Introduction
Periodontitis is a common inflammatory disease that
results in irreversible destruction of periodontal tissues,
including the periodontal ligament, alveolar bone and root
cementum, and is the primary reason for tooth loss in adults.
Currently, there are numerous challenges for treating periodontitis
in clinical practice. A number of treatment approaches have been
developed in the past decades and the canonical treatment of
periodontal bone defects includes guided tissue regeneration
(1), bone grafts (2), and application of enamel matrix
derivative (3). However, these
conventional therapies cannot be regarded as best-practice
regenerative techniques due to their limited success and
unpredictable prognosis.
Adult periodontal ligament stem cells (PDLSCs) were
first discovered in 2004 (4).
Since then, stem cell-based periodontal tissue regeneration
treatment has attracted increasing attention in the field of
dentistry. Furthermore, as an important cell type for periodontal
tissue regeneration, PDLSCs have been reported to exhibit the
ability to differentiate into osteoblasts and cementoblasts in
vitro (5). However, the number
and sources of autologous PDLSCs are limited, thereby hampering
their clinical application. It is of great urgency to produce novel
bioactive substances with the ability to enhance the proliferation,
differentiation and osteogenic-associated gene/protein expression
of PDLSCs.
In 2000, the French researcher Choukroun et
al developed a straightforward open-access platelet-rich fibrin
(PRF) protocol (6,7). Choukroun's platelet-rich fibrin (PRF)
has been considered as a second-generation platelet concentrate
(6–8). When activated by thrombin and other
triggers, platelets release a large number of growth factors,
chemokines, and cytokines, which can interact with target protein
receptors and induce cell proliferation, cell differentiation, and
bone formation. As an autologous biomaterial, synthetic collagen
PRF has been applied in a number of medical fields, including oral
and maxillofacial surgery (9),
plastic surgery, and dental implantology (10–13).
PRF is fibrin in a three-dimensional structure that
contains a number of growth factors, including platelet-derived
growth factor AB and transforming growth factor β1 (TGF-β1), and
these are continuously released from the PRF matrix for >7 days
(14). A number of studies have
reported that PRF stimulates the proliferation and differentiation
of a variety of cell types in vitro (15,16).
Studies have demonstrated that platelet-rich plasma can promote the
proliferation of bone mesenchymal stem cells, fat source stem cells
and skeletal muscle satellite cells as well as osteogenic
differentiation (17). However,
there is little recent evidence regarding the effects of PRF
exudates on the proliferation and differentiation of human PDLCs
(hPDLCs).
The objective of the present study was to assess the
biological effects of different concentrations of PRF exudate on
hPDLCs by measuring cell proliferation, alkaline phosphatase (ALP)
activity, as well as osteocalcin (OCN), runt-related
transcription factor 1 (RUNX2) and osterix (OSX) gene
expression.
Materials and methods
Preparation of hPRF exudates
The present study was approved by the Ethics
Committee of the Jilin University Health Science Center (Jilin,
China). In accordance with this committee, the hPRF exudate was
prepared from three healthy male donors who had visited the
outpatient clinic at the Jilin University Health Science Center
between March 2017 and August 2017. They were nonsmokers and
nondrinkers with age range 22–30 years, and gave their informed
consent. Patient blood samples (50 ml) were used to produce hPRF
according to an existing protocol (8). Briefly, the samples were centrifuged
at 750 × g for 12 min at 10°C. A white PRF clot formed between the
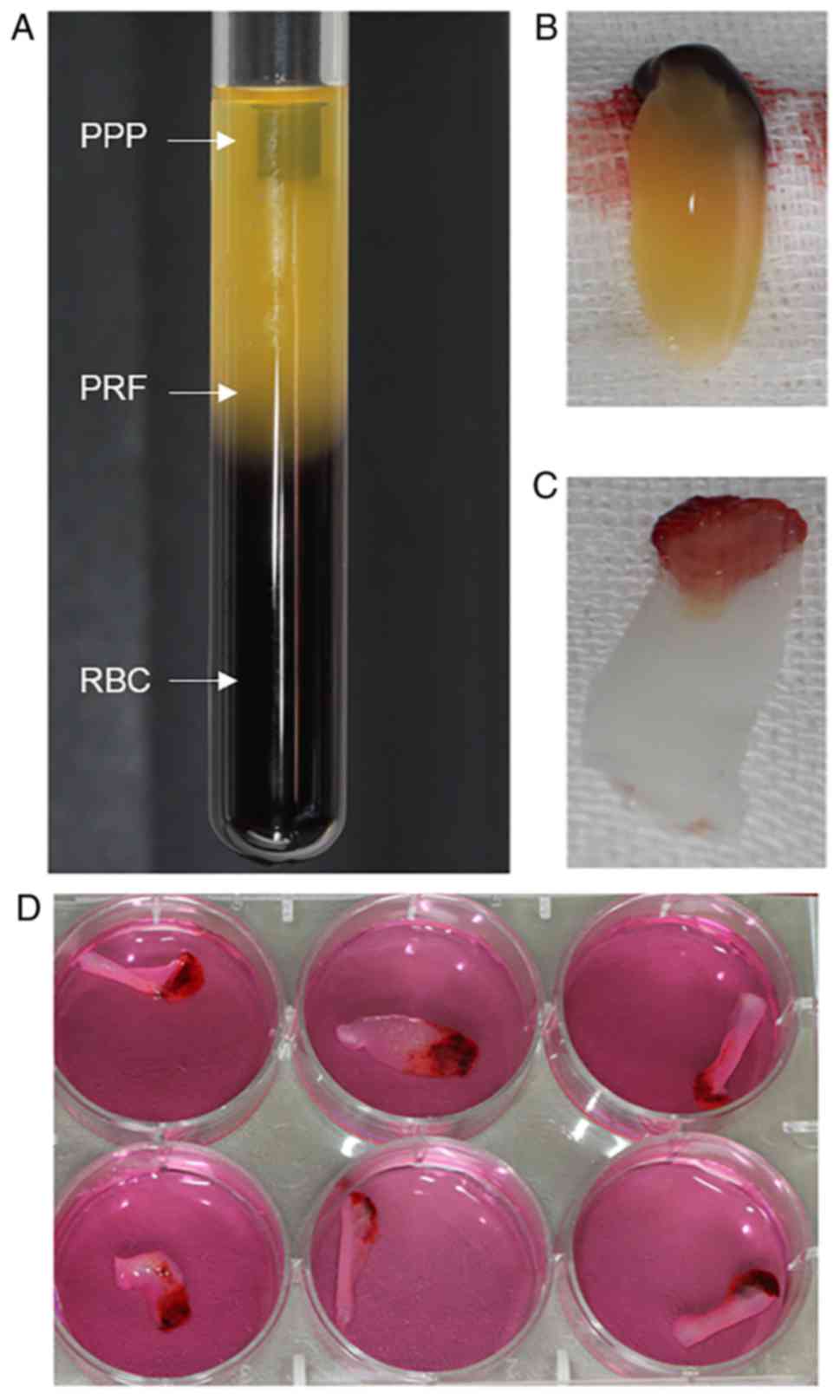
acellular plasma and red blood cells (RBCs; Fig. 1A), and the clot was held by sterile
forceps and separated from the RBCs using scissors (Fig. 1B). The clot was placed on the grid
of an endo box and compressed by the endo box cover. Following 1
min of applied pressure, the PRF clot was converted into PRF
membrane and the exudate was collected in the tray of the endo box
(Fig. 1C).
