Introduction
The severity and frequency of joint loading are
principal factors for the development of joint destruction, which
is characterized by the articular cartilage damage. Actually,
excessive motion and load on the joint cause the articular
cartilage damage (1–4). Thus, sports with repetitive impact
and torsional loading on the joints enhance the risk of articular
cartilage degeneration, and result in the clinical symptoms of
osteoarthritis (4). The
pathological process of osteoarthritis leads to the degradation and
functional loss of joint cartilage. Notably, in the experimental
osteoarthritis models the early changes of the cartilage metabolism
can be detected before the appearance of morphological changes of
cartilage (2).
Thus, a number of biomarkers with reliability and
sensitivity have been developed as indicators of cartilage and bone
metabolism in subjects with joint and bone disorders (5). In this context, sports-related
mechanical loading on the joints is shown to modulate the
metabolism (turnover) of matrices of bone as well as cartilage in
humans, and these changes can be detected by using biomarkers
(1–4). Namely, type II collagen is a major
component of cartilage matrix (6),
and fragments of type II collagen have been used as biomarkers for
cartilage metabolism (breakdown and synthesis of cartilage matrix).
A C-terminal telopeptide (CTX-II) is cleaved during breakdown of
type II collagen (7), whereas a
neoepitope (C2C) is cleaved from the C terminus of the 3/4 piece of
degraded type II collagen (8).
Thus, both CTX-II and C2C are utilized as markers for type II
collagen degradation. In contrast, a C-terminal type II procollagen
peptide (CPII) is localized in newly formed type II collagen and
cleaved during processing of synthesized type II procollagen; thus,
CPII can be utilized as a marker for type II collagen synthesis
(9). Additionally, a cross-linked
N-terminal telopeptides of type I collagen (NTx) can be utilized as
a marker for type I collagen degradation in bone (bone resorption)
(5).
Nutritional supplements containing such as
glucosamine, chondroitin and collagen are often administered for
‘joint health’ of sports-related cartilage injuries
(osteoarthritis) in athletes (10–13).
Among these substances, glucosamine, an amino monosaccharide, has
been widely used to prevent or treat osteoarthritis in humans
(14–17). Glucosamine is a component of
glycosaminoglycans in the connective and cartilage tissues, and
contributes to maintaining the elasticity, flexibility and strength
of these tissues. Several clinical studies revealed the significant
symptom-relieving and structure-modifying effects of glucosamine in
osteoarthritis (14–17). Interestingly, glucosamine
suppresses the degradation and augments the synthesis of
glycosaminoglycans (proteoglycans) in vitro (18,19).
In addition, glucosamine suppresses the expression of
collagen-degrading enzymes (matrix metalloproteinases) but enhances
the expression of type II collagen in chondrocytes in vitro
(20,21). Based on these findings, glucosamine
is expected to exert a chondroprotective action by maintaining
proteoglycans as well as type II collagen in the articular
cartilage.
Among sports with various different frequency and
intensity of joint loading, soccer is categorized as a
representative with high levels of repetitive impact and torsional
loading on the joint (4). Thus, to
investigate the effect of glucosamine on articular cartilage in
endurance athletes, we previously measured the levels of biomarkers
for type II collagen degradation (CTX-II) and synthesis (CPII) in
an open-label (unblinded) study using soccer players (22). The results indicated that
glucosamine administration (1.5 g and 3 g/day for 3 months)
significantly decreased the CTX-II level without affecting the CPII
level, suggesting that glucosamine exerts a chondroprotective
action in endurance athletes (soccer players) by preventing type II
collagen degradation but maintaining type II collagen synthesis.
However, the effect of glucosamine has not yet been confirmed in a
randomized controlled trial. Thus, in the present study, we
performed a randomized double-blind placebo-controlled trial, and
determined the levels of type II collagen degradation (CTX-II and
C2C) and synthesis (CPII) markers before and after the
administration of a placebo or glucosamine (2 g/day)-containing
supplement for 4 months (16 weeks) to soccer players.
Materials and methods
Study design
A prospective randomized double-blind
placebo-controlled, parallel-group comparative study was designed
to assess the actions of a glucosamine-containing supplement and a
placebo diet on the cartilage metabolism (type II collagen
degradation and synthesis) in healthy soccer players without
symptoms of joint disorders. Additionally, the safety of the test
supplement was evaluated. The study was registered at the UMIN
Clinical Trials Registry (trial no. UMIN000023852) on August 31,
2016, and performed from August 2016 to February 2017 at Juntendo
University, Japan. The study protocol with the title of ‘Evaluation
of a jelly-type functional food on the bone and cartilage
metabolism in athletes’ (protocol number: 20160703) was approved on
August 10, 2016 by the Ethics Committee of The Japan Society of
Vascular Medicine and Rheology (Tokyo, Japan), and the study was
conducted in accordance with the principles of the amended
Declaration of Helsinki and ‘Ethical Guidelines for Epidemiological
Research’ (established by the Japanese Government in 2008). Written
informed consent was obtained from all participants prior to their
enrollment in the study. The whole design of the study consisted of
a 3-week run-in (screening) period, a 16-week (4-month)
intervention period, and a 8-week follow-up period without
intervention. Subjects were screened at a baseline visit by a
symptom questionnaire and routine laboratory tests. Additionally,
laboratory tests were performed at weeks 0, 4, 8, 12 and 16 during
the intervention, and 4 and 8 weeks after the intervention for the
subjects.
Subjects
Selection criteria contained the following: i)
Players belonging to the soccer team of Juntendo University School
of Health and Sports Science; ii) nutritionally healthy adults with
18 years of age or older and iii) individuals who can participate
in the study, comprising of 8 times of urine collection, 2 times of
blood sampling and intake of test supplement for 4 months, with the
test period of about 7 months.
