Introduction
Breast cancer, one of the most common types of
cancer in women, is associated with high mortality (1). Treatment for breast cancer includes
hormonal therapy, chemotherapy, radiotherapy, targeted therapy,
surgery and various combinations of these strategies. However, the
prognosis of certain subtypes remains poor (2).
Autophagy is a homeostatic cellular self-digestive
process responsible for degrading unnecessary or dysfunctional
cellular organelles and proteins in all living cells (3). Although autophagy promotes a cell
survival response, it also serves a role in cell death (4). Previous studies have demonstrated
that autophagic cell death is triggered by numerous signaling
pathways including adenosine monophosphate-activated protein kinase
pathway (5), mammalian target of
rapamycin (mTOR) pathway (6), and
mitogen-activated protein kinase (MAPK)/extracellular
signal-regulated kinase (ERK)1/2 pathway (7).
Medicinal plants and their extracts are commonly
used to prevent and treat numerous diseases, including cancer. The
World Health Organization has reported that ~4 million people (80%
of the population in developing countries), depend on medicinal
plants for primary healthcare (8).
Pristimerin, a quinonemethide triterpenoid compound, has long been
used as an anti-inflammatory, antioxidant, antimalarial and
insecticidal agent (9).
Pristimerin also possesses promising clinical potential as a
therapeutic and chemopreventive agent for numerous types of cancer,
including colon cancer (10),
prostate cancer (11), ovarian
cancer (12) and breast cancer
(13). Pristimerin induces cell
death via several mechanisms, including proteasome inhibition
(14), caspase activation
(15), inhibition of the human
epidermal growth factor receptor 2 (HER2) (13), inhibition of protein kinase
B/nuclear factor-κB/mTOR signaling (12), cell cycle arrest (12) and inhibition of migration and
invasion (10). However, the
effect of pristimerin on autophagy in human breast cancer has not
been reported yet to the best of our knowledge.
The present study aimed to evaluate whether
pristimerin induces autophagy in human breast cancer cells. The
results of the present study demonstrated that pristimerin
inhibited cell proliferation by autophagy induction. The effects of
pristimerin were enhanced when combined with paclitaxel treatment
through suppression of ERK1/2/p90 ribosomal S6 kinase (p90RSK)
signaling, which in turn activated autophagy. These results
indicated that pristimerin has potential to treat breast cancer
through autophagy and combination therapy can enhance
paclitaxel-induced anticancer activities.
Materials and methods
Chemicals
Pristimerin was obtained from Sigma-Aldrich (Merck
KGaA, Darmstadt, Germany) and dissolved in dimethyl sulfoxide
(DMSO) to give a stock solution of 100 mM and stored at −20°C in
aliquots. Paclitaxel was gifted by Boryung Co., Ltd. (Seoul,
Korea). Dulbecco's modified Eagle medium (DMEM), fetal bovine serum
(FBS) and penicillin/streptomycin were obtained from GE Healthcare
Life Sciences HyClone (Logan, UT, USA). Trypsin/EDTA was purchased
from Gibco™ (Thermo Fisher Scientific, Inc., Waltham, MA, USA). The
following primary antibodies were used: Rabbit polyclonal
anti-human light chain (LC) 3-I/II (1:1,000; cat. no. 4108), rabbit
polyclonal anti-human phosphorylated (phospho)-p44/p42 MAPK
(ERK1/2; Thr202/Tyr204; 1:1,000; cat. no. 9101), rabbit monoclonal
anti-human P-90RSK (1:1,000; cat. no. 9355), rabbit polyclonal
anti-human phospho-p90RSK (Ser380; 1:1000; cat. no. 9314) were
purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA)
and rabbit polyclonal anti-human ERK (1:1,000; cat. no. sc-94),
mouse monoclonal anti-human p62 (1:1,000; sc-48389), rabbit
polyclonal anti-human beclin1 (1:1,000; cat. no. sc-11427) and
rabbit polyclonal anti-human GAPDH (1:1,000; cat. no. sc-25778)
were obtained from Santa Cruz Biotechnology, Inc., (Dallas, TX,
USA). Horseradish peroxidase-conjugated anti-mouse and anti-rabbit
antibodies were purchased from BD Biosciences, Pharmingen (San
Diego, CA, USA). SuperSignal® West Pico Chemiluminescent
Substrate was purchased from Pierce (Thermo Fisher Scientific,
Inc.). The Cell Counting Kit-8 (CCK-8) was purchased from Dojindo
Molecular Technologies, Inc., (Kumamoto, Japan) and the Autophagy
Detection kit (cat. no. ab139484) was purchased from
Abcam® (Cambridge, MA, USA). 3-MA, ceramide C6 and all
other reagents were obtained from Sigma-Aldrich (Merck KGaA).
