, Tokyo, Japan) to observe the changes in the shape of
cells, and then examined by flow cytometry. Following incubation
with FDR and MFA for 24 and 48 h, apoptotic cell death was examined
using a phycoerythrin (PE) CellEvent™ Caspase-3/7 Green Flow
Cytometry Assay kit (Thermo Fisher Scientific, Inc.).
Alternatively, after incubation with FDR and MFA for 12 and 24 h,
apoptotic cell death was examined using the MitoProbe™
(5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl-benzimidazol-carbocyanine
iodide; JC-1) assay kit (Thermo Fisher Scientific, Inc.) by flow
cytometry and the ApoAlert reagent kit (Thermo Fisher Scientific,
Inc.), according to the manufacturer's protocols. A total of 10,000
events were acquired for each sample treated with FDR and MFA, and
analyzed using the FACScan™ automated flow cytometer system (BD
Biosciences, Franklin Lakes, NJ, USA) with C6 Plus software version
1.0.264.21 (BD Biosciences) and WinMDI software version 2.7 (kindly
made available by Dr Joe Trotter; The Scripps Research Institute,
La Jolla, CA).
Statistical analysis
Differences in cell inhibition among the various
dosage groups were compared using an analysis of variance with a
factorial design and one-way analysis of variance (ANOVA).