Exclusion criteria contained the following: i)
Current diagnosis and medication of bone or cartilage disorders
including arthritis, fracture and distortion; ii) history of
surgical treatment of gastric disorders, or current diagnosis of
gastric disorders; iii) routine use of dietary supplements
containing glucosamine or any other constituents of the test
supplement, which likely influence cartilage and bone metabolism;
iv) medication likely to influence cartilage and bone metabolism;
v) hypersensitivity or allergy to the test components; vi)
diagnosis or current medication of disorders including
malignancies, hypertension (atherosclerosis), cardiac, renal,
thyroid, lung and hepatic disorders; vii) pregnant women, nursing
mothers or women intending to have children during the study
periods; viii) blood donation >400 ml within 4 months prior to
the study; ix) participation in any other clinical studies within
one month prior to enrollment; and x) the presence of any clinical
conditions judged by the medical investigators to preclude the
participation of subjects in the study.
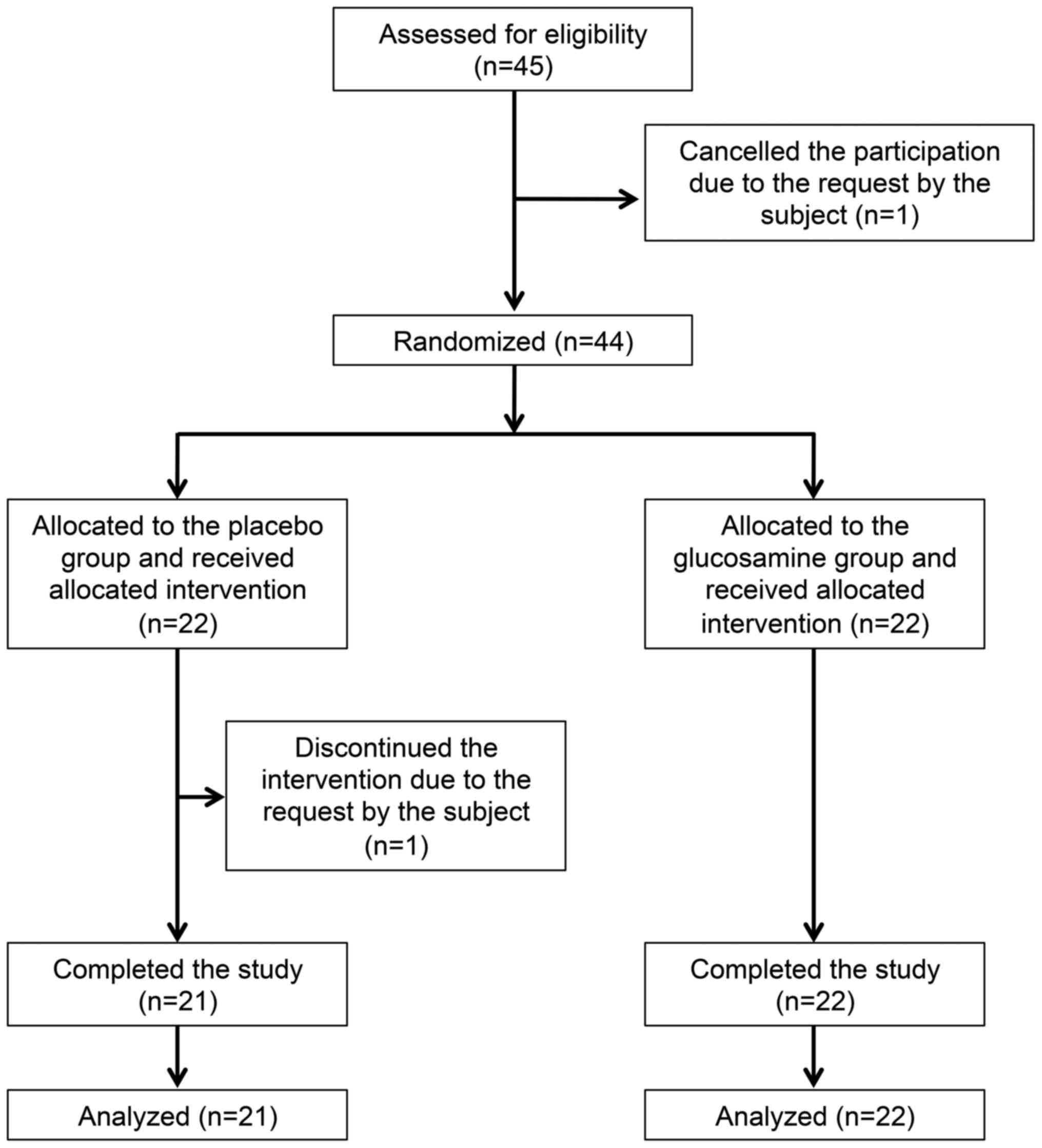
Following the assessment of 45 subjects for
eligibility, one subject declined to participate of his own
volition. Finally, 44 male players [aged 18–22 years; mean age,
20.1±1.1 years (mean ± SD)] were enrolled as eligible subjects. All
subjects were actively training for soccer during the study period:
They performed the training session four times a week (from Tuesday
to Friday) for approximately 2 h/day and played the official match
every Saturday or Sunday. The research co-coordinators randomly
assigned the eligible subjects to receive a placebo (22 subjects in
the placebo group) or glucosamine-containing supplement (22
subjects in the glucosamine group; Fig. 1) using a table of random numbers.
The allocation table was sealed, and all research staffs (which
included medical investigators, clinical service staffs, analyzers
of urine and blood samples, and other research staffs) and
participants were blinded to the allocation during the test period.
