Introduction
Thyroid cancer is the most common tumour of the
endocrine system, and its incidence rate has dramatically increased
over the past several decades (1).
Thyroid cancer incidence is rapidly increasing in the USA; in 2017,
its estimated annual diagnosis rate and annual mortality rate were
56,870 people and 2,010 cases, respectively (2). Papillary thyroid carcinoma (PTC)
accounts for the majority of thyroid cancers and generally exhibits
favourable prognosis (3). However,
the recurrence rate is approximately 15% in 3 years (4). Recurrent PTC has limited therapeutic
options and poor prognosis, and this disease is currently the
leading cause of death in patients with PTC. Therefore, our
understanding of the mechanisms underlying the pathogenesis of PTC
should be improved, and further effective therapeutic approaches
should be developed.
Long non-coding RNAs (lncRNAs) are a diverse class
of RNAs with a length of more than 200 nucleotides that do not
encode proteins (5). Genome-wide
transcriptional analysis has revealed the high prevalence of
lncRNAs in all transcripts (5).
lncRNAs are important epigenetic modulators with sensory, guiding
and scaffolding capacities (6).
Thus, lncRNAs have gained increasing attention as a critical
regulator of normal physiology and disease development,
particularly in proliferation and migration (7). lncRNA expression is dysregulated in
many diseases affecting different tissue types (7). lncRNAs can be detected in various
human body fluids, such as serum and plasma, suggesting that
lncRNAs may serve as new biomarkers for disease diagnosis and
prognosis without invasive procedures (8).
Urothelial carcinoma-associated 1 (UCA1) is a lncRNA
that shows potential as a biomarker for cancer. The UCA1 gene is
located at chromosome 19p-13.12 and dysregulated in breast cancer,
oesophageal cancer, gastric cancer and bladder cancer (9–13).
UCA1 depletion can attenuate the migratory ability of various
cancers (9–13), whereas UCA1 overexpression can
enhance ovarian cancer cell migration and invasion (14), suggesting that UCA1 functions as a
pivotal regulator of cellular migration and invasion. Nevertheless,
up to now, there is no relevant report about the relationship
between UCA1 and the progression of PTC. Therefore, the role and
underlying mechanism of UCA1 in PTC should be elucidated.
Previous studies have showed that miR-204 plays a
protective role by inhibiting thyroid cancer cell proliferation and
may identify new targets for anti-cancer treatment (15,16).
Moreover, in the recent studies has showed that UCA1 can directly
regulated the expression of miR-204 in prostate cancer, colorectal
cancer and oesophageal cancer (9–11,17).
However, the correlation of UCA1 and miR-204 remains elusive in
PTC. Therefore, there is a vital necessity to illustrate the
relationship of UCA1 and miR-204 in PTC.
In this study, we reported an interaction between
UCA1 and miR-204, which regulates PTC cell growth by targeting
BRD4. Our study demonstrated that the UCA1/miR-204/BRD4 signalling
pathway might be a novel therapeutic target for patients with
PTC.
Materials and methods
Cell culture and tissue
collections
The human PTC cell lines (TPC-1 and BCPAP) and
immortal human thyroid follicular cell line (Nthy-ori3-1) were
obtained from the Cell Bank of China (Shanghai, China). These cell
lines were authenticated via short-tandem repeat profiling
performed by BMR Genomics. BCPAP usually known as a PTC cell line
is derived from a poorly differentiated PTC, however, recent study
has showed that the mRNA expression profile of BCPAP were closer to
anaplastic thyroid cancer cells (18). In this study, BCPAP serves as
poorly differentiated papillary thyroid cancer cell line to further
confirm the experiments results of TPC-1 and not affect the
outcomes of this study. The cells were cultured in DMEM (HyClone;
GE Healthcare Life Sciences, Shanghai, China) supplemented with 10%
foetal bovine serum (HyClone; GE Healthcare Life Sciences) in a
humidified atmosphere with 5% CO2 and humidified atmosphere of 95%
at 37°C. Human PTC specimens and their adjacent normal thyroid
tissues were obtained from 30 patients who had PTC and who had
undergone surgery at the Jining Medical University. All tissue
samples were immediately frozen in liquid N and stored at −80°C
until RNA extraction. Written informed consent was obtained from
each patient, and the study protocol and consent procedures were
approved by the Ethics Committee of the Jining Medical University
(Shandong, China).
Cell transfection
LV3 (H1/GFP&Puro) vector was synthesized for
Lv-shRNAUCA1 (Guangzhou RiboBio Co., Ltd., Guangzhou, China). A
nontarget scrambled oligonucleotide served as the negative control.
The shRNA sequences used in the present study were as follows:
shUCA1, 5′-GCCACCUACAUUAAAGCUAdTdT-3′ and shcontrol,
5′-CAGUACUUUUGUGUAGUACAA-3′. miR-204 mimic (miR10000265-1-5),
miR-NC (miR01201-1-5), inhibitor (miR20022693-1-5) and anti-NC
(miR02201-1-5) were obtained from Guangzhou RiboBio Co., Ltd..
Transfection was performed by using Lipofectamine 2000 reagent
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
according to the manufacturer's protocols.
Western blot analysis
Equal amounts of proteins (30 µg) from the lysates
of the cells were subjected to electrophoresis through a 10% SDS
PAGE (Beyotime Institute of Biotechnology, Haimen, China) at 80 V
for 30 min and at 100 V for 1.5 h. The proteins were then
transferred onto polyvinylidene difluoride membranes. After
blocking in 5% skimmed milk, the membranes were then incubated with
the following diluted primary antibodies: Rabbit polyclonal BRD4
(ab84776, 1:500, Abcam, Cambridge, MA, USA), mouse monoclonal GAPDH
(AF0006, 1:1,000, Beyotime Institute of Biotechnology) overnight at
4°C and peroxidase-coupled secondary antibody (1:2,000; cat. no.