Cell line and cell culture
The MDA-MB-231 human breast cancer cell line was
purchased from American Type Culture Collection (Manassas, MD,
USA). Cells were grown in DMEM supplemented with 10% (v/v) FBS,
penicillin (100 U/ml)/streptomycin (100 µg/ml) at 37°C in a
humidified CO2 (5%)-controlled incubator.
Cell viability assay
Cells were seeded into 96-well microplates at a
density of 5×103 cells/ml and allowed to attach for 24
h. Pristimerin (1, 2.5, 5 and 10 µM) and paclitaxel (6, 12, 24 and
30 µM) were added to the medium at various concentrations.
Following treatment, the cell cytotoxicity and/or proliferation was
assessed using the CCK-8 assay. CCK-8 (10 µl) was added to each
well and incubated for 3 h at 37°C; cell proliferation and
cytotoxicity were assessed by measuring the absorbance at a
wavelength of 450 nm using a microplate reader (Corning, Inc.,
Corning, NY, USA). A total of three replicated wells were used per
experimental condition.
Western blotting analysis
Cells were harvested using Trypsin-EDTA, washed
twice with cold phosphate buffered saline (PBS), lysed with lysis
buffer (10 mM Tris, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% TritonX-100,
0.5% NP-40, 1 mM PI, 1 mM DTT and 1 mM PMSF), placed on ice for 1 h
with occasional vortexing and centrifuged at 13,000 × g for 10 min
at 4°C to collect the supernatant. A Pierce BCA Protein Assay kit
(Pierce; Thermo Fisher Scientific Inc.) was used to determine
protein concentration. Cell lysates (50 µg) were subjected to 8,
10, and 15% SDS-PAGE and transferred to a polyvinylidene difluoride
membrane. Blots were blocked with 5% skim milk in PBS containing
0.05% Tween-20 (PBST) for 1 h at 25°C and then incubated with
primary antibodies (1:1,000) overnight at 4°C. After washing with
PBST, membranes were incubated with anti-rabbit horseradish
peroxidase-conjugated IgG (1:3,000) at room temperature for 2 h and
visualized with enhanced chemiluminescence. Band intensity was
quantified by densitometry using ImageJ (version 1.52) software
(National Institutes of Health, Bethesda, MD, USA) and was
normalized to loading controls. Quantification value was expressed
as the fold change vs. band numbered 1.0.
Autophagy detection assay
Autophagy determination was performed using an
Autophagy Detection kit according to the manufacturer's protocol.
According to product overview by the company, the Autophagy
Detection kit can measure autophagic vacuoles and monitor
autophagic flux in live cells using a novel dye that selectively
labels autophagic vacuoles. The dye has been optimized through
identification of titratable functional moieties that allow for
minimal staining of lysosomes while exhibiting bright fluorescence
upon incorporation into pre-autophagosomes, autophagosomes and
autolysosomes (autophagolysosomes). Cells were seeded into 8-well
chamber slides at a density of 1×10 cells/ml and treated with
indicated drugs. Following drug treatment, cells were washed with
1X assay buffer, following incubation with 100 µl microscopy dual
detection reagent for 30 min at 37°C in the dark. Following the
incubation, cells were washed with 1X assay buffer to remove
unbound detection reagent and examined using a confocal microscope
(LSM5; Carl Zeiss AG, Oberkochen, Germany).
Statistical analysis
All results presented were confirmed in at least
three independent experiments. Data were presented as the mean ±
standard deviation. Statistical differences were analyzed by
one-way analysis of variance followed by a Tukey test using
IBM® SPSS® Statistics Version 24.0 (IBM
Corp., Armonk, NY, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Pristimerin enhances cell death and
activates autophagy in MDA-MB-231 cells
Exposure to pristimerin for 24 h significantly
inhibited growth of MDA-MB-231 cells in a concentration-dependent
manner compared with control treatment with a vehicle (P<0.05;
Fig. 1A). Whether
pristimerin-mediated inhibition of MDA-MB-231 cells originated from
autophagy was further examined. Autophagy can be accurately
measured by assessing the expression of microtubule-associated
protein light chain 3 (LC3), namely the conversion of LC3-I to
LC3-II using western blot analysis. Following treatment with
pristimerin at 10 µM for 24 h, the ratio of LC3-II/LC3-I as well as
LC3-II levels were increased (Fig.
1B). Co-treatment with 2 mM 3-MA (an autophagy inhibitor)
inhibited LC3-II accumulation induced by 10 µM pristimerin
(Fig. 1C) and promoted cell
viability (Fig. 1D). These results
suggested that pristimerin exposure induced autophagic cytotoxicity
in MDA-MB-231 cells whereas inhibition of pristimerin-induced
autophagy by 3-MA increased cell viability.
