Introduction
Ulcerative colitis (UC) is a chronic, idiopathic,
inflammatory bowel disease (IBD) of the colon (1,2). The
primary symptoms of UC are abdominal pain and bloody diarrhea
(3). The direct causes of UC are
unknown, but immune system dysfunction has been implicated in the
pathogenesis of UC (4). T cell
responses have been intensively explored in UC. Upon activation,
naive CD4+T cells differentiate into T helper 1 (Th1),
Th2 and Th17 cells. The mucosal levels of interferon γ (IFNγ, a Th1
cell produced cytokine) was increased in patients with UC compared
with those in normal control (5).
Interleukin-4 (IL-4, a Th2 cell produced cytokine) was more
frequently detected in UC than in inflammatory controls (6). By using an oxazolone colitis model,
Fuss et al (7),
demonstrated that UC has an increased Th2-oriented immune response.
IL-17 (a Th17 cell produced cytokine) mRNA was increased in biopsy
specimens from UC (8,9). IL-23, the key cytokine that promotes
Th17 cells to produce IL17 (10),
differentially regulates the Th1/Th17 balance in UC (11).
Approximately 50% of patients with UC can be treated
with a number of medications, including 5-aminosalicylic acid (ASA)
drugs, such as sulfasalazine and mesalamine (2,3,12).
Patients who fail to respond to 5-ASA drugs are treated with
steroids (13), azathioprine
(14) and infliximab (15), which may have serious toxicity
(16). Additional medical
therapies for patients failing 5-ASA drugs are needed.
Andrographis paniculata (AP), an important herbal medicine,
has been used to treat inflammatory and infectious diseases
(17–19). Andrographolide is the main active
component of AP. AP extracts and andrographolide possess
immunostimulatory (20,21), anti-cancer (20,22),
antiviral (23) and antibacterial
activities (24). AP extract
(HMPL-004) showed similar efficacy to mesalamine for UC (25,26).
Andrographolide sulfonate, a derivative of andrographolide, could
inhibit Th1/Th17 responses and improve experimental colitis
(27). However, whether
andrographolide affects the T cell responses of UC patients has not
been explored.
In the present study, peripheral blood mononuclear
cells (PBMCs) isolated from UC patients were treated with various
concentrations of andrographolide. Then, the effects of
andrographolide on Th cell differentiation were investigated.
Further experiments with PBMCs from healthy donors confirmed these
findings.
Materials and methods
Isolation, culture and treatment of
PBMCs
The present study received ethical approval from the
Ethics Committee of Zhejiang Hospital. Blood samples were collected
from 3 UC patients and 3 age-match healthy donors after written
informed consent was obtained from all participants.
Clinicopathological data of UC patients are listed in Table I. PBMCs were freshly isolated from
blood samples by gradient centrifugation with lymphocyte cell
separation media (Cedarlane Laboratories, Ontario, Canada) and
grown in RPMI-1640 (HyClone; GE Healthcare Life Sciences, Logan,
UT, USA) containing 10% fetal bovine serum (FBS; Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) and
penicillin/streptomycin. The PBMCs were maintained in a 37°C
incubator with 5% CO2.
 | Table I.Clinicopathological data of patients
with UC (n=3). |
Table I.
Clinicopathological data of patients
with UC (n=3).
| Characteristic | Patient data |
|---|
| Sex | Male |
| Mean age at operation
(years) | 32 |
| Duration of disease
(years) | 4.8 |
| Extent of
disease | Total colitis |
PBMCs from UC patients were randomly treated with
dimethyl sulfoxide (DMSO; Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) and 10, 20 or 30 µg/ml of andrographolide (AG;
Sigma-Aldrich; Merck KGaA). PBMCs from healthy donors were randomly
divided into three groups: Control group, treated with DMSO; IL-23
group, treated with 50 ng/ml IL-23 (Sigma-Aldrich; Merck KGaA); and
IL-23+AG group, treated with 20 µg/ml andrographolide for 2 h and
then treated with 50 ng/ml IL-23. After 48 h of culture, the
culture media were collected for enzyme-linked immunosorbent assay
(ELISA), and PBMCs were harvested for flow cytometry and Western
blot analysis.
ELISA assay
The culture media were collected and the
concentrations of IFNγ, IL-4, IL-23 and IL-17A were measured by
using commercial ELISA kits (Bio-swap, Shanghai, China) according
to the manufacturer's instructions. Optic densities were measured
at 450 nm, and the concentrations of cytokines were calculated
according to a standard curve.