A0216; Beyotime Institute of Biotechnology) at room temperature for
2 h. Specific bands were visualised on an autoradiographic film
using an enhanced chemiluminescence reagent (Nanjing KeyGen Biotech
Co., Ltd., Nanjing, China).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA from cells and tissues was isolated with a
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. First-strand cDNA was
generated by reverse transcribing the total cellular RNA with M-MLV
reverse transcriptase (Promega Corporation, Madison, WI, USA). The
SYBR Premix Ex Taq™ kit (Takara Bio, Inc., Otsu, Japan) was used
according to the manufacturer's instructions, and RT-qPCR was
performed and analysed by using the iQ5 detection system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The GAPDH served as the
internal control for detection. The qPCR conditions were applied
for detecting mRNAs: 95°C for 30 sec, followed by 40 cycles of 95°C
for 30 sec, 60°C for 30 sec and 72°C for 30 sec. Relative mRNA
expression was analyzed as the inverse log of ∆∆Cq and was
normalized to the reference (19).
UCA1: Forward 5′-TTTGCCAGCCTCAGCTTAAT-3′, Reverse
5′-TTGTCCCCATTTTCCATCAT-3′; miR-204 Forward 5′-GTCCCTGTGTCATCCT-3′,
Reverse 5′-CAGTGCAGGGTCCGAGGTAT-3′; U6 Forward
5′-TGCGGGTGCTCGCTTCGCAGC-3′, Reverse 5′-CCAGTGCAGGGTCCGAGGT-3′;
BRD4 Forward 5′-CATGGACATGAGCACAATCA-3′, Reverse
5′-TCATGGTCAGGAGGGTTGTA-3′; GAPDH Forward
5′-CTGACCTGCCGTCTAGAAA-3′, Reverse 5′-GTGGTGTGACTTAGAGGGG-3′.
Cell viability assay
Cell viability was examined using CCK-8 assay.
Briefly, TPC-1 and BCPAP cells transfected with either shUCA1 or
shcontrol were plated onto 96-well plates with 3,000 cells/well.
After culturing at an indicated time (0, 24, 48 and 72 h), 10 µl of
CCK-8 solution was added into each well at 37°C. After 3 h,
absorbance was determined using a microplate spectrophotometer at a
wavelength of 450 nm.
Colony formation assay
The TPC-1 and BCPAP cells transfected with either
shUCA1 or shcontrol (500 cells/well) were placed into 6-well
plates. After 1 week, the colonies were fixed with methanol and
stained with 0.1% crystal violet for 20 min, and the images of the
stained colonies were captured by using a CKX41 light microscope
(Thermo Labsystems, Vantaa, Finland). The number of the colonies
was counted by using the images.
Migration and invasion assay
Migration and invasion ability was examined by using
Transwell assay. For the invasion assay, the upper sides of the
filters were coated with 50 µl of Matrigel (BD Biosciences,
Franklin Lakes, NJ, USA). Indicated cells were plated at a density
of 5×104 per well in the upper chamber without serum.
Cells were incubated for 12 h for the migration assay and 24 h for
the invasion assay. After the cells were incubated at the exact
time at 37°C, the inserts were washed with PBS, and cells on the
upper surface of the insert were removed with a cotton swab. Cells
adhering to the lower surface were fixed with 4% formaldehyde for
20 min, stained with 0.1% crystal violet solution and imaged using
a CKX41 light microscope.
Dual luciferase reporter assay
TPC-1 and BCPAP cells cultured in 24-well plates
were cotransfected with luciferase reporter plasmids (wt-BRD4,
mut-BRD4 containing miR-204 binding site, wt-UCA1 and mut-UCA1
containing miR-204 binding site) and miRNA mimics or inhibitor.
After 48 h, luciferase activity was measured via dual-luciferase
reporter assay system according to the manufacturer's instruction
(Promega Corporation).
In vivo assay
All the procedures were carried out in accordance to
the Guide for the Care and Use of Laboratory Animals issued by the
Institutional Animal Care and Use Committee of the Jining Medical
University (Jining, China). Five-week-old female BALB⁄c nude mice
were purchased from the Yangzhou Laboratory Animal Centre and
maintained in a SPF environment. Subcutaneous tumour xenografting
was performed by subcutaneously injecting mice with 100 µl of PBS
containing 5×105 BCPAP cells that had been transfected
with either shUCA1 or shcontrol. Tumour volume (mm3) was
calculated every 7 days by using the following equation: V = 0.5 ×
length × width2. After 42 days, the mice were
euthanized, and the tumors were isolated, weighed, photographed and
then processed for further study.
Immunohistochemistry
Paraffin-embedded sections of tumor tissue (4 µm
thick) were deparaffinized in xylene, rehydrated via graded alcohol
solutions, blocked in methanol containing 3% hydrogen peroxide for
10 min at room temperature, and then incubated with mouse BRD4
antibody (ab84776, 1:500; Abcam, Cambridge, MA, USA) at 4°C
overnight. Following rinsing with PBS solution, biotinylated goat
anti-rabbit serum IgG (ab6785, 1:2,000; Abcam, Cambridge, MA, USA)
was used as secondary antibodies and streptavidin peroxidase
complex reagent were applied for 1 h at room temperature. Finally,
the sections were incubated in a 3,3′-diaminobenzidine solution at
room temperature for 10 min and then counterstained with
hematoxylin for 3 min at room temperature. Ten randomly selected
visual fields per section were examined under a light microscope to
evaluate the BRD4 expression.
Statistical analysis
All experiments were performed three times. The data
were presented as the mean ± standard deviation. Differences
between two groups were assessed using two-tailed Student's t-test.
Data from >2 groups were analyzed using one way analysis of
variance with the post hoc Tukey's test. Statistical analyses were
performed using SPSS version 13.0 (SPSS, Inc., Chicago, IL, USA).
Differences were considered statistically significant when
P<0.05.
