Introduction
Lung cancer is the leading cause of tumor
malignancy-associated mortality worldwide. The morbidity of lung
cancer has continuously increased and the overall
five-year-survival rate is ~15% (1), which poses a threat to public
health.
Lung cancer, which is also termed lung carcinoma,
may be divided into four major types: Small-cell lung carcinoma,
adenocarcinoma, squamous cell carcinoma and large cell carcinoma.
The latter three types are recognized collectively as non-small
cell lung cancer, which accounts for ~85% of all lung cancers
(2,3). At present, surgical operation is the
most preferred and primary treatment for lung cancer at an early
stage, which can completely remove the non-metastatic lesions to
improve the long-term survival rate of patients (4,5).
However, the majority of lung cancers are detected at a late stage
of tumor development, and in these cases the opportunity for
surgery is lost and non-surgical treatments are relied upon,
including chemotherapy, radiotherapy and targeted therapy. In
recent years, non-surgical treatment of lung cancer has been
associated with characteristics of chemotherapeutic toxicity, drug
resistance and insensitivity (6,7).
Therefore, the development of novel anti-lung cancer drugs with
high efficiency and low toxicity is essential.
Natural plants are an important source of novel
pharmaceutical ingredients, and certain plants possess natural
anticancer agents. Screening and investigating novel compounds from
plants is an important strategy by which novel anticancer drugs
with high efficiency and low toxicity may be identified (8). Isofraxidin is a coumarin compound
that primarily exists in plants such as Sarcandraglabra and
Acanthopanaxsenticosus (9).
Isofraxidin has been reported to exhibit antibacterial,
antioxidant, antidepressant and anti-inflammatory properties
(10–12). Recently, isofraxidin was
demonstrated to exert antitumor effects on two human colorectal
adenocarcinoma cell lines, as the proliferation of HT-29 and SW-480
cells was inhibited by exposure to isofraxidin (9). Metastasis is the major factor
associated with the recurrence and mortality of patients with lung
cancer. Yamazaki and Tokiwa (13)
indicated that isofraxidin may reduce the expression of matrix
metallopeptidase 7 (MMP7) protein, thus inhibiting the invasive
ability of liver cancer cells; however, to the best of our
knowledge, the effects of isofraxidin on lung cancer and the
associated mechanisms have not previously been reported. The
present study investigated the effect of isofraxidin on the
proliferation, apoptosis, migration and invasion of A549 lung
cancer cells, as well as the underlying mechanisms, which provided
theoretical and data support for the clinical application of
isofraxidin in lung cancer.
Materials and methods
Reagents
Isofraxidin (Fig.
1A) was purchased from Shanghai Yuan Ye Biotechnology Co., Ltd.
(Shanghai, China). Isofraxidin was dissolved in dimethyl sulfoxide
(DMSO) as the stock solution, which was stored at 4°C. The working
solution of isofraxidin was diluted with Dulbecco's modified
Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) prior to use (the final DMSO concentration was
<0.5% in all cultures).
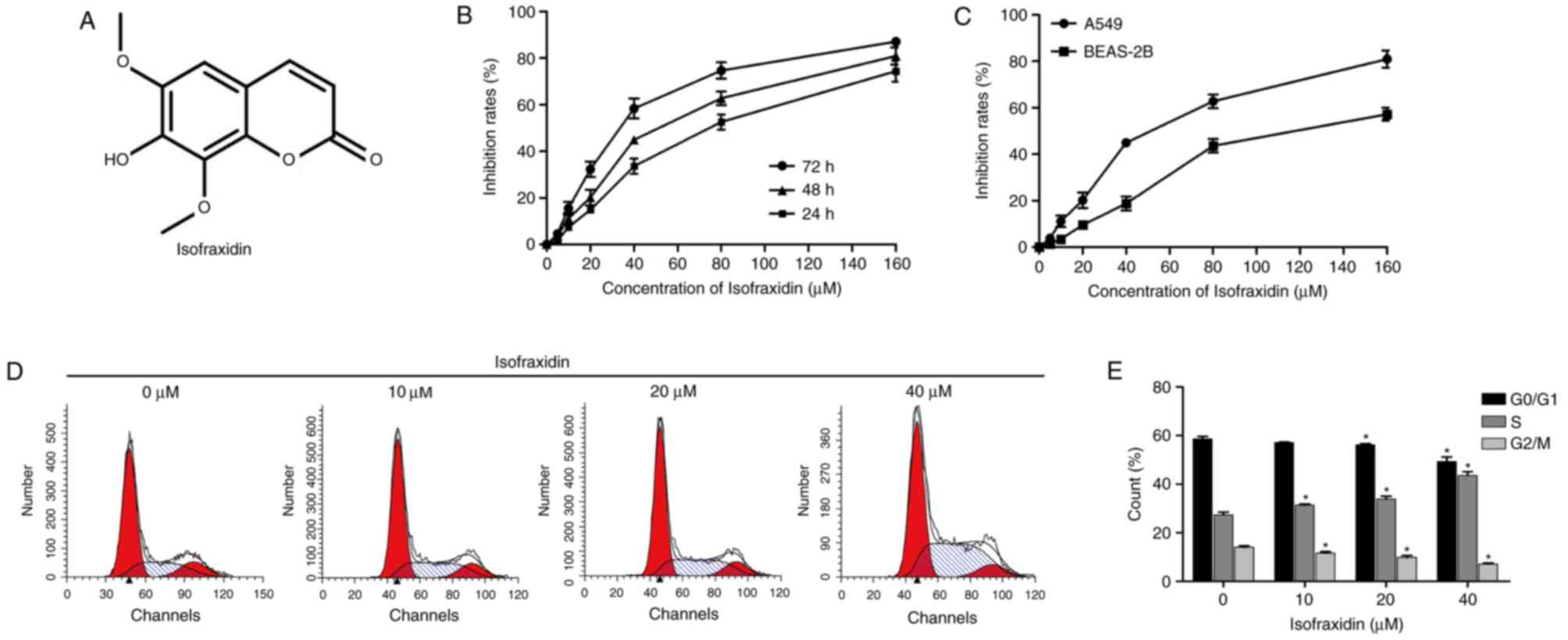 | Figure 1.Isofraxidin inhibits the proliferation
of A549 human non-small cell lung cancer cells. (A) Chemical
structure of isofraxidin. (B) Cell proliferation of A549 cells
exposed to isofraxidin at different concentrations for 24, 48 and
72 h was detected with a CCK-8 kit. (C) Cell proliferation of A549
and BEAS-2B cells after 48 h treatment with isofraxidin at
different concentrations was detected with a CCK-8 kit. (D)
Representative flow cytometry graphs indicating cell cycle
distribution of A549 cells following 48 h treatment with
isofraxidin at different concentrations. (E) Percentage of A549
cells in each cell cycle stage after 48 h treatment with
isofraxidin at different concentrations was calculated: 0 µM
(G0/G1, 58.63±0.94%; G2/M, 13.97±0.74%; S, 27.38±1.14%), 20 µM
(G0/G1, 57.08±0.24%; G2/M, 11.62±0.58%; S, 31.30±0.52%), 40 µM
(G0/G1, 56.25±0.46%; G2/M, 9.85±0.72%; S, 33.90±1.15%), 60 µM
(G0/G1, 49.32±1.87%; G2/M, 7.08±0.39%; S, 43.60±1.56%). *P<0.05
vs. 0 µM isofraxidin. CCK-8, Cell Counting kit-8. |
Cell lines and culture
A549 human lung cancer and BEAS-2B human normal lung
epithelial cell lines were purchased from the Cell resource center
of Shanghai institute of life sciences, Chinese academy of sciences
(Shanghai, China). Cells were maintained in DMEM supplemented with
10% fetal bovine serum (FBS; Hyclone; GE Healthcare Life Sciences,
Logan, UT, USA) and 100 U/ml penicillin and streptomycin in a
humidified atmosphere containing 5% CO2 at 37°C. Cells
were cultured to a confluency of 80% and were subsequently digested
with 0.25% trypsin and seeded into new plates at the required
density.
