Introduction
Psoriasis is a chronic inflammatory autoimmune
disease that predominantly affects the skin (1). The pathogenesis of psoriasis is
complex, and involves genetic, immunological and environmental
factors (2). The development of
the disease is characterized by continuous relapses. In addition,
patients with psoriasis tend to suffer from the disease for their
entire lives (3). At present,
psoriasis is incurable; however, it may be controlled with
medication. Topical corticosteroid agents are the most effective
therapeutic approach for the treatment of this disease. Treatment
with systemic therapies, including numerous monoclonal antibodies
and inhibitors, are often used to treat patients with psoriasis
resistant to topical therapy or alternative therapies; however, the
adverse side effects of systemic therapies including nausea,
gastrointestinal intolerance, hyperlipidaemia should not be
underestimated (4,5). Considering this, phytomedicine has
been considered as an alternative therapy for the treatment of
patients with psoriasis, due to its safety and tolerability
(6).
It is reported that the active ingredients of
Aloe vera could be used as antibacterials and wound-healing
promoters. Aloe polysaccharide (APS) is the main bioactive
component of Aloe vera (7).
Traditionally, Aloe vera has been used to treat patients
with skin disorders. Numerous studies have revealed that Aloe
vera exhibits anti-inflammatory and antioxidant effects
(8,9). Previous studies investigating whether
Aloe vera exhibits therapeutic effects when administered to
patients with psoriasis have generated mixed results (10,11).
It has been well established that tumor necrosis
factor (TNF)-α is involved in direct and indirect regulation of
numerous genes involved in immune and inflammatory responses
(12). In patients with psoriasis,
the serum levels of TNF-α are associated with disease activity.
During the development of psoriasis, TNF-α is secreted by T cells
and has an important role in the pathogenic process (13). Despite previous studies suggesting
that Aloe vera may represent a promising therapeutic agent
for the treatment of patients with psoriasis (6,14,15).
To the best of our knowledge, the molecular mechanism underlying
the involvement of TNF-α and APS in treatment of psoriasis has not
yet been investigated.
Nuclear factor (NF)-κB has an important role in
inflammation via induction of the transcription of proinflammatory
genes (16). Members of the
NF-κB/Rel family include NF-κB1 (p50/p105), NF-κB2 (p52/p100), p65
(RelA), RelB and c-Rel. p65 can form active heterodimers with a p50
or p52 subunit containing transactivation domains (17). In its inactive form, NF-κB is
sequestered in the cytoplasm associated with IκB. Upon stimulation,
IκB is degraded, which then triggers phosphorylation of p65
(18). A previous study revealed
that there is a significant overexpression of phosphorylated
(p)-p65 in the epidermis of psoriatic plaques compared with normal
skin (19). Furthermore, it has
been demonstrated that Aloe vera can directly inhibit NF-κB
activation in peripheral blood mononuclear cells (20); however, whether APS can alleviate
the symptoms of psoriasis via inactivation of NF-κB is largely
unknown. The study was designed to examine whether APS inhibited
TNF-α-induced proliferation of keratinocytes through NF-κB
signaling pathway.
Materials and methods
Cell culture and agents
HaCaT cells (Shanghai Blowing Applied Biotechnology
Co., Ltd., Shanghai, China; STR profile confirmed as appropriate)
were cultured in Dulbecco's modified Eagle's medium supplemented
with 10% fetal bovine serum, and 1% penicillin and streptomycin
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), and were incubated
at 37°C in a humidified incubator containing 5% CO2.
Recombinant human TNF-α was purchased from BioLegend, Inc. (San
Diego, CA, USA). APS (75.6%) was supplied by the Chinese Academy of
Sciences, Shanghai Institute of Materia Medica (Shanghai,
China).