Results
UCA1 is upregulated in PTC tissues and
cell lines
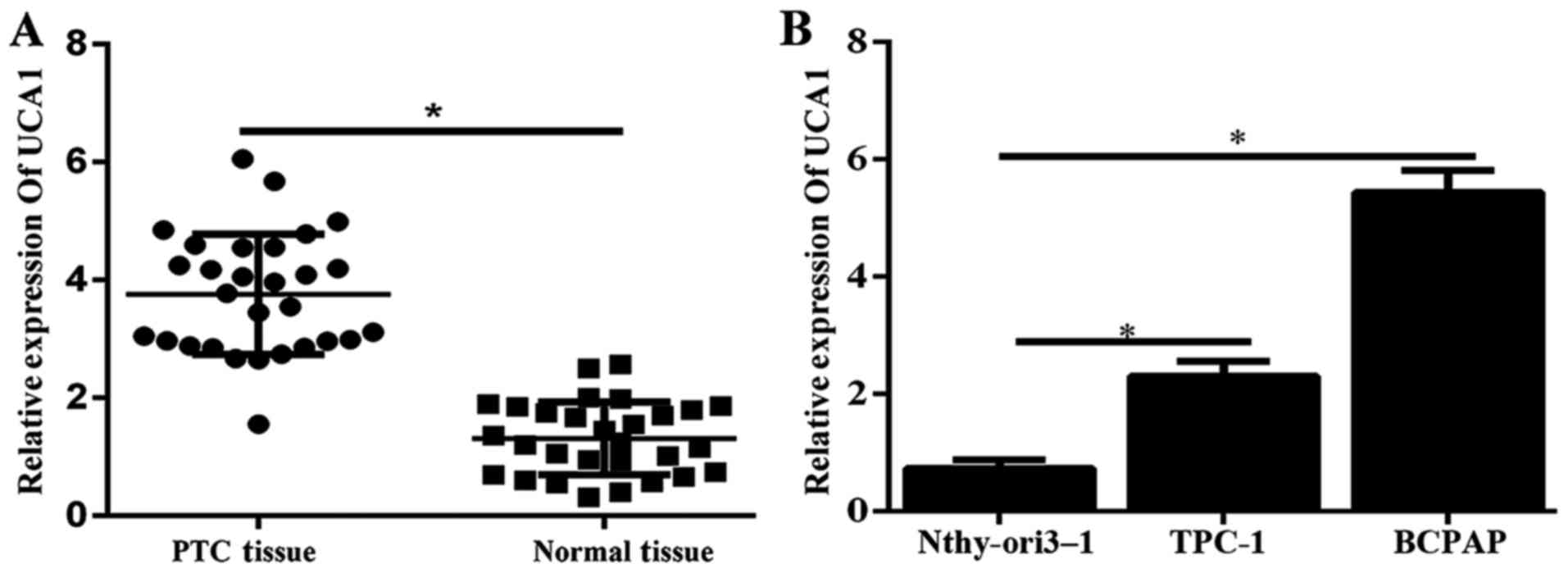
To evaluate the role of UCA1 in PTC, we first
detected the expression level of UCA1 in 30 pairs of human PTC
specimens and corresponding adjacent non-tumor tissues were
determined by using qPCR. Results illustrated that the expression
of UCA1 was significantly higher in tumor tissues (Fig. 1A). Furthermore, the expression
level of UCA1 in PTC cell lines (TPC-1 and BCPAP) was significantly
enhanced compared to that in the human immortal follicular thyroid
cell Nthy-ori3-1 (Fig. 1B).
Therefore, UCA1 might play a critical role in the progression of
PTC.
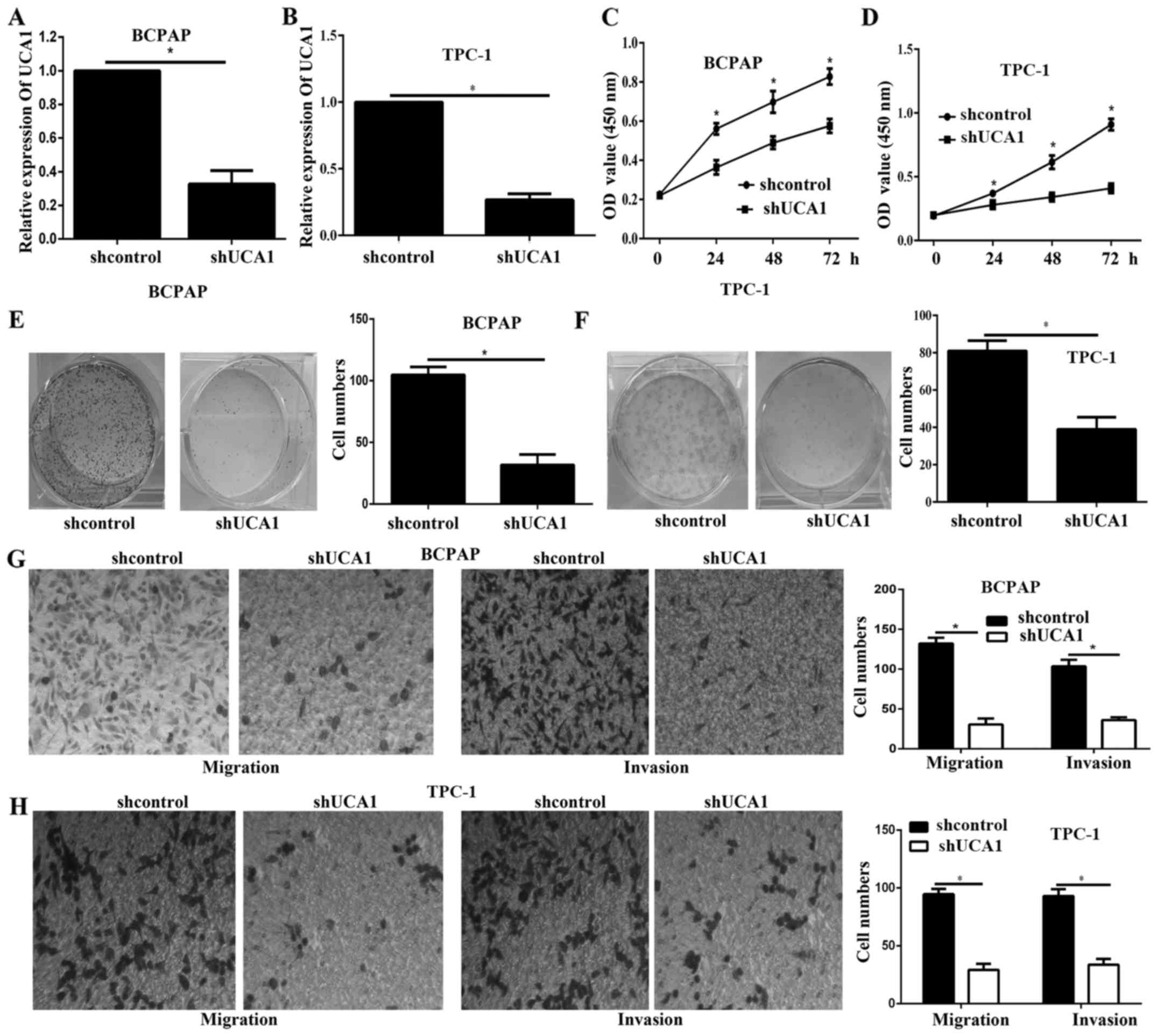
UCA1 silencing decreased cell
viability, proliferation in PTC cell lines
UCA1 knockdown was achieved by transfection with
shUCA1 in BCPAP and TPC-1, as verified via qPCR assays (Fig. 2A and B). The CCK-8 results showed
that the cell viability of BCPAP and TPC-1 cells was significantly
decreased after UCA1 knockdown (Fig.