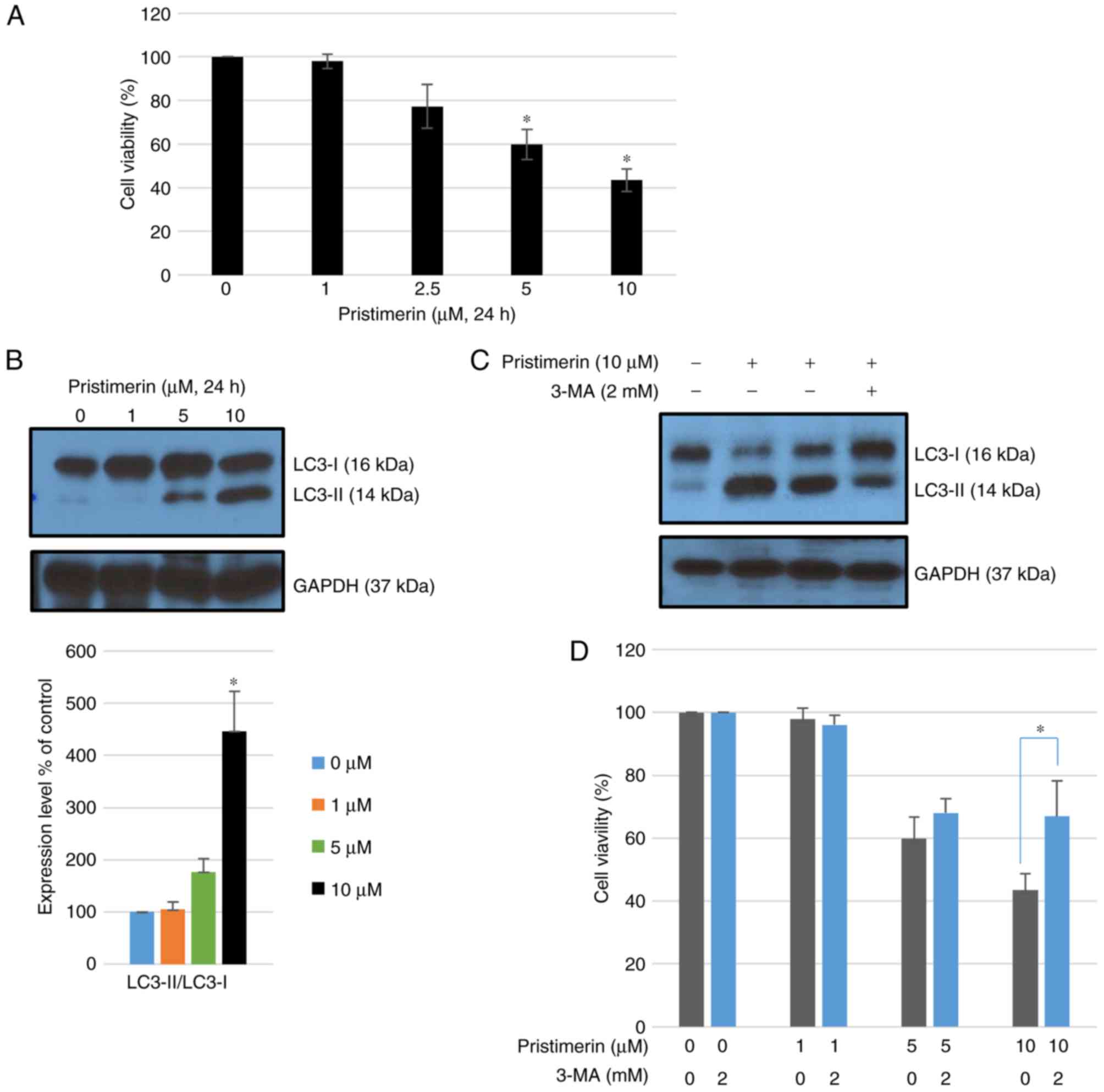 | Figure 1.Pristimerin-induced cell death is
associated with activation of autophagy. (A) Cell viability
following treatment with pristimerin (0, 1, 2.5, 5 and 10 µM) in
MDA-MB-231 human breast cancer cells. *P<0.05 vs. pristimerin (0
µM). (B) MDA-MB-231 breast cancer cells were treated with 0, 1, 5
and 10 µM pristimerin for 24 h and expression levels of LC3-I and
LC3-II were detected by western blot analysis. GAPDH was used as a
loading control. Values are expressed as the mean ± standard
deviation. *P<0.05 vs. control. (C) Cells were pre-incubated
with or without 3-MA (2 mM) for 3 h and then incubated with
pristimerin (10 µM) for 24 h, following which LC3-I and LC3-II
levels were detected by western blotting analysis. (D) Cells were
pre-incubated with or without 3-MA (2 mM) for 3 h and then
incubated with 0, 1, 5 and 10 µM pristimerin, and cell viability
was detected using a Cell Counting Kit-8 assay. Data are expressed
as the mean ± standard deviation. *P<0.05 vs. pristimerin single
treatment. 3-MA, 3-methyladenine; LC3-I, light chain 3. |
Paclitaxel induces autophagy in
MDA-MB-231 cells
To examine the inhibitory effect of paclitaxel on
the proliferation of MDA-MB-231 cells breast cancer cells, various
concentrations (0, 6, 12, 24 and 30 µM) of paclitaxel were
evaluated by CCK-8 viability assay. Paclitaxel exhibited no
significant toxicity to MDA-MB-231 cells at any concentration
evaluated up to 24 µM (Fig. 2A).