Differences in apoptosis rates among the groups were compared using
a paired t-test, depending on data distribution. Two-way ANOVA
followed by the Turkey's post hoc test was used. All analyses were
performed using SPSS version 15.0 software SPSS, Inc. (Chicago, IL,
USA). P≤0.05 was considered to indicate a statistically significant
difference. All values are expressed as the mean ± standard
error.
Results
Inhibition of CD19-CAR-T cells by
chemotherapeutic agents
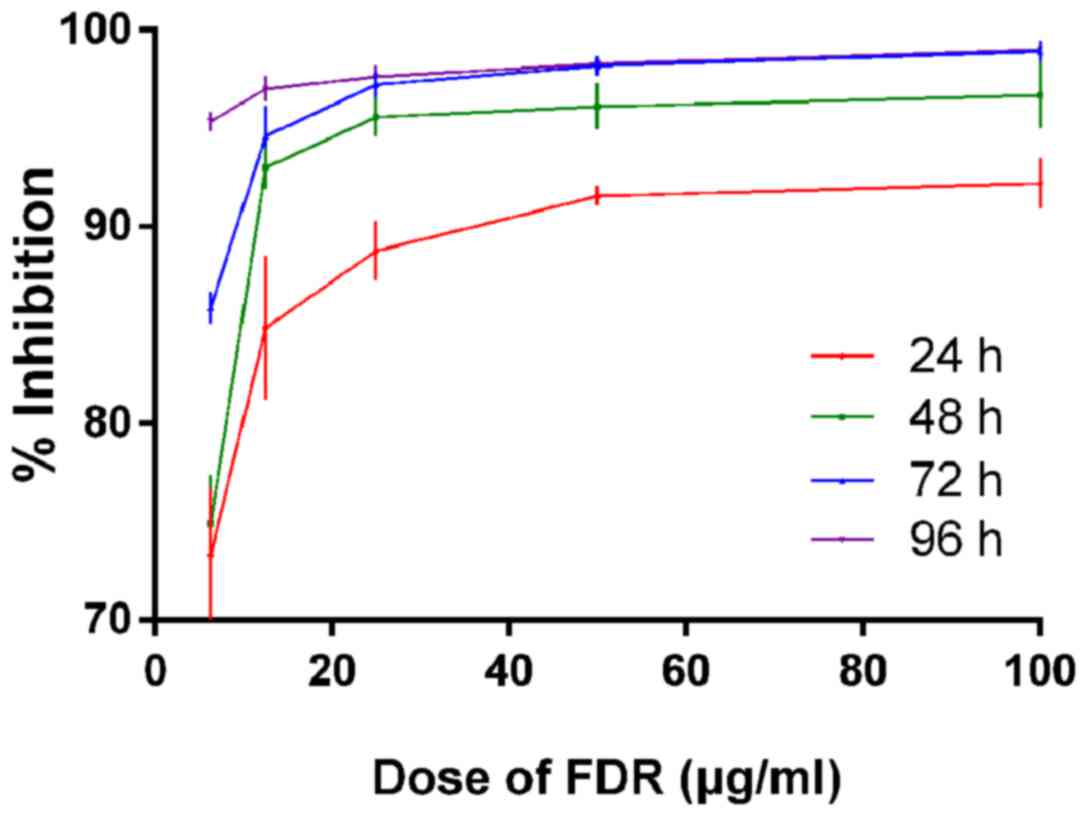
Both FDR and MFA were demonstrated to inhibit the
viability of CD19-CAR-T cells. The inhibition ratio increased with
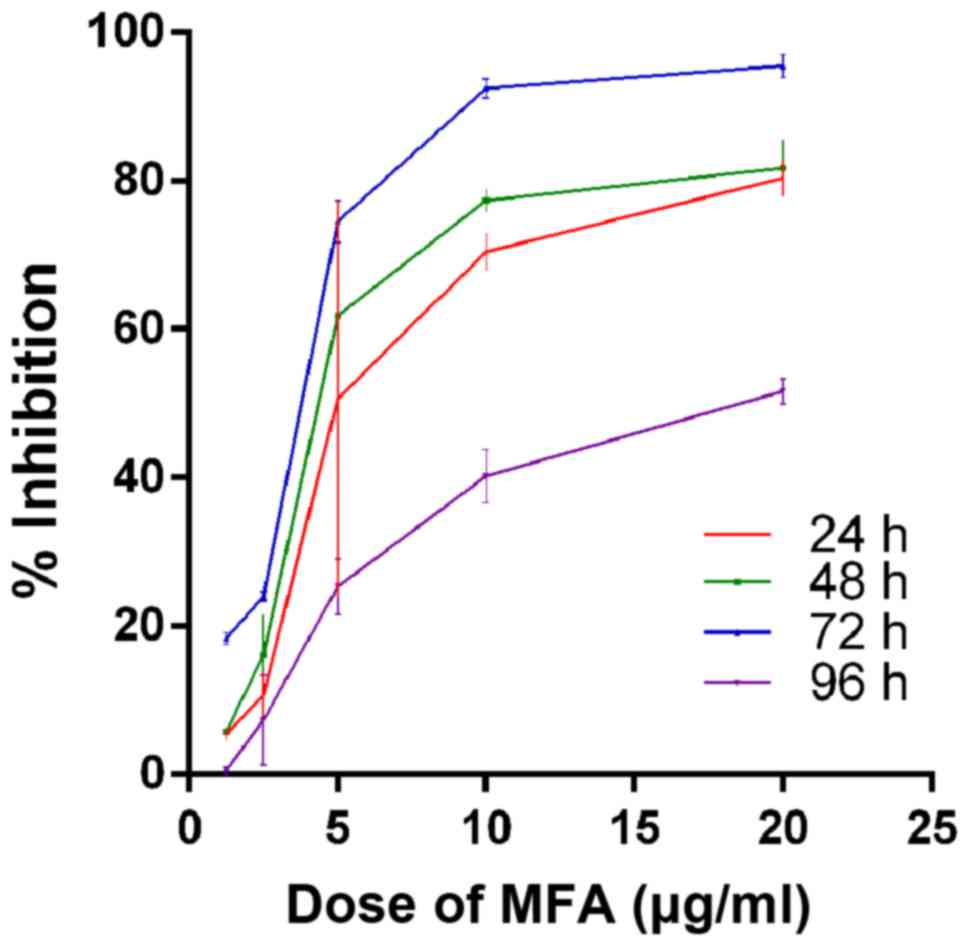
increasing drug dosage and incubation time (P<0.05; Fig. 1). With MFA the inhibition ratio
increased with increasing drug dosage and incubation time within 72
h (Fig. 2). At 96 h, there was
≥90% inhibition of CD19-CAR T cells by FDR at 1.55 µg/ml. The 50%
inhibition ratio (IC50) of CD19-CAR-T cells treated with
FDR for 24 h was 1.55 µg/ml (data not shown). At a dose of 10 µg/ml
MFA, the % inhibition was >90% at 72 h; however, the %
inhibition was lowest at 96 h. Based on the % inhibition at 72 h,
the IC50 was 3.34 µg/ml (Fig. 2).