2C and D). In addition, the colony formation assay results
illustrated that the proliferation of UCA1 silencing cells was
remarkably decreased (Fig. 2E and
F). The Transwell assay illustrated that the migration and
invasion of UCA1 silencing cells was significantly decreased
(Fig. 2G and H).
Previous study has showed that BRD4 is involved in
the progression of PTC (20). BRD4
silencing decreased the tumor viability, proliferation in
vitro and in vivo (20). Here we also monitored the protein
levels of BRD4 in BCPAP and TPC-1 cells, in response to UCA1
silencing. The western blot and qPCR results showed that UCA1
silencing significantly decreased the expression level of BRD4
(Fig. 3A-D). These results
illustrated that UCA1 might enhance PTC progression via the
activation of BRD4.
miR-204 could negatively regulate BRD4
expression
To discover the underlying mechanism by which UCA1
regulated PTC progression, TargetScan (http://www.targetscan.org/vert_71/) was used. BRD4 was
predicted to be a target of miR-204. miR-204 has been demonstrated
to serve as tumor suppressor gene in some kinds of cancer including
thyroid cancer (15,16). Moreover, recent studies have showed
that UCA1 can directly regulate the expression of miR-204 in some
types of cancer (9,11,17).
BCPAP and TPC-1 cells were transfected with miR-204 mimic, miR-NC,
miR-204 inhibitor or NC-inhibitor respectively. The expression
level of miR-204 was examined via qPCR (Fig. 4A and B). The results showed that
the mRNA expression of BRD4 was significantly decreased by miR-204
overexpression while enhanced by miR-204 inhibition (Fig. 4C and D). The protein expression
levels of BRD4 in BCPAP and TPC-1 cell lines in response to miR-204
change were then examined by using Western blot. Results showed
that the protein expression of BRD4 was decreased by miR-204 mimic
while increased by miR-204 inhibitor (Fig. 4E and F). These data showed that
miR-204 could negatively regulate BRD4 expression in PTC cell
lines.
UCA1 competed with BRD4 for miR-204
binding
We demonstrated that miR-204 negatively regulated
BRD4 expression; then we illustrated the correlation between UCA1
and miR-204 in PTC. The expression level of UCA1 in response to
miR-204 inhibition and overexpression in BCPAP and TPC-1 cell was
examined via qPCR. Results showed that UCA1 was inhibited by
miR-204 overexpression while enhanced by miR-204 inhibition
(Fig. 5A and B). In addition, qPCR
data illustrated that the expression of miR-204 was remarkably
enhanced while UCA1 expression was downregulated (Fig. 5C and D). These results demonstrated