However, high concentrations of paclitaxel (over 48 µM)
demonstrated strong toxicity (data not shown). Paclitaxel can
modulate autophagy (16,17), although its mode of action remains
controversial. In the present study, the effect of various
concentrations of paclitaxel on autophagy in MDA-MB-231 cells was
examined. Following treatment with paclitaxel at 12, 24, or 30 µM
for 24 h, the ratio of LC3-II/LC3-I was significantly increased
(P<0.05; Fig. 2B). These
results demonstrated that paclitaxel induced autophagy in
MDA-MB-231 cells without demonstrating significant
cytotoxicity.
Involvement of autophagy in the
additive action of pristimerin in combination with paclitaxel
As illustrated in Fig.
3A, combination treatment with 10 µM pristimerin and 24 µM
paclitaxel additively inhibited cell viability. Further experiments
were performed to observe whether paclitaxel influenced
autophagic-cell death following treatment combined with
pristimerin. Cells were pretreated with paclitaxel for 2 h and then
treated with pristimerin for another 24 h. It was observed that the
ratio of LC3-II/LC3-I as well as LC3-II levels were significantly
additively increased (P<0.05; Fig.
3B). Chemical inhibition of autophagy using 3-MA significantly
inhibited LC3-II accumulation induced by the combination of
pristimerin and paclitaxel (P<0.05; Fig. 3B). Autophagy was further assessed
by a detection assay where a population of green detection
reagent-labeled vesicles co-localized with LC3, a specific
autophagosome marker. It was revealed that paclitaxel or
pristimerin treatment for 24 h resulted in the appearance of green
detection reagent while their combination exhibited stronger green
fluorescence (Fig. 3C). Autophagy
inhibitor (3-MA) inhibited the autophagy reaction (Fig. 3C). Co-treatment with 3-MA
significantly increased cell viability, even in the presence of a
combination of pristimerin and paclitaxel (P<0.05; Fig. 3D). These results suggested that
pristimerin enhanced the paclitaxel-induced growth inhibition of
MDA-MB-231 cells by enhancing cytotoxic autophagic cell death.
Regulation of ERK1/2 signaling
contributes to pristimerin-induced autophagy in MDA-MB-231
cells
It is well known that ERK1/2 and autophagy are
closely linked (18). Whether
pristimerin treatment of MDA MB-231 cells could affect the ERK 1/2
signaling pathway was investigated. Cells were treated with various
concentrations of pristimerin for 24 h, following which,
phospho-ERK 1/2 and p90RSK, one of the potentially important
substrates of ERK, were assessed by western blot analysis.
Pristimerin dose-dependently inhibited the phosphorylation of
ERK1/2 and p90RSK (Fig. 4A). It
was also observed that beclin 1 expression and p62 degradation,
both autophagic proteins, were increased by pristimerin treatment
(Fig. 4A). Paclitaxel treatment
(24 µM) alone also inhibited ERK1/2/p90RSK phosphorylation levels
and increased the beclin 1 expression and p62 degradation (Fig. 4A). Combined treatment of
pristimerin and paclitaxel additively inhibited ERK1/2
phosphorylation and significantly increased the ratio of
LC3-II/LC3-I (P<0.05; Fig. 4B).
