Introduction
Carbon monoxide (CO) is a toxic gas produced by the
incomplete combustion of fossil fuels (1,2). It
is a cause of significant morbidity and mortality worldwide with no
specific antidote. Although both normobaric and hyperbaric oxygen
are used as a common treatment, neurological sequelae are common in
survivors of CO poisoning (3,4). In
the USA, CO poisoning accounts for 50,000 referrals to emergency
departments and causes 334 deaths annually (5). In Iran, the improper use of gasoline
and natural gas appliances cause a significant number of CO
poisoning cases (6). Reports from
different parts of Iran have shown that the mortality and morbidity
rates were higher compared to other parts of the world (6). The majority of cases of CO poisoning
occur in the colder months of the year due to the use of fossil
fuels in heating appliances (7,8).
Therefore, CO poisoning is regarded as one of the most challenging
cases of poisoning in Iranian health system. The pathophysiology of
CO poisoning centers on the production of carboxyhemoglobin, which
reduces the oxyhemoglobin concentration and consequently diminishes
tissue oxygen delivery (9,10). Since CO affinity to hemoglobin is
approximately 230–270-fold higher than that of O2 to
hemoglobin, even at low CO concentrations, the carboxyhemoglobin
concentration becomes sufficiently high to induce toxicity
(9). The clinical manifestations
of CO poisoning are non-specific (i.e., headaches, fatigue,
confusion, nausea, dizziness, visual problems, chest pain,
shortness of breath, loss of consciousness and seizures) and they
are principally associated with the deleterious effects of
normobaric on the brain and heart (11,12).
Magnesium sulfate (MS) is used for the treatment of
several conditions, including eclampsia, pre-eclampsia and the
prevention of torsade de pointes (13). It has attracted the interest of
scientists due to its protective properties against cerebral
ischemia/reperfusion (I/R) (14–16),
as it has been shown to reduce brain cell necrosis, apoptosis and
oxidative stress levels (17–21).
Moreover, MS is inexpensive, widely available, is simple to
administer and lacks severe adverse drug reactions for common uses
in the treatment of pre-eclampsia, eclampsia and torsade de pointes
(22,23).
B-cell lymphoma-2 (Bcl2) controls mitochondrial
membrane permeability in order to impede apoptotic signal
transduction, whereas Bcl2-associated-X protein (Bax), as a
pro-apoptotic factor, disrupts mitochondrial membrane potential and
induces caspase-3 activation, leading to irreversible apoptosis
(24). As shown by recent
literature, the Bax/Bcl2 ratio alone stands as an index of cell
apoptosis or survival (25–28).
In addition, in our previous studies using a model of CO poisoning,
a clear connection between apoptosis and the Bax/Bcl2 ratio was
proven by TUNEL assay and caspase activity measurements (29). Moreover, Akt is regarded as a
pro-survival factor (30,31), whose activation induces
phosphorylation at different sites. Activated Akt influences a
number of factors involved in apoptosis, either by transcription
regulation or direct phosphorylation, yielding favorable effects
against ischemia-induced apoptosis. Thus, chemicals capable of
inducing Akt expression/activity may be used in the treatment of
I/R injury (2,32–35).
Considering the importance of CO poisoning and with
regard to the promising properties of MS, in the present study, we
examined the effects of MS on CO-induced cerebral injury in
rats.
Materials and methods
Animals
In the present study, 25 male Wistar rats (8–10
weeks old; weight, 200–250 g), were obtained from the Animal House
of Zabol University of Medical Sciences (Zabol, Iran). The animals
were kept under standard conditions (at 25°C with a 12 h/12 h
light/dark cycle) and were allowed access to food and water ad
libitum. The present study was approved by the Ethics Committee of
Zabol University of Medical Sciences (approval no.
ZBMU.1.REC.1394.112). All animals were treated in accordance with
the guidelines for the Care And Use Of Laboratory Animals prepared
by the Animal Research Ethics Committee of Zabol University of
Medical Sciences and in conformity with EU Directive 2010/63/EU for
animal experiments. The animals were randomly divided into 5 groups
namely, the intact group (rats that were not exposed to CO), the
control (rats that were exposed to CO and received normal saline)
and 3 MS-treated groups (rats that were exposed to CO and received
MS 75, 150 and 300 mg/kg). CO poisoning was induced by exposing the
animals to CO at 3,000 ppm for 60 min, as previously described
(36). Immediately following the
exposure period, the first dose of MS (or normal saline for the
control group) was administered intraperitoneally (i.p.) and the
next 4 doses were administered on the next 4 consecutive days on a
daily basis (a total of 5 doses of MS).