Following completion of the study, the allocation table was made
available for analysis of the data. During the intervention, one
subject discontinued the study of his own volition in a placebo
group. Thus, 43 subjects completed the study (mean age 20.1±1.1
years; 21 subjects in the placebo group and 22 subjects in the
glucosamine group), and were finally judged to be eligible for
assessment of the efficacy of test supplement (Fig. 1 and Table I). Moreover, to improve the clarity
regarding the effect of test supplement, the subjects with less
variation of exercise loading, based on the changes of type II and
type I collagen degradation markers (CTX-II and NTx, respectively)
(5), were analyzed. Thus, 2
subjects (one subject in a placebo group and one subject in a
glucosamine group) with augmented exercise loading during the test
period, as evidenced by the increased levels of both CTX-II and NTx
(>1.8-fold), were excluded, and 41 subjects with less variation
of exercise loading (mean age, 20.2±1.1 years; 20 subjects in the
placebo group, 21 subjects in the glucosamine group) were evaluated
(Table II).
 | Table I.Baseline characteristics of the
subjects in the placebo and glucosamine groups. |
Table I.
Baseline characteristics of the
subjects in the placebo and glucosamine groups.
| Characteristics | Placebo (n=21) | Glucosamine
(n=22) | P-value |
|---|
| Ages (years) | 20.1±1.2 | 20.2±1.0 | 0.48 |
| Height (cm) | 173.0±5.5 | 174.9±4.9 | 0.70 |
| Weight (kg) | 67.1±6.0 | 68.9±5.1 | 0.56 |
| BMI
(kg/cm2) | 22.4±1.1 | 22.5±1.1 | 0.92 |
| NTx (nmol BCE/mmol
Cr) | 99.0±27.7 | 77.0±26.6 | 0.85 |
| CTX-II (ng/mmol
Cr) | 1460.9±1036.5 | 1248.6±657.9 | 0.04 |
| CPII (ng/mmol
Cr) | 3294.2±1866.7 | 2837.2±1990.3 | 0.78 |
| C2C (ng/ml) | 22.3±7.0 | 20.9±9.5 | 0.18 |
 | Table II.Baseline characteristics of the
subjects in the placebo and glucosamine groups, with less variation
in exercise loading during the test period. |
Table II.
Baseline characteristics of the
subjects in the placebo and glucosamine groups, with less variation
in exercise loading during the test period.
|
Characteristics | Placebo (n=20) | Glucosamine
(n=21) | P-value |
|---|
| Ages (years) | 20.2±1.2 | 20.2±1.0 | 0.45 |
| Height (cm) | 173.0±5.5 | 175.1±5.1 | 0.70 |
| Weight (kg) | 67.1±6.0 | 69.0±5.3 | 0.54 |
| BMI
(kg/cm2) | 22.4±1.1 | 22.5±1.2 | 0.85 |
| NTx (nmol BCE/mmol
Cr) | 101.9±25.0 | 77.9±26.9 | 0.76 |
| CTX-II (ng/mmol
Cr) | 1510.1±1038.0 | 1250.7±674.1 | 0.06 |
| CPII (ng/mmol
Cr) | 3356.6±1892.6 | 2826.8±2038.8 | 0.75 |
| C2C (ng/ml) | 22.3±7.2 | 20.7±9.7 | 0.20 |
Intervention and subject
assignment
The participants were instructed not to alter their
lifestyles, including habits of eating, sleeping and drinking
alcohol, and especially exercise training. They were also
instructed not to start taking any dietary supplements during the
test period, or not to stop taking dietary supplements if they
already started taking dietary supplements. The test supplement was
manufactured in a jelly type (180 g in an aluminum pouch with a
spout) by Toppan Packaging Service Co., Ltd., (Tokyo, Japan) and
contained 2,000 mg glucosamine hydrochloride, 1,800 mg arginine,
600 mg lysine hydrochloride, 400 mg alanine, 400 mg glycine, 10 mg
hesperidin, 0.2 mg β-cryptoxanthin and a vehicle consisting of 14 g
maltodextrin, 4 g fructose, 4 g glucose and 5 g sucrose, whereas
the placebo diet (180 g) contained only a vehicle consisting of 14
g maltodextrin, 5 g fructose, 4 g glucose and 11 g sucrose.
Subjects were randomly assigned to receive a 2,000
mg glucosamine-containing supplement (glucosamine group) or a
placebo containing only a vehicle (placebo group). All subjects
were instructed to take the test supplement or placebo once a day
within 30 min after exercise for 16 weeks. The daily dose of
glucosamine (2,000 mg/day) was determined based on the results of
the previous study (22).
Adherence to the intervention was evaluated on the basis of
consumption record in the study diary.
Second void of morning urine was collected after an
overnight fast at a baseline visit, weeks 0, 4, 8, 12, and 16
during the intervention, and 4 and 8 weeks after the intervention.
Serum was collected from the subjects in a fasting state at weeks 0
and 16 during the intervention. Serum and urine samples were
immediately used for routine laboratory tests; alternatively, serum
and urine samples were aliquoted and stored at −80°C until the
assays of CTX-II, C2C and CPII.
Evaluation of efficacy and safety
To evaluate the effect of test supplement on the
cartilage metabolism, urine and serum samples collected at weeks 0
and 16 during the intervention were used for the assays of type II
collagen degradation (urine CTX-II and serum C2C) and synthesis
(urine CPII) markers. Urine CTX-II was measured using a Urine
Cartilaps (CTX-II) EIA kit (Immunodiagnostic Systems Limited,
Boldon, UK), which detects a C-terminal telopeptide (CTX-II) of
type II collagen (7). Serum C2C
was measured using a Collagen Type II Cleavege ELISA kit (IBEX
Pharmaceuticals Inc., Montreal, Canada), which detects a neoepitope
created by the collagenase cleavage of type II collagen (8). Urine CPII was measured using a
Procollagen type II C-propeptide ELISA kit (IBEX Pharmaceuticals),
which detects a C-terminal propeptide of newly formed type II
procollagen (C-propeptide, also referred as CPII) (9).