Cell counting kit-8 (CCK-8) assay
Cell proliferation was evaluated via a CCK-8 assay
(Beyotime Institute of Biotechnology, Haimen, China). A549 and
BEAS-2B cells were seeded in 96-well plates at a concentration of
5×103/100 µl per well and incubated overnight at 37°C.
The media were replaced with fresh media containing different
concentrations of isofraxidin (0–160 µM) and cells were cultured
for 24, 48 and 72 h at 37°C. Subsequently, 10 µl CCK-8 was added
and cells were incubated for 4 h in the dark at 37°C. The optical
density (OD) at 450 nm was recorded by a microplate reader. The
inhibitory rate (IR) of isofraxidin on cell proliferation was
calculated using the following formula: IR
(%)=(1-ODtreatedgroup/ODcontrol)×100.
Cell cycle assay
Flow cytometric analysis was performed to determine
the effects of isofraxidin on the cell cycle of A549 cells. A total
of 4.5×105 A549 cells per 6 cm dish were exposed to
isofraxidin (0, 10, 20 and 40 µM) at 37°C for 48 h and subsequently
digested with EDTA-free trypsin. Cells were collected by
centrifugation (1,000 × g, 4°C, 5 min), fixed with cold 75% ethanol
and incubated overnight at 4°C. Cells were then stained with
propidium iodide [100 µg/ml; Multi Sciences (Lianke) Biotech, Co.,
Ltd., Hangzhou, China] at 25°C for 30 min and flow cytometric
analysis was performed immediately after staining (First-generation
Attune flow cytometer, Thermo Fisher Scientific, Inc.; Attune
cytometric software, v2.1.0, Thermo Fisher Scientific, Inc.).
Hoechst 33342 staining
Hoechst 33342 (Beyotime Institute of Biotechnology,
Shanghai, China) staining was used to detect the apoptosis of A549
cells treated with isofraxidin. A total of 1.5×105 A549
cells per well of 6-well plate were exposed to isofraxidin (0, 10,
20 and 40 µM) at 37°C for 48 h, followed by fixing with 4%
formaldehyde for 20 min at room temperature. Cells were
subsequently stained with Hoechst 33342 (10 µg/ml) at 25°C in the
dark for 20 min and cell apoptosis was immediately evaluated with a
fluorescent microscope (magnification, ×100). Three fields of view
were employed for analysis of apoptotic rate.
Migration and invasion assays
Transwell inserts (EMD Millipore, Billerica, MA,
USA) were utilized for A549 cell migration and invasion assays.
Cells were diluted with serum-free DMEM at the logarithmic growth
phase and 5×104 cells in 200 µl serum-free DMEM were
added in each upper chamber. A total of 500 µl DMEM medium
containing 20% FBS was added into the lower chambers. In the
invasion assay, Matrigel was also added to the upper well according
manufacturer's protocol. After 1 h at 37°C, isofraxidin was added
to reach a concentration of 0, 2.5, 5 and 10 µM. The concentrations
of isofraxidin in upper and lower wells were in parallel. Following
incubation at 37°C for 48 h, the cells on the upper membrane were
removed by cotton swab. The cells at the bottom of the membrane
were fixed with 4% formaldehyde at 25°C for 20 min, stained with
10% crystal violet at 25°C for 10–15 min and photographed under a
light microscope (magnification, ×200). Three fields of view were
analyzed per sample.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RT-qPCR was used to determine the transcript levels
of the invasion-associated proteins MMP-2, MMP-9. A549 cells were
exposed to isofraxidin (0, 10, 20 and 40 µM) for 48 h at 37°C and
the total RNA of cells was extracted using TRIzol reagent (Thermo
Fisher Scientific, Inc., Waltham, MA, USA). RT of isolated RNA was
conducted (PrimeScript™ RT reagent kit; cat. no. RR037A, Takara
Biotechnology Co., Ltd., Beijing, China) and amplified using the
qPCR [ABI 7900HT Fast Real-Time PCR, (Applied Biosystems; Thermo
Fisher Scientific, Inc.); SYBR-Green qPCR Master mix, cat. no.
638320; Takara Biotechnology Co., Ltd.]. Thermocycling conditions
for qPCR: 95°C, 5 sec; 55°C, 20 sec; 72°C, 30 sec; 45 cycles. The
2−ΔΔCq method was employed for normalization. GAPDH was
used as the control. Primer sequences used were as follows: MMP-2
forward, 5′-AGCGAGTGGATGCCGCCTTTAA-3′ and reverse,
5′-CATTCCAGGCATCTGCGATGAG-3′; MMP-9 forward,
5′-GCCACTACTGTGCCTTTGAGTC-3′ and reverse,
5′-CCCTCAGAGAATCGCCAGTACT-3′; and GAPDH forward,
5′-GTCTCCTCTGACTTCAACAGCG-3′ and reverse,
5′-ACCACCCTGTTGCTGTAGCCAA-3′.