a dual regulation between UCA1 and miR-204. According to online
tools, UCA1 shared a same binding site in miR-204 with BRD4. Then
luciferase assays were performed to discover the correlations
between miR-204 and UCA1. Luciferase reporter gene vectors (wt-BRD4
3′UTR, mut-BRD4 3′UTR containing a 6 bp mutation on miR-204 binding
site in the 3′UTR of BRD4, wt-UCA1, and mut-UCA1 containing a 6 bp
mutation on miR-204 binding site in UCA1) were constructed and
co-transfected into BCPAP and TPC-1 cells with miR-204 mimics or
miR-204 inhibitor (Fig. 5E). The
luciferase assays results showed that the luciferase activity of
wt-UCA1 and wt-BRD4 vectors was remarkably decreased by miR-204
mimics, enhanced by miR-204 inhibitor; after mutation in the
predicted binding sites of miR-204, the changes of the luciferase
activity were abolished (Fig. 5F and
G). These data suggested that UCA1 and BRD4 both could bind to
miR-204. Given the consistency of the binding site(s), UCA1 might
compete with BRD4 for miR-204 binding, so that to attenuate the
inhibitory effect of miR-204 on BRD4 expression.
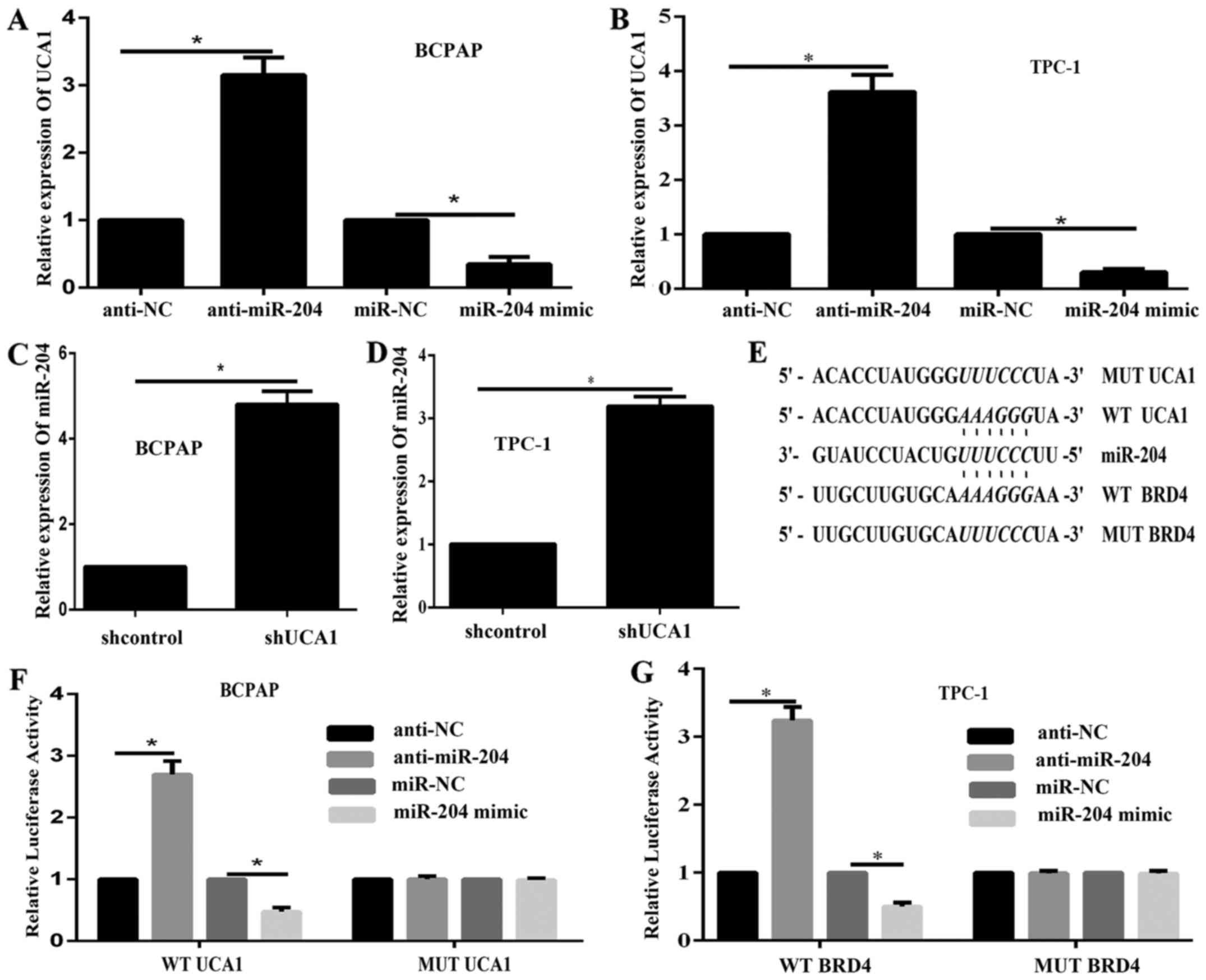 | Figure 5.UCA1 competes with BRD4 for miR-204
binding. (A and B) The expression of UCA1 in response to miR-204
changes in (A) BCPAP and (B) TPC-1 cells was examined via RT-qPCR.
(C and D) miR-204 expression in response to UCA1 silencing in (C)
BCPAP and (D) TPC-1 cells was examined by RT-qPCR. (E) A WT or MUT
UCA1 (wt-UCA1 and mut-UCA1 containing 6 bp mutation in the
predicted binding sites of miR-204) or BRD4 3′UTR (wt-BRD4 3′UTR
and mut-BRD4 3′UTR containing a 6 bp mutation in the predicted
binding sites of miR-204) luciferase reporter gene vector was
constructed. (F and G) Following overnight culture, cells were
co-transfected with the indicated vectors and miR-204 mimics or
miR-204 inhibitor, respectively. Luciferase assays were performed
48 h following transfection to determine the luciferase activity.
*P<0.05, as indicated. UCA1, urothelial carcinoma-associated 1;
sh-, short hairpin RNA; BRD4, bromodomain containing 4; miR,
microRNA; NC, negative control; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; WT, wild
type; MUT, mutant; UTR, untranslated region. |
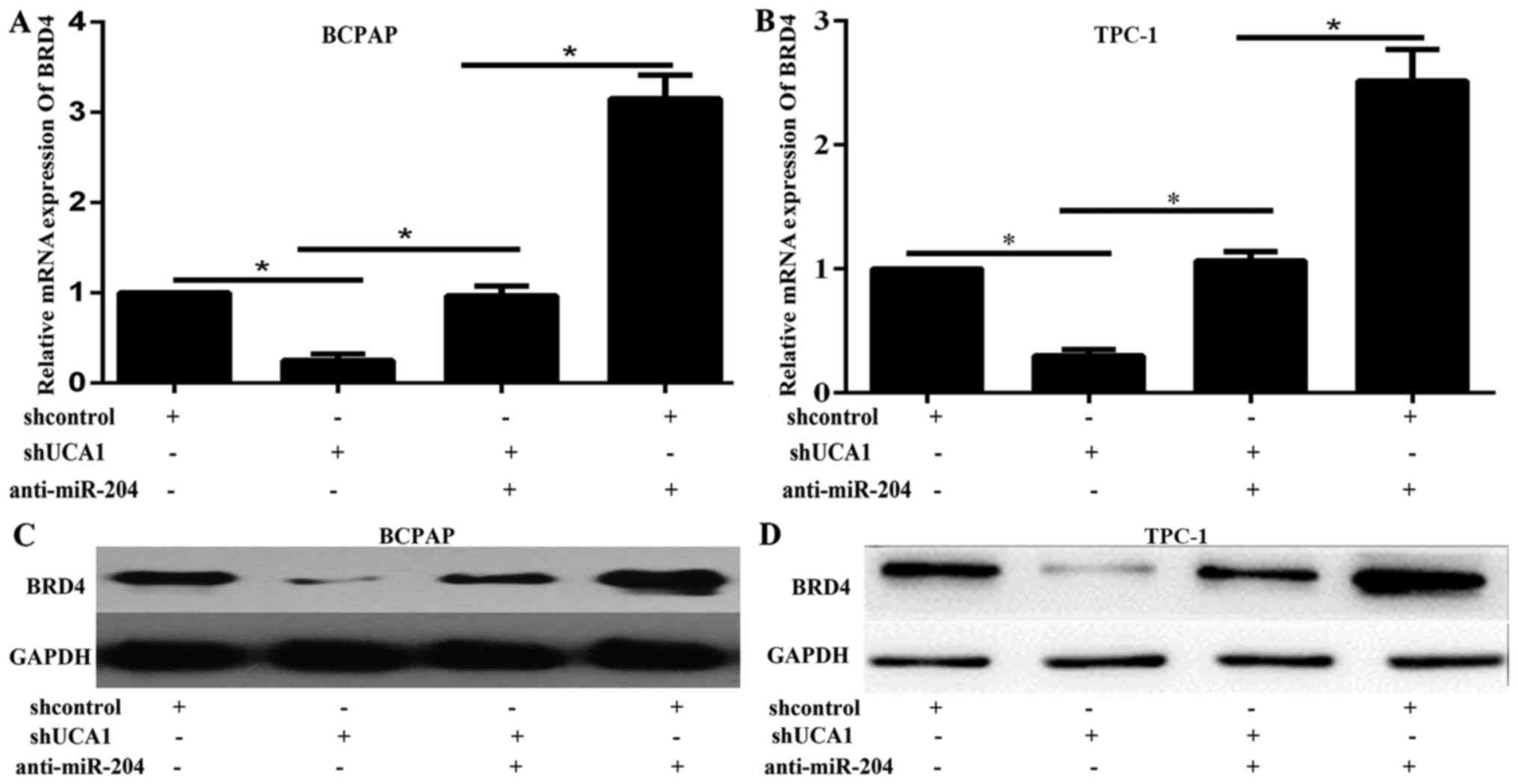
UCA1 regulated BRD4 expression via
miR-204
To validate whether UCA1 regulated BRD4 expression
through miR-204, we cotransfected BCPAP and TPC-1 cells with
miR-204 inhibitor and shUCA1, and examined the expression of BRD4
by western blot and qPCR. The results showed that the expression of
protein and mRNA of BRD4 were increased by miR-204 inhibition,
decreased by shUCA1 (Fig. 3A-D).