Chemicals
Protein kinase B (Akt; cat. no. 4685S; dilution,
1/1,000), Bcl2-associated-X (cat. no. 2772S; dilution, 1/1,000),
Bcl2 (cat. no. 2876S; dilution, 1/1,000) and anti-β-actin (cat. no.
4967S; dilution, 1/1,000) antibodies and secondary rabbit antibody
(anti-rabbit IgG, HRP-linked; cat. no. 7074S) were all purchased
from Cell Signaling Technology Inc. (Danvers, MA, USA). The
Coomassie (Bradford) Protein Assay kit was purchased from Thermo
Fisher Scientific, Inc. (Waltham, MA, USA). A CO capsule (99.999%
purity) was obtained from Darman Gas (Tehran, Iran). Thiobarbituric
acid was obtained from Sigma-Aldrich; Merck KGaA (Darmstadt,
Germany) and MS was purchased from Pasteur Institute (Tehran,
Iran).
Study design and treatments
For CO poisoning induction, the rats were placed in
a 12-liter airtight Plexiglas container which was connected via
polyethylene glycol (PEG) tubes to oxygen and CO capsules. The CO
concentration was continuously monitored by a CO analyzer (TPI 707
Carbon Monoxide Analyzer; TPI Korea Co., Anyang, Korea) and had a
constant level of 3,000±100 ppm for 1 h. Subsequently, the animals
were exposed to ambient air and MS was injected (i.p.) at 3 doses
(75, 150 and 300 mg/kg). On the 5th day, at 2 h after the final
injection, the animals were anesthetized by an intraperitoneal
administration of ketamine (90 mg/kg) and xylazine (10 mg/kg) and
sacrificed. The brain samples were then collected and harvested for
further evaluation. Moreover, for western blot analysis and
malondialdehyde (MDA) assay, the harvested samples were preserved
in cryotubes and stored at −80°C, as previously described (37).
Carboxyhemoglobin level
assessment
Within 30 min following exposure CO, blood samples
were obtained from the tail of the animals. The serum
carboxyhemoglobin concentration was measured using a
spectrophotometer calibrated for rat blood (Jenway 6305; Bibby
Scientific Ltd., Staffordshire, UK), to ensure the induction of CO
poisoning (38).
Histopathological examinations
For histopathological evaluation, serial brain
sections (5-µm-thick; corresponding to bregma −3.3 cm) according to
a histological atlas (39) were
obtained. The samples were placed in microtubes containing 10%
formalin for fixation and 24 h later, they were sent to the
Pathology Department of Amiralmomenin Hospital (Zabol, Iran).
Following H&E staining, pathological insults were evaluated
based on the severity of the injury, by a pathologist who was
blinded to the grouping and treatments. The findings were
categorized into 3 grades of mild (dispersed necrotic cells and/or
lymphatic infiltration) (Fig. 1B),
moderate (necrotic unifocal and/or bifocal area) (Fig. 1C), and severe (more than two
necrotic areas) (Fig. 1D)
insults.
Bax, Bcl2 and Akt protein expression
assessment
For the determination of Akt, Bax, Bcl2 and β-actin
expression, western blot analysis was performed. For this purpose,
first, approximately 200 mg of harvested whole brain samples which
were kept at −80°C, were weighed, homogenized using a mechanical
homogenizer, sonicated and centrifuged using a refrigerated
centrifuge at 10,000 × g at 4°C for 10 min. The supernatants were
then collected, the protein contents were measured using the
Coomassie (Bradford) Protein Assay kit which was purchased from
Thermo Fisher Scientific, Inc. and samples were placed in a hot
bath (boiling water) for the denaturation of proteins.