Western blot analysis
A549 cells were treated with isofraxidin (0, 10, 20
and 40 µM) for 48 h at 37°C and collected. Cells lysis was
conducted on ice for 30 min using lysis buffer, which consisted of
a proteinase inhibitor mix, phosphatase inhibitor,
phenylmethylsulfonyl fluoride and radioimmunoprecipitation assay
buffer (1:1:1:100; Beyotime institute of Biotechnology, Shanghai,
China). The supernatant was collected following centrifugation,
10,000 × g, 30 min, 4°C, the loading buffer was added and the
mixture was boiled for 5 min. The BCA method was used to determine
the protein concentration and 10 µg protein was loaded per loading
well. Samples were analyzed by 10% SDS-PAGE and transferred
electrophoretically to polyvinylidene difluoride (PVDF) membranes.
The membranes were blocked with 10% skim milk solution at 25°C for
1 h and incubated overnight at 4°C with the following primary
antibodies: Rabbit anti-epidermal growth factor receptor (EGFR)
antibody (cat. no. 4267S), rabbit anti-phosphorylated (p)-EGFR
(pY1068) antibody (cat. no. 3777S), mouse anti-AKT antibody (cat.
no. 2920S), mouse anti-p-AKT (pS473) antibody (cat. no. 4060S),
rabbit anti-extracellular signal-regulated kinase (ERK) antibody
(cat. no. 4695S), rabbit anti-p-ERK (pT202/Y204) antibody (cat. no.
4370S), rabbit anti-Bcl-2-associated X (Bax) antibody (cat. no.
5023T), rabbit anti-Bcl-2 antibody (cat. no. 4223S), rabbit
anti-MMP-9 antibody (cat. no. 13667T), rabbit anti-MMP2 antibody
(cat. no. 40994S), rabbit anti-GAPDH antibody (HRP Conjugate; cat.
no. 8884), which were purchased from Cell Signaling Technology,
Inc., (Danvers, MA, USA). All primary antibodies were diluted in
1:1,000 proportion. GAPDH was used as the loading control.
Following washing with TBST buffer (with 0.1% Tween-20), the PVDF
membranes were incubated with species-specific horseradish
peroxidase-conjugated secondary antibodies [anti-mouse IgG,
horseradish peroxidase (HRP)-linked antibody; cat. no. 7076;
anti-rabbit IgG, HRP-linked antibody; cat. no. 7074; Cell Signaling
Technology, Inc., (Danvers, MA, USA), 1:5,000 dilution] at 25°C for
1 h. The membranes were visualized using Pierce™ ECL Plus Western
Blotting Substrate (Thermo Fisher Scientific, Inc.). Image Pro Plus
6.0 (Media Cybernetics, Inc., Rockville, MD, USA) was used for
densitometry.
In vivo xenograft study
All procedures involving animals were approved by
the Ethics Committee of the Jingzhou Central Hospital Affiliated to
Tongji Medical College of Huazhong University of Science and
Technology (Jingzhou, China). A total of 18 male BALB/c nude mice
were purchased from the CAVENS Laboratory Animal Company
(Changzhou, China; http://www.cavens.com.cn/). The average weight of the
mice was ~18 g. They were maintained under specific pathogen-free
conditions at 20–25°C (Humidity: 50–60%; 12-h light dark cycle).
A549 cells in PBS at 4×106/150 µl per mouse were
injected subcutaneously into the 4-week-old nude mice. The mice
were returned to their cages for continuous feeding. Ad
libitum food and water was available. The tumor formation was
regularly analyzed. The visible subcutaneous tumor was inspected by
the naked eye. The tumor-bearing mice were separated into three
groups: Negative control group (n=6 mice), isofraxidin-treated
group (5 mg/kg, n=6 mice) and cisplatin-treated (Shanghai
Yuanyeshengwu, Shanghai, China) positive control group (5 mg/kg,
n=6 mice). Drugs were delivered daily by intraperitoneal injection
for 21 days. All mice were sacrificed following the treatment
period and the body weights and tumor volumes were measured. The
expression levels of p-EGFR, p-AKT and p-ERK were detected by
immunohistochemical staining as described previously (14).
Statistical analysis
All the assays were repeated in triplicate, data
were processed using SPSS 17.0 statistical software (SPSS, Inc.,
Chicago, IL, USA) and the results are presented as the mean ±
standard deviation. The quantitative experiments were analyzed
using analysis of variance. Dunnett's test was used as a post-hoc
test. Statistical graphs were produced using GraphPad Prism 5
software (GraphPad Software, Inc., La Jolla, CA, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
Isofraxidin inhibits the proliferation
of the A549 cell line
To determine the effect of isofraxidin on the
proliferation of A549 cells and the optimal dosage, the cells were
treated with isofraxidin at concentrations of 0, 5, 10, 20, 40, 80
and 160 µM for 24, 48 and 72 h. The results revealed that
isofraxidin exhibited a dose- and time-dependent inhibitory effect
on the proliferation of A549 cells (Fig. 1B), with half-maximal inhibitory
concentration (IC50) values of 28.76±3.16, 42.71±4.05
and 75.16±3.42 µM following 72, 48 and 24 h of treatment,
respectively. Furthermore, a lower rate of inhibition was observed
in BEAS-2B normal lung epithelial cells following isofraxidin
treatment compared with A549 cells (Fig. 1C), with an IC50 value of
85.32±2.34 µM after 48 h of treatment, indicating that within a
certain dosage range, isofraxidin exhibited a lower toxicity
towards normal lung epithelial cells compared with cancerous cells.
Additionally, alterations in the cell cycle were analyzed by flow
cytometry, the results of which demonstrated that following
treatment with isofraxidin for 48 h, the cell cycle of A549 cells
was markedly altered, particularly at concentrations of 20 and 40
µM, compared with the control cells (Fig. 1D and E). The percentage of cells in
S phase increased in a dose-dependent manner (Fig. 1D and E), indicating that
isofraxidin inhibited the proliferation of A549 cells by arresting
cells in S phase.