Apoptosis of CD19-CAR-T cells treated
with FDR and MFA
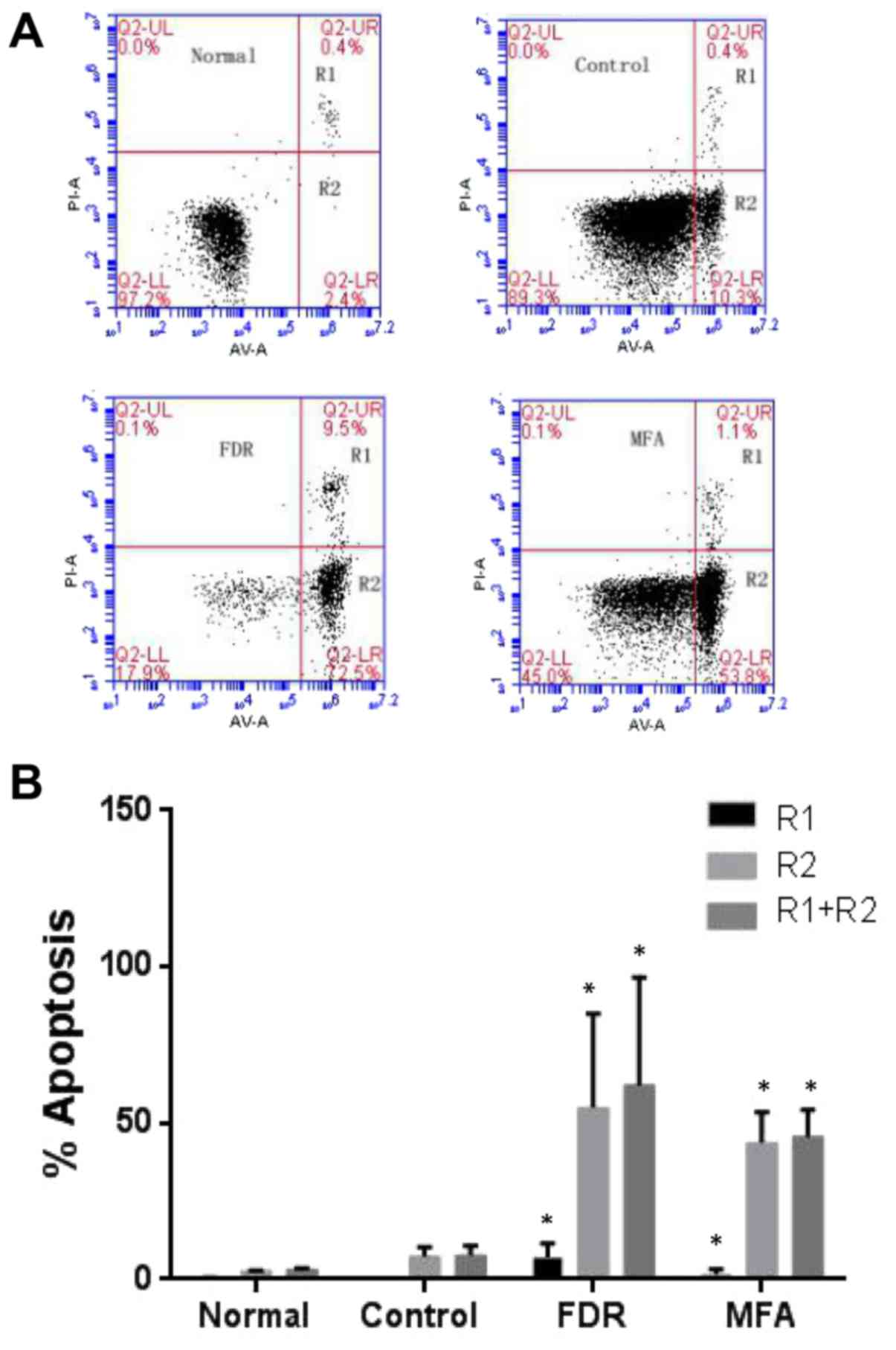
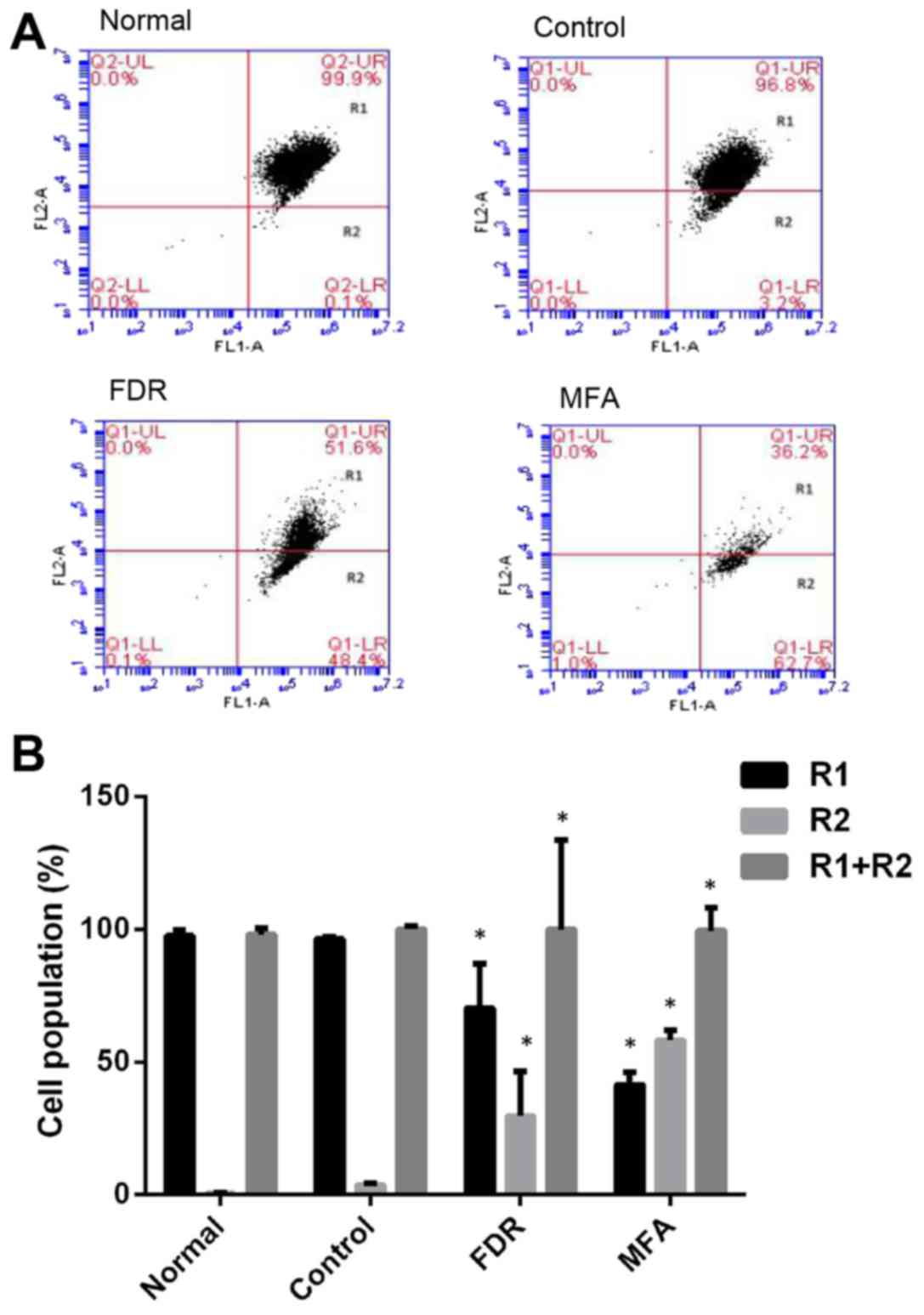
Following treatment with FDR and MFA for 24 h, early
apoptotic CD19-CAR-T cells assumed irregular shapes and increased
cell debris, due to eversion of phosphatidylserine in the cell
membrane. The data demonstrated that the number of early apoptotic
cells [Annexin V positive, propidium iodide negative, (R2 segment
of the plots)] and dead cells [Annexin V positive (R1+R2 sections
of the plots)] increased following treatment with FDR and MFA
(P<0.05 vs. control group; Fig.