In order to determine the total Akt, Bax, Bcl2 and
β-actin levels, 5–10 µl of supernatant was loaded into 12% SDS page
wells and proteins were separated using gel electrophoresis
(Bio-Rad power supply, 120 v for 1.5 h; Bio-Rad, Hercules, CA,
USA). At the end of the electrophoresis period, proteins were
transferred (Bio-Rad power supply, 350 mA, 25–45 min; Bio-Rad) to a
PVDF membrane using transfer buffer (25 mM Tris, 1.2 mM glycine,
and 20% methanol; pH 8.0). The membrane was washed 3 times (each
time for 5 min) with Tris-buffered saline (TBS). After blocking in
5% non-fat milk in TBST (0.5% Tween-20, 137 mM NaCl, 20 mM Tris–HCl
pH 7.5) overnight at 4°C, the membranes were incubated with the
primary antibodies for 1 h at room temperature on a rocker. The
membrane was then washed 5 times (each time for 5 min) in washing
buffer, in order to remove any unbound conjugate proteins. The
samples were then treated with the secondary antibody for 1 h at
room temperature, washed thoroughly with TBST and visualized by
means of 500–1,000 µl of enhanced chemiluminescence (Pierce,
Rockford, IL, USA) to visualize the blots using Syngene ChemiDoc
(Syngene, Frederick, MD, USA). Eventually, blot analysis was
carried out using GeneTools software.
Thiobarbituric acid reactive
substances (TBARS) assay
MDA is a product of lipid peroxidation that can be
measured by spectrophotometric methods (40,41).
In this study, the brain samples (200 mg) were homogenized in cold
1.15% potassium chloride to yield a 10% homogenate. Subsequently,
0.5 ml of the 10% homogenate was mixed with 3 ml of phosphoric acid
1% w/v, boiled for 45 min at 95°C and centrifuged at 12,000 × g for
10 min. After cooling to room temperature, 4 ml n-butanol was added
and the reaction mixture was vortexed. The absorbance of the
supernatant was measured at 532 nm (40) using a spectrophotometer (Jenway
6305; Bibby Scientific Ltd.) and the level of MDA was expressed as
nmol per gram of wet tissue.
Statistical analysis
Data were analyzed using SPSS version 16 software
(SPSS, Inc., Chicago, IL, USA). One-way analysis of variance
followed by Tukey's post hoc test, was used for data analysis.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Carboxyhemoglobin concentration and
the effect of MS on CO-induced brain histological insults
The mean blood carboxyhemoglobin concentration was
70±8% in the CO-exposed rats. Brain histopathological evaluations
revealed that MS treatment decreased the number and intensity of
brain insults in the CO-poisoned rats. As shown in Fig. 1, brain samples were stained with
H&E and brain insults were categorized as mild (dispersed
necrotic cells and/or lymphocytic infiltration), moderate (necrotic
cells with low foci) and severe (multi foci necrosis). As shown in
Table I, in the control group
(normal saline-treated), 2 out of the 5 animals exhibited mild
insults, 1 out of 5 had moderate insults and 2 out of 5 had severe
insults, whereas in the group treated with MS at 300 mg/kg, 1 out
of 5 had mild and moderate insults and no animal showed severe
insults. Furthermore, in the animals treated with MS at 75 and 150
mg/kg, the number and severity of insults were decreased. Taken
together, these results demonstrated that as compared to the
control animals, all MS doses reduced CO-induced damage to the
brain tissues (Table I).
 | Table I.Histological findings in intact rats
and in rats exposed to CO at 3,000 ppm for 1 h and treated with
normal saline (control) and MS at 75, 150 and 300 mg/kg. |
Table I.
Histological findings in intact rats
and in rats exposed to CO at 3,000 ppm for 1 h and treated with
normal saline (control) and MS at 75, 150 and 300 mg/kg.