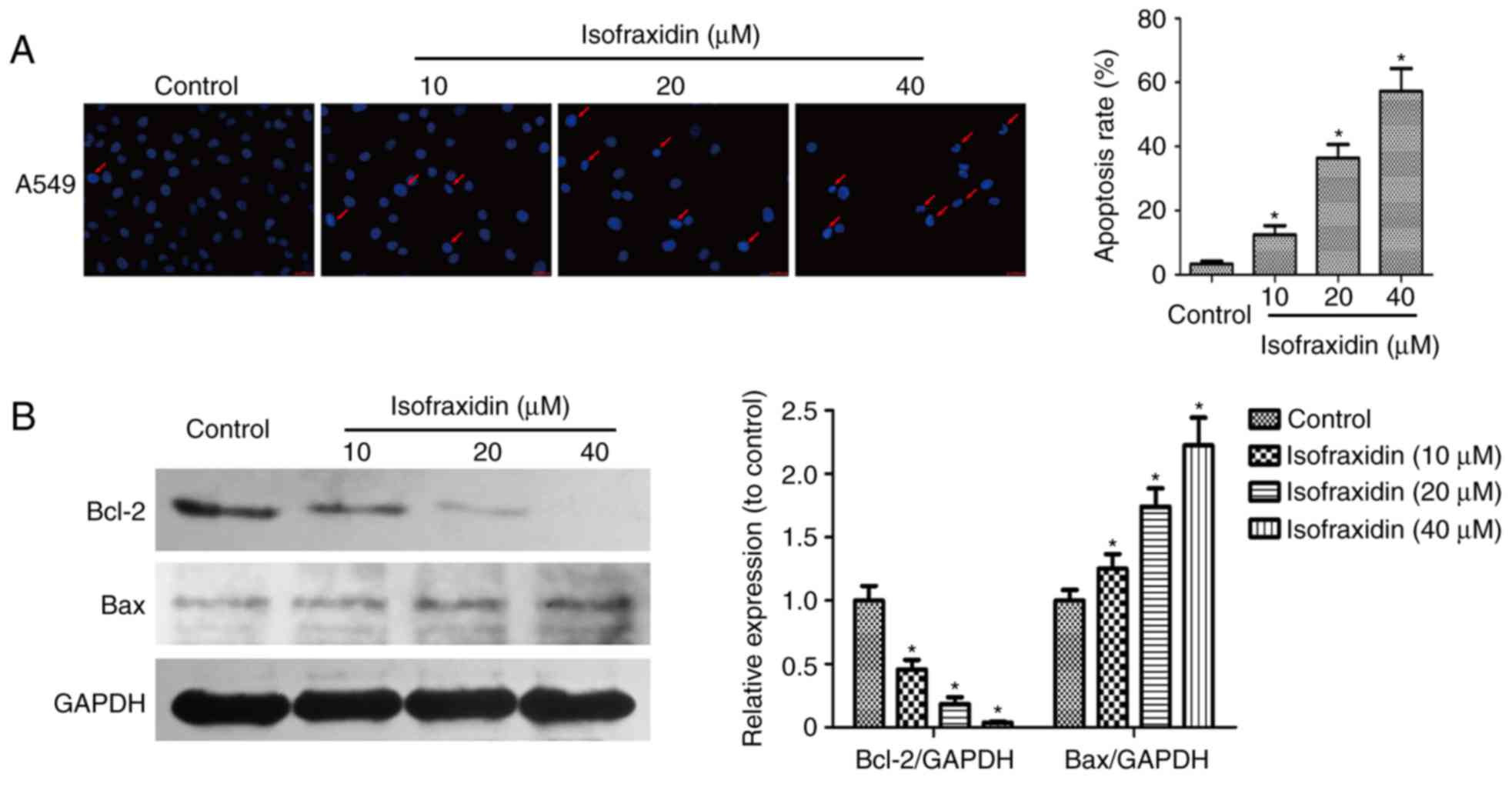
Isofraxidin induces apoptosis in A549
cells in a dose-dependent manner
Drug-induced apoptosis was microscopically observed
by Hoechst 33342 staining. A549 cells exposed to isofraxidin (0,
10, 20 and 40 µM) for 48 h were fixed, stained and observed under a
microscope. The results demonstrated that the nuclei of cells in
the control group were uniformly stained and the fluorescence
intensity was not as strong compared with cells treated with
isofraxidin. Increased fluorescence intensity in
isofraxidin-treated cells appeared to be dose-dependent (Fig. 2A). When treated with 40 µM
isofraxidin, nuclear pyknosis and crescent-shaped nuclei were
observed, indicating that isofraxidin may induce the apoptosis of
A549 cells in a dose-dependent manner (Fig. 2A). To determine the expression of
two apoptosis-associated proteins, Bcl-2 and Bax, western blot
analysis was performed. The ill-defined band of lower molecular
weight is assumed to be nonspecific. The results revealed that the
expression of Bax was significantly upregulated, while the
expression Bcl-2 was significantly downregulated, in response to
isofraxidin treatment in a dose-dependent manner (Fig. 2B). This result further indicated
that isofraxidin may induce the apoptosis of A549 cells.