Cytokine staining and flow
cytometry
The treated PBMCs were centrifuged at 1,000 rpm for
10 min. The pellet was resuspended in cultured media supplemented
with PMA (100 ng/ml; Sigma-Aldrich; Merck KGaA)/ionomycin (1 µg/ml;
Sigma-Aldrich; Merck KGaA) and monensin (1 µg/ml; Shanghai Aladdin
Bio-Chem Technology Co., Shanghai, China) and plated onto 24-well
plates (0.5×105 cells/well). After incubation at 37°C
for 4 h, the PBMCs were collected, resuspended in
phosphate-buffered saline (PBS) and labeled with anti-CD4-FITC
(BioLegend, Inc., San Diego, CA, USA) for 1 h at 4°C. Subsequently,
the cells were fixed with 2% formaldehyde and permeabilized with
0.1% Triton X-100 in PBS. Intracellular cytokine staining was then
performed with anti-IFNγ-APC, anti-IL-4-PE or anti-IL-17A-PE
(BioLegend, Inc.) for 1 h. The cells were detected by using flow
cytometry (BD Biosciences, Franklin Lakes, NJ, USA). The
proportions of CD4+IFNγ+ cells, CD4+IL-4+ cells and CD4+IL-17A+
cells (right upper quadrant) in CD4+ cells (right upper and lower
quadrant) were calculated.
Western blot analysis
PBMCs was lysed in RIPA buffer and then centrifuged
at 12,000 rpm for 20 min. The supernatant was collected, and the
protein concentrations were quantified by using a BCA method. An
equal amount of protein (30 µg) from each sample was loaded onto
10% sodium dodecyl sulfate (SDS)-polyacrylamide gels and
transferred onto a nitrocellulose blotting membrane (EMD Millipore,
Billerica, MA, USA). Following incubation with 5% skim milk at 4°C
for 1 h, the membranes were incubated with anti-T-bet (cat. no.
Ab91109, 1:500; Abcam, Cambridge, MA, USA), anti-GATA-3 (cat. no.
Ab106625, 1:1,000; Abcam), anti-ROR-γt (cat.no. Ab78007, 1:1,500;
Abcam) and anti-GAPDH (cat. no. 5174, 1:2,000; Cell Signaling
Technology, Danvers, MA, USA) antibodies at 4°C overnight. Then,
the membrane was washed three times with TBST buffer and incubated
with horseradish peroxidase conjugated secondary antibody (Beyotime
Institute of Biotechnology, Shanghai, China) for 1 h.
Immunoreactive bands were detected using an ECL detection kit
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) and semi-quantified
by ImageJ software (http://rsb.info.nih.gov/ij/, National Institutes of
Health, Bethesda, MD, USA).
Statistical analysis
Data are expressed as the means ± standard deviation
(SD). Statistical analysis was performed with GraphPad Prism
software (v6.0, San Diego, CA, USA). One-way analysis of variance
with a Tukey's post hoc test was performed. P<0.05 was
considered to indicate a statistically significant difference.
Results
Effects of andrographolide on the
release of IFNγ, IL-4, IL-23 and IL-17A in PBMCs from UC
patients
To examine the effect of andrographolide on the
production of Th cell-specific cytokines, PBMCs were isolated from
three UC patients and treated with 10, 20 or 30 µg/ml of
andrographolide. The concentrations of cytokines in the culture
media were determined by an ELISA assay. After 48 h of treatment,
andrographolide decreased IFNγ, IL-23 and IL-17A but increased IL-4
in a dose-dependent manner (Fig.
1).