3). There were significant differences between the FDR or
MFA-treated, control and normal groups (P<0.05 vs. control
group; Fig. 3).
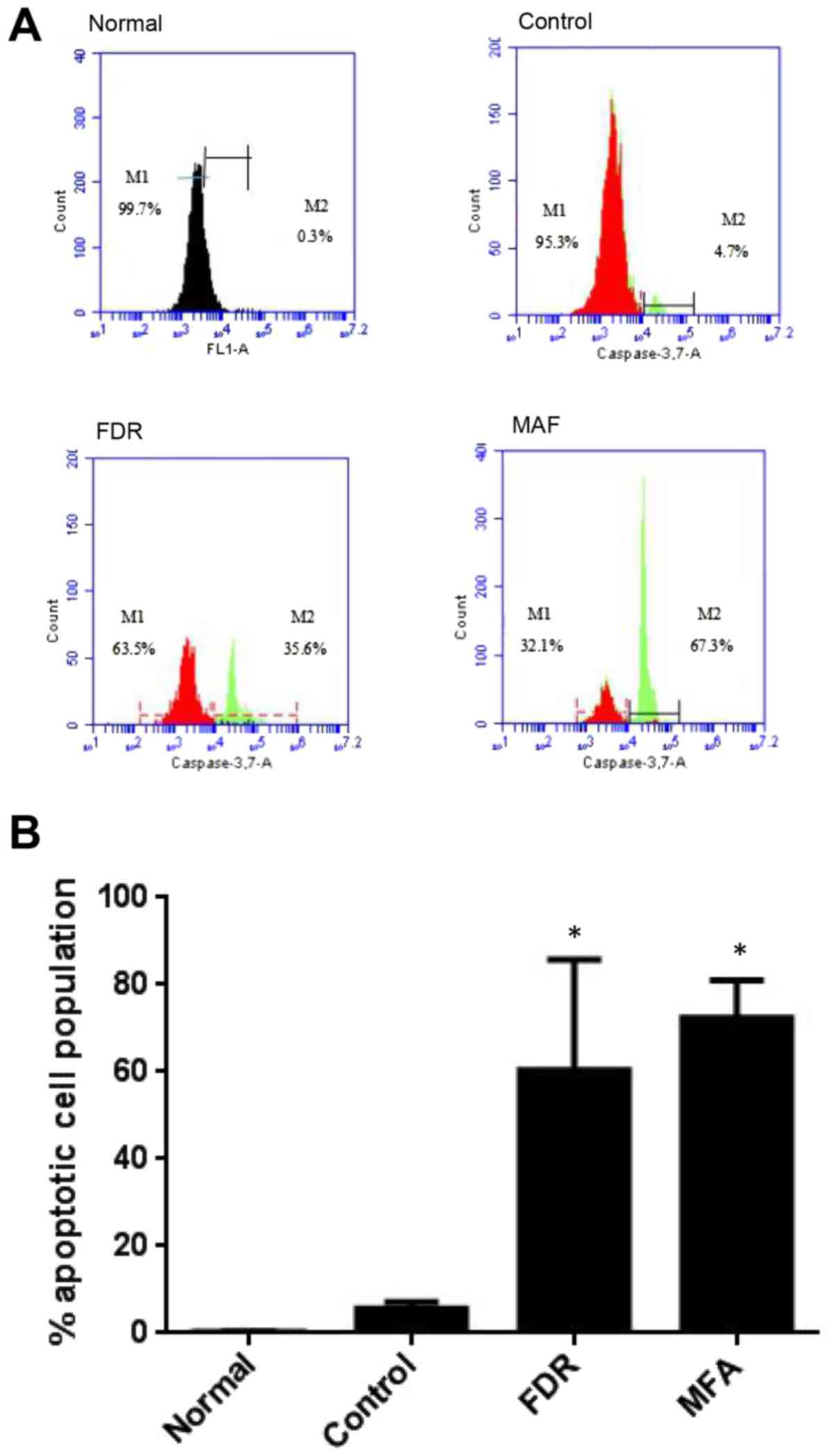
The expression of active caspase-3/7 (M2 gate;
Fig. 4), a downstream effector of
the apoptotic signaling cascade, was significantly increased in
CD19-CAR-T cells after treatment with FDR and MFA (P<0.05 vs.
control group; Fig. 4) compared
with the control group. Apoptosis of CD19-CAR-T cells treated with
FDR occurred within 48 h, and within 24 h MFA treatment.
Mitochondria with normal membrane potential (Δψm)
concentrates JC-1 into aggregates (red fluorescence in FL2),
whereas in depolarized mitochondria, JC-1 forms monomers (green
fluorescence in FL1). Compared with normal CD19-CAR-T cells, FDR
and MFA-treated groups had greater proportions of cells containing
JC-1 monomers (R2 gate; green fluorescence), suggesting a drop in
Δψm (P<0.05 vs. control group; Fig.
5). Next, CD19-CAR-T cells were incubated with FDR for 24 h, or
MFA for 12 h, and the fluorescence of Δψm altered from red to green
(P<0.05 vs. control group; Fig.
5). Together, these results suggested that FDR and MFA induced
apoptosis of CD19-CAR-T cells.
Discussion
FDR and MFA are chemotherapeutic agents most
commonly used as a conditioning regimen prior to acute leukemia
therapy. Lukenbill and Kalaycio (9) reported that T cells may be inhibited
by FDR for up to 3 years, with the lowest concentration of T cells
observed in the first year, which then recover to a normal level in