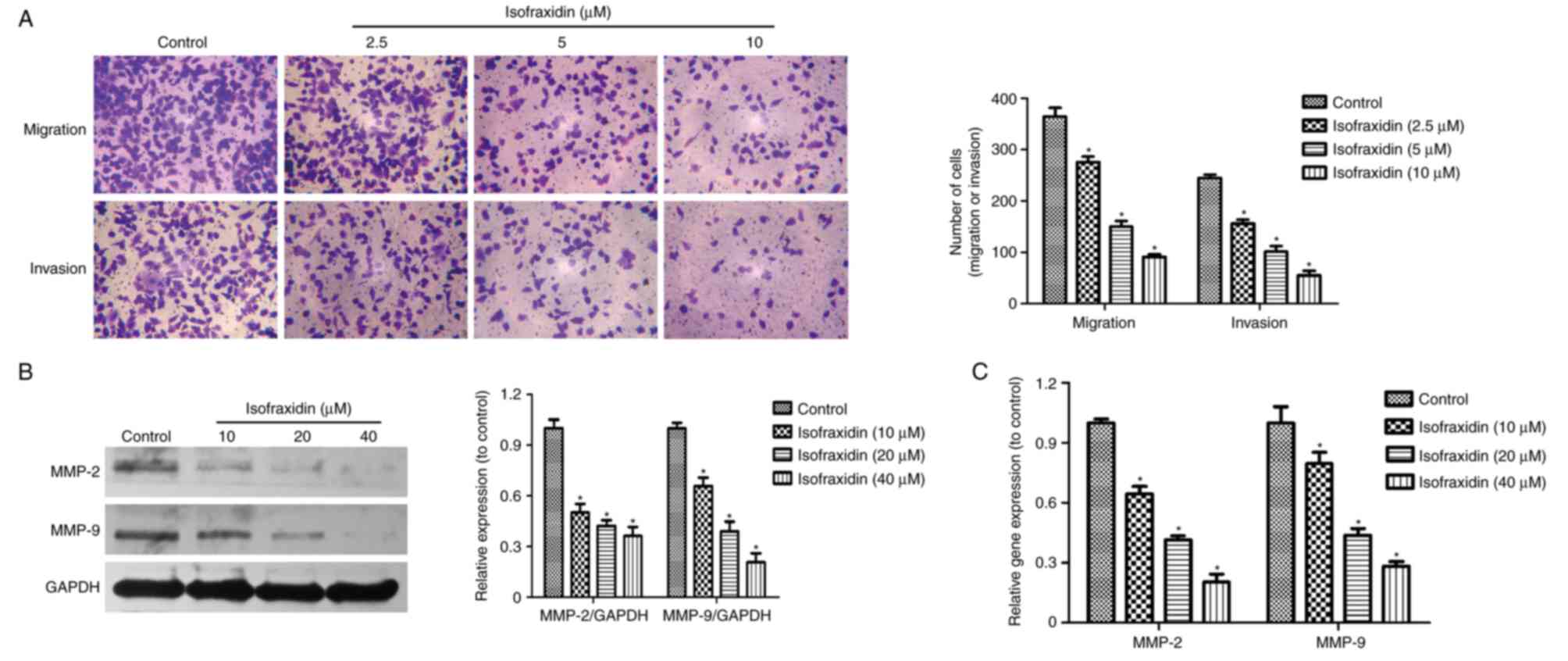
Isofraxidin suppresses the migration
and invasion of A549 cells
Metastasis is closely associated with the prognosis
and recurrence of cancer (15,16).
To confirm the effects of isofraxidin on cell motility, Transwell
migration and invasion assays were conducted. A549 cells were
treated with isofraxidin at relatively low concentrations (0, 2.5,
5 and 10 µM) for 48 h, low concentrations of isofraxidin have
little inhibitory effect on cell proliferation, which may affect
the cell migration and invasion. The results revealed that the
increase of isofraxidin concentration led to a decreased number of
migratory/invasive cells, indicating that the migration and
invasion of A549 cells was significantly inhibited compared with
the corresponding controls (Fig.
3A). Western blot and RT-qPCR analyses of the expression of two
invasion-associated genes, MMP-2 and MMP-9, revealed that the
expression levels of MMP-2 and MMP-9 were significantly reduced
following treatment with isofraxidin compared with control cells
(Fig. 3B and C), which further
confirmed the inhibitory effects of isofraxidin on the invasion of
A549 cells.
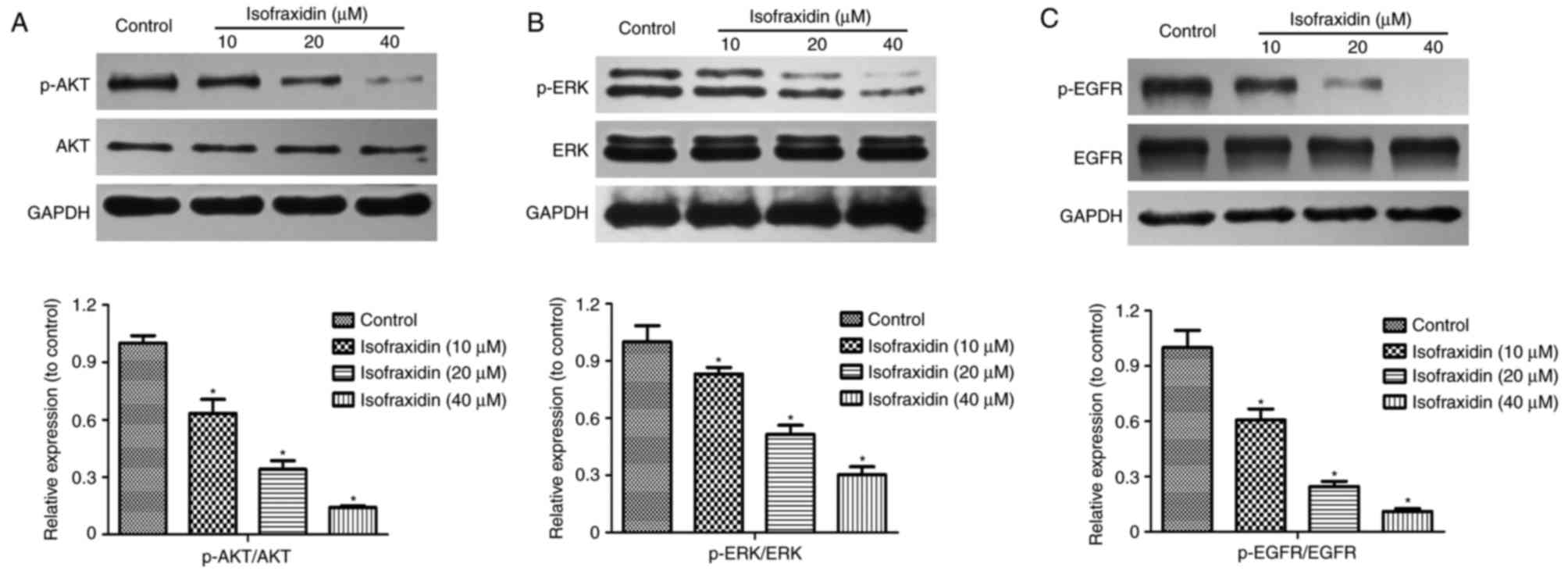
Isofraxidin inhibits A549 cell
proliferation, migration and invasion by inhibiting EGFR
phosphorylation
Phosphoinositide 3-kinase (PI3K)/AKT and
mitogen-activated protein kinase (MAPK)/ERK signaling pathways are
important in the proliferation, apoptosis, invasion and migration
of tumor cells (17–19). The alterations in p-AKT and p-ERK
expression levels in A549 cells following isofraxidin treatment
were detected by western blotting. The results demonstrated that
the expression levels of p-AKT and p-ERK following treatment with
isofraxidin was significantly inhibited in a dose-dependent manner
(Fig. 4A and B). PI3K/AKT and
MAPK/ERK signaling pathways are primarily regulated by receptor
tyrosine kinases (RTKs) and EGFR is the principal member of the
RTKs. Western blot analysis revealed that following treatment with
isofraxidin, the expression levels of p-EGFR were significantly
downregulated (Fig. 4C).