The promotive effect of miR-204 inhibition on the expression of
BRD4 could be partially abolished by shUCA1 (Fig. 3A-D). These results demonstrated
that UCA1 could regulate BRD4 expression through miR-204.
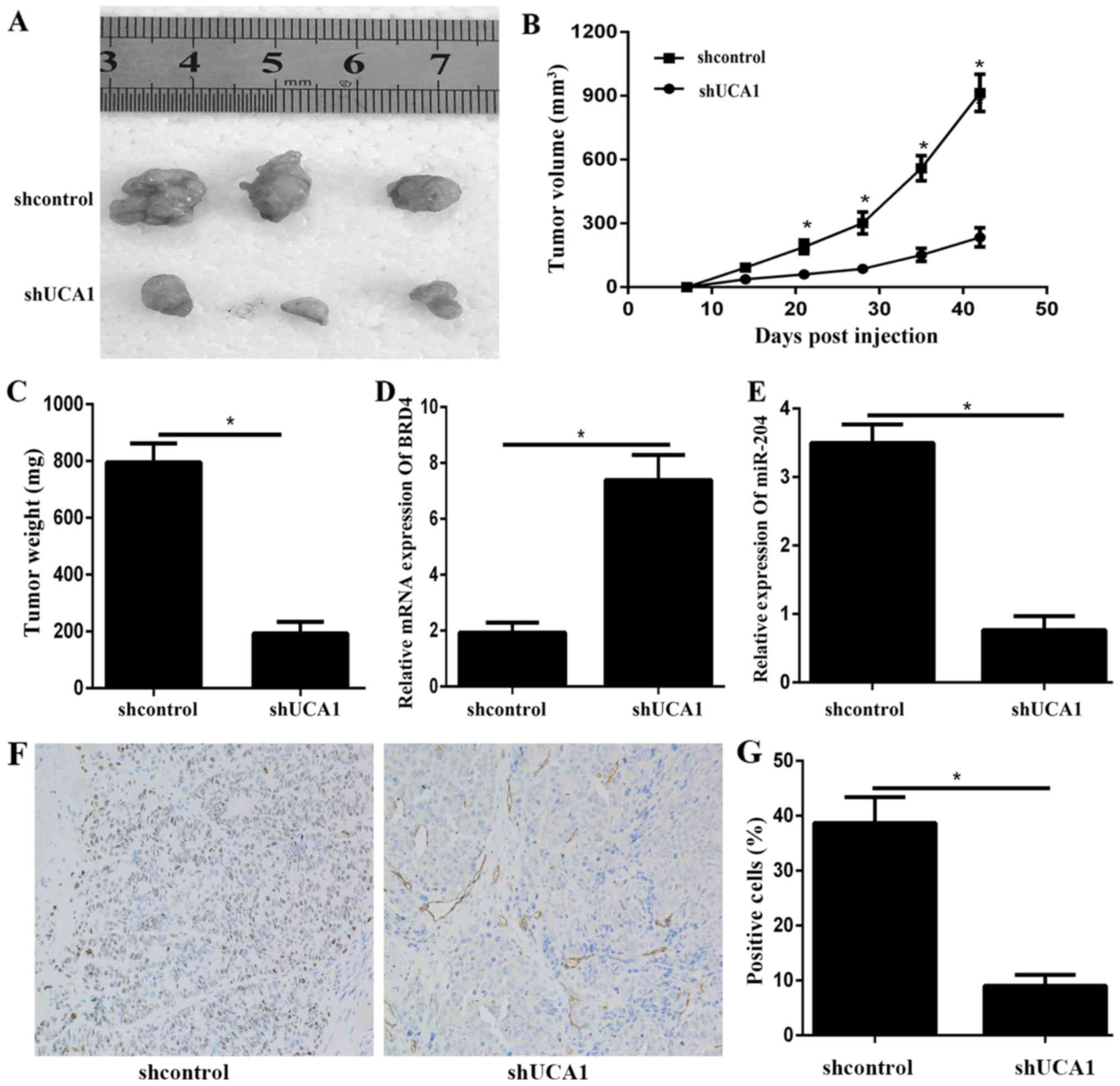
Knockdown of UCA1 inhibits tumor
growth in vivo
To further illustrate the effects of UCA1 on the PTC
growth in vivo, we applied a xenograft model in which the
BCPAP cells transfected with shUCA1 or shNC were subcutaneously
injected into the flanks of athymic mice and measured the tumor in
a period time. The results showed that tumors formed by transfected
shUCA1 grew more slowly than those formed by transfected shNC
(Fig. 6A-C). Furthermore, qPCR
results showed that the BRD4 expression levels in the shUCA1 tumour
xenografts were lower than those in the control xenografts
(Fig. 6D). In addition, the qPCR
results showed that the expression level of miR-204 was
significantly downregulated in shUCA1 transfected tumor compared to
shNC transfected tumor (Fig. 6E).
At last, histologic analysis showed that shUCA1 tumors had
significantly fewer BRD4 positive cells than the shNC tumors
(Fig. 6F and G). The in
vivo results also demonstrated that UCA1 might regulate BRD4
via miR-204.
Discussion
In the present study, we confirmed the oncogenic
role of UCA1 in the progression of PTC. Initially, we demonstrated
that lncRNA UCA1 was significantly increased in PTC cell lines and
tissues. Then, we showed that UCA1 silencing inhibited the
viability, proliferation, migration and invasion of PTC cells in
vitro and in vivo. Finally, we observed that UCA1 could
sponge miR-204 and modulate BRD4 expression.