the third year. Essentially, CD19-CAR-T cells may be inhibited by
FDR, as shown in the experiments of the present study where an
increase in the inhibition ratio with time was observed. Therefore,
when deciding upon a regimen, the time point must be considered
both before and after bone marrow transplantation to optimize the
effects of CD19-CAR-T cell infusion.
Previous research has indicated that activities of
regulatory T cells (Tregs) may be inhibited by administration of
cyclophosphamide (10). MFA is an
analogue of cyclophosphamide, which is a known inhibitor of T cells
(11). The results of the present
study revealed that inhibition of cell viability in MFA-treated
CD19-CAR-T cells was increased after 72 h. Prior to infusion of
CD19-CAR-T cells, the interval time between MFA and CD19-CAR-T
cells must be ≥3 days. Therefore, after bone marrow
transplantation, MFA may be used to kill CD19-CAR-T cells remaining
after 3 days.
The pathogenesis of several diseases by detection of
apoptosis of peripheral blood leukocytes has been reported in many
clinical studies (12–15). At different developmental stages of
lymphocytes, the inhibition ratio increases with time (16). The present study is the first to
reveal that FDR and MFA induce apoptosis of CD19-CAR-T cells, as
confirmed by the eversion of phosphatidylserine in the cell
membrane using the Annexin V and propidium iodide apoptosis kit,
which is currently a robust method for measuring early apoptosis.