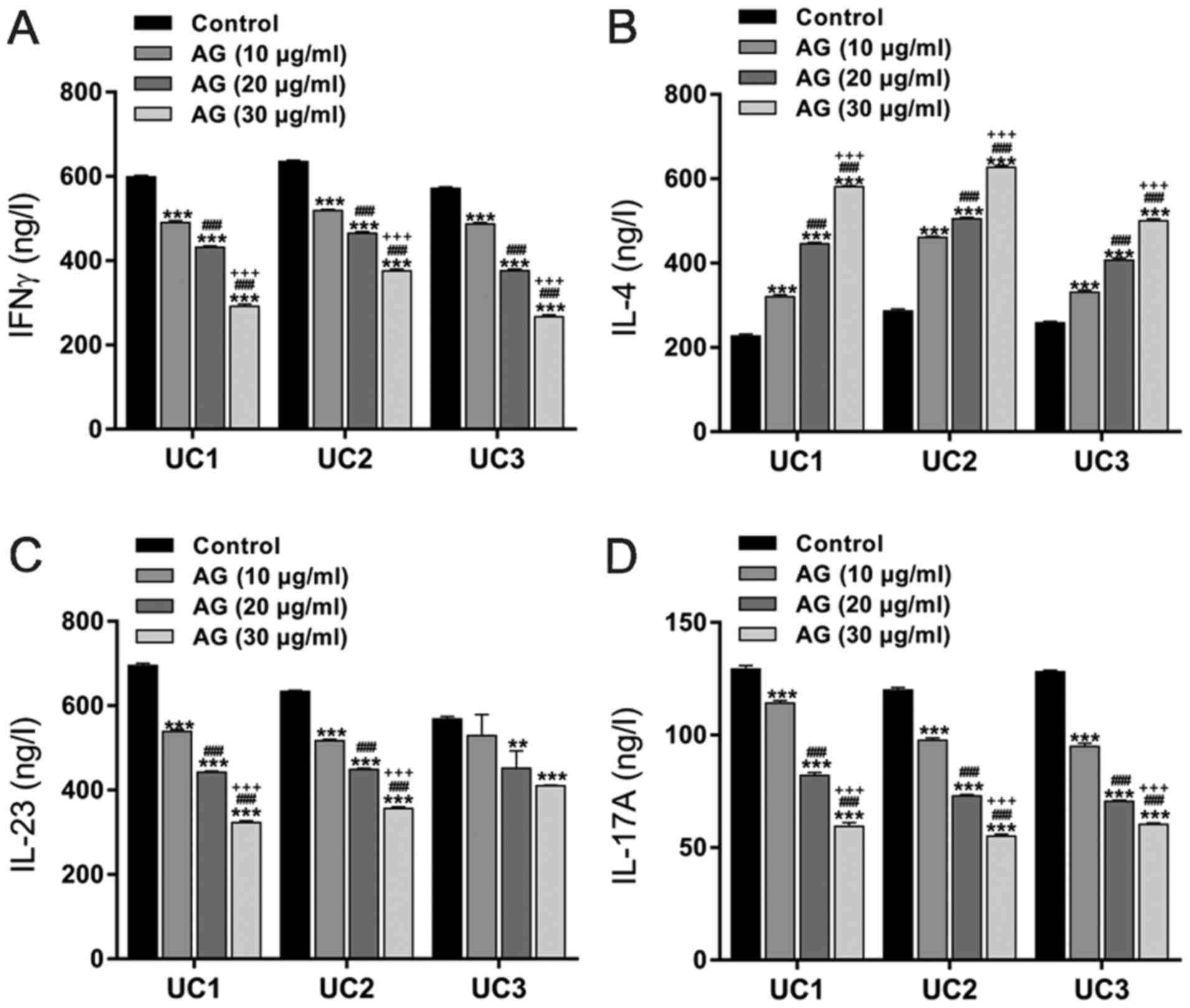 | Figure 1.Effects of AG on the release of
cytokines in PBMCs from patients with UC. PBMCs from 3 UC patients
(UC1, UC2 and UC3) were randomly treated with 10, 20 or 30 µg/ml of
AG. Cells treated with DMSO served as negative controls. After 48 h
of treatment, the culture media were collected, and the
concentrations of (A) IFNγ, (B) IL-4, (C) IL-23 and (D) IL-17A were
assessed by ELISA assay. **P<0.01 and ***P<0.001 vs. the
control; ###P<0.001 vs. AG (10 µg/ml);
+++P<0.001 vs. AG (20 µg/ml). AG, andrographolide;