Cell viability assay
The viability of HaCaT cells was investigated using
Cell Counting Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan). Cells were seeded into a 96-well plate at a
density of 5.0×103 cells/well and were incubated for 24
h at 37°C in 5% CO2. Subsequently, cells were treated
with TNF-α (10 ng/ml), various concentrations of APS (20–80 µg/ml)
or a combination of TNF-α (10 ng/ml) and APS (20, 40 and 80 µg/ml)
for 24 h at 37°C in 5% CO2. CCK-8 solution (10 µl) was
then added to each well, and the plates were incubated for an
additional 2 h at 37°C. The control group was treated with an equal
amount of PBS. Absorbance values at 450 nm were determined for each
well using a microplate reader. Cell proliferation rate=(OD24
h-OD0 h)/OD0 h ×100%. All assays were
carried out in triplicate.
ELISA
HaCaT cells were treated with either TNF-α or a
combination of TNF-α and various concentrations of APS, and
supernatants were collected 24 h post-treatment. Concentrations of
interleukin (IL)-8 (cat. no. D8000C) and IL-12 (cat. no. M1270)
were determined using ELISA kits (RayBiotech, Inc., Norcross, GA,
USA). Briefly, 100 µl of each standard and supernatant sample were
added to a 96-well plate coated with anti-Human IL-8 or IL-12 and
incubated overnight at 4°C with gentle agitation. Plates were
subsequently washed four times with wash buffer. Wells were then
incubated for 1 h with IL-8 or IL-12 specific biotinylated
antibodies at room temperature, and were rinsed four times with
wash buffer. Following this, cells were treated with diluted
streptavidin solution (100 µl/well) for 45 min at room temperature,
washed four times with wash buffer, and further incubated for 30
min at room temperature with 100 µl TMB One-Step substrate reagent
in the dark. The plates were quenched with stop solution and
absorbance values were detected at 450 nm using a PowerWave XS
spectrophotometer (BioTek Instruments, Inc., Winooski, VT, USA).
ELISA analyses for each sample were repeated in triplicate. All
assays were carried out in triplicate.
Western blotting
Protein lysates were prepared using
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology, Haimen, China) with a protease/phosphatase inhibitor
cocktail (Cell Signaling Technology, Inc., Danvers, MA, USA).
Antibodies for IL-8 (cat. no. 94853S; 1:1,000), NF-κB inhibitor-α
(IκBα; cat. no. 4814S; 1:1,000), NF-κB (p65; cat. no. 8242S;
1:1,000) purchased from Cell Signaling Technology, Inc, and IL-12
(cat. no. ab9992; 1:1,000) purchased from Abcam, (Cambridge, MA,
USA). GAPDH mouse monoclonal antibody (cat. no. D190636; 1:1,000)
was purchased from Sangon BioTech Co., Ltd. (Shanghai, China). The
protein concentrations were determined through BCA Protein Assay
kit (Vazyme, Piscataway, NJ, USA). Samples with equal amounts of
protein (25 µg) were fractionated on 10% SDS-PAGE and were then
transferred to polyvinylidene fluoride membranes. Following
blocking with 5% non-fat milk for 1.5 h at 25°C, the membranes were
incubated with primary antibodies for at 4°C overnight. Membranes
were then washed with PBS and incubated with goat anti Rabbit IgG
horseradish peroxidase (cat. no. ab6721; 1:1,000) for another 2 h
at 25°C. Blots were developed with SuperSignal West Femto Maximum
Sensitivity Substrate (Thermo Fisher Scientific, Inc., Waltham, MA,
USA) and the images were obtained by ImageQuant LAS 4000 (GE
Healthcare Life Sciences, Little Chalfont, UK). All assays were
carried out in triplicate.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Extraction of RNA from cell lysates was performed
using an RNeasy kit (Qiagen GmbH, Hilden, Germany). RNA was then
subjected to cDNA synthesis using PrimeScript RT reagent kit
(Takara Bio, Inc., Otsu, Japan). Synthesized cDNA was analyzed by
RT-qPCR using SYBR Premix Ex Taq (Takara Bio, Inc.) according to
manufacturer's protocol, the PCR conditions consisted of 95°C for
30 sec, followed by 40 cycles of amplification (95°C for 3 sec and
60°C for 30 sec). Bio-Rad CFX96 Touch™ Real-Time PCR Detection
system (Bio-Rad Laboratories, Inc., Hercules, CA, USA) was
performed. The fold-change was determined as 2−∆∆Cq
using β-actin as an internal control (21). The primer sequences are as follows:
NF-κB (p65), forward 5′-ATCAATGGCTACACAGGA-3′, reverse
5′-CCCGTGAAATACACCTCA-3′; and β-actin forward
5′-CATGTACGTTGCTATCCAGGC-3′ and reverse
5′-CTCCTTAATGTCACGCACGAT-3′. All assays were carried out in
triplicate.