CTX-II, C2C and CPII were measured in duplicates on
the same microtiter plate. Concentrations of urine CTX-II and CPII
were corrected by urinary creatinine (Cr), and expressed as ng/mmol
Cr.
Cross-linked N-terminal telopeptides of type I
collagen (NTx) is excreted in urine during bone degradation
(degradation of type I collagen), and can be used as markers of
bone turnover (resorption) (5).
Urinary NTx and creatinine were measured by LSI Medience
Corporation (Tokyo, Japan), based on the ELISA (NTx) and enzyme
assay (creatinine), respectively, and the concentrations of NTx was
expressed as nmol BCE (bone collagen equivalent)/mmol Cr, after
correction with urinary creatinine.
Safety and tolerability were assessed throughout the
study, based on the incidence and severity of intervention-related
adverse events (side effects), as well as abnormal changes in
laboratory tests, including hematology, biochemical profile and
urinalysis. Change in the physical conditions and use of
pharmaceutical products were also recorded in a diary by the
participants.
Statistical analysis
Values are expressed as the means ± standard
deviation (SD). In the baseline characteristics of subjects,
parameters were analyzed by Student's t-test (Prism 5; GraphPad
Software, San Diego, CA, USA) between the placebo and glucosamine
groups. The levels of CTX-II, C2C, CPII and NTx were compared
between weeks 0 and 16 in the placebo or glucosamine group, and
between the placebo and glucosamine groups at weeks 0 and 16 during
the intervention by two-way analysis of variance with Tukey's post
hoc test. Additionally, safety data were compared between the
placebo and glucosamine groups by Student's t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Characterization of study groups
Table I shows the
baseline characteristics of 43 subjects (21 subjects in the placebo
group; 22 subjects in the glucosamine group), who completed the
study and fulfilled the eligibility criteria. The baseline
characteristics included age, physiological characteristics (body
height, body weight and body mass index) and levels of biomarkers
for type II collagen metabolism (CTX-II, C2C and CPII). There were
no significant differences in these parameters between the placebo
and glucosamine groups at the baseline, except for CTX-II level,
which was significantly lower in the glucosamine group compared
with the placebo group (P=0.04). Adherence to the allotted dietary
supplement (a placebo or a glucosamine-containing supplement) was
100% among the 43 subjects who completed the study.
Assessment of cartilage metabolism
using type II collagen degradation and synthesis markers
Next, we examined the effect of test supplement on
cartilage metabolism using type II collagen degradation (CTX-II and
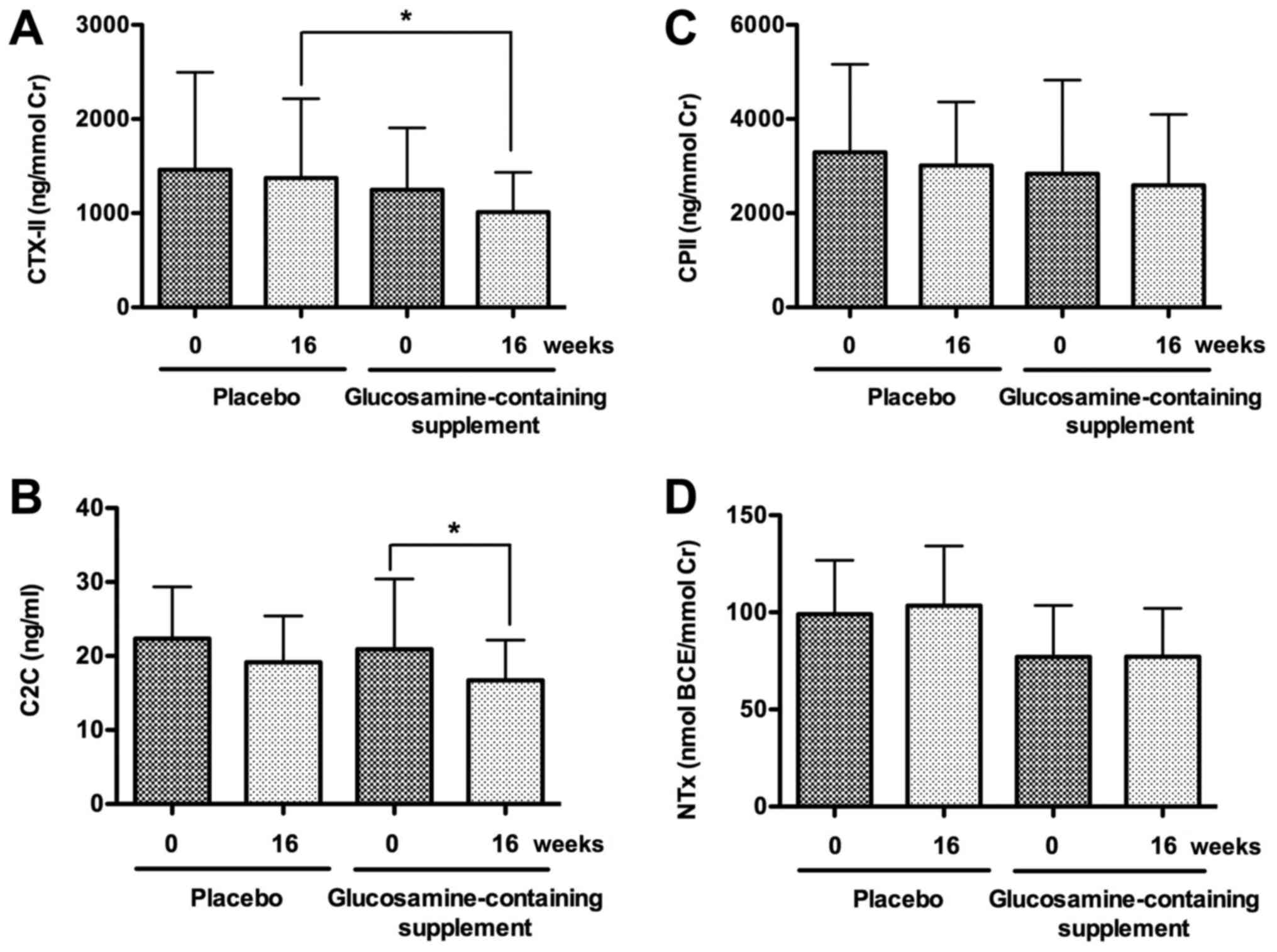
C2C) and synthesis (CPII) markers. Interestingly, urine CTX-II
level substantially decreased in the glucosamine group but not in
the placebo group after the intervention for 16 weeks (P=0.05;
Fig. 2A). Moreover, CTX-II level
was significantly lower in the glucosamine group than that in the
placebo group at week 16 during the intervention (P<0.05;
Fig. 2A). Similarly, serum C2C
level significantly decreased in the glucosamine group but not in
the placebo group after the intervention for 16 weeks (P<0.05),
although C2C levels were not significantly different between the
placebo and glucosamine groups at week 0 or 16 during the
intervention (Fig. 2B). In
contrast, urine CPII levels were not significantly different
between weeks 0 and 16 during the intervention in the placebo or
glucosamine group, and between the placebo and glucosamine groups
at week 0 or 16 during the intervention (Fig. 2C).