PBMC, peripheral blood mononuclear cells; UC, ulcerative colitis;
IFN, interferon; IL, interleukin. |
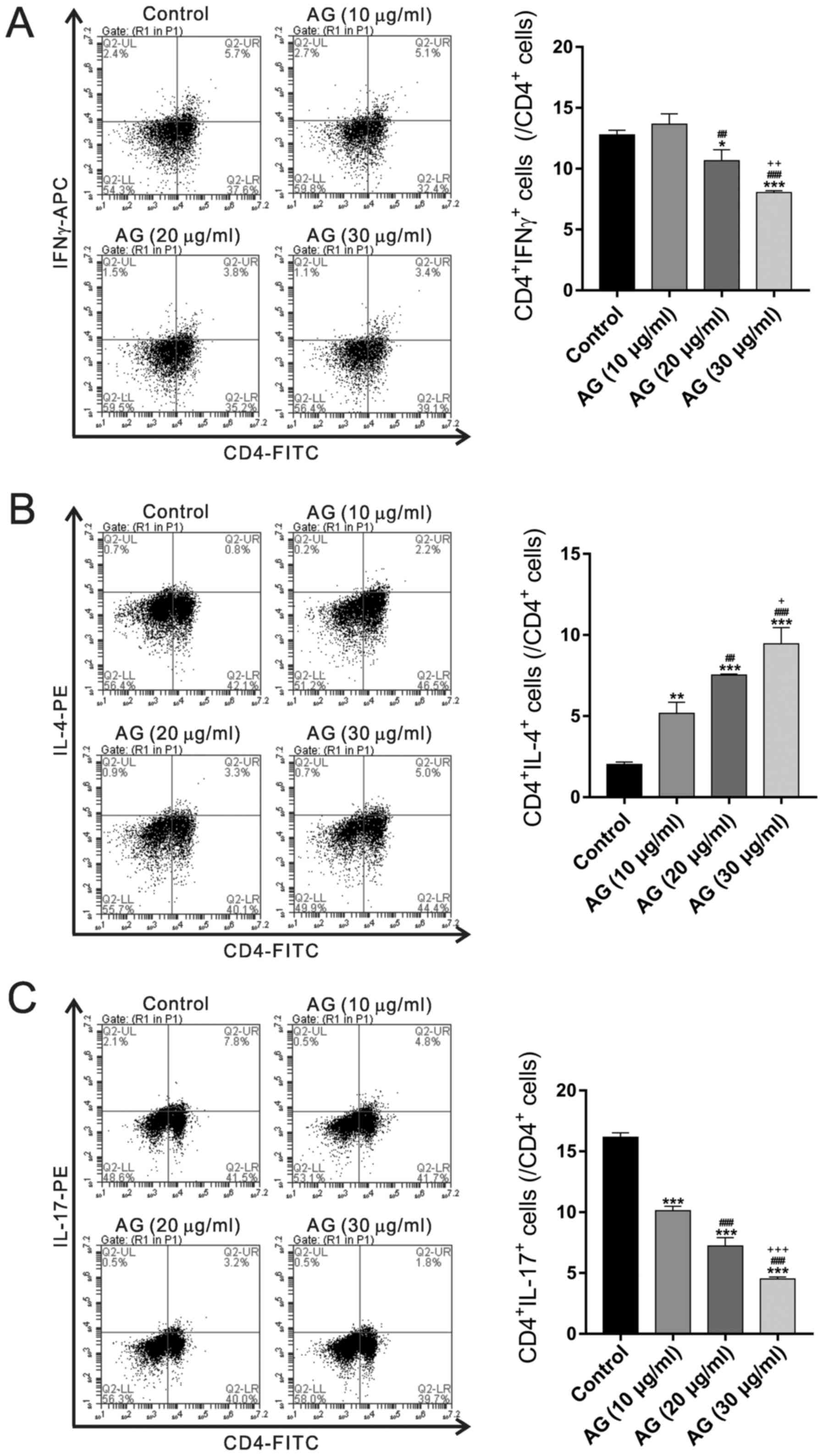
Effects of andrographolide on Th cell
subset in PBMCs from UC patients
To analyze the effect of andrographolide on subtypes
of Th cell populations, PBMCs treated with andrographolide were
stained with IFNγ, IL-4 and IL-17A in CD4+ T cells, which are the
respective signature cytokines of Th1, Th2 and Th17 cells.
Andrographolide treatment resulted in a decreased percentage of Th1
and Th17 cells and an increased proportion of Th2 cells (Fig. 2).