Statistical analysis
All data were analyzed using Prism 5.0 software
(GraphPad Software Inc., La Jolla, CA, USA) and expressed as the
means ± standard error of the mean. Significant differences between
groups were statistically analyzed using one-way analysis of
variance followed by the Newman-Keuls method. P<0.05 was
considered to indicate a statistically significant difference. All
assays were carried out in triplicate in the present study.
Results
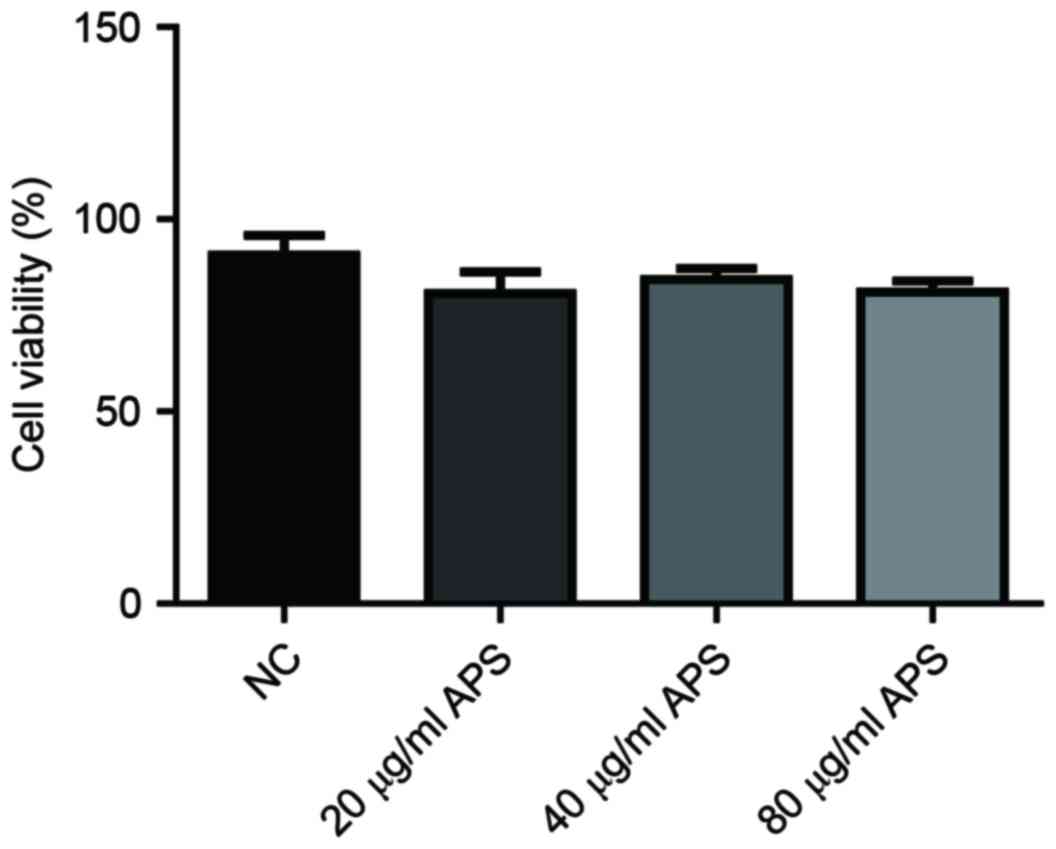
Treatment with APS has no marked
effects on the viability of HaCaT keratinocytes
The viability of HaCaT cells was investigated to
determine the effects of APS on HaCaT keratinocytes. The results
demonstrated that compared with in the negative control (NC) group,
cell viability was not altered following treatment with APS (20, 40
and 80 µg/ml) (Fig. 1).