| Treatment | No injury | Mild | Moderate | Severe |
|---|
| Intact | 5/5 | 0/5 | 0/5 | 0/5 |
| Normal saline | 0/5 | 2/5 | 1/5 | 2/5 |
| MS 75 mg/kg | 1/5 | 1/5 | 2/5 | 1/5 |
| MS 150 mg/kg | 3/5 | 0/5 | 1/5 | 1/5 |
| MS 300 mg/kg | 3/5 | 1/5 | 1/5 | 0/5 |
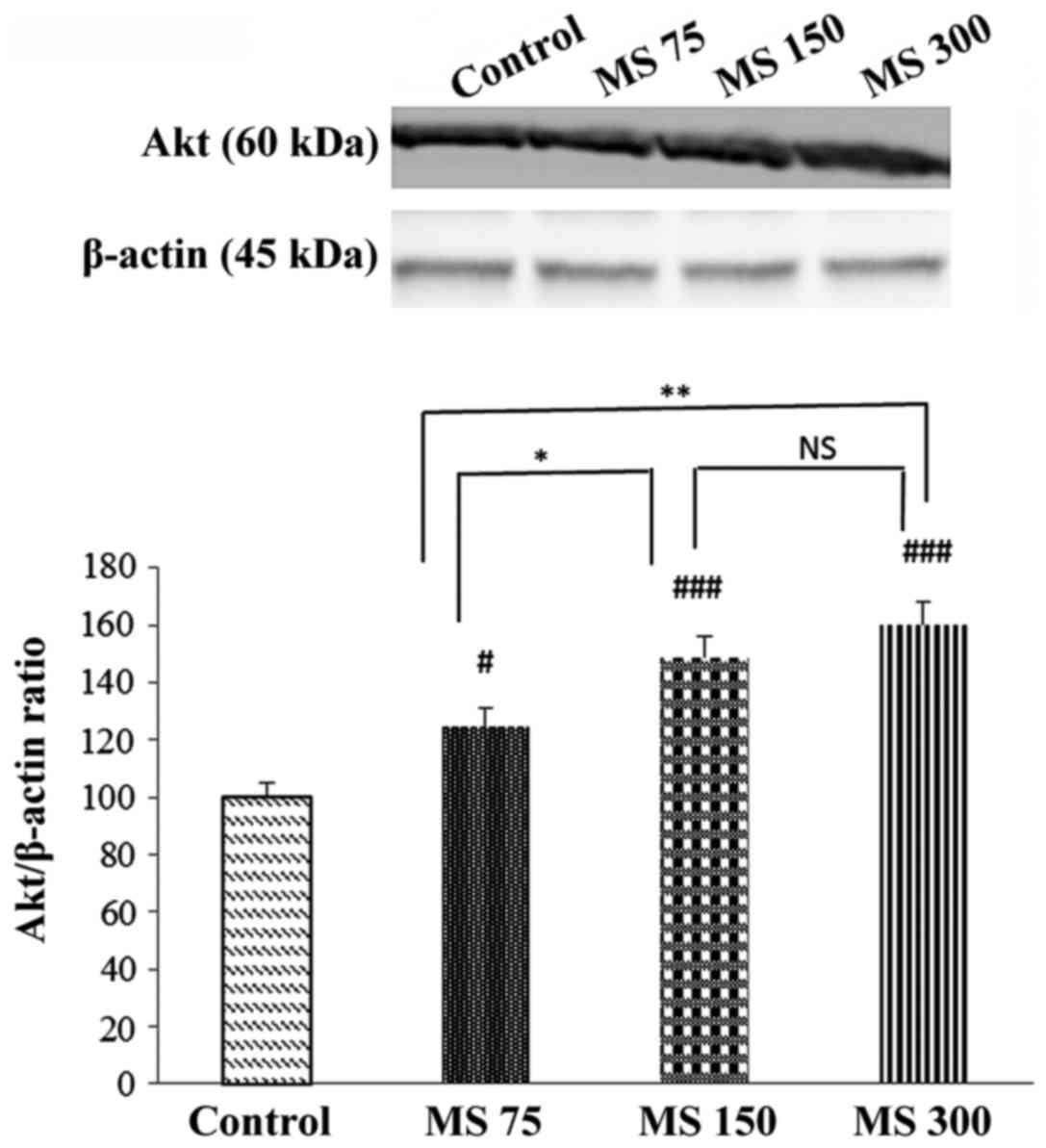
Effects of MS on Akt protein levels in
CO-poisoned rats
As depicted in Fig.
2, the expression levels of Akt, as a pro-survival protein,
significantly increased following treatment with MS as compared to
the control group (normal saline-treated rats). In addition,
significant differences were observed between the MS 300 and MS 75
(P<0.01), and between the MS 150 and MS 75 (P<0.05) groups.
Furthermore, the differences between MS treatment at 150 and 300 mg
and the control groups were significant (for both cases
P<0.001).
Effect of MS on Bax/Bcl2 protein ratio
in brain cells following treatment with MS in CO-poisoned rats
Compared to the control group, in the MS treatment
groups, Bax protein expression levels were decreased, while Bcl2
(an anti-apoptotic protein) expression levels were increased
(Fig. 3). It was evident that
following treatment with MS at 150 and 300 mg/kg, the Bax/Bcl2
ratio was decreased in comparison to the control group (P<0.01
and P<0.001, respectively). Based on our