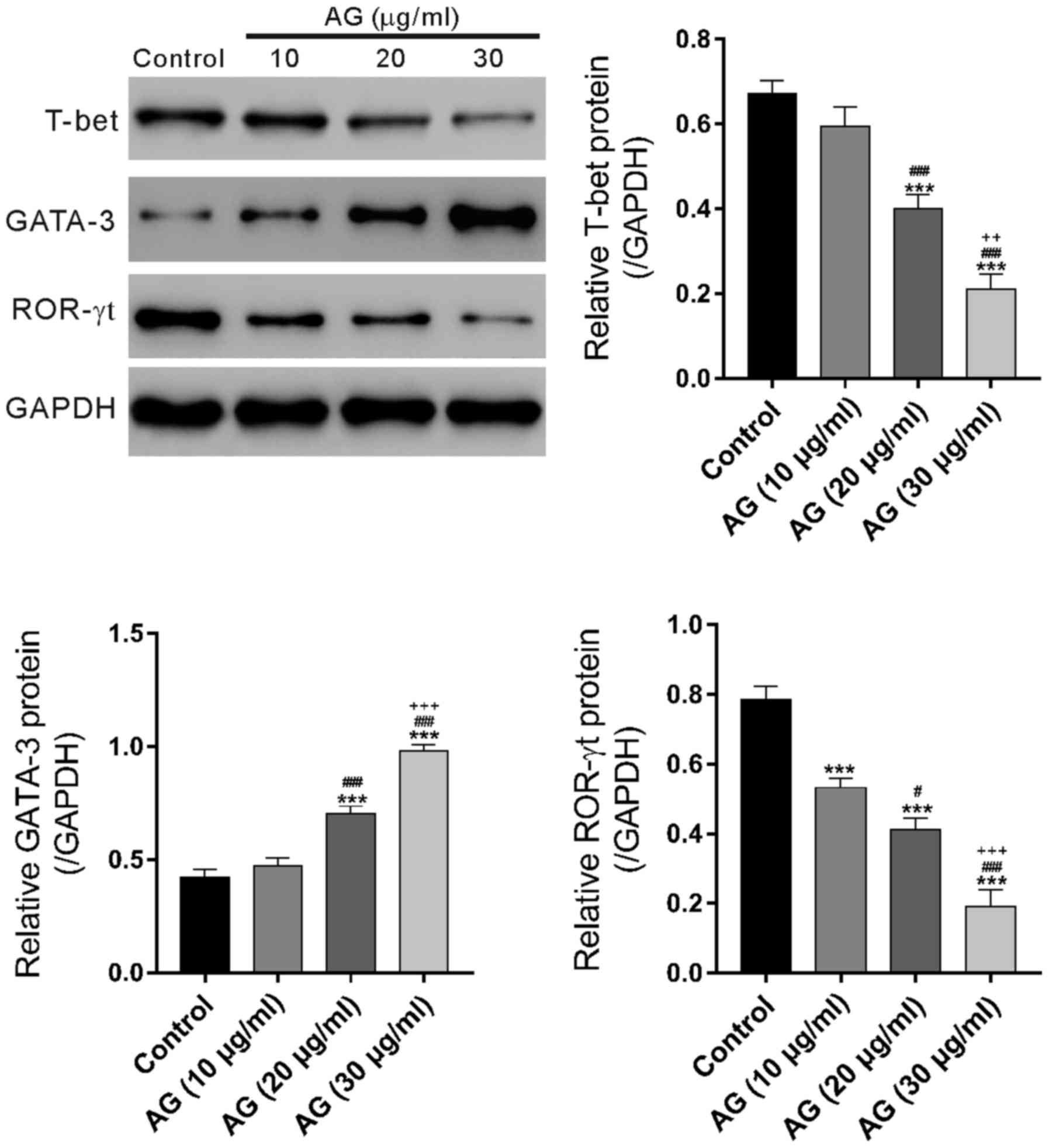
Effects of andrographolide on the
protein levels of T-bet, GATA3 and ROR-γt in PBMCs from UC
patients
The protein expression levels of the transcription
factors, T-bet, GATA-3 and ROR-γt, of the T lymphocytes were
measured, and the results showed that T-bet and ROR-γt expression
was decreased (n = 3); however, GATA-3 expression was increased
after andrographolide treatment (n=3, Fig. 3).
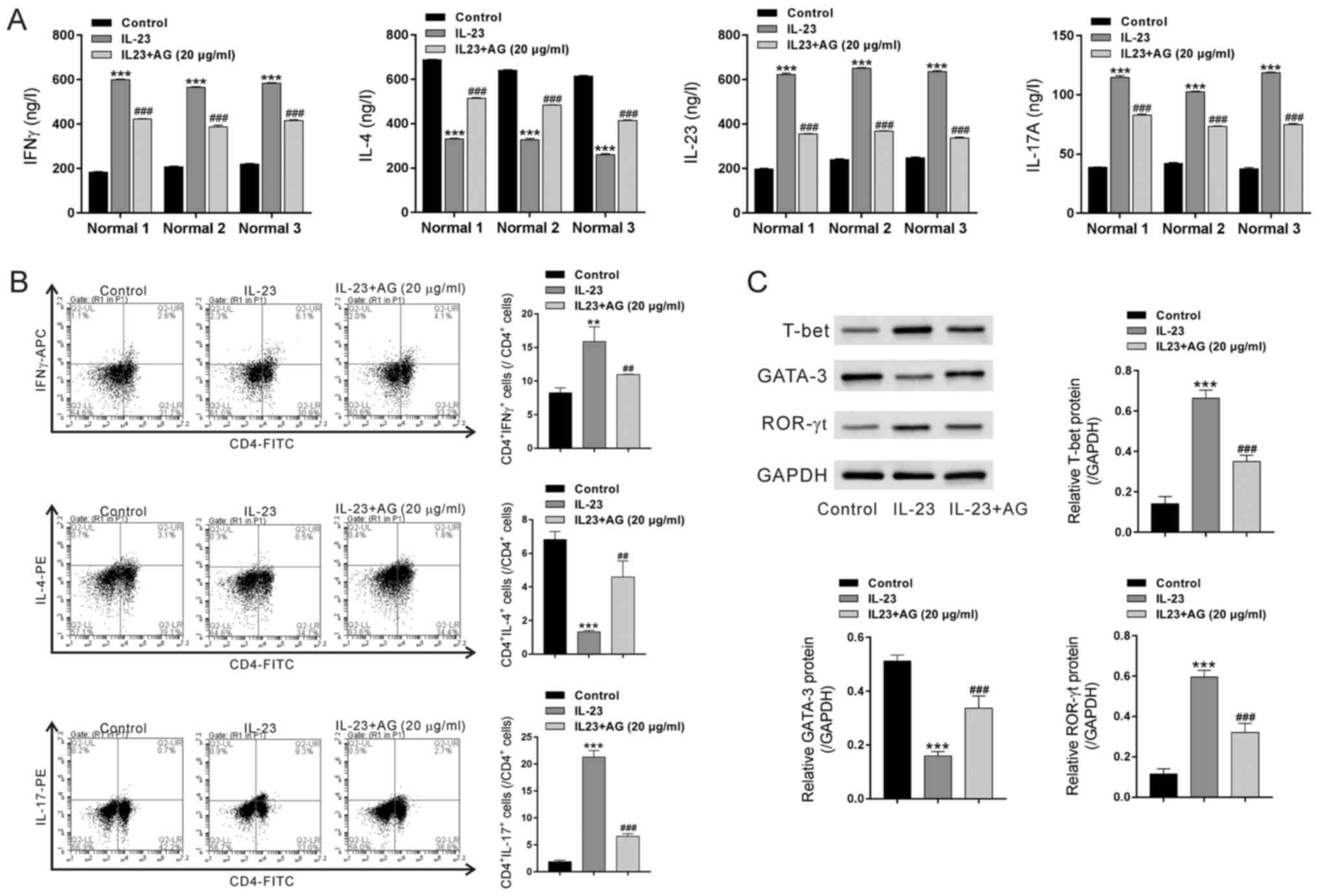
Effects of andrographolide on
IL-23-treated PBMCs from healthy donors
We next explored the effects of andrographolide
pretreatment on IL-23-treated PBMCs from healthy donors. As shown
in Fig. 4, IL-23 treatment
significantly increased the concentrations of IFNγ, IL-23 and
IL-17A but decreased the concentrations of IL-4. IL-23 exposure
caused a notable increase in the percentages of IFNγ+CD4+ cells and
IL-17+CD4+ but a decrease in the percentages of IL-4+CD4+ cells.
Additionally, IL-23 treatment significantly increased the protein
levels of T-bet and ROR-γt but reduced GATA-3 expression.
Pretreatment with andrographolide significantly rescued the effects
of IL-23 on PBMCs.
Discussion
Th1, Th2 and Th17 immune responses have been
associated with the pathology of UC (5–7). AP
extract (HMPL-004) was efficient for UC treatment (25,26).
In the present study, we examined whether andrographolide, the main
active component of AP, affected T cell responses of UC
patients.
First, PBMCs isolated from UC patients were treated
with various concentrations of andrographolide. The concentrations
of IFNγ (a Th1 cell produced cytokine), IL-23 and IL-17A (Th17 cell
produced cytokine) in the culture medium, the percentages of Th1