APS inhibits TNF-α induced
proliferation of HaCaT keratinocytes
Psoriasis is characterized by excessive
proliferation of keratinocytes (14,15);
therefore, the majority of treatment strategies to alleviate
psoriasis are via suppression of the proliferation of
keratinocytes. To investigate the effects of APS on psoriasis,
HaCaT cells were treated with various concentrations of APS (20, 40
and 80 µg/ml) for 24 h. Furthermore, TNF-α is involved in the
overproliferation of keratinocytes (22). The results of the present study
demonstrated that HaCaT cells treated with 10 ng/ml TNF-α exhibited
a significantly increased rate of cell proliferation compared with
in the NC group (Fig. 2). In
addition, the results revealed that TNF-α-stimulated cells treated
with APS exhibited significantly decreased rates of cell
proliferation in a dose-dependent manner compared with cells
treated with TNF-α alone (Fig. 2).
These results suggested that APS inhibited the inflammatory
factor-induced proliferation of HaCaT keratinocytes.
APS decreases TNF-α-induced production
of inflammatory factors
Inhibition of TNF-α regulates numerous inflammatory
factors; therefore, TNF-α inhibitors have been approved for the
treatment of patients with psoriasis (23). Following treatment with 10 ng/ml
TNF-α, or a combination of 10 ng/ml TNF-α and increasing
concentrations of APS (20, 40 and 80 µg/ml), for 24 h, IL-8 and
IL-12 protein expression levels were detected in HaCaT cell
lysates. The results of western blotting revealed that TNF-α
treatment significantly enhanced the protein expression levels of
IL-8 and IL-12 compared with in the NC group (Fig. 3A-C). Furthermore, cells
additionally treated with APS exhibited significantly decreased
levels of IL-8 and IL-12 in a dose-dependent manner compared with
in cells treated with TNF-α alone (Fig. 3A-C).
APS suppresses TNF-α-induced secretion
of inflammatory factors
Following treatment with TNF-α, cells exhibited
enhanced levels of secreted IL-8 and IL-12 into the culture medium
compared with in the NC group (Fig. 4A
and B). In addition, in TNF-α-stimulated cells treated with
increasing concentrations of APS, the secretion of IL-8 and IL-12
into the culture medium was significantly decreased in a
dose-dependent manner compared with in cells treated with TNF-α
alone (Fig. 4A and B).
APS suppresses TNF-α-induced
enhancement of p65 mRNA expression
NF-κB functions as a linker for dysregulated
crosstalk between keratinocytes and immune cells in the
pathogenesis of psoriasis (24).
In patients with psoriasis, NF-κB signaling is normally activated
by TNF-α; therefore, TNF-α inhibitory drugs such as etanercept may
decrease p-p65 levels and subsequently attenuate symptoms of the
disease (25). Following treatment
with TNF-α, HaCaT cells exhibited increased expression levels of
p65 mRNA compared with in the NC group; however; administration of
APS attenuated this effect in a dose-dependent manner (Fig. 5).
APS downregulates TNF-α-induced NF-κB
activity
Notably, following treatment with TNF-α, the protein
expression levels of p-p65 were significantly increased in HaCaT
cells compared with in the NC group; however, this effect was
significantly attenuated following treatment with APS in a
dose-dependent manner (Fig. 6A and
B). In addition, HaCaT cells treated with TNF-α exhibited
significantly decreased levels of IκBα, an inhibitory protein of
NF-κB; however, this effect was significantly attenuated following
treatment with APS in a dose-dependent manner (Fig. 6A and C).
Discussion
Pharmacological treatments available at present for
patients with psoriasis are not satisfactory due to adverse side
effects and unfavorable outcomes. Phytomedicines, including Aloe
vera, indigo naturalis and Mahonia aquifolium, are
therefore considered to represent alternative therapeutic
approaches for the treatment of patients with psoriasis (26).
Aloe vera is a traditional medicinal agent
used for the treatment of dermatological diseases. Numerous studies
have investigated the application of Aloe vera for the
treatment of psoriasis (6,14,15).