Furthermore, we examined the effect of test
supplement on bone metabolism using type I collagen degradation
marker (NTx). Urine NTx levels were not significantly different
between weeks 0 and 16 during the intervention in the placebo or
glucosamine group, and between the placebo and glucosamine groups
at week 0 or 16 during the intervention (Fig. 2D).
Assessment of cartilage metabolism in
subjects with less variation of exercise loading
It has been reported that the level of CTX-II (type
II collagen degradation in cartilage) significantly correlates with
that of NTx (type I collagen degradation in bone) in endurance
athletes, and the levels of CTX-II and NTx reflect the exercise
loading (3,13,23).
Thus, in order to make the effect of the test supplement more
clear, 2 subjects (one subject in the placebo group and one subject
in the glucosamine group) were excluded, because both CTX-II and
NTx levels (exercise loading) were markedly increased
(>1.8-fold) in these subjects during the intervention, and these
changes may affect the efficacy of test supplement. Thus, the rest
of 41 subjects with less variation of exercise loading, based on
the changes of type II (CTX-II) and type I collagen (NTx)
degradation markers, were evaluated (mean age, 20.2±1.1 years; 20
subjects in the placebo group, 21 subjects in the glucosamine
group; Table II).
Table II presents
the baseline characteristics of these subjects, including age,
physiological characteristics (body height, body weight and body
mass index) and levels of biomarkers for type II collagen
metabolism (CTX-II, C2C and CPII). There were no significant
differences in these parameters between the placebo and glucosamine
groups at the baseline. Thus, the effect of test supplement on
cartilage metabolism was evaluated using these subjects at weeks 0
and 16 during the intervention.
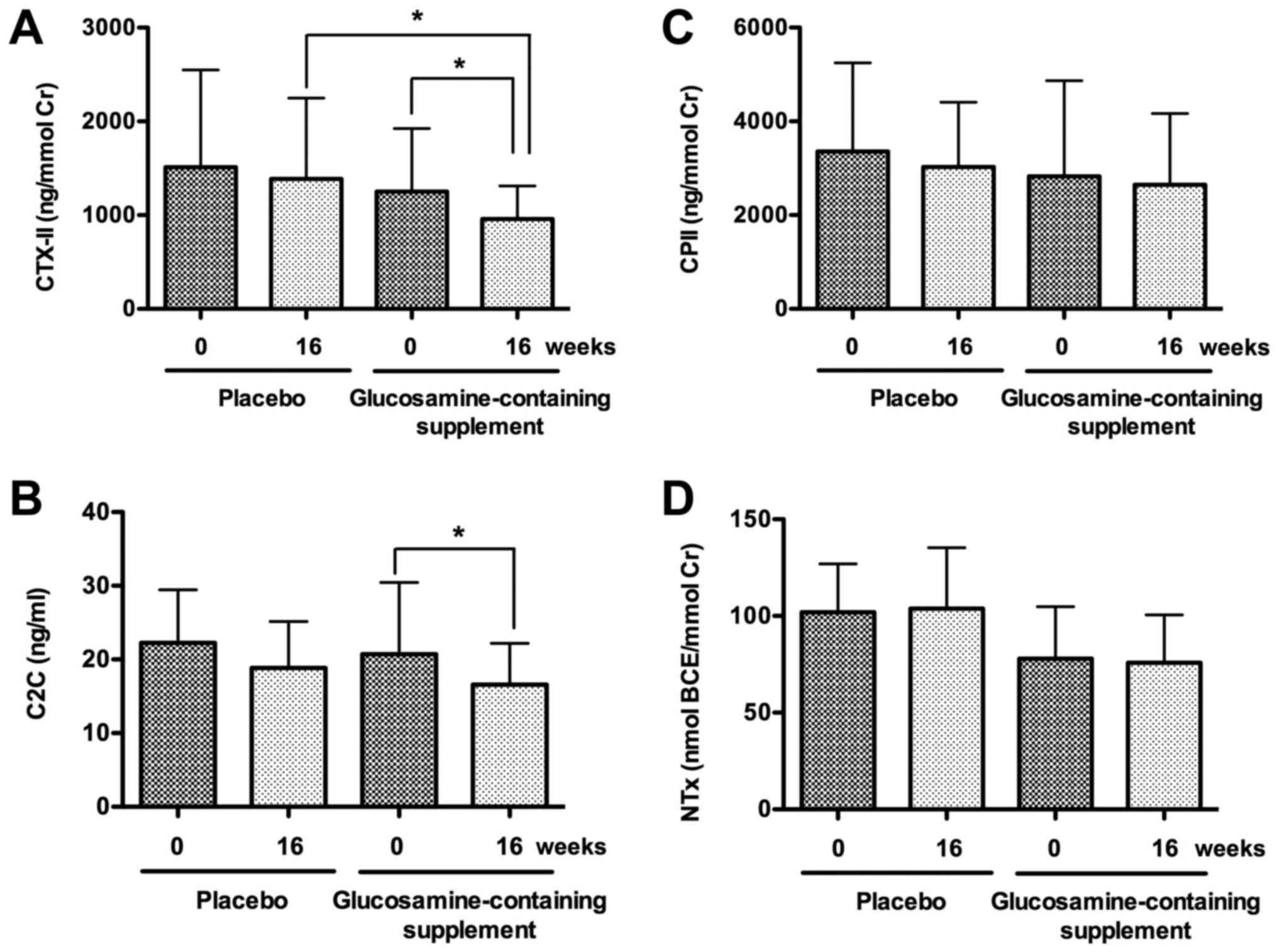
The results indicated that urine CTX-II level
significantly decreased in the glucosamine group but not in the
placebo group after the intervention for 16 weeks (P<0.01);
moreover, CTX-II level was significantly lower in the glucosamine
group than that in the placebo group at week 16 during the
intervention (P<0.05; Fig. 3A).
Similarly, serum C2C level significantly decreased in the
glucosamine group but not in the placebo group after the
intervention for 16 weeks (P<0.05), although C2C levels were not
significantly different between the placebo and glucosamine groups
at week 0 or 16 during the intervention (Fig. 3B). In contrast, urine CPII levels
were not significantly different between weeks 0 and 16 during the
intervention in the placebo or glucosamine group, and between the
placebo and glucosamine groups at week 0 or 16 during the
intervention (Fig. 3C). Similarly,
urine NTx levels were not significantly different between weeks 0
and 16 during the intervention in the placebo or glucosamine group,
and between the placebo and glucosamine groups at week 0 or 16
during the intervention (Fig.
3D).
Assessment of safety and
tolerability
Among 44 enrolled subjects, all subjects in the
placebo group (n=22) and the glucosamine group (n=22) experienced
essentially no adverse events during and after the intervention