Therefore, isofraxidin may inhibit the PI3K/AKT and MAPK/ERK
signaling pathways by reducing the levels p-EGFR to hinder the
development of lung cancer cells.
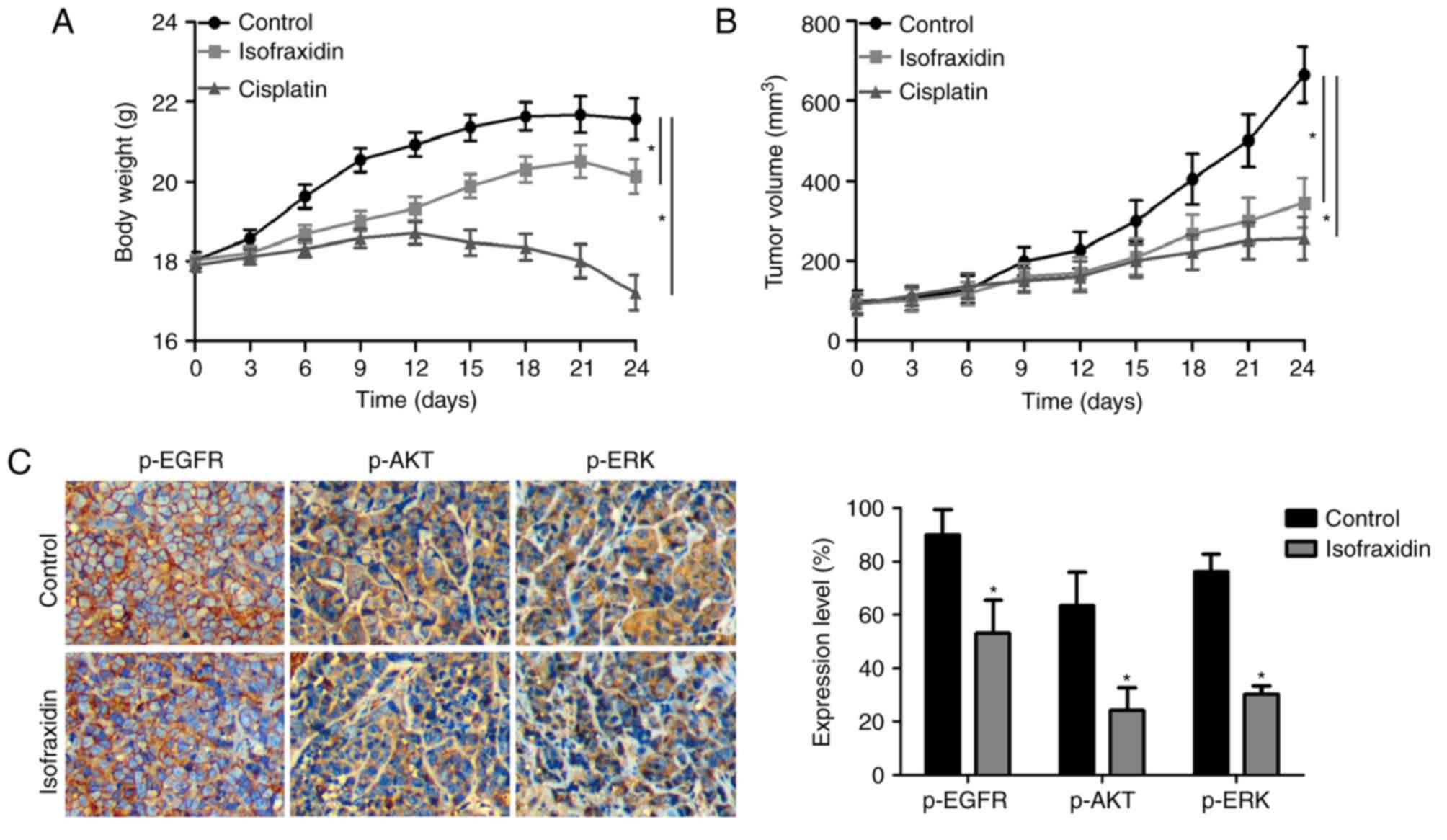
Isofraxidinsuppresses tumor growth in
vivo
To determine whether isofraxidin has potential
therapeutic value for the treatment of lung carcinoma, the effects
of isofraxidin on A549 cells were investigated in vivo in
the present study using a xenograft mouse model. The weights of
tumor-bearing nude mice are presented in Fig. 5A. Compared with in the positive
control group, isofraxidin treatment reduced the body weight of
nude mice less, with body weights more similar to those in the
negative control group, indicating the potentially low toxicity and
side effects of isofraxidin. The volume of the xenografts of
samples collected after 21 days of treatment via intraperitoneal
injection are presented in Fig.
5B. Compared with in the negative control group, isofraxidin
significantly reduced the volume of the xenografts. Furthermore,
Immunohistochemical detection demonstrated that following treatment
with isofraxidin, the expression levels of p-EGFR, p-AKT and p-ERK
were significantly downregulated compared with in the control group
(Fig. 5C), which was consistent
with the aforementioned in vitro results of the current
study. These data indicated that isofraxidin suppressed the growth
of xenografts in mice.
Discussion
Natural compounds have frequently been used to treat
cancers. At present, there are >3,000 anticancer drugs that are
derived from plants, including vinblastine, vincristine, etoposide,
paclitaxel and camptothecin (8).
Isofraxidin is a coumarin compound that primarily exists inceratin
plants, including Sarcandraglabra and
Acanthopanaxsenticosus (9).
Antibacterial, antioxidant, antidepressant, anti-inflammation and
antitumor effects of isofraxidin have previously been reported
(20). As one of the most common
types of malignant tumor, lung cancer is associated with certain
characteristics, including rapid development and high metastasis,
which is associated with high mortality. To the best of our
knowledge, the effect of isofraxidin on lung cancer has not
previously been reported; however, the present study revealed for
the first time that isofraxidin may inhibit the proliferation,
migration and invasion of A549 cells, and induce cell apoptosis, by
inhibiting the EGFR signaling pathway.