Choonhakarn et al (6)
revealed that Aloe vera markedly reduces the clinical
symptoms exhibited by patients with psoriasis. In the present
study, it was revealed that APS, the main bioactive component of
Aloe vera, did not have a significant effect on the
viability of HaCaT keratinocytes. However, treatment with APS was
revealed to suppress TNF-α-induced increases in HaCaT cell
proliferation in a dose-dependent manner. These results suggested
that treatment with APS inhibited excessive proliferation of
keratinocytes without affecting normal skin cells.
The majority of studies that have investigated the
clinical effects of Aloe vera have not determined the
underlying mechanism. Dysregulation of TNF-α has previously been
revealed to be involved in the development of psoriasis and is
considered to represent a major therapeutic target (27). In the present study, it was
demonstrated that APS attenuated the effects induced following
treatment with TNF-α. TNF-α has been revealed to induce the
production of numerous cytokines, such as IL-8 and IL12, and is
associated with a cytokine network involved in the pathogenesis of
psoriasis (28). In addition,
TNF-α, IL-8 and IL-12 represent commonly expressed cytokines in
patients with psoriasis (29,30).
The results of ELISA and western blotting demonstrated that HaCaT
cells treated with APS exhibited decreased IL-8 and IL-12
expression in a dose-dependent manner, which had otherwise been
induced by treatment with TNF-α. This result is in agreement with
the results of the cell viability assay, which revealed that APS
was associated with proinflammatory cytokines and may suppress the
proliferation of TNF-α-treated keratinocytes in a dose-dependent
manner via the suppression of TNF-α signaling.
It has been well established that NF-κB is involved
in the regulation of proinflammatory gene expression via induction
of the transcription of cytokines, including TNF-α (31). The TNF-α-activated NF-κB signaling
pathway is involved in inflammatory processes and forms a positive
feedback loop (32). In the
present study, the protein expression levels of p-p65 and the mRNA
expression levels of p65 were detected. Notably, the expression
levels of p-p65 in HaCaT cells were significantly increased
following treatment with TNF-α; however, treatment with APS was
revealed to reverse this effect in a dose-dependent manner.
Notably, p65 mRNA expression was also significantly decreased in
response to APS in a dose-dependent manner compared with in cells
treated with TNF-α alone. NF-κB is sequestered in the cytoplasm by
its inhibitors, such as IκBα and IκBβ (33). Knockout of IκBα in lymphocytes and
keratinocytes may result in the features of psoriasis (34). In HaCaT cells treated with TNF-α,
the protein expression levels of IκBα were decreased; however,
treatment with APS was revealed to attenuate this effect in a
dose-dependent manner. Therefore, these results suggested that the
regulation of NF-κB activity by APS may be via enhancement of IκBα
protein expression. The results of the present study revealed that
APS inhibited the NF-κB signaling pathway via regulation of p65
activity and p65 mRNA expression. These results provided additional
information regarding the mechanism underlying the effects of APS
on psoriasis treatment.
In conclusion, the results of the present study
demonstrated that APS may represent a potential therapeutic agent
for the treatment of patients with psoriasis. Administration of APS
suppressed the proliferation of keratinocytes induced by treatment
with TNF-α. Furthermore, APS treatment NF-κB signaling pathways
induced with TNF-α in proliferating keratinocytes. Therefore, the
efficacy of APS administration for the treatment of patients with
psoriasis appeared to rely on its anti-inflammatory activity. Based
on these results, APS may be a promising drug candidate of treat
psoriasis for its potential clinical applications.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural
Science Youth Foundation of Jiangsu (grant no. BK20130277), and the
Jiangsu Provincial Key Laboratory of Molecular Biology for Skin
Disease and STIs.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
HL and KC designed the experiments. LP, LX, XS and
JJ performed the experiments. HL and KC wrote the main manuscript
text. All authors reviewed the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Menter A, Gottlieb A, Feldman SR, Van
Voorhees AS, Leonardi CL, Gordon KB, Lebwohl M, Koo JY, Elmets CA,
Korman NJ, et al: Guidelines of care for the management of
psoriasis and psoriatic arthritis: Section 1. Overview of psoriasis
and guidelines of care for the treatment of psoriasis with
biologics. J Am Acad Dermatol. 58:826–850. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lin AM, Rubin CJ, Khandpur R, Wang JY,
Riblett M, Yalavarthi S, Villanueva EC, Shah P, Kaplan MJ and Bruce
AT: Mast cells and neutrophils release IL-17 through extracellular
trap formation in psoriasis. J Immunol. 187:490–500. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Carey W, Glazer S, Gottlieb AB, Lebwohl M,
Leonardi C, Menter A, Papp K, Rundle AC and Toth D: Relapse,
rebound, and psoriasis adverse events: An advisory group report. J
Am Acad Dermatol. 54 4 Suppl 1:S171–S181. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Menter A and Griffiths CE: Current and
future management of psoriasis. Lancet. 370:272–284. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sala M, Elaissari A and Fessi H: Advances
in psoriasis physiopathology and treatments: Up to date of
mechanistic insights and perspectives of novel therapies based on
innovative skin drug delivery systems (ISDDS). J Control Release.