period. Furthermore, the physical measurement parameters (body
weight and body mass index) and laboratory tests (urinalysis,
hematology and blood chemistry) did not show any significant
changes from the baseline during and after the intervention in the
two groups.
Discussion
Glucosamine is now used as a functional dietary
supplement to relieve the symptoms of osteoarthritis, based on its
significant symptom-modifying action on osteoarthritis revealed by
the clinical trials (14–17). Notably, the in vitro studies
have indicated that glucosamine likely exhibits chondroprotective
action by inhibiting the degradation and stimulating the synthesis
of proteoglycans in the cartialge (18,19).
Biomarkers can be used to evaluate the
pathophysiological conditions of joint disorders (5). Markers are basically derived form the
constituents of cartilage, such as aggrecan, chondroitin sulfate
and collagens (5,6). Among these constituents, type II
collagen is a major constituent of articular cartilage, and the
catabolism and anabolism of articular type II collagen are
essentially involved in joint disorders; thus the components of
type II collagen are recognized as the most important biomarkers
for joint disorders (such as osteoarthritis) (24).
In an open-study, we previously evaluated the effect
of glucosamine administration (1.5 g and 3 g/day for 3 months) on
the cartilage metabolism in soccer players by using biomarkers for
type II collagen-degradation (CTX-II) and -synthesis (CPII)
(22), since soccer players
repetitively expose their joints to impact and torsional loading
and have a potential risk of articular cartilage degeneration
(4). Furthermore, we evaluated the
bone metabolism in soccer players by using a degradation maker
(NTx) of type I collagen, a major component of bone collagen
(5), since subchondral bone
remodeling (accompanied with degradation of type I collagen) is
developed during progression of joint disorders (25,26).
Moreover, we compared the basic levels of type II collagen
degradation (urine CTX-II) and synthesis (urine CPII) markers and
type I collagen degradation (urine NTx) marker between soccer
players and non-athlete controls (22). The results indicated that the basic
levels of CTX-II and NTx but not CPII are significantly higher in
soccer players than in non-athlete controls, suggesting that type
II collagen and type I collagen degradation is enhanced in soccer
players with excessive motion and loading on the joints.
Importantly, glucosamine administration (1.5 g and 3 g/day for 3
months) significantly reduced the level of CTX-II but not CPII and
NTx, suggesting that glucosamine exerts a chondroprotective action
on athletes (soccer players) by mainly suppressing type II collagen
degradation. However, the effect of glucosamine has not yet been
confirmed in a randomized controlled trial. Thus, in the present
study, we performed a randomized double-blind placebo-controlled
trial, and measured the biomarker levels of type II collagen
degradation (CTX-II and C2C) and synthesis (CPII), and type I
collagen degradation (NTx) in soccer players, before and after the
administration of a placebo or glucosamine (2 g/day)-containing
supplement for 16 weeks.