The proliferation, apoptosis, migration and invasion
of tumor cells involves a series of signaling pathways, making it a
complex process with particular mechanisms that require further
investigation. Bcl-2 family proteins are the most important
regulatory factors in the mitochondrial apoptosis pathway and are
made up of two primary groups: Apoptosis-inducing (apoptotic)
proteins, including Bax, Bcl-2 antagonist/killer 1 and
peptidyl-tRNA hydrolase 2, andapoptosis-inhibiting (antiapoptotic)
proteins, including Bcl-2 and Bcl-2-like 1 (21–23).
It was previously reported that isofraxidin significantly reduced
the expression of the antiapoptotic protein Bcl-2 in colon cancer
cells and increased the expression of the apoptotic
proteinscaspase-3, caspase-9 and Bax (9). Therefore, the present study detected
the effect of isofraxidin on the expression of Bcl-2 protein family
members. The results revealed that isofraxidin promoted the
expression of Bax but reduced the expression of Bcl-2 in a
dose-dependent manner, indicating that isofraxidin may induce the
apoptosis of A549 cells.
Patients with cancer succumb to the disease
primarily due to the recurrence of malignant tumors and metastasis,
and the key process resulting in the invasion and metastasis of
malignant tumors is the degradation of the extracellular matrix
(ECM). MMPs are the major enzymes that degrade the components of
the ECM (24–26). In the process of developing safe
and effective anticancer drugs, analysis of the antimetastatic
ability of the drug is particularly important; the inhibition of
MMP activity is also regarded as one aim of cancer treatment. It
has been demonstrated that isofraxidin may suppress the invasion of
hepatocellular carcinoma by inhibiting the expression of MMPs
(13). Therefore, the present
study investigated the effect of isofraxidin on expression of the
MMP family proteins and the results revealed that the expression
levels of MMP-2 and MMP-9 were significantly reduced following
treatment with isofraxidin. The results of the present study
indicated that isofraxidin was able to suppress the migration and
invasion of A549 cells, as confirmed by Transwell migration and
invasion assays.
PI3K/AKT and MAPK/ERK signaling pathways are
important in the proliferation, apoptosis, migration and invasion
of tumor cells. Numerous studies have reported that AKT may affect
cell proliferation and apoptosis by regulating the Bcl-2
family-associated proteins (27,28).
The results of the present study revealed that isofraxidin reduced
the expression of p-AKT in A549 cells; therefore, isofraxidin may
affect the expression of the Bcl-2 protein family by inhibiting the
AKT signaling pathway, leading to the subsequent inhibition of the
proliferation of A549 cells and the induction of apoptosis. ERK has
been reported to regulate the expression of MMP family-associated
proteins, thus affecting cell migration and invasion (29,30).
The present study also revealed that isofraxidin reduced the
expression of p-ERK in A549 cells, indicating that isofraxidin may
inhibit the expression of MMP-2 and MMP-9 proteins by inhibiting
the ERK signaling pathway, subsequently inhibiting the migration
and invasion of A549 cells.
RTKs are glycoproteins that span the cell membrane
and bind a growth factor within its surface, which controls the
transduction of cellular signals (31). There are numerous members of the
RTK family, including EGFR, fibroblast growth factor receptor,
vascular endothelial growth factor receptor and platelet-derived
growth factor receptor (32). EGFR
is considered to be among the most important members of the RTK
family and its importance has been demonstrated by the clinical
application of EGFR-targeting drugs, including gefitinib and
afatinib (33).
Based on the effect of isofraxidin on PI3K/AKT and
MAPK/ERK signaling pathways in the present study, isofraxidin may
affect the activity of EGFR in lung cancer cells. The results of
the present study revealed that the phosphorylation level of EGFR
in lung cancer cells was significantly inhibited following
treatment with treatment isofraxidin.
In conclusion, the present study confirmed the
inhibitory effect of isofraxidin on lung cancer development. The
results indicated that isofraxidin may inhibit the development of
lung cancer cells by inhibiting the phosphorylation of EGFR and
inhibiting the PI3K/AKT and MAPK/ERK signaling pathways.
Furthermore, BEAS-2B human normal lung epithelial cells exhibited
low sensitivity to isofraxidin within a certain dose range,
indicating the potentially low toxicity and side effects of
isofraxidin in the treatment of lung cancer. These results indicate
the potential of isofraxidin as a novel candidate for anti-lung
cancer chemotherapy.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data and materials described in this manuscript
are available from the correspondence author upon reasonable
request.
Authors' contributions
HZ and QQF performed the experiments. JHG and JPM
analyzed the data and wrote the manuscript.
Ethics approval and consent to
participate
All procedures involving animals were approved by
the Ethics Committee of the Jingzhou Central Hospital Affiliated to
Tongji Medical College of Huazhong University of Science and
Technology (Jingzhou, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chang A: Chemotherapy, chemoresistance and
the changing treatment landscape for NSCLC. Lung Cancer. 71:3–10.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lamb YN and Scott LJ: Osimertinib: A
review in T790M-positive advanced non-small cell lung cancer.
Target Oncol. 12:555–562. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Goss GD and Spaans JN: Epidermal growth
factor receptor inhibition in the management of squamous cell
carcinoma of the lung. Oncologist. 21:205–213. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Salmenkivi K and Knuuttila A: Diagnostics
of non-small cell lung carcinoma. Duodecim. 130:701–704. 2014.(In
Finnish). PubMed/NCBI
|
|
5
|
Rosenzweig K: Stereotactic body radiation
therapy as an alternative to surgery in early-stage non-small-cell
lung cancer. Oncology (Williston Park). 31:492–498. 2017.PubMed/NCBI
|
|
6
|
Wan Y, Yuan Y, Pan Y and Zhang Y:
Antitumor activity of high-dose pulsatile gefitinib in
non-small-cell lung cancer with acquired resistance to epidermal
growth factor receptor tyrosine kinase inhibitors. Exp Ther Med.
13:3067–3074. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang K and Yuan Q: Current mechanism of
acquired resistance to epidermal growth factor receptor-tyrosine
kinase inhibitors and updated therapy strategies in human nonsmall
cell lung cancer. J Cancer Res Ther. 12 Suppl:C131–C137. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pan L, Chai H and Kinghorn AD: The
continuing search for antitumor agents from higher plants.