The PRF exudate was centrifuged at 500 × g for 5 min
at 10°C to obtain exudate without RBCs, which were precipitated.
The exudate was filtered using a 0.22 µm sterile syringe filter
unit (EMD Millipore, Billerica, MA, USA; cat. no. 01730). Pooled
PRF exudates were stored at −80°C prior to use. The original
concentration of RF exudate was defined as 100% and various
concentrations were obtained by dilution with minimal essential
medium α (α-MEM; Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA). A total of three PRF exudate concentrations were used in
the present study: 100% (E1), 20% (E2) and 4% (E3).
hPDLC culture
A total of 10 healthy and noncarious premolars from
2 female and 3 male donors aged 13–18 years old, who had received
orthodontic treatment at the Oral and Maxillofacial Surgery
Department of the Stomatology School of Jilin University
(Changchun, China) between March 2017 and August 2017, were
obtained with informed consent. Periodontal ligaments were gently
scraped from the middle third of the tooth-root surface with a
sharp scalpel, minced with ophthalmic scissors and rinsed with
α-MEM (Gibco; Thermo Fisher Scientific, Inc.). These explants were
cultured in α-MEM supplemented with 15% fetal bovine serum (FBS;
Thermo Fisher Scientific Inc.) and 1% streptomycin and penicillin
(Gibco; Thermo Fisher Scientific, Inc.), and the cultured pieces
were then incubated at 37°C in an environment containing 5%
CO2. Examination by inverted light microscopy (Olympus
Corporation, Tokyo, Japan) was carried out daily, and the medium
was changed every three days. When the cell colony-formation units
reached 80% confluence, the cells were transferred to a
75-cm2 flask and this was defined as passage 1. The same
procedure was carried out repeatedly to produce multiple passages.
Feeding was continued according to the cell-culturing protocol and
only cells prior to passage 6 were used in the present study.
Immunocytochemistry staining
hPDLCs at passage 3 (0.05×106) were
seeded into 24-well plates and covered in advance with 14-mm
diameter circular coverslips and incubated for 48 h. The cells were
then rinsed 3 times with 0.01 M phosphate-buffered saline (PBS) and
then fixed with 4% paraformaldehyde for 20 min at room temperature.
Following washing with PBS, 0.25% Triton X-100 was added to the
24-well plates, which were incubated at 37°C for 15 min. Endogenous
peroxidase activity was eliminated by incubation with 3%
H2O2 for 10 min at room temperature. Cells
were then incubated with 1% bovine serum albumin (Gibco; Thermo
Fisher Scientific, Inc.) and 22.52 mg/ml glycine in PBS + 0.1%
Tween 20 for 30 min at room temperature to block unspecific binding
of antibodies. The cells were then incubated with anti-vimentin
(1:100; cat. no. ab24525; Abcam, Cambridge, MA, USA) and
anti-cytokeratin (1:200; cat. no. AM06387SU-N; OriGene
Technologies, Inc., Beijing, China) primary antibodies overnight at
4°C. Secondary goat-anti-rabbit, goat-anti-mouse and goat
anti-chicken IgG were AlexaFluor 488 (cat. no. A-11008; Invitrogen;
Thermo Fisher Scientific, Inc.), 568 (cat. no. A-11004; Invitrogen;
Thermo Fisher Scientific, Inc.), and 647 (cat. no. A-21449;
Invitrogen; Thermo Fisher Scientific, Inc.) labeled, respectively;
and used in various combinations at a 1:1,000 dilution. The SP
immunohistochemistry assay kit (OriGene Technologies, Inc.) was
used for immunocytochemical staining according to the
manufacturer's protocol and the diaminobenzidine kit (OriGene
Technologies, Inc.) was used to stain positive cells. An inverted
phase-contrast microscope (IX73; Olympus Corporation, Tokyo, Japan)
was used to observe stained hPDLCs at magnifications of ×20 or
×40.
Proliferation analysis using the Cell
Counting Kit-8 (CCK-8) assay
CCK-8 (Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan) was used to assay PRF exudate for its effects on
hPDLC proliferation. hPDLCs (2×103/100 µl/well) were
seeded into each well of 96-well plates containing 10% FBS complete
medium and incubated for 24 h. Next, 50 µl medium supplemented with
4, 20, or 100% concentration of PRF exudate were added to each well
and incubated for 24, 48, or 72 h. Cell proliferation was
calculated using the CCK-8 at specified experimental time points.
The kit reagent (10 µl) was added to the culture medium of each
well. Following a 90 min incubation, the absorbance at a wavelength
of 450 nm was detected using an automatic microplate reader
(Infinite 200 PRO; Tecan Group Ltd., Mannedorf, Switzerland). The
well containing medium and CCK-8 solution minus seeded cells was
used as the blank control. The assay was performed in duplicate and
the trial repeated six times under the same conditions.
Assay of ALP activity
hPDLCs (500 µl) were seeded into each well of
24-well plates (Corning Incorporated, Corning, NY, USA) at a
density of 1×104/well in 10% FBS complete medium and
incubated for 24 h. Next, the cells were exposed to PRF exudate at
various concentrations [100% (E1), 20% (E2) or 4% (E3)] at 7 or 14
days. At the given time points, the cells were lysed with 0.1%
Triton X-100 and the lysates were centrifuged at 8,000 × g for 10
min at 4°C. Supernatant (50 µl/well) was added to 96-well plates
and the ALP activity was examined using the ALP assay kit (Nanjing
Jiancheng Bioengineering Institute, Nanjing, China). The optical
density values were read ata wavelength of 520 nm with an automatic
microplate reader (Infinite200 PRO; Tecan Group Ltd.).