In the initial analysis of all the subject, who
completed the study and fulfilled the eligibility criteria
(Table I), urine CTX-II level
substantially decreased in the glucosamine group but not in the
placebo group after the intervention for 16 weeks (P=0.05; Fig. 2A). Unexpectedly, CTX-II level in
the glucosamine group was also significantly lower than that in the
placebo group at week 16 during the intervention (P<0.05;
Fig. 2A). Likewise, serum C2C
level significantly decreased in the glucosamine group but not in
the placebo group after the intervention for 16 weeks (P<0.05;
Fig. 2B). In contrast, urine CPII
level as well ad urine NTx level was not significantly changed
after the intervention in both the placebo or glucosamine groups
(Fig. 2C and D).
In the second analysis, in order to make the effect
of the test supplement more clear, 41 subjects with less variation
of exercise loading, based on the changes of type II (CTX-II) and
type I collagen (NTx) degradation markers, were evaluated (Table II). The results revealed that
urine CTX-II level significantly decreased in the glucosamine group
but not in the placebo group after the intervention for 16 weeks
(P<0.05) (Fig. 3A).
Furthermore, CTX-II level in the glucosamine group significantly
decreased compared with the placebo group after the intervention
for 16 weeks (P<0.05; Fig. 3A).
Also, serum C2C level significantly decreased in the glucosamine
group but not in the placebo group after the intervention for 16
weeks (P<0.05; Fig. 3B). In
contrast, the levels of urine CPII as well as urine NTx were not
significantly changed even after the intervention in both the
placebo and glucosamine groups (Fig.
3C and D). Thus, the present randomized controlled study
revealed that glucosamine administration (2 g/day for 16 weeks)
significantly reduces the levels of CTX-II and C2C but not CPII and
NTx, confirming that glucosamine exerts a chondroprotective action
on athletes (soccer players) by suppressing type II collagen
degradation (as assessed by CTX-II and C2C, type II collagen
degradation markers).
In this context, it has been reported that
glucosamine suppresses the production of matrix metalloproteinase
(MMP)-13, a major type II collagen-degradaing enzyme, from
chondrocytes and synoviocytes in vitro (20,21)
and reduces the serum level of MMP-3 in sera of patients with
rheumatoid arthritis (27). Based
on these findings, it is interesting to speculate that glucosamine
inhibits MMP production, thereby suppressing type II collagen
degradation (as evidenced by the reduction of CTX-II and C2C
levels) in vivo. In contrast, glucosamine administration did
not basically affect the levels of CPII as well as NTx, suggesting
no effect of glucosamine administration on the levels of type II
collagen synthesis (CPII) and type I collagen degradation (NTx) in
soccer players. Importantly, glucosamine has been reported to
enhance the expression of type II collagen in chondrocytes in
vitro (21); however, in the
present study, the increase of type II collagen synthesis (as
evaluated by CPII) could bot detected in soccer players (Figs. 1C and 2C). This is probably due to the fact that
type II collagen synthesis (as evaluated by CPII) was slightly
increased in soccer players than in non-athlete controls (22,23),
although the increase is not significant; thus, the CPII level can
not be further enhanced by glucosamine administration.
In summary, this is the first randomized
double-blind placebo-controlled study to demonstrate the potential
effect of oral administration of glucosamine administration on the
cartilage metabolism in healthy individuals (soccer players). The
efficacy and safety of glucosamine administration suggest that a
dietary supplement containing glucosamine (2 g/day for 16 weeks)
can be safely administered and potently exerts a chondroprotective
action on healthy individuals (soccer players) without symptoms of
joint disorders by improving the type II collagen metabolism
(suppressing type II collagen degradation but not affecting type II
collagen synthesis) in the cartilage, without any adverse effects.
Thus, glucosamine-containing supplement, as a functional food
(28), can be considered a
potential candidate for maintaining or caring the joint health of
healthy individuals without joint disorders.
Acknowledgements
The authors would like to thank Mr. Motoki Kanayama,
a manager of the soccer team of Juntendo University School of
Health and Sports Science for helping the authors collect the urine
samples and performing this randomized controlled study.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
AT and IN designed the research; AT, TH and MY
performed the clinical study; AT and IN analyzed the data; AT and
IN prepared the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was registered at the UMIN Clinical Trials
Registry (trial no. UMIN000023852) on August 31, 2016, and
performed from August 2016 to February 2017 at Juntendo University,
Japan. The study protocol with the title of ‘Evaluation of a
jelly-type functional food on the bone and cartilage metabolism in
athletes’ (protocol no: 20160703) was approved on August 10, 2016
by the Ethics Committee of The Japan Society of Vascular Medicine
and Rheology (Tokyo, Japan), and the study was conducted in
accordance with the principles of the amended Declaration of
Helsinki and ‘Ethical Guidelines for Epidemiological Research’
(established by the Japanese Government in 2008). Written informed
consent was obtained from all participants prior to their
enrollment in the study.
Patient consent for publication
All the participants enrolled in the present study
provided written informed consent for the publication of any
associated data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Roos H, Dahlberg L, Hoerrner LA, Lark MW,
Thonar EJ, Shinmei M, Lindqvist U and Lohmander LS: Markers of
cartilage matrix metabolism in human joint fluid and serum: The
effect of exercise. Osteoarthritis Cartilage. 3:7–14. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Qi C and Changlin H: Effects of moving
training on histology and biomarkers levels of articular cartilage.
J Surg Res. 135:352–363. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
O'Kane JW, Hutchinson E, Atley LM and Eyre
DR: Sport-related differences in biomarkers of bone resorption and
cartilage degradation in endurance athletes. Osteoarthritis
Cartilage. 14:71–76. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Buckwalter JA and Lane NE: Athletics and
osteoarthritis. Am J Sports Med. 25:873–881. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rousseau JC and Delmas PD: Biological
markers in osteoarthritis. Nat Clin Pract Rheumatol. 3:346–356.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Poole AR: Biochemical/immunochemical
biomarkers of osteoarthritis: Utility for prediction of incident or
progressive osteoarthritis. Rheum Dis Clin North Am. 29:803–818.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Christgau S, Garnero P, Fledelius C, Moniz
C, Ensig M, Gineyts E, Rosenquist C and Qvist P: Collagen type II
C-telopeptide fragments as an index of cartilage degradation. Bone.