Co-treatment with an ERK activator (ceramide C6) increased
phosphorylation of ERK and inhibited LC3-II accumulation in
combination treatment of pristimerin and paclitaxel (Fig. 4B). These results suggested that
pristimerin-induced autophagy was associated with ERK1/2 signaling
and paclitaxel additively enhanced pristimerin-induced autophagic
cell death in MDA-MB-231 cells.
 | Figure 4.Pristimerin-induced autophagy is
regulated by ERK1/2 signaling in MDA-MB-231 cells. (A) Cells were
treated with pristimerin and paclitaxel for 24 h and expression
levels of p-ERK1/2, ERK1/2, p-p90RSK, p90RSK, beclin 1, and p62
were detected by western blot analysis. GAPDH was used as a loading
control. *P<0.05 vs. pristimerin (0 µM) (Lane no. 1) and
#P<0.05 vs. paclitaxel (0 µM) (Lane no. 5). (B) Cells
were treated with pristimerin and paclitaxel and expression levels
of p-ERK1/2, ERK1/2, LC3-II/LC3-I were detected by western blot
analysis. Cells were pretreated with ERK activator ceramide C6 for
3 h prior to combined treatment with pristimerin and paclitaxel.
GAPDH was used as a loading control. Data are expressed as the mean
± standard deviation. *P<0.05 vs. paclitaxel (lane no. 3),
&P<0.05 vs. pristimerin (lane no. 2) and
##P<0.01 vs. pristimerin and paclitaxel combined
treatment without ceramide C6 (lane no. 4). p-ERK, phosphorylated
extracellular signal-regulated kinase; LC3, light chain 3; p90RSK,
p90 ribosomal S6 kinase. |
Discussion
Breast cancer is a complicated and heterogeneous
disease with a number of biomarkers, including estrogen receptor,
progesterone receptor, HER2 and triple-negative breast cancer
(19). Each of them has different
treatment strategies and prognosis (19). A high mortality rate is associated
with breast cancer patients (19).
Increasing effort has been focused on the identification of novel
anti-breast cancer agents. Medicinal plants and their extracts are
commonly used to prevent and treat a number of diseases, including
cancer. Developing novel therapeutic agents from plants with fewer
side-effects and high efficacy is a promising strategy to reduce
the mortality rate of breast cancer.
Pristimerin, a quinonemethide triterpenoid, has been
isolated from several plants including Maytenus chuchuhuasca
and M. ilicifolia in South Africa (20). Promising anticancer activities of
pristimerin have been emphasized in terms of its therapeutic
potential for breast cancer (21).
Previous studies have demonstrated that pristimerin is involved in
apoptotic cell death of MDA-MB-231 (15) and SKBR3 human breast cancer cells
(13). It was demonstrated that
the apoptotic activity of pristimerin and pristimerin induced
apoptosis in MDA-MB-231 cells, as expected. However, the effect of
pristimerin on autophagy in human breast cancer has not been fully
understood. Certain studies have reported that triterpenoids can
cause cell death by autophagy, including cimigenol (KY17) (22), 2α, 3α,
24-thrihydroxyurs-12-en-28-oicacid (23), ursolic acid (24) and cucurbitane (25). In the present study, the autophagic
effect of pristimerin on MDA-MB-231 human breast cancer cells was
examined.
Autophagy has been established as a type of
programmed cell death involving self-destruction characterized by
distinct morphological and biochemical features. Autophagy is
generally considered to be pro-survival associated, or
cytoprotective under stressful conditions such as g-radiation and
chemotherapy (26). However, it is
frequently activated in response to a number of environmental
stresses, thereby leading to cell death (27). LC3 is considered to be a strong
marker of autophagy. The conversion of LC3-I to LC3-II and LC3
puncta usually demonstrate an activation of autophagy (28). In the present study,
pristimerin-induced autophagy in MDA-MB-231 human breast cancer
cells was examined using western blot analysis. As demonstrated in
the results, LC3-II/LC3-I levels were increased, which indicated
that induction of autophagy was concentration-dependent. This
autophagy induction has the same pattern as pristimerin-induced
cell death. Furthermore, it was observed that autophagy inhibition
by 3-MA partially decreased pristimerin-induced cytotoxicity and
undermined LC3-II levels. These data suggested that
pristimerin-induced autophagy can serve as a cell death
pathway.
Paclitaxel is isolated from the bark of the yew
tree. It inhibits the growth of tumor cells. It is an important
therapeutic drug in the treatment of a number of types of cancer,
including breast cancer (29). It
is known to stabilize microtubules during DNA synthesis, thereby
suppressing mitosis of cancer cells. Paclitaxel is capable of
inducing mitochondria-mediated apoptosis involving
caspase-dependent (via caspase-3) and caspase-independent pathways
(via apoptosis inhibitory factor) (30). Apoptosis is frequently closely
associated with autophagy in cancer (31). Since autophagy has a housekeeping
role in clearing damaged organelles and eliminating intracellular
pathogens, autophagy is generally regarded as a survival mechanism.
On the other hand, autophagy has a key role in tumorigenesis,
progression and oncotherapy (30).