BRD4 is an epigenome reader and member of the
bromodomain and extra-terminal family of proteins, which consist of
two bromodomains in tandem and an extra-terminal domain. BRD4
promotes cell cycle progression, regulates cell growth and
transcription and plays a critical role in the tumour progression
of various cancers via different molecular mechanisms (21–28).
BRD4 inhibition with JQ1 significantly prevents PTC progression
in vitro and in vivo (20). The BRD4 expression in breast cancer
is regulated by miR-599 (29).
However, how BRD4 is aberrantly dysregulated in PTC is unclear, and
the relation between miRNA and BRD4 has yet to be established.
miR-204 plays a protective role by inhibiting thyroid cancer cell
proliferation and may identify new targets for anti-cancer
treatment (15). In the present
study, luciferase assay and Western blot analysis confirmed that
BRD4 was a direct target gene of miR-204.
lncRNAs are transcribed RNA molecules with a length
of more than 200 nucleotides that lack a substantial protein-coding
potential. They can regulate protein-coding genes at epigenetic,
transcriptional and post-transcriptional levels and participate in
physiological processes. Various lncRNAs are frequently aberrantly
expressed in cancers, and their differential expression is closely
related to tumour progression; therefore, they serve as oncogenes
or tumor suppressor genes (28).
Further studies should be conducted to clarify the biological
function and molecular mechanisms of lncRNAs in tumour
progression.
UCA1 is an identified lncRNA that promotes tumor
progression in a wide range of tumour types, including bladder
cancer, breast cancer, hepatocellular carcinoma, colorectal cancer
and gastric cancer (9,30–32).
However, the role of UCA1 in PTC has yet to be illustrated.
Initially, we investigated the mRNA expression of UCA1 in PTC
tissues and cell lines and found that UCA1 expression was
significantly higher in PTC tissues and cell lines than in normal
tissues. Moreover, UCA1 levels in PTC tissues were positively
correlated with advanced clinical stages and lymph node metastasis,
but not with age, gender, or tumor size (data not shown). These
data suggested that UCA1 functioned as an oncogene in PTC, and this
finding is consistent with previous results on other tumors
(9,30–32).
To investigate the molecular mechanism by which UCA1 affects PTC
proliferation and migration, we monitored the protein level of BRD4
when the UCA1 expression was downregulated by using shUCA1 in
thyroid cancer. We showed that UCA1 silencing reduced the BRD4
protein expression, indicating the involvement of BRD4 in the
regulation of UCA1 cell proliferation and invasion. A previous
study demonstrated that lncRNAs act as competing endogenous RNAs to
mediate miRNAs, and lncRNA-miRNA interaction regulates tumour
progression through the regulation of oncogenes or tumour
suppressor genes (33). UCA1 is
related to miR-204 via the regulation of different downstream
target genes (9–11). In the present study, we revealed
the interaction between UCA1 and miR-204 in PTC for the first time;
that is, UCA1 and miR-204 could negatively regulate each other. In
this reciprocal negative regulation loop, the overexpression of
UCA1 represses the expression of miR-204 to upregulate the
expression of BRD4, thereby enhancing thyroid cancer cell growth
and migration and invasion. On the other hand, the miR-204
represses the expression of UCA1 and decrease the expression of
BRD4, so that to inhibit the tumor growth and metastasis. The
equilibrium might depend on the tumour microenvironment and the
type of cancer. We also showed that miR-204 could directly control
the BRD4 expression. Given this result, we investigated whether
miR-204 could regulate UCA1 and BRD4 through direct targeting. Dual
luciferase assays revealed that UCA1 could bind to miR-204. miR-204
could also bind to the 3′UTR of BRD4, suggesting that UCA1 might
compete with BRD4 for miR-204 binding to inhibit miR-204, to
promote BRD4 expression and to affect PTC progression. To further
discover whether BRD4 could regulate the expression of UCA1 and
miR-204, we used siBRD4 to knockdown the expression of BRD4, and
did not discover that BRD4 could regulate the expression of miR-204
and UCA1 in PTC cell lines (data not shown). These data
demonstrated that BRD4 was a downstream target gene of UCA1/miR-204
axis, and illustrated that drug resistance to BRD4 might select the
upstream gene UCA1 or miR-204 as alternative therapy target.
In conclusion, the UCA1/miR-204/BRD4 axis plays a
critical role in PTC cell proliferation and invasion and shows
potential for therapeutic applications in patients with thyroid
cancer.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Foundation of
Shandong People and Family Planning Commission (grant no.
2015WSA07008).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
DL and DH conceived and designed the experiments.
CC, JC, ZH and YW conducted all of the experiments. YW wrote and
revised the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Jining Medical University (Jining, China). All
patients provided written informed consent.
Patient consent for publication
All patients provided written informed consent for
the publication of any associated data and accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Maniakas A, Davies L and Zafereo ME:
Thyroid disease around the World. Otolaryngol Clin North Am.
51:631–642. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xing M: Molecular pathogenesis and
mechanisms of thyroid cancer. Nat Rev Cancer. 13:184–199. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lupoli R, Cacciapuoti M, Tortora A, Barba
L, Verde N, Romano F, Vastarella M, Fonderico F, Masone S, Milone
M, et al: Clinical outcome in differentiated thyroid carcinoma and
microcarcinoma. Int J Surg. 12 Suppl 1:S148–S151. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mercer TR and Mattick JS: Structure and
function of long noncoding RNAs in epigenetic regulation. Nat
Struct Mol Biol. 20:300–307. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lin CY and Xu HM: Novel perspectives of
long non-coding RNAs in esophageal carcinoma. Carcinogenesis.