Osteogenic differentiation
induction
hPDLCs (1×104/500 µl/well) were seeded
into each well of 24-well plates in normal medium until they
reached 60–70% confluence. The medium was then replaced with four
different media: Standard medium (10% FBS) or standard medium
supplemented with three different concentrations of PRF exudate:
100% (E1), 20% (E2), or 4% (E3). The cells in the three
experimental groups with E1, E2 and E3 were all maintained in
osteogenic differentiation medium [Dulbecco's Modified Eagle Medium
(DMEM) supplemented with 10% FBS, 50 nM dexamethasone, 0.2 mM
ascorbic acid, 10 mM b-glycerophosphate and 100 units/ml
penicillin-streptomycin]. Each well in the 24-well plate contained
a specific concentration as follows: DMEM only, E1, E2, or E3. In
addition, the three experimental groups with E1, E2 and E3 also
contained mineralization-induction medium (α-MEM containing 10%
FBS, 50 mg/ml ascorbic acid, 10 mmol/l β-glycerol phosphate and
10−8 M dexamethasone). Only the control group containing
DMEM alone did not contain mineralization-induction medium. The
medium was changed every 3 days.
Alizarin red S staining
Formation of the mineralized nodules was detected
and quantified following 14 days by an Alizarin red-based assay.
This assay was carried out according to the previous protocol with
minor modifications (18).
Briefly, hPDLCs were fixed with 95% ethanol for 15 min at room
temperature. The cells were then washed twice with dH2O
and stained with 0.1% alizarin red S solution (pH 4.1) for 20 min
at room temperature. Next, the cells were washed three times with
dH2O. To semi-quantify the content of mineralized matrix
nodules, 100 mM cetyl pyridinium chloride was added to the 24-well
plates to dissolve and release calcium-combined alizarin red S into
solution. The optical density values were read at 570 nm, which
represented the relative quantity of mineralization nodules. A
total of 14 days post-osteogenic induction, mineralized nodules
were observed by inverted phase-contrast microscopy (IX73;
magnification, ×20; Olympus Corporation, Tokyo, Japan).
Western blotting
Cell lysates were prepared in
radioimmunoprecipitation buffer (150 mM NaCl, 0.1% SDS, 1 mM PMSF,
10 mM Tris-Cl, pH 7.4, 1% sodium deoxycholate and 1% Triton X-100).
The cells were treated with four different media for 3, 5, or 7
days. The cell lysates were incubated for 30 min on ice, then
clarified by centrifugation at 6,000 × g for 10 min at 4°C. The
protein contents were quantified with a bicinchoninic acid assay.
Protein samples (20 µl) were denatured and resolved by 10%
SDS-PAGE, transferred onto a polyvinylidene difluoride membrane
(EMD Millipore), and run at 300 mA for 2 h. The membranes were
blocked by incubating with 5% non-fat milk at room temperature for
1 h, then incubated with primary anti-RUNX2 antibody (1:1,000; cat.
no. 12556; Cell Signaling Technology) overnight at 4°C. The primary
antibody was then removed by washing the membrane three times in
Tris-buffered saline containing 0.1% Tween 20 (TBST) and incubated
with horseradish peroxidase-goat anti-rabbit immunoglobulin G
(1:500; cat. no. 10285-1-AP; Proteintech Group) for 1 h at room
temperature. Following three washes with TBST, the protein bands
were visualized using the Enhanced Chemiluminescence kit (GE
Healthcare, Chicago, IL, USA) and exposed to X-ray film. β-actin
(1:1,000; cat. no. A5441; Sigma-Aldrich; Merck KGaA) was used as
internal reference.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
hPDLCs (1×105/2 ml/well) were seeded into
each well of 6-well plates in standard medium until they reached
60–70% confluence. The cells were treated with four different
media. The control group was osteogenic-inducing medium [DMEM plus
50 µg/ml ascorbic acid and 10 mM β-sodium glycerophosphate
(Sigma-Aldrich; Merck KGaA)]. The three experimental groups were
treated with osteogenic-inducing medium plus a PRF exudate dilution
(E1, E2 or E3). RNA was extracted using TRIzol®
(Invitrogen; Thermo Fisher Scientific, Inc.) and reverse
transcribed into cDNA on the 3rd, 5th and 7th days. cDNA synthesis
was performed with 1 µg total RNA using SuperScript II reverse
transcriptase and random hexamer primers (cat. no. 18064014;
Invitrogen; Thermo Fisher Scientific, Inc.). The temperature
protocol used was as follows: Room temperature for 10 min and then
37°C for 60 min; followed by 85°C for 5 min.
An aliquot (2 µl) of each sample was used for
quantitative (q) PCR determination of the expression of the
osteogenic genes OCN, RUNX2 and OSX using the
SYBR® Premix Ex Taq™ II kit (Takara Bio,
Inc., Otsu, Japan). qPCR primers were designed to span an intron so
that only RNA-specific amplification was possible. PCR was
performed in a Rotor-Gene thermocycler as follows: 95°C for 3 min,
40 cycles of 95°C for 3 sec and 60°C for 60 sec. Each sample was
tested in triplicate and fold differences in gene expression were
calculated using the 2−ΔΔCq method (19) with normalization to human β-actin.
The primer design is presented in Table I.
 | Table I.Primers used for reverse
transcription-quantitative polymerase chain reaction analysis. |
Table I.
Primers used for reverse
transcription-quantitative polymerase chain reaction analysis.
| Gene | Primer sequences
(5′-3′) | PCR bp |
|---|
| β-actin |
| F |
AGAAAATCTGGCACCACACC | 139 |
| R |
GGGGTGTTGAAGGTCTAAA |
|
| Osteocalcin |
| F |
GGCGCTACCTGTATCAATGG | 106 |
| R |
TCAGCCAACTCGTCACAGTC |
|
| RUNX2 |
| F |
CACCATGTCAGCAAAACTTCTT | 96 |
| R |
TCACGTCGCTCATTTTGC |
|
| Osterix |
| F |
TGCTTGAGGAGGAAGTTCAC | 148 |
| R |
AGGTCACTGCCCACAGAGTA |
|
Statistical analysis
Data are presented as the mean ± standard deviation
of 3–4 independent experiments, and statistical analyses were
performed with SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA).