Paclitaxel can induce autophagy in human osteosarcoma cells (MG-63)
(30), non-small cell lung cancer
cells (A549) (16) and cervical
cancer (HeLa) (32). In the
present study, paclitaxel treatment promoted autophagy in
MDA-MB-231 cells at concentrations over 12 µM, and did not
demonstrate cytotoxicity at 12 µM. However, higher concentrations
of paclitaxel (48 and 60 µM) demonstrated strong cytotoxicity along
with autophagy induction (data not shown). A relatively high
concentration of paclitaxel was used in the present experiment
compared with other studies. There may be certain differences in
drug use. Paclitaxel was obtained for intravenous use from Boryung
Co., Ltd. (Seoul, Korea). Other investigations purchased the drug
from Sigma-Aldrich; Merck KGaA. For unknown reasons, in the present
experiment, MDA-MB-231 cancer cell lines did not respond to low
concentrations at all, in contrary to results of other papers. It
was demonstrated that another previous study also used a high
concentration of paclitaxel (33).
The clinical use of paclitaxel is frequently limited due to
acquisition of anticancer drug resistance (34). Therefore, combined treatment is
often used to enhance the effectiveness of chemotherapy and avoid
chemo-resistance to a single agent. All single anticancer agents
could similarly be used at reduced concentrations when they are
combined with others to synergistically induce cancer cell death
(35). Pristimerin in combination
with taxol can synergistically induce the death of cervical cancer
cells (35). In the present study,
pristimerin additively enhanced paclitaxel-induced cell death by
autophagic induction in MDA-MB-231 cells. To the best of our
knowledge, the present study is the first to propose that autophagy
in breast cancer cells may be stimulated by pristimerin alone, as
well as in combination with paclitaxel.
In the present experimental data (not shown),
pristimerin-induced apoptotic activity was increased by addition of
paclitaxel. The mechanism involved in these effects is still being
investigated. There is a complex crosstalk between autophagy and
apoptosis (31). It is frequently
unclear which specific interactions may contribute to cancer cell
death. Cancer cell death in this experiment is not the effect of
only one mechanism, namely autophagy. However, autophagy may be one
of the mechanisms that contribute to cancer cell death, which can
be detected by a number of methods.
GTPase HRas/Raf proto-oncogene serine/threonine
protein kinase/Dual specificity mitogen-activated protein kinase
kinase mek/ERK pathway serves an important role in autophagy. ERK
phosphorylates and inhibits TSC1/TSC2 which then activates C1 and
induces autophagy (36). Recently,
it has been demonstrated that a synthetic antihepatitis drug
(Bicyclol) can induce autophagy via the ERK signaling pathway in
HepG2 hepatocellular carcinoma cells (37). To understand the signaling cascade
that mediates the autophagic effect of pristimerin on MDA-MB-231
cells, modulation of the activation of ERK1/2 by pristimerin was
examined. Pristimerin treatment suppressed phospho-ERK1/2 and
phospho-p90RSK levels in a dose-dependent manner, although without
affecting total ERK1/2 and total p90RSK expression. The function of
pristimerin on ERK regulation remains controversial. A previous
study suggested that pristimerin can decrease the level of p-ERK1/2
and mTOR to induce cell death in SKBR3 breast cancer cells
(13). However, another study
demonstrated that ERK phosphorylation is not altered by pristimerin
treatment in HeLa cervical cancer cells (35). In the present study, ERK1/2
inhibition by pristimerin, paclitaxel, or the combination was
confirmed to induce autophagy (increased p62 degradation and
increased beclin1 expression). These effects were reversed by
treatment with an ERK activator. These results suggested that
pristimerin-induced autophagy served as a cell death pathway via
ERK1/2 inhibition, and that non-toxic doses of paclitaxel can
additively enhance these activities.
In conclusion, the results of the present study
elucidated the anti-cancer mechanism of pristimerin, and
demonstrated that non-toxic paclitaxel doses induced autophagy in
breast cancer cells. The current study provides sufficient evidence
that an autophagy inducer may be used as an adjuvant modality
during anti-cancer pharmacological treatment.
Acknowledgements
Not applicable.
Funding
The present study was supported by research fund of
Chungnam National University (grant no. 2015088201).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YL and JN designed the study and prepared the
manuscript. ML, EC and JP performed the experiments and analyzed
the data. JS and JL were involved in the study conception and
design and revised the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zagouri F, Sergentanis TN, Tsigginou A,
Dimitrakakis C, Zografos GC, Dimopoulos MA and Psaltopoulou T:
Female breast cancer in Europe: Statistics, diagnosis and treatment
modalities. J Thorac Dis. 6:589–590. 2014.PubMed/NCBI
|
|
2
|
Majeed W, Aslam B, Javed I, Khaliq T,
Muhammad F, Ali A and Raza A: Breast cancer: Major risk factors and
recent developments in treatment. Asian Pac J Cancer Prev.