239:182–202. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Choonhakarn C, Busaracome P,
Sripanidkulchai B and Sarakarn P: A prospective, randomized
clinical trial comparing topical aloe vera with 0.1% triamcinolone
acetonide in mild to moderate plaque psoriasis. J Eur Acad Dermatol
Venereol. 24:168–172. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Das S, Mishra B, Gill K, Ashraf MS, Singh
AK, Sinha M, Sharma S, Xess I, Dalal K, Singh TP and Dey S:
Isolation and characterization of novel protein with anti-fungal
and anti-inflammatory properties from Aloe vera leaf gel. Int J
Biol Macromol. 48:38–43. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Davis RH, DiDonato JJ, Johnson RW and
Stewart CB: Aloe vera, hydrocortisone, and sterol influence on
wound tensile strength and anti-inflammation. J Am Podiatr Med
Assoc. 84:614–621. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Baradaran A, Nasri H, Nematbakhsh M and
Rafieian-Kopaei M: Antioxidant activity and preventive effect of
aqueous leaf extract of Aloe Vera on gentamicin-induced
nephrotoxicity in male Wistar rats. Clin Ter. 165:7–11.
2014.PubMed/NCBI
|
|
10
|
Seyger MM, van de Kerkhof PC, van
Vlijmen-Willems IM, de Bakker ES, Zwiers F and de Jong EM: The
efficacy of a new topical treatment for psoriasis: Mirak. J Eur
Acad Dermatol Venereol. 11:13–18. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Paulsen E, Korsholm L and Brandrup F: A
double-blind, placebo-controlled study of a commercial Aloe vera
gel in the treatment of slight to moderate psoriasis vulgaris. J
Eur Acad Dermatol Venereol. 19:326–331. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yarilina A, Park-Min KH, Antoniv T, Hu X
and Ivashkiv LB: TNF activates an IRF1-dependent autocrine loop
leading to sustained expression of chemokines and STAT1-dependent
type I interferon-response genes. Nat Immunol. 9:378–387. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gibellini L, De Biasi S, Bianchini E,
Bartolomeo R, Fabiano A, Manfredini M, Ferrari F, Albertini G,
Trenti T, Nasi M, et al: Anti-TNF-α drugs differently affect the
TNFα-sTNFR system and monocyte subsets in patients with psoriasis.
PLoS One. 11:e01677572016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Miroddi M, Navarra M, Calapai F, Mancari
F, Giofrè SV, Gangemi S and Calapai G: Review of clinical
pharmacology of Aloe vera L. in the treatment of psoriasis.
Phytother Res. 29:648–655. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dhanabal SP, Dwarampudi Priyanka L,
Muruganantham N and Vadivelan R: Evaluation of the antipsoriatic
activity of Aloe vera leaf extract using mouse tail model
psoriasis. Phytother Res. 26:617–619. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Baldwin AS Jr: The NF-kappa B and I kappa
B proteins: New discoveries and insights. Annu Rev Immunol.