36:1255–1262. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huarte M: The emerging role of lncRNAs in
cancer. Nat Med. 21:1253–1261. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xie H, Ma H and Zhou D: Plasma HULC as a
promising novel biomarker for the detection of hepatocellular
carcinoma. Biomed Res Int. 2013:1361062013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang S, Dong X, Ji T, Chen G and Shan L:
Long non-coding RNA UCA1 promotes cell progression by acting as a
competing endogenous RNA of ATF2 in prostate cancer. Am J Transl
Res. 9:366–375. 2017.PubMed/NCBI
|
|
10
|
Wang X, Yang B and Ma B: The
UCA1/miR-204/Sirt1 axis modulates docetaxel sensitivity of prostate
cancer cells. Cancer Chemother Pharmacol. 78:1025–1031. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jiao C, Song Z, Chen J, Zhong J, Cai W,
Tian S, Chen S, Yi Y and Xiao Y: lncRNA-UCA1 enhances cell
proliferation through functioning as a ceRNA of Sox4 in esophageal
cancer. Oncol Rep. 36:2960–2966. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang ZQ, Cai Q, Hu L, He CY, Li JF, Quan
ZW, Liu BY, Li C and Zhu ZG: Long noncoding RNA UCA1 induced by SP1
promotes cell proliferation via recruiting EZH2 and activating AKT
pathway in gastric cancer. Cell Death Dis. 8:e28392017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang X, Gao Z, Liao J, Shang M, Li X, Yin
L, Pu Y and Liu R: lncRNA UCA1 inhibits esophageal squamous-cell
carcinoma growth by regulating the Wnt signaling pathway. J Toxicol
Environ Health A. 79:407–418. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang F, Zhou J, Xie X, Hu J, Chen L, Hu Q,
Guo H and Yu C: Involvement of SRPK1 in cisplatin resistance
related to long non-coding RNA UCA1 in human ovarian cancer cells.
Neoplasma. 62:432–438. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu ZY, Wang SM, Chen ZH, Huv SX, Huang K,
Huang BJ, Du JL, Huang CM, Peng L, Jian ZX and Zhao G: MiR-204
regulates HMGA2 expression and inhibits cell proliferation in human
thyroid cancer. Cancer Biomark. 15:535–542. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu L, Wang J, Li X, Ma J, Shi C, Zhu H,
Xi Q, Zhang J, Zhao X and Gu M: MiR-204-5p suppresses cell
proliferation by inhibiting IGFBP5 in papillary thyroid carcinoma.
Biochem Biophys Res Commun. 457:621–626. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bian Z, Jin L, Zhang J, Yin Y, Quan C, Hu
Y, Feng Y, Liu H, Fei B, Mao Y, et al: LncRNA-UCA1 enhances cell
proliferation and 5-fluorouracil resistance in colorectal cancer by
inhibiting miR-204-5p. Sci Rep. 6:238922016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Saiselet M, Floor S, Tarabichi M, Dom G,
Hébrant A, van Staveren WC and Maenhaut C: Thyroid cancer cell
lines: An overview. Front Endocrinol (Lausanne).
3:1332012.PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gao X, Wu X, Zhang X, Hua W and Zhang Y,
Maimaiti Y, Gao Z and Zhang Y: Inhibition of BRD4 suppresses tumor
growth and enhances iodine uptake in thyroid cancer. Biochem
Biophys Res Commun. 469:679–685. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang YH, Sui XM, Sui YN, Zhu QW, Yan K,
Wang LS, Wang F and Zhou JH: BRD4 induces cell migration and
invasion in HCC cells through MMP-2 and MMP-9 activation mediated
by the Sonic hedgehog signaling pathway. Oncol Lett. 10:2227–2232.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Segura MF, Fontanals-Cirera B,
Gaziel-Sovran A, Guijarro MV, Hanniford D, Zhang G, González-Gomez
P, Morante M, Jubierre L, Zhang W, et al: BRD4 sustains melanoma
proliferation and represents a new target for epigenetic therapy.
Cancer Res. 73:6264–6276. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Puissant A, Frumm SM, Alexe G, Bassil CF,
Qi J, Chanthery YH, Nekritz EA, Zeid R, Gustafson WC, Greninger P,
et al: Targeting MYCN in neuroblastoma by BET bromodomain
inhibition. Cancer Discov. 3:308–323. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Patel AJ, Liao CP, Chen Z, Liu C, Wang Y
and Le LQ: BET bromodomain inhibition triggers apoptosis of
NF1-associated malignant peripheral nerve sheath tumors through Bim
induction. Cell Rep. 6:81–92. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ott CJ, Kopp N, Bird L, Paranal RM, Qi J,
Bowman T, Rodig SJ, Kung AL, Bradner JE and Weinstock DM: BET
bromodomain inhibition targets both c-Myc and IL7R in high-risk
acute lymphoblastic leukemia. Blood. 120:2843–2852. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lockwood WW, Zejnullahu K, Bradner JE and
Varmus H: Sensitivity of human lung adenocarcinoma cell lines to
targeted inhibition of BET epigenetic signaling proteins. Proc Natl
Acad Sci USA. 109:19408–19413. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Andrieu G, Tran AH, Strissel KJ and Denis
GV: BRD4 regulates breast cancer dissemination through
Jagged1/Notch1 signaling. Cancer Res. 76:6555–6567. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
He Y, Meng XM, Huang C, Wu BM, Zhang L, Lv
XW and Li J: Long noncoding RNAs: Novel insights into hepatocelluar
carcinoma. Cancer Lett. 344:20–27. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang Y, Sui Y, Zhu Q and Sui X:
Hsa-miR-599 suppresses the migration and invasion by targeting BRD4
in breast cancer. Oncol Lett. 14:3455–3462. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang F, Ying HQ, He BS, Pan YQ, Deng QW,
Sun HL, Chen J, Liu X and Wang SK: Upregulated lncRNA-UCA1
contributes to progression of hepatocellular carcinoma through
inhibition of miR-216b and activation of FGFR1/ERK signaling
pathway. Oncotarget. 6:7899–7917. 2015.PubMed/NCBI
|
|
31
|
Zheng Q, Wu F, Dai WY, Zheng DC, Zheng C,
Ye H, Zhou B, Chen JJ and Chen P: Aberrant expression of UCA1 in
gastric cancer and its clinical significance. Clin Transl Oncol.
17:640–646. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu M, Xing LQ and Liu YJ: A three-long
noncoding RNA signature as a diagnostic biomarker for
differentiating between triple-negative and non-triple-negative
breast cancers. Medicine (Baltimore). 96:e62222017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li JH, Zhang SQ, Qiu XG, Zhang SJ, Zheng
SH and Zhang DH: Long non-coding RNA NEAT1 promotes malignant
progression of thyroid carcinoma by regulating miRNA-214. Int J
Oncol. 50:708–716. 2017. View Article : Google Scholar : PubMed/NCBI
|