15:3353–3358. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lum JJ, Bauer DE, Kong M, Harris MH, Li C,
Lindsten T and Thompson CB: Growth factor regulation of autophagy
and cell survival in the absence of apoptosis. Cell. 120:237–248.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mizushima N, Levine B, Cuervo AM and
Klionsky DJ: Autophagy fights disease through cellular
self-digestion. Nature. 451:1069–1075. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mihaylova MM and Shaw RJ: The AMPK
signalling pathway coordinates cell growth, autophagy and
metabolism. Nat Cell Biol. 13:1016–1023. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shi WY, Xiao D, Wang L, Dong LH, Yan ZX,
Shen ZX, Chen SJ, Chen Y and Zhao WL: Therapeutic metformin/AMPK
activation blocked lymphoma cell growth via inhibition of mTOR
pathway and induction of autophagy. Cell Death Dis. 3:e2752012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Corcelle E, Djerbi N, Mari M, Nebout M,
Fiorini C, Fénichel P, Hofman P, Poujeol P and Mograbi B: Control
of the autophagy maturation step by the MAPK ERK and p38: Lessons
from environmental carcinogens. Autophagy. 3:57–59. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang B, Zhu R, Tian S, Wang Y, Lou S and
Zhao H: Jatamanvaltrate P induces cell cycle arrest, apoptosis and
autophagy in human breast cancer cells in vitro and in vivo. Biomed
Pharmacother. 89:1027–1036. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dirsch VM, Kiemer AK, Wagner H and Vollmar
AM: The triterpenoid quinonemethide pristimerin inhibits induction
of inducible nitric oxide synthase in murine macrophages. Eur J
Pharmacol. 336:211–217. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yousef BA, Hassan HM, Guerram M, Hamdi AM,
Wang B, Zhang LY and Jiang ZZ: Pristimerin inhibits proliferation,
migration and invasion and induces apoptosis in HCT-116 colorectal
cancer cells. Biomed Pharmacother. 79:112–119. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu YB, Gao X, Deeb D, Pindolia K and
Gautam SC: Role of telomerase in anticancer activity of pristimerin
in prostate cancer cells. J Exp Ther Oncol. 11:41–49.
2015.PubMed/NCBI
|
|
12
|
Yousef BA, Guerram M, Hassan HM, Hamdi AM,
Zhang LY and Jiang ZZ: Pristimerin demonstrates anticancer
potential in colorectal cancer cells by inducing G1 phase arrest
and apoptosis and suppressing various pro-survival signaling
proteins. Oncol Rep. 35:1091–1100. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee JS, Yoon IS, Lee MS, Cha EY, Thuong
PT, Diep TT and Kim JR: Anticancer activity of pristimerin in
epidermal growth factor receptor 2-positive SKBR3 human breast
cancer cells. Biol Pharm Bull. 36:316–325. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang H, Landis-Piwowar KR, Lu D, Yuan P,
Li L, Reddy GP, Yuan X and Dou QP: Pristimerin induces apoptosis by
targeting the proteasome in prostate cancer cells. J Cell Biochem.
103:234–244. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu CC, Chan ML, Chen WY, Tsai CY, Chang FR
and Wu YC: Pristimerin induces caspase-dependent apoptosis in
MDA-MB-231 cells via direct effects on mitochondria. Mol Cancer
Ther. 4:1277–1285. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Klimaszewska-Wisniewska A,
Halas-Wisniewska M, Tadrowski T, Gagat M, Grzanka D and Grzanka A:
Paclitaxel and the dietary flavonoid fisetin: A synergistic
combination that induces mitotic catastrophe and autophagic cell
death in A549 non-small cell lung cancer cells. Cancer Cell Int.
16:102016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Veldhoen RA, Banman SL, Hemmerling DR,
Odsen R, Simmen T, Simmonds AJ, Underhill DA and Goping IS: The
chemotherapeutic agent paclitaxel inhibits autophagy through two
distinct mechanisms that regulate apoptosis. Oncogene. 32:736–746.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Corcelle E, Djerbi N, Mari M, Nebout M,
Fiorini C, Fénichel P, Hofman P, Poujeol P and Mograbi B: Control
of the autophagy maturation step by the MAPK ERK and p38: Lessons
from environmental carcinogens. Autophagy. 3:57–59. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Holliday DL and Speirs V: Choosing the
right cell line for breast cancer research. Breast Cancer Res.