29:209–215. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Poole AR, Ionescu M, Fitzcharles MA and
Billinghurst RC: The assessment of cartilage degradation in vivo:
Development of an immunoassay for the measurement in body fluids of
type II collagen cleaved by collagenases. J Immunol Methods.
294:145–153. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shinmei M, Ito K, Matsuyama S, Yoshihara Y
and Matsuzawa K: Joint fluid carboxy-terminal type II procollagen
peptide as a marker of cartilage collagen biosynthesis.
Osteoarthritis Cartilage. 1:121–128. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schwenk TL and Costley CD: When food
becomes a drug: Nonanabolic nutritional supplement use in athletes.
Am J Sports Med. 30:907–916. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gorsline RT and Kaeding CC: The use of
NSAIDs and nutritional supplements in athletes with osteoarthritis:
Prevalence, benefits, and consequences. Clin Sports Med. 24:71–82.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ostojic SM, Arsic M, Prodanovic S, Vukovic
J and Zlatanovic M: Glucosamine administration in athletes: Effects
on recovery of acute knee injury. Res Sports Med. 15:113–124. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nagaoka I: Joint health of athletes and
the chondroprotective action of glucosamine. Juntendo Med J.
63:104–114. 2017. View Article : Google Scholar
|
|
14
|
McAlindon TE, LaValley MP, Gulin JP and
Felson DT: Glucosamine and chondroitin for treatment of
osteoarthritis: A systematic quality assessment and meta-analysis.
JAMA. 283:1469–1475. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Reginster JY, Deroisy R, Rovati LC, Lee
RL, Lejeune E, Bruyere O, Giacovelli G, Henrotin Y, Dacre JE and
Gossett C: Long-term effects of glucosamine sulphate on
osteoarthritis progression: A randomized, placebo-controlled
clinical trial. Lancet. 357:251–256. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pavelká K, Gatterová J, Olejarová M,
Machacek S, Giacovelli G and Rovati LC: Glucosamine sulfate use and
delay of progression of knee osteoarthritis: A 3-year, randomized,
placebo-controlled, double-blind study. Arch Intern Med.
162:2113–2123. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nagaoka I: Recent aspect of the
chondroprotective and anti-inflammatory actions of glucosamine, a
functional food. Juntendo Med J. 60:580–587. 2014. View Article : Google Scholar
|
|
18
|
Fenton JI, Chlebek-Brown KA, Peters TL,
Caron JP and Orth MW: Glucosamine HCl reduces equine articular
cartilage degradation in explant culture. Osteoarthritis Cartilage.
8:258–265. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gouze JN, Bordji K, Gulberti S, Terlain B,
Netter P, Magdalou J, Fournel-Gigleux S and Ouzzine M:
Interleukin-1beta down-regulates the expression of
glucuronosyltransferase I, a key enzyme priming glycosaminoglycan
biosynthesis: Influence of glucosamine on
interleukin-1beta-mediated effects in rat chondrocytes. Arthritis
Rheum. 44:351–360. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nakamura H, Shibakawa A, Tanaka M, Kato T
and Nishioka K: Effects of glucosamine hydrochloride on the
production of prostaglandin E2, nitric oxide and metalloproteases
by chondrocytes and synoviocytes in osteoarthritis. Clin Exp
Rheumatol. 22:293–299. 2004.PubMed/NCBI
|
|
21
|
Derfoul A, Miyoshi AD, Freeman DE and Tuan
RS: Glucosamine promotes chondrogenic phenotype in both
chondrocytes and mesenchymal stem cells and inhibits MMP-13
expression and matrix degradation. Osteoarthritis Cartilage.
15:646–655. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yoshimura M, Sakamoto K, Tsuruta A,
Yamamoto T, Ishida K, Yamaguchi H and Nagaoka I: Evaluation of the
effect of glucosamine administration on biomarkers for cartilage
and bone metabolism in soccer players. Int J Mol Med. 24:487–494.
2009.PubMed/NCBI
|
|
23
|
Nagaoka I, Tsuruta A and Yoshimura M:
Evaluation of cartilage and bone metabolism in collegiate athletes
belonging to various sports clubs by analyzing type II collagen
degradation and synthesis and type I collagen degradation. Juntendo
Med J. 64 Suppl 1:122–127. 2018.
|
|
24
|
Garnero P, Piperno M, Gineyts E, Christgau
S, Delmas PD and Vignon E: Cross sectional evaluation of
biochemical markers of bone, cartilage, and synovial tissue
metabolism in patients with knee osteoarthritis: Relations with
disease activity and joint damage. Ann Rheum Dis. 60:619–626. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Radin EL and Rose RM: Role of subchondral
bone in the initiation and progression of cartilage damage. Clin
Orthop Relat Res. 1–40. 1986.
|
|
26
|
Bettica P, Cline G, Hart DJ, Meyer J and
Spector TD: Evidence for increased bone resorption in patients with
progressive knee osteoarthritis: Longitudinal results from the
Chingford study. Arthritis Rheum. 46:3178–3184. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nakamura H, Masuko K, Yudoh K, Kato T,
Kamada T and Kawahara T: Effects of glucosamine administration on
patients with rheumatoid arthritis. Rheumatol Int. 27:213–218.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Martirosyan DM and Singh J: A new
definition of functional food by FFC: What makes a new definition
unique? Functi Foods Health Dis. 5:209–223. 2015.
|