In the present study, after the cells were incubated with the two
chemotherapeutic agents for 24 h, lipid membrane rollover by
phosphatidylserine to the outer membrane was detected by the FITC
Annexin V/Dead Cell Apoptosis kit, which suggested apoptosis of
CD19-CAR-T cells.
According to the mitochondrial apoptotic pathway
theory (17), red fluorescence
changing to green fluorescence indicates early apoptosis of
CD19-CAR-T cells. The results of the present study revealed that
MFA induced apoptosis of CD19-CAR-T cells earlier than FDR.
However, these results may have been influenced by the dosage and
the different underlying mechanism of actions of the drugs.
A change in caspase-3/7 activity indicates an
important role in the mitochondrial apoptotic singling pathway.
Activated caspase-3 cleaves the corresponding nuclear cytoplasmic
substrates, resulting in apoptosis. Caspase-3 may also activate
caspase-7 (18), which further
promotes apoptosis (19). The
results of the present study showed that caspase-3/7 activity, an
indicator of early apoptosis in CD19-CAR-T cells, significantly
increased after treatment with FDR and MFA.
In conclusion, this study may be the first to
demonstrate that FDR and MFA added separately inhibited the
activities of CD19-CAR-T cells, as confirmed by the CCK-8 assay.
These findings lay the foundation for future cell immunotherapies
and the sequencing of therapeutic regimens. Apoptotic cell death of
CD19-CAR-T cells warrants further research for the advancement of
cellular immunotherapies. The present study focused on the cellular
level. Future studies should establish animal models with a B-cell
malignant cell line to test whether the activities of CD19-CAR-T
cells were affected by FDR and MFA treatment.
Acknowledgements
The authors would like to thank Mr. Guangchao Li
(Bio-Gene Technology, Ltd., Guangzhou, China) for technical
support.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. U1401221) and the
Guangzhou Science and Technology Program (grant no.
201508020258).
Availability of data and materials
The datasets generated and/or analyzed during the
current study are not publicly available due to ongoing research
but are available from the corresponding author on reasonable
request.
Authors' contributions
WY was a major contributor in writing the manuscript
and the design and performance of the experiments; FP, GL and CZ
were responsible for collecting samples; WD was in charge of
manufacture and tested the CD19-CART cells; XW, YH, MY and XF
designed experiments; HL and ZP wrote the article and analyzed the
data; and, CL was responsible for paper guidance, writing of the
manuscript and designing the study. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
FDR
|
fludarabine
|
|
MFA
|
mafosfamide
|
|
CAR
|
chimeric antigen receptor
|
|
CCK-8
|
Cell Counting kit 8
|
|
OD
|
optical density
|
|
FITC
|
fluorescein isothiocyanate
|
|
CD19
|
cluster of differentiation 19
|
References
|
1
|
Lee SY, Olsen P, Lee DH, Kenoyer AL, Budde
LE, O'Steen S, Green DJ, Heimfeld S, Jensen MC, Riddell SR, et al:
Preclinical optimization of a CD20-specific chimeric antigen
receptor vector and culture conditions. J Immunother. 41:19–31.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Davila ML and Brentjens R: Chimeric
antigen receptor therapy for chronic lymphocytic leukemia: What are
the challenges? Hematol Oncol Clin North Am. 27:341–353. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Deniger DC, Switzer K, Mi T, Maiti S,
Hurton L, Singh H, Huls H, Olivares S, Lee DA, Champlin RE and
Cooper LJ: Bispecific T-cells expressing polyclonal repertoire of
endogenous γδ T-cell receptors and introduced CD19-specific
chimeric antigen receptor. Mol Ther. 21:638–647. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhou X, Di Stasi A, Tey SK, Krance RA,
Martinez C, Leung KS, Durett AG, Wu MF, Liu H, Leen AM, et al:
Long-term outcome after haploidentical stem cell transplant and
infusion of T cells expressing the inducible caspase 9 safety
transgene. Blood. 123:3895–3905. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kochenderfer JN, Dudley ME, Feldman SA,
Wilson WH, Spaner DE, Maric I, Stetler-Stevenson M, Phan GQ, Hughes
MS, Sherry RM, et al: B-cell depletion and remissions of malignancy
along with cytokine-associated toxicity in a clinical trial of
anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood.