The cell proliferation and ALP activity assays were analyzed by
one-way analysis of variance (ANOVA) and Tukey's multiple
comparison tests The western blotting and Alizarin red staining
assay data were analyzed using two-way ANOVA followed by
Bonferroni's post-hoc comparisons test for independent samples.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Characterization of hPDLCs
hPDLCs grew from the explant following 7–10 days of
culture, a spindle shape was observed and certain cells were
distributed in a circinate alignment pattern with rapid
proliferation (Fig. 1A and B). The
PRF exudate obtained was a clear, yellowish fluid. Each 50-ml blood
sample produced 4.5 ml of PRF exudate. The cells were vimentin
positive (Fig. 1C) and keratin
negative (Fig. 1D), according to
immunocytochemistry staining, which indicated that the primary
cells were of mesenchymal origin.
Effects of PRF exudate on PDLC
proliferation
The objective of the present study was to examine
the impact of various concentrations of hPRF exudate on the
proliferation of hPDLCs in vitro. The hPRF was obtained
according to the previous protocol (Fig. 2) (8). The cells were exposed to varying
concentrations of PRF exudate for 24, 48, or 72 h. The viability
and proliferation of hPDLCs were determined by using a CCK-8 kit
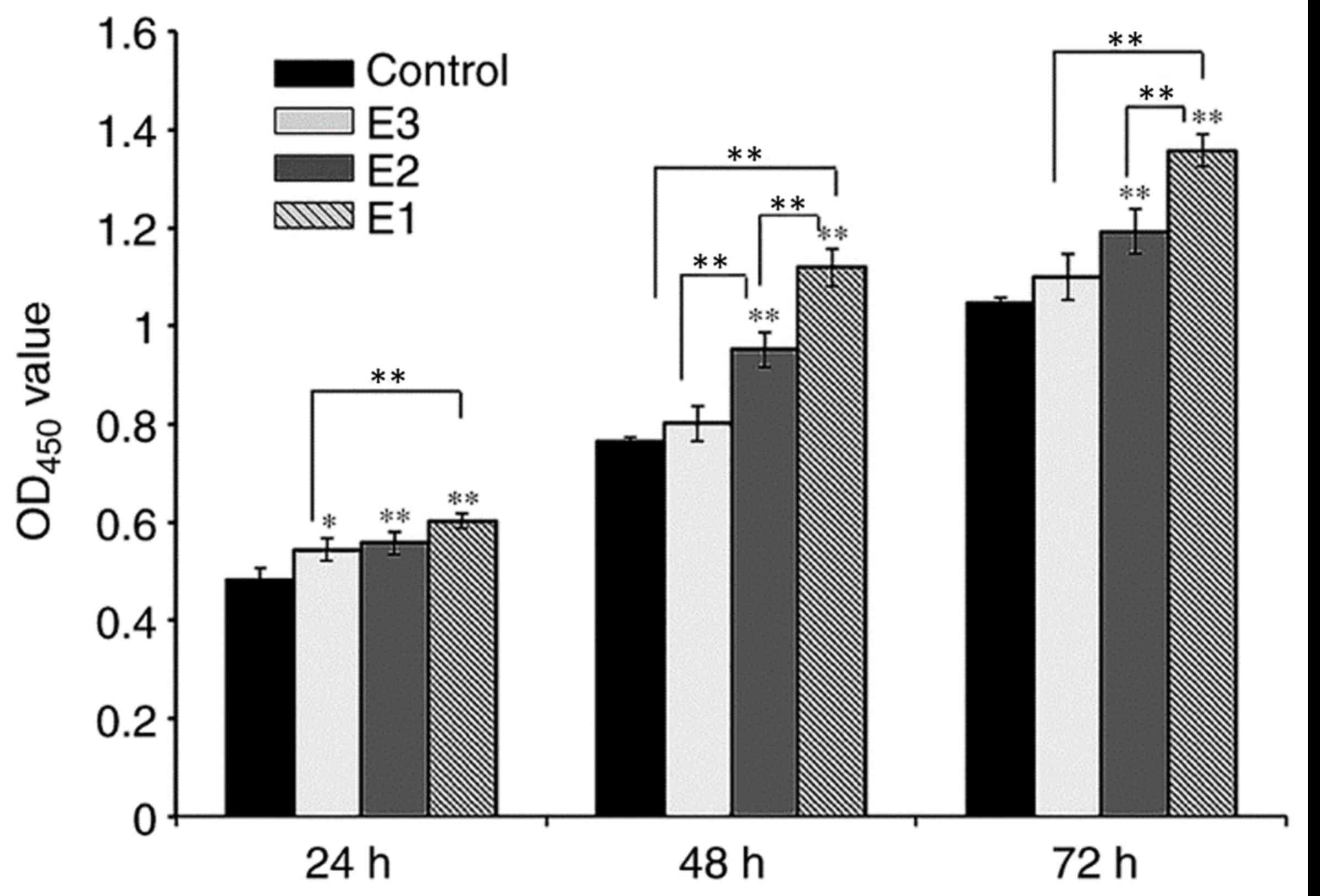
assay (Fig. 3). On day 1, the
difference between the control and experimental groups was
significant (P<0.05), while the difference between the E1 and E2
experimental groups was not significant (P>0.05), and the
difference between E1 and E3 experimental groups was significant
(P<0.01; Fig. 3). On days 2 and
3, when compared with the control group, the proliferation rate of
groups E1 and E2 increased significantly (P<0.01). The PRF
exudates in group E3 also enhanced the proliferation of hPDLCs,
although there was no significant difference between group E3 and
the control group (P>0.05). On days 2 and 3, the difference
between groups E1 and E2 was significant (P<0.05) as was the
difference between groups E1 and E3 (P<0.05), while the
difference between groups E2 and E3 was only significant at the day
2 time interval (P<0.01).
ALP activity
Following 7 or 14 days of culture, the hPDLCs
cultured in the E3 group demonstrated the highest levels of ALP
activity compared with the other experimental groups and the
differences between the control group, and the E2 and E3
experimental groups were significant (P<0.01) at all time
intervals compared with the control (Fig. 4). Furthermore, the ALP activity of
hPDLCs in the E1 group on days 7 and 14 were also upregulated,
although the difference was not significant (P>0.05). As
expected, ALP activity in all groups progressively increased over
time in culture.