and Th17 cells, and the protein levels of T-bet (a transcription
factor directing Th1 lineage commitment (28)) and ROR-γt (Th17 lineage-specific
transcription factor (29)) were
significantly decreased by andrographolide treatment. These data
suggested that andrographolide could inhibit Th1/Th17 response.
These findings were consistent with those of a previous study of
andrographolide sulfonate in mice (27). In contrast, the concentrations of
IL-4 (a Th2 cell produced cytokine) in the culture medium, the
percentages of Th2 cells, and the protein levels of GATA-3 (Th2
lineage-specific transcription factor (30)) were significantly increased by
andrographolide treatment. The present study demonstrated that
andrographolide could inhibit Th1/Th17 responses and enhance the
Th2 response of PBMCs from UC patients.
Recently, increasing evidence has established
correlative links between the association of IL-23/IL-17 axis and
the frequency of several human autoimmune or immune-mediated
inflammatory diseases, such as Crohn disease, psoriasis and
spondyloarthritis (31–33). Then, we treated PBMCs from healthy
donors with IL-23 to induce a Th17 response. IL-23 treatment
significantly increased Th1/Th17 responses but decreased the Th2
response, as indicated by the concentrations of specific cytokines,
the percentages of Th cell subsets, and the levels of specific
transcription factors. More importantly, andrographolide
pretreatment rescued the effects of IL-23. These data suggested
that andrographolide might effectively treat other IL-23-mediated
diseases. Further studies are needed to investigate the therapeutic
effects of andrographolide on such diseases.