119:2709–2720. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Haso W, Lee DW, Shah NN, Stetler-Stevenson
M, Yuan CM, Pastan IH, Dimitrov DS, Morgan RA, FitzGerald DJ,
Barrett DM, et al: Anti-CD22-chimeric antigen receptors targeting
B-cell precursor acute lymphoblastic leukemia. Blood.
121:1165–1174. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Porter DL, Levine BL, Kalos M, Bagg A and
June CH: Chimeric antigen receptor-modified T cells in chronic
lymphoid leukemia. N Engl J Med. 365:725–733. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Grupp SA, Kalos M, Barrett D, Aplenc R,
Porter DL, Rheingold SR, Teachey DT, Chew A, Hauck B, Wright JF, et
al: Chimeric antigen receptor-modified T cells for acute lymphoid
leukemia. N Engl J Med. 368:1509–1518. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lukenbill J and Kalaycio M: Fludarabine: A
review of the clear benefits and potential harms. Leuk Res.
37:986–994. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kanakry CG, Ganguly S, Zahurak M,
Bolaños-Meade J, Thoburn C, Perkins B, Fuchs EJ, Jones RJ, Hess AD
and Luznik L: Aldehyde dehydrogenase expression drives human
regulatory T cell resistance to posttransplantation
cyclophosphamide. Sci Transl Med. 5:211ra1572013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Goldstein M, Roos WP and Kaina B:
Apoptotic death induced by the cyclophosphamide analogue
mafosfamide inhuman lymphoblastoid cells: Contribution of DNA
replication, transcription inhibition and Chk/p53 signaling.
Toxicol Appl Pharmacol. 229:20–32. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Alemán M, Beigier-Bompadre M, Borghetti C,
de la Barrera S, Abbate E, Isturiz M and Sasiain MC: Activation of
peripheral blood neutrophils from patients with active advanced
tuberculosis. Clin Immunol. 100:87–95. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Smith JA and Weidemann MJ: Further
characterization of the neutrophil oxidative burst by flow
cytometry. J Immunol Methods. 162:261–268. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jung YJ, Kim YJ, Kim LH, Lee SO, Park BL,
Shin HD and Lee HS: Putative association of Fas and FasL gene
polymorphisms with clinical outcomes of hepatitis B virus
infection. Intervirology. 50:369–376. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Maloy KJ, Salaun L, Cahill R, Dougan G,
Saunders NJ and Powrie F: CD4+CD25+ T(R)
cells suppress innate immune pathology through cytokine-dependent
mechanisms. J Exp Med. 197:111–119. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hua YY, Wang XS, Zhang Y, Yao CG, Zhang XM
and Xiong ZA: Intense picosecond pulsed electric fields induce
apoptosis through a mitochondrial-mediated pathway in HeLa cells.
Mol Med Rep. 5:981–987. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
El Kebir D and Filep JG: Role of
neutrophil apoptosis in the resolution of inflammation.
ScientificWorldJournal. 10:1731–1748. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Stanczyk J, Ospelt C, Gay RE and Gay S:
Synovial cell activation. Curr Opin Rheumatol. 18:262–267. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang H, Gao G, Clayburne G and Schumacher
HR: Elimination of rheumatoid synovium in situ using a Fas ligand
‘gene scalpel’. Arthritis Res Ther. 7:R1235–R1243. 2005. View Article : Google Scholar : PubMed/NCBI
|