14:649–683. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tak PP and Firestein GS: NF-kappaB: A key
role in inflammatory diseases. J Clin Invest. 107:7–11. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hayden MS and Ghosh S: Signaling to
NF-kappaB. Genes Dev. 18:2195–2224. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lizzul PF, Aphale A, Malaviya R, Sun Y,
Masud S, Dombrovskiy V and Gottlieb AB: Differential expression of
phosphorylated NF-kappaB/RelA in normal and psoriatic epidermis and
downregulation of NF-kappaB in response to treatment with
etanercept. J Invest Dermatol. 124:1275–1283. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vijayalakshmi D, Dhandapani R, Jayaveni S,
Jithendra PS, Rose C and Mandal AB: In vitro anti inflammatory
activity of Aloe vera by down regulation of MMP-9 in peripheral
blood mononuclear cells. J Ethnopharmacol. 141:542–546. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lima JF, Carvalho J, Pinto-Ribeiro I,
Almeida C, Wengel J, Cerquera L, Figueiredo C, Oliveira C and
Azevedo NF: Targeting miR-9 in gastric cancer cells using locked
nucleic acid oligonucleotides. BMC Mol Biol. 19:62018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mizuno K, Morizane S, Takiguchi T and
Iwatsuki K: Dexamethasone but not tacrolimus suppresses
THF-α-induced thymic stromal lymphopoitin expression in lesional
keratinocytes of atopic dermatitis model. J Dermatol Sci. 80:45–53.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kyle S, Chandler D, Griffiths CE,
Helliwell P, Lewis J, McInnes I, Oliver S, Symmons D and McHugh N:
British Society for Rheumatology Standards Guidelines Audit Working
Group (SGAWG): Guideline for anti-TNF-alpha therapy in psoriatic
arthritis. Rheumatology (Oxford). 44:390–397. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lowes MA, Bowcock AM and Krueger JG:
Pathogenesis and therapy of psoriasis. Nature. 445:866–873. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yan S, Xu Z, Lou F, Zhang L, Ke F, Bai J,
Liu Z, Liu J, Wang H, Zhu H, et al: NF-κB-induced microRNA-31
promotes epidermal hyperplasia by repressing protein phosphatase 6
in psoriasis. Nat Commun. 6:76522015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Deng S, May BH, Zhang AL, Lu C and Xue CC:
Plant extracts for the topical management of psoriasis: A
systematic review and meta-analysis. Br J Dermatol. 169:769–782.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sato K, Takaishi M, Tokuoka S and Sano S:
Involvement of TNF-α converting enzyme in the development of
psoriasis-like in a mose model. PLoS One. 9:e1124082014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gudjonsson JE, Johnston A, Sigmundsdottir
H and Valdimarsson H: Immunopathogenic mechanisms in psoriasis.
Clin Exp Immunol. 135:1–8. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sidhom E, Pilmane M and Kisis J: Local
antimicrobial, protease and cytokine defense systems in psoriatic
skin. Indian J Dermatol Venereol Leprol. 82:284–291. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kauffman CL, Aria N, Toichi E, McCormick
TS, Cooper KD, Gottlieb AB, Everitt DE, Frederick B, Zhu Y, Graham
MA, et al: A phase I study evaluating the safety, pharmacokinetics,
and clinical response of a human IL-12 p40 antibody in subjects
with plaque psoriasis. J Invest Dermatol. 123:1037–1044. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chandel NS, Trzyna WC, McClintock DS and
Schumacker PT: Role of oxidants in NF-kappa B activation and
TNF-alpha gene transcription induced by hypoxia and endotoxin. J
Immunol. 165:1013–1021. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bouwmeester T, Bauch A, Ruffner H, Angrand
PO, Bergamini G, Croughton K, Cruciat C, Eberhard D, Gagneur J,
Ghidelli S, et al: A physical and functional map of the human
TNF-alpha/NF-kappa B signal transduction pathway. Nat Cell Biol.
6:97–105. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Baeuerle PA and Henkel T: Function and
activation of NF-kappa B in the immune system. Annu Rev Immunol.
12:141–179. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Goldminz AM, Au SC, Kim N, Gottlieb AB and
Lizzul PF: NF-kappaB: An essential transcription factor in
psoriasis. J Dermatol Sci. 69:89–94. 2013. View Article : Google Scholar : PubMed/NCBI
|