13:2152011. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shirota O, Morita H, Takeya K and Itokawa
H: Cytotoxic aromatic triterpenes from Maytenus ilicifolia and
Maytenus chuchuhuasca. J Nat Prod. 57:1675–1681. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Salminen A, Lehtonen M, Suuronen T,
Kaarniranta K and Huuskonen J: Terpenoids: Natural inhibitors of
NF-kappaB signaling with anti-inflammatory and anticancer
potential. Cell Mol Life Sci. 65:2979–2999. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dai X, Liu J, Nian Y, Qiu MH, Luo Y and
Zhang J: A novel cycloartane triterpenoid from Cimicifuga induces
apoptotic and autophagic cell death in human colon cancer HT-29
cells. Oncol Rep. 37:2079–2086. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang D, Gao C, Li R, Zhang L and Tian J:
TEOA, a triterpenoid from Actinidia eriantha, induces autophagy in
SW620 cells via endoplasmic reticulum stress and ROS-dependent
mitophagy. Arch Pharm Res. 40:579–591. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hen S, Zhang Y, Zhang R, Tu X and Gong X:
Ursolic acid induces autophagy in U87MG cells via ROS-dependent
endoplasmic reticulum stress. Chem Biol Interact. 218:28–41. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Weng JR, Bai LY, Chiu CF, Hu JL, Chiu SJ
and Wu CY: Cucurbitane Triterpenoid from Momordica charantia
Induces Apoptosis and Autophagy in Breast Cancer Cells, in Part,
through Peroxisome Proliferator-Activated Receptor γ Activation.
Evid Based Complement Alternat Med. 2013:9356752013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen HY and White E: Role of autophagy in
cancer prevention. Cancer Prev Res (Phila). 4:973–983. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Denton D, Nicolson S and Kumar S: Cell
death by autophagy: Facts and apparent artefacts. Cell Death
Differ. 19:87–95. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kabeya Y, Mizushima N, Ueno T, Yamamoto A,
Kirisako T, Noda T, Kominami E, Ohsumi Y and Yoshimori T: LC3, a
mammalian homologue of yeast Apg8p, is localized in autophagosome
membranes after processing. EMBO J. 19:5720–5728. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dank M, Budi L, Piko B, Mangel L, Erfan J,
Cseh J, Ruzsa A and Landherr L: First-line bevacizumab-paclitaxel
in 220 patients with metastatic breast cancer: Results from the
AVAREG study. Anticancer Res. 34:1275–1280. 2014.PubMed/NCBI
|
|
30
|
Guo Y, Huang C, Li G, Chen T, Li J and
Huang Z: Paxilitaxel induces apoptosis accompanied by protective
autophagy in osteosarcoma cells through hypoxia-inducible factor-1α
pathway. Mol Med Rep. 12:3681–3687. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zambrano J and Yeh ES: Autophagy and
apoptotic crosstalk: Mechanism of therapeutic resistance in
HER2-positive breast cancer. Breast Cancer (Auckl). 10:13–23.
2016.PubMed/NCBI
|
|
32
|
Chi EY, Viriyapak B, Kwack HS, Lee YK, Kim
SI, Lee KH and Park TC: Regulation of paclitaxel-induced programmed
cell death by autophagic induction: A model for cervical cancer.
Obstet Gynecol Sci. 56:84–92. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Qu C, Zhang W, Zheng G, Zhang Z, Yin J and
He Z: Metformin reverses multidrug resistance and
epithelial-mesenchymal transition (EMT) via activating
AMP-activated protein kinase (AMPK) in human breast cancer cells.
Mol Cell Biochem. 386:63–71. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Koziara JM, Whisman TR, Tseng MT and
Mumper RJ: In-vivo efficacy of novel paclitaxel nanoparticles in
paclitaxel-resistant human colorectal tumors. J Control Release.
112:312–319. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Eum DY, Byun JY, Yoon CH, Seo WD, Park KH,
Lee JH, Chung HY, An S, Suh Y, Kim MJ and Lee SJ: Triterpenoid
pristimerin synergizes with taxol to induce cervical cancer cell
death through reactive oxygen species-mediated mitochondrial
dysfunction. Anticancer Drugs. 22:763–773. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ma L, Chen Z, Erdjument-Bromage H, Tempst
P and Pandolfi PP: Phosphorylation and functional inactivation of
TSC2 by Erk implications for tuberous sclerosis and cancer
pathogenesis. Cell. 121:179–193. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang Y, Nie H, Zhao X, Qin Y and Gong X:
Bicyclol induces cell cycle arrest and autophagy in HepG2 human
hepatocellular carcinoma cells through the PI3K/AKT and
Ras/Raf/MEK/ERK pathways. BMC Cancer. 16:7422016. View Article : Google Scholar : PubMed/NCBI
|


