Alizarin red staining and
semi-quantification of mineralized matrix nodules
To detect the formation of mineralized matrix
nodules, alizarin red staining was performed (Fig. 5A). Following 14 days of osteogenic
induction, the mineralized nodules were observed by inverted
phase-contrast microscopy and the number of nodules was increased
in the MM + E1 group compared with the MM group. However, it was
hard to detect mineralized nodules in the control group.
 | Figure 5.Alizarin red staining of periodontal
ligament cells and semi-quantification of mineralized nodules. Four
kinds of media: Control medium, E1, MM and E1 + MM. Scale bar, 200
µm. (A) Following 14 days of osteogenic induction, the number of
mineralized nodules in the E1 + MM group was much greater than in
the MM group. Furthermore, the E1 group exhibited an increased
number of mineralized nodules compared with the control group. (B)
The relative mineralized nodule content in the E1 + MM group was
increased compared with the MM group. In addition, compared with
the control group, the relative mineralized nodule content also
increased. **P<0.01; PRF, platelet-rich fibrin; MM,
mineralization-inducing medium; E1, 100% PRF exudate medium. |
Furthermore, the formation level of mineralized
matrix nodules were quantified following 14 days of induction
(Fig. 5B). The absorbance values
at 570 nm revealed that extracellular calcium deposition in the E1
group and the MM + E1 group was significantly increased compared
with the control group or MM group, respectively (P<0.05).
Effect of PRF on the expression of
osteogenic-associated genes
The results of the expression levels of OCN,
OSX (also known as SP7) and RUNX2 genes were
recorded on the 3rd, 5th, and 7th days. It was observed that on the
3rd day, the expression of osteogenic-associated genes RUNX2
and the later marker OSX, were all upregulated in the PRF
treatment groups vs. the corresponding control and MM group, and
the differences became significant by the 5th day (Fig. 6A and B). Following the addition of
the PRF exudate, the expression level of the other late marker,
OCN, decreased on the 3rd day, but was upregulated in the
experimental groups during the following period and was enhanced
significantly by the 7th day (P<0.01; Fig. 6C).
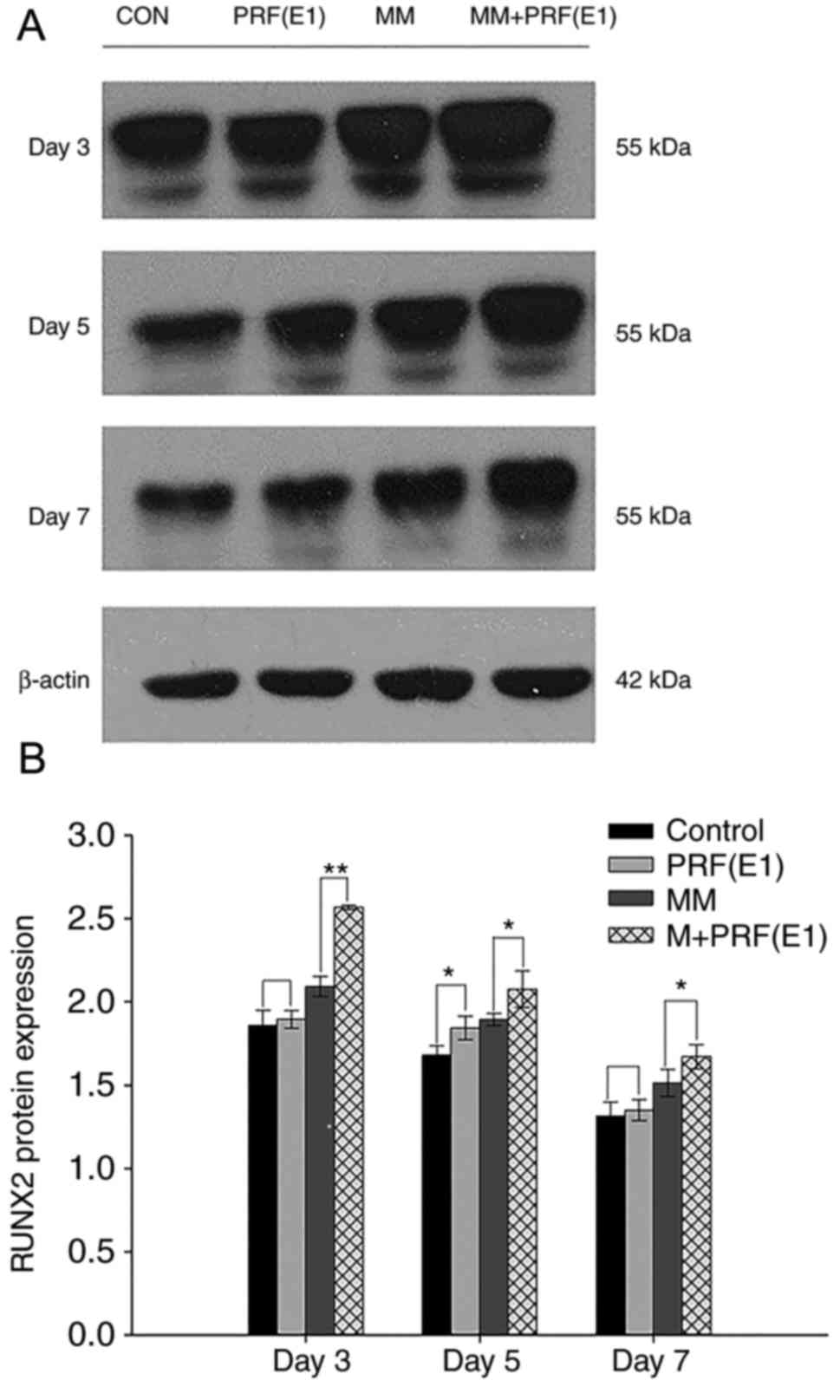
Western blotting was performed to examine the effect
of PRF exudate on hPDLC differentiation and confirm the qPCR
results. Cells were cultured in four varied media for 3, 5, or 7
days. As demonstrated in Fig. 7A and
B, compared with the control group and the MM group, the
protein levels of RUNX2 were upregulated in the E1-treated group
and the MM + E1-treated group at different time points.