In conclusion, the present study explored the
effects of andrographolide on the Th1/Th2/Th17 responses of PBMCs
from UC patients and IL-23-treated-PBMCs from healthy donors. These
results suggest that andrographolide can be an effective candidate
for the treatment of IL-23-mediated diseases.
Acknowledgments
Not applicable.
Funding
This study was financially supported through grants
from the Natural Science Foundation of Zhejiang Province
(LQ15H030005), Medical and Health Science and Technology Plan of
Zhejiang Province (2015KYA011) and the Natural Science Foundation
of Zhejiang Province (Y17H030031).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
QZ and PZ conceived and designed the study. QZ, JZ,
XC, YF, WW, FZ and QH performed the experiments. QZ and PZ wrote
the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study received ethical approval from the
Ethics Committee of Zhejiang Hospital and written informed consent
was obtained from all participants.
Consent for publication
Written informed consent was obtained from all
participants for the publication of their data and any accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Baumgart DC and Sandborn WJ: Inflammatory
bowel disease: Clinical aspects and established and evolving
therapies. Lancet. 369:1641–1657. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Baumgart DC and Carding SR: Inflammatory
bowel disease: Cause and immunobiology. Lancet. 369:1627–1640.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kornbluth A and Sachar DB: Practice
Parameters Committee of the American College of Gastroenterology:
Ulcerative colitis practice guidelines in adults: American College
Of Gastroenterology, Practice Parameters Committee. Am J
Gastroenterol. 105:501–524. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Akiho H, Yokoyama A, Abe S, Nakazono Y,
Murakami M, Otsuka Y, Fukawa K, Esaki M, Niina Y and Ogino H:
Promising biological therapies for ulcerative colitis: A review of
the literature. World J Gastrointest Pathophysiol. 6:219–227. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Masuda H, Iwai S, Tanaka T and Hayakawa S:
Expression of IL-8, TNF-alpha and IFN-gamma m-RNA in ulcerative
colitis, particularly in patients with inactive phase. J Clin Lab
Immunol. 46:111–123. 1995.PubMed/NCBI
|
|
6
|
Inoue S, Matsumoto T, Iida M, Mizuno M,
Kuroki F, Hoshika K and Shimizu M: Characterization of cytokine
expression in the rectal mucosa of ulcerative colitis: Correlation
with disease activity. Am J Gastroenterol. 94:2441–2446. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fuss IJ, Heller F, Boirivant M, Leon F,
Yoshida M, Fichtner-Feigl S, Yang Z, Exley M, Kitani A, Blumberg
RS, et al: Nonclassical CD1d-restricted NK T cells that produce
IL-13 characterize an atypical Th2 response in ulcerative colitis.
J Clin Invest. 113:1490–1497. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fujino S, Andoh A, Bamba S, Ogawa A, Hata
K, Araki Y, Bamba T and Fujiyama Y: Increased expression of
interleukin 17 in inflammatory bowel disease. Gut. 52:65–70. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Annunziato F, Cosmi L, Santarlasci V,
Maggi L, Liotta F, Mazzinghi B, Parente E, Filì L, Ferri S, Frosali
F, et al: Phenotypic and functional features of human Th17 cells. J
Exp Med. 204:1849–1861. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Korn T, Bettelli E, Oukka M and Kuchroo
VK: IL-17 and Th17 cells. Annu Rev Immunol. 27:485–517. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kobayashi T, Okamoto S, Hisamatsu T,
Kamada N, Chinen H, Saito R, Kitazume MT, Nakazawa A, Sugita A,
Koganei K, et al: IL23 differentially regulates the Th1/Th17
balance in ulcerative colitis and Crohn's disease. Gut.
57:1682–1689. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Harbord M, Eliakim R, Bettenworth D,
Karmiris K, Katsanos K, Kopylov U, Kucharzik T, Molnár T, Raine T,
Sebastian S, et al: Third European evidence-based consensus on
diagnosis and management of ulcerative colitis. Part 2: Current
management. J Crohns Colitis. 11:769–784. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Truelove SC and Witts LJ: Cortisone in
ulcerative colitis; final report on a therapeutic trial. Br Med J.
2:1041–1048. 1955. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Leung Y, Panaccione R, Hemmelgarn B and
Jones J: Exposing the weaknesses: A systematic review of
azathioprine efficacy in ulcerative colitis. Dig Dis Sci.