Phytochem Lett. 3:1–8. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shen P, Wang HG, Li MM, Ma QY, Zhou CW,
Pan F and Xie R: Isofraxidin inhibited proliferation and induced
apoptosis via blockage of Akt pathway in human colorectal cancer
cells. Biomed Pharmacother. 92:78–85. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Niu X, Xing W, Li W, Fan T, Hu H and Li Y:
Isofraxidin exhibited anti-inflammatory effects in vivo and
inhibited TNF-α production in LPS-induced mouse peritoneal
macrophages in vitro via the MAPK pathway. Int Immunopharmacol.
14:164–171. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Khan S, Riaz N, Afza N, Malik A,
Aziz-ur-Rehman, Iqbal L and Lateef M: Antioxidant constituents from
Cotoneaster racemiflora. J Asian Nat Prod Res. 11:44–48. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Deyama T, Nishibe S and Nakazawa Y:
Constituents and pharmacological effects of Eucommia and Siberian
ginseng. Acta Pharmacol Sin. 22:1057–1070. 2001.PubMed/NCBI
|
|
13
|
Yamazaki T and Tokiwa T: Isofraxidin, a
coumarin component from Acanthopanax senticosus, inhibits matrix
metalloproteinase-7 expression and cell invasion of human hepatoma
cells. Biol Pharm Bull. 33:1716–1722. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qian L, Li X, Ye P, Wang G, Dai W, Liu Y,
Gao Q and Shen G: Oxymatrine induces apoptosis and inhibits
invasion in Gallbladder carcinoma via PTEN/PI3K/AKT pathway.
Cytotechnology. 70:83–94. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hu S, Ran Y, Chen W, Zhang Y and Xu Y:
MicroRNA-326 inhibits cell proliferation and invasion, activating
apoptosis in hepatocellular carcinoma by directly targeting LIM and
SH3 protein 1. Oncol Rep. 38:1569–1578. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bai L, Lin G, Sun L, Liu Y, Huang X, Cao
C, Guo Y and Xie C: Upregulation of SIRT6 predicts poor prognosis
and promotes metastasis of non-small cell lung cancer via the
ERK1/2/MMP9 pathway. Oncotarget. 7:40377–40386. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Y, Wan D, Zhou R, Zhong W, Lu S and
Chai Y: Geraniin inhibits migration and invasion of human
osteosarcoma cancer cells through regulation of PI3K/Akt and ERK1/2
signaling pathways. Anticancer Drugs. 28:959–966. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li W, Sun D, Lv Z, Wei Y, Zheng L, Zeng T
and Zhao J: Insulin-like growth factor binding protein-4 inhibits
cell growth, migration and invasion, and downregulates COX-2
expression in A549 lung cancer cells. Cell Biol Int. 41:384–391.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang L, Ge C, Zhao F, Zhang Y, Wang X,
Yao M and Li J: NRBP2 overexpression increases the chemosensitivity
of hepatocellular carcinoma cells via Akt signaling. Cancer Res.
76:7059–7071. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Niu X, Wang Y, Li W, Mu Q, Li H, Yao H and
Zhang H: Protective effects of Isofraxidin against
lipopolysaccharide-induced acute lung injury in mice. Int
Immunopharmacol. 24:432–439. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Edlich F: BCL-2 proteins and apoptosis:
Recent insights and unknowns. Biochem Biophys Res Commun. Jul
1–2017.(Epub ahead of print). PubMed/NCBI
|
|
22
|
Zhou L, Cai X, Han X, Xu N and Chang DC:
CDK1 switches mitotic arrest to apoptosis by phosphorylating
Bcl-2/Bax family proteins during treatment with microtubule
interfering agents. Cell Biol Int. 38:737–746. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Spencer SL and Sorger PK: Measuring and
modeling apoptosis in single cells. Cell. 144:926–939. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hu X, Li D, Zhang W, Zhou J, Tang B and Li
L: Matrix metalloproteinase-9 expression correlates with prognosis
and involved in ovarian cancer cell invasion. Arch Gynecol Obstet.
286:1537–1543. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: Regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang L, Wu H, Wang L, Zhang H, Lu J, Liang
Z and Liu T: Asporin promotes pancreatic cancer cell invasion and
migration by regulating the epithelial-to-mesenchymal transition
(EMT) through both autocrine and paracrine mechanisms. Cancer Lett.
398:24–36. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang L, Liu Y, Wang M, Qian Y, Dong X, Gu
H, Wang H, Guo S and Hisamitsu T: Quercetin-induced apoptosis of
HT-29 colon cancer cells via inhibition of the Akt-CSN6-Myc
signaling axis. Mol Med Rep. 14:4559–4566. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang H, Chen B, Duan B, Zheng J and Wu X:
miR-205 suppresses cell proliferation, invasion, and metastasis via
regulation of the PTEN/AKT pathway in renal cell carcinoma. Mol Med
Rep. 14:3343–3349. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chan LP, Liu C, Chiang FY, Wang LF, Lee
KW, Chen WT, Kuo PL and Liang CH: IL-8 promotes inflammatory
mediators and stimulates activation of p38 MAPK/ERK-NF-κB pathway
and reduction of JNK in HNSCC. Oncotarget. 8:56375–56388. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cepeda MA, Evered CL, Pelling JJH and
Damjanovski S: Inhibition of MT1-MMP proteolytic function and
ERK1/2 signalling influences cell migration and invasion through
changes in MMP-2 and MMP-9 levels. J Cell Commun Signal.
11:167–179. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gur S, Sikka SC, Abdel-Mageed AB, Elmageed
Abd ZY, Rezk B, Pankey E, Kadowitz PJ and Hellstrom WJ: Imatinib
mesylate (gleevec) induces human corpus cavernosum relaxation by
inhibiting Receptor Tyrosine Kinases (RTKs): Identification of new
RTK targets. Urology. 82(745): e11–6. 2013.PubMed/NCBI
|
|
32
|
Puri N and Salgia R: Synergism of EGFR and
c-Met pathways, cross-talk and inhibition, in non-small cell lung
cancer. J Carcinog. 7:92008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu TC, Jin X, Wang Y and Wang K: Role of
epidermal growth factor receptor in lung cancer and targeted
therapies. Am J Cancer Res. 7:187–202. 2017.PubMed/NCBI
|