Discussion
PRF has been described as a second-generation
platelet concentrate, since it is produced naturally without using
an anticoagulant. Platelet-rich plasma contains various growth
factors, coagulation factors, adhesion molecules, cell factors,
chemokines and binding elements (20). When the platelets are activated,
the α granules release a large variety of growth factors (21) that promote the proliferation and
differentiation of mesenchymal stem cells (22). The PRF clot forms an active fibrin
matrix with a complex three-dimensional architecture, in which most
of the platelets and leukocytes are trapped. Compared with
platelet-rich plasma, PRF obtained by means of Choukroun's protocol
does not decompose quickly following application; instead, the
active PRF membrane is slowly remodeled in a similar way to a
natural blood clot, and releases large amounts of factors and other
matrix glycoproteins for at least 7 days.
The goal of the present study was to assess the
in vitro effect of the soluble materials released from PRF
membranes on the proliferation of hPDLCs. In this study, PRF
demonstrated no cytotoxic impact on hPDLCs, according to the CCK-8
assay, which is considered to be more sensitive compared with
assays based on tetrazolium salts, including MTT (23). Furthermore, the result is
consistent with previous studies in other cell types (24,25).
Earlier studies proved that PRF stimulates proliferation of human
bone mesenchymal stem cells, human osteoblast-derived osteosarcoma
(SaOS2) cells, human keratinocyte-derived carcinoma (KB) cells and
human fibroblast-derived lung carcinoma (MRC5) cells in a similar
way (26,27). However, there has been little study
regarding the effects of PRF on hPDLCs until now. In the present
study, the results of the CCK-8 assay demonstrated that PRF
promotes the proliferation of hPDLCs. The higher proliferation rate
occurred when cells were treated with concentrations E1 (100%) or
E2 (20%). For the lowest concentration, E3 (4%), the improved
effect on cell proliferation was weak or not significant. In other
words, PRF stimulated hPDLC proliferation in a dose-dependent
manner, even though there was not always a significant difference.
These data are consistent with the results reported in previous
studies (28–30).
The enhanced effects on cell proliferation can be
explained as follows. PRF releases platelet-derived growth factor
(31), which is the primary growth
factor in PRF and has been demonstrated to have potent mitogenic
effects on stem cells, osteoblasts and dental pulp cells, and also
promotes angiogenesis and collagen synthesis. Furthermore, other
growth factors trapped in the PRF matrix (32), including insulin-like growth
factor, vascular endothelial growth factor and basic fibroblast
growth factor facilitate periodontal ligament stem cell and Schwann
cell proliferation (31,33), although these cells are fewer in
number. Therefore, all of these growth factors together enhance the
proliferation of hPDLCs.
In addition to the measurement of proliferation in
the present study, the osteogenic differentiation of hPDLCs was
also assessed by analyzing ALP activity, the formation of
mineralized nodules and expression of the early-phase
osteoblast-associated protein RUNX2.
ALP is a membrane enzyme that hydrolyzes phosphate
ions and allows for the formation of hydroxyapatite crystals and
the enhancement of mineralization (34). It is also regarded as a marker of
osteoblastic differentiation at an early phase. Alizarin red is a
marker that directly reflects the osteogenic differentiation of
human umbilical-cord-derived mesenchymal stem cells (35). In this study, ALP activity was
increased following stimulation with PRF. Similar effects have been
reported in which PRF enhances ALP activity in osteoblasts, bone
marrow mesenchymal stem cells and dentritic cell precursors
(20). These results demonstrated
that PRF contributes to the differentiation of hPDLSCs within a
short period.
RUNX2 is a bone transcription factor and is one of
the homologs of the Drosophila runt protein family. It was
previously demonstrated that RUNX2 serves a significant role in
osteogenesis and acts as an early transcriptional regulator in the
process of osteogenic differentiation. Several studies have
observed that RUNX2 gene-deficient mice exhibit a complete
block in bone formation and osteoblast differentiation (36,37).
Additionally, RUNX2 is essential for mesenchymal cell
differentiation into an osteoblastic lineage (36). In accordance with the ALP result in
the present study, the expression of RUNX2 was upregulated by PRF
treatment. Based on the results that the expression of RUNX2 is
increased following induction by PRF, it can be inferred that PRFs
affects osteogenesis by promoting the differentiation of hPDLSCs
into the osteogenic lineage.
Following analysis of this result, which growth
factor contributes to the effect observed in the present study was
investigated. RUNX2 is the primary binding protein induced by
TGF-β1 and bone morphogenetic protein 2. In addition, TGF-β is one
of the major growth factors demonstrated in PRF and it is also
involved in the regulation of multi-lineage differentiation of
cells through the mothers against decapentaplegic homolog (SMAD)
and non-SMAD pathways, including the mitogen-activated protein
kinase, phosphoinositol-3 kinase-protein kinase B, and Rho-like
GTPase signaling pathways (38–40).
For example, TGF-β promotes the differentiation of stem cells into
cardiomyocytes, neurocytes, hepatic stellate cells, dendritic cells
and other cell types (41).
In addition to the function of growth factors in
PRF, it is worthwhile to investigate the role of the leukocytes
that are released by PRF. To a certain extent, cell cultures that
are treated with PRF membrane or exudate are a co-culture with
leukocytes. However, most of the literature concerning platelet
concentrates ignores the impact of these leukocytes, although a
previous study has discussed the issue concerning the immune and
antimicrobial properties of platelet concentrates (42).
PRF, due to its particular architecture, low expense
and ease of production, may be a good choice for periodontal tissue
regeneration. The promotion of the proliferation and
differentiation of hPDLSCs also indicates the possibility of
producing a PRF-hPDLSC conglomerate with several clots of PRF and a
relatively small number of hPDLSCs. Although it has been
demonstrated that PRF significantly facilitates hPDLSC
proliferation and osteogenic differentiation, the ability to
simulate clinical conditions may be limited, and the precise
mechanism has yet to be proven. Finally, a limitation of the
present study is that the blood samples and hPDLCs were not
collected from the same donors, and the immune incompatibility may
have an adverse effect on the experimental results.
In conclusion, we evaluated the effects of PRF
exduate on the proliferation, osteogenic differentiation and
mineralization of hPDLCs in vitro and the results showed
that PRF exduate could enhance hPDLC adhesion, proliferation and
induce the differentiation of hPDLC into mineralized tissue
formation cell; thereby contribute to the main processes of
periodontal tissue regeneration. PRF exduate may therefore provide
potential benefits for periodontal tissue engineering; contributing
to the primary processes of periodontal tissue regeneration. For
economical and biological reasons, PRF exduate has greater clinical
benefits than analogous growth factors. Furthermore, PRF exduate
may be used to improve the early healing process of periodontal
infrabony defects in clinical practice, as it may attenuate
angiogenesis and prevent infectious activity. Future studies should
investigate the underling mechanism of the association between PRF
exduate and the promotion of osteogenic differentiation and
mineralization of hPDLCs, as well as the effect of PRF exudate on
the regeneration of soft and hard periodontal tissue following
infection-induced inflammation.