53:1455–1461. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rutgeerts P, Sandborn WJ, Feagan BG,
Reinisch W, Olson A, Johanns J, Travers S, Rachmilewitz D, Hanauer
SB, Lichtenstein GR, et al: Infliximab for induction and
maintenance therapy for ulcerative colitis. N Engl J Med.
353:2462–2476. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lichtenstein GR, Abreu MT, Cohen R and
Tremaine W: American Gastroenterological Association: American
Gastroenterological Association Institute technical review on
corticosteroids, immunomodulators, and infliximab in inflammatory
bowel disease. Gastroenterology. 130:940–987. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Poolsup N, Suthisisang C, Prathanturarug
S, Asawamekin A and Chanchareon U: Andrographis paniculata in the
symptomatic treatment of uncomplicated upper respiratory tract
infection: Systematic review of randomized controlled trials. J
Clin Pharm Ther. 29:37–45. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Coon JT and Ernst E: Andrographis
paniculata in the treatment of upper respiratory tract infections:
A systematic review of safety and efficacy. Planta Med. 70:293–298.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Saxena RC, Singh R, Kumar P, Yadav SC,
Negi MP, Saxena VS, Joshua AJ, Vijayabalaji V, Goudar KS,
Venkateshwarlu K and Amit A: A randomized double blind placebo
controlled clinical evaluation of extract of Andrographis
paniculata (KalmCold) in patients with uncomplicated upper
respiratory tract infection. Phytomedicine. 17:178–185. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kumar RA, Sridevi K, Kumar NV, Nanduri S
and Rajagopal S: Anticancer and immunostimulatory compounds from
Andrographis paniculata. J Ethnopharmacol. 92:291–295. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Puri A, Saxena R, Saxena RP, Saxena KC,
Srivastava V and Tandon JS: Immunostimulant agents from
Andrographis paniculata. J Nat Prod. 56:995–999. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rajagopal S, Kumar RA, Deevi DS,
Satyanarayana C and Rajagopalan R: Andrographolide, a potential
cancer therapeutic agent isolated from Andrographis paniculata. J
Exp Ther Oncol. 3:147–158. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wiart C, Kumar K, Yusof MY, Hamimah H,
Fauzi ZM and Sulaiman M: Antiviral properties of ent-labdene
diterpenes of Andrographis paniculata nees, inhibitors of herpes
simplex virus type 1. Phytother Res. 19:1069–1070. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Singha PK, Roy S and Dey S: Antimicrobial
activity of Andrographis paniculata. Fitoterapia. 74:692–694. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sandborn WJ, Targan SR, Byers VS, Rutty
DA, Mu H, Zhang X and Tang T: Andrographis paniculata extract
(HMPL-004) for active ulcerative colitis. Am J Gastroenterol.
108:90–98. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tang T, Targan SR, Li ZS, Xu C, Byers VS
and Sandborn WJ: Randomised clinical trial: Herbal extract HMPL-004
in active ulcerative colitis-a double-blind comparison with
sustained release mesalazine. Aliment Pharmacol Ther. 33:194–202.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu W, Guo W, Guo L, Gu Y, Cai P, Xie N,
Yang X, Shu Y, Wu X, Sun Y and Xu Q: Andrographolide sulfonate
ameliorates experimental colitis in mice by inhibiting Th1/Th17
response. Int Immunopharmacol. 20:337–345. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Szabo SJ, Kim ST, Costa GL, Zhang X,
Fathman CG and Glimcher LH: A novel transcription factor, T-bet,
directs Th1 lineage commitment. Cell. 100:655–669. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ayyoub M, Deknuydt F, Raimbaud I, Dousset
C, Leveque L, Bioley G and Valmori D: Human memory
FOXP3+ Tregs secrete IL-17 ex vivo and constitutively
express the TH17 lineage-specific transcription factor
RORγt. PNAS. 106:8635–8640. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zheng W and Flavell RA: The transcription
factor GATA-3 is necessary and sufficient for Th2 cytokine gene
expression in CD4 T cells. Cell. 89:587–596. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Neurath MF: IL-23: A master regulator in
Crohn disease. Nat Med. 13:26–28. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Iwakura Y and Ishigame H: The IL-23/IL-17
axis in inflammation. J Clin Invest. 116:1218–1222. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Di Cesare A, Di Meglio P and Nestle FO:
The IL-23/Th17 axis in the immunopathogenesis of psoriasis. J
Invest Dermatol. 129:1339–1350. 2009. View Article : Google Scholar : PubMed/NCBI
|