Acknowledgements
The authors are grateful to the patients and their
families for participating in the study.
Funding
This work was supported by grant from the People's
Hospital of Longhua (Shenzhen, China) and the Health and Family
Planning Commission of Shenzhen Municipality (grant no.
SZFZ2018035).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
XL, HY and BW conceived of and designed the study.
XL, YZ and HL performed cell culture, immunostaining and
proliferation analysis. XL and YZ performed osteogenic
differentiation induction, quantitative polymerase chain reaction
and western blotting. HY, ZZ and ZY provided reagents and performed
data interpretation. XL, HY and BW performed data analysis and
wrote the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Jilin University Health Science Center (Jilin,
China), and subjects provided informed consent.
Patient consent for publication
Patients gave their informed consent.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Villar CC and Cochran DL: Regeneration of
periodontal tissues: Guided tissue regeneration. Dent Clin North
Am. 54:73–92. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Reynolds MA, Aichelmann-Reidy ME and
Branch-Mays GL: Regeneration of periodontal tissue: Bone
replacement grafts. Dent Clin North Am. 54:55–71. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pietruska M, Pietruski J, Nagy K, Brecx M,
Arweiler NB and Sculean A: Four-year results following treatment of
intrabony periodontal defects with an enamel matrix derivative
alone or combined with a biphasic calcium phosphate. Clin Oral
Investig. 16:1191–1197. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Seo BM, Miura M, Gronthos S, Bartold PM,
Batouli S, Brahim J, Young M, Robey PG, Wang CY and Shi S:
Investigation of multipotent postnatal stem cells from human
periodontal ligament. Lancet. 364:149–155. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Silvério KG, Rodrigues TL, Coletta RD,
Benevides L, Da Silva JS, Casati MZ, Sallum EA and Nociti FH Jr:
Mesenchymal stem cell properties of periodontal ligament cells from
deciduous and permanent teeth. J Periodontol. 81:1207–1215. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dohan DM, Choukroun J, Diss A, Dohan SL,
Dohan AJ, Mouhyi J and Gogly B: Platelet-rich fibrin (PRF): A
second-generation platelet concentrate. Part III: Leucocyte
activation: A new feature for platelet concentrates. Oral Surg Oral
Med Oral Pathol Oral Radiol Endod. 101:e51–e55. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Choukroun J, Diss A, Simonpieri A, Girard
MO, Schoeffler C, Dohan SL, Dohan AJ, Mouhyi J and Dohan DM:
Platelet-rich fibrin (PRF): A second-generation platelet
concentrate. Part IV: Clinical effects on tissue healing. Oral Surg
Oral Med Oral Pathol Oral Radiol Endod. 101:e56–e60. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Choukroun J, Diss A, Simonpieri A, Girard
MO, Schoeffler C, Dohan SL, Dohan AJ, Mouhyi J and Dohan DM:
Platelet-rich fibrin (PRF): A second-generation platelet
concentrate. Part V: histologic evaluations of PRF effects on bone
allograft maturation in sinus lift. Oral Surg Oral Med Oral Pathol
Oral Radiol Endod. 101:299–303. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Diss A, Dohan DM, Mouhyi J and Mahler P:
Osteotome sinus floor elevation using Choukroun's platelet-rich
fibrin as grafting material: A 1-year prospective pilot study with
microthreaded implants. Oral Surg Oral Med Oral Pathol Oral Radiol
Endod. 105:572–579. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Park SI, Lee HR, Kim S, Ahn MW and Do SH:
Time-sequential modulation inexpression of growth factors from
platelet-rich plasma (PRP) on the chondrocyte cultures. Mol Cell
Biochem. 361:9–17. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Albanese A, Licata ME, Polizzi B and
Campisi G: Platelet-rich plasma (PRP) in dental and oral surgery:
From the wound healing to bone regeneration. Immun Ageing.
10:232013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee JW, Kwon OH, Kim TK, Cho YK, Choi KY,
Chung HY, Cho BC, Yang JD and Shin JH: Platelet-rich plasma:
Quantitative assessment of growth factor levels and comparative
analysis of activated and inactivated groups. Arch Plast Surg.
40:530–535. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Simonpieri A, Del Corso M, Sammartino G
and Dohan Ehrenfest DM: The relevance of Choukroun's platelet-Rich
fibrin and metronidazole during complex maxillary rehabilitations
using bone allograft. Part 1: A new grafting protocol. Implant
Dent. 18:102–111. 2009.
|
|
14
|
Dohan Ehrenfest DM, de Peppo GM, Doglioli
P and Sammartino G: Slow release of growth factors and
thrombospondin-1 in Choukroun's platelet-rich fibrin (PRF): A gold
standard to achieve for all surgical platelet concentrates
technologies. Growth Factors. 27:63–69. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dohan Ehrenfest DM, Diss A, Odin G,
Doglioli P, Hippolyte MP and Charrier JB: In vitro effects of
Choukroun's PRF (platelet-rich fibrin) on human gingival
fibroblasts, dermal prekeratinocytes, preadipocytes, and
maxillofacial osteoblasts in primary cultures. Oral Surg Oral Med
Oral Pathol Oral Radiol Endod. 108:341–352. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
He L, Lin Y, Hu X, Zhang Y and Wu H: A
comparative study of platelet-rich fibrin (PRF) and platelet-rich
plasma (PRP) on the effect of proliferation and differentiation of
rat osteoblasts in vitro. Oral Surg Oral Med Oral Pathol Oral
Radiol Endod. 108:707–713. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tavakolinejad S, Khosravi M, Mashkani B,
Ebrahimzadeh-Bideskan A, Sanjar-Mossavi N, Parizadeh MR and
Hamidi-Alamdari D: The effect of human platelet-rich plasma on
adipose-derived stem cell proliferation and osteogenic
differentiation. Iran Biomed J. 18:151–157. 2014.PubMed/NCBI
|
|
18
|
Yu J, Deng Z, Shi J, Zhai H, Nie X, Zhuang
H, Li Y and Jin Y: Differentiation of dental pulp stem cells into
regular-shaped dentin-pulp complex induced by tooth germ cell
conditioned medium. Tissue Eng. 12:3097–3105. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gassling V, Hedderich J, Açil Y, Purcz N,
Wiltfang J and Douglas T: Comparison of platelet rich fibrin and
collagen as osteoblast-seeded scaffolds for bone tissue engineering
applications. Clin Oral Implants Res. 24:320–328. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xie XT, Zhang CQ and Tuan RS: Biology of
platelet-rich plasma and its clinical application in cartilage
repair. Arthritis Res Ther. 16:204–211. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jia X, Pan J, Li X, Li N, Han Y, Feng X
and Cui J: Bone marrow mesenchymal stromal cells ameliorate
angiogenesis and renal damage via promoting PI3k-Akt signaling
pathway activation in vivo. Cytotherapy. 18:838–845. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
González-Fernández ML, Pérez-Castrillo S,
Sánchez-Lázaro JA, Prieto-Fernández JG, López-González ME,
Lobato-Pérez S, Colaço BJ, Olivera ER and Villar-Suárez V:
Assessment of regeneration in meniscal lesions by use of
mesenchymal stem cells derived from equine bone marrow and adipose
tissue. Am J Vet Res. 77:779–788. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
An S, Ling J, Gao Y and Xiao Y: Effects of
varied ionic calcium and phosphate on the proliferation, osteogenic
differentiation and mineralization of human periodontal ligament
cells in vitro. J Periodontal Res. 47:374–382. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang FM, Yang SF, Zhao JH and Chang YC:
Platelet-rich fibrin increases proliferation and differentiation of
human dental pulp cells. J Endod. 36:1628–1632. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chang IC, Tsai CH and Chang YC:
Platelet-rich fibrin modulates the expression of extracellular
signal-regulated protein kinase and osteoprotegerin in human
osteoblasts. J Biomed Mater Res A. 95:327–332. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Clipet F, Tricot S, Alno N, Massot M,
Solhi H, Cathelineau G, Perez F, De Mello G and Pellen-Mussi P: In
vitro effects of Choukroun's platelet-rich fibrin conditioned
medium on 3 different cell lines implicated in dental implantology.
Implant Dent. 21:51–56. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Han J, Meng HX, Tang JM, Li SL, Tang Y and
Chen ZB: The effect of different platelet-rich plasma
concentrations on proliferation and differentiation of human
periodontal ligament cells in vitro. Cell Prolif. 40:241–252. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xia L, Zhang Z, Chen L, Zhang W, Zeng D,
Zhang X, Chang J and Jiang X: Proliferation and osteogenic
differentiation of human periodontal ligament cells on akermanite
and β-TCP bioceramics. Eur Cell Mater. 22:68–83. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao YH, Zhang M, Liu NX, Lv X, Zhang J,
Chen FM and Chen YJ: The combined use of cell sheet fragments of
periodontal ligament stem cells and platelet-rich fibrin granules
for avulsed tooth reimplantation. Biomaterials. 34:5506–5520. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dohan Ehrenfest DM, Doglioli P, de Peppo
GM, Del Corso M and Charrier JB: Choukroun's platelet-rich fibrin
(PRF) stimulates in vitro proliferation and differentiation of
human oral bone mesenchymal stem cell in a dose-dependent way. Arch
Oral Biol. 55:185–194. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang X, Zhang Y, Choukroun J, Ghanaati S
and Miron RJ: Effects of an injectable platelet-rich fibrin on
osteoblast behavior and bone tissue formation in comparison to
platelet-rich plasma. Platelets. 29:48–55. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yu Y, Mu J, Fan Z, Lei G, Yan M, Wang S,
Tang C, Wang Z, Yu J and Zhang G: Insulin-like growth factor 1
enhances the proliferation and osteogenic differentiation of human
periodontal ligament stem cells via ERK and JNK MAPK pathways.
Histochem Cell Biol. 137:513–525. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xu FT, Li HM, Yin QS, Liang ZJ, Huang MH,
Chi GY, Huang L, Liu DL and Nan H: Effect of activated
autologousplatelet-rich plasma on proliferation and
osteogenicdifferentiation of human adipose-derived stem cells in
vitro. Am J Transl Res. 7:257–270. 2015.PubMed/NCBI
|
|
35
|
Pereira Lopes FR, Lisboa BC, Frattini F,
Almeida FM, Tomaz MA, Matsumoto PK, Langone F, Lora S, Melo PA,
Borojevic R, et al: Enhancement of sciatic nerve regeneration after
vascular endothelial growth factor (VEGF) gene therapy. Neuropathol
Appl Neurobiol. 37:600–612. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Roohani-Esfahani SI, No YJ, Lu Z, Ng PY,
Chen Y, Shi J, Pavlos NJ and Zreiqat H: A bioceramic with enhanced
osteogenic properties to regulate the function of osteoblastic and
osteocalastic cells for bone tissue regeneration. Biomed Mater.
11:35–38. 2016. View Article : Google Scholar
|
|
37
|
Fowlkes JL, Bunn RC, Liu L, Wahl EC,
Coleman HN, Cockrell GE, Perrien DS, Lumpkin CK Jr and Thrailkill
KM: Runt-related transcription factor 2 (RUNX2) and RUNX2-related
osteogenic genes are down-regulated throughout osteogenesis in type
1 diabetes mellitus. Endocrinology. 149:1697–1704. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Marie PJ: Transcription factors
controlling osteoblastogenesis. Arch Biochem Biophys. 473:98–105.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen L, Zou X, Zhang RX, Pi CJ, Wu N, Yin
LJ and Deng ZL: IGF1 potentiates BMP9-induced osteogenic
differentiation in mesenchymal stem cells through the enhancement
of BMP/Smad signaling. BMB Rep. 49:122–127. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sağsöz H, Liman N and Alan E: Expression
of vascular endothelial growth factor receptors and their ligands
in rat uterus during the postpartum involution period. Biotech
Histochem. 90:361–374. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Corrado A, Neve A and Cantatore FP:
Expression of vascular endothelial growth factor in normal,
osteoarthritic and osteoporotic osteoblasts. Clin Exp Med.
13:81–84. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang MK, Sun HQ, Xiang YC, Jiang F, Su YP
and Zou ZM: Different roles of TGF-β in the multi-lineage
differentiation of stem cells. World J Stem Cells. 4:28–34. 2012.
View Article : Google Scholar : PubMed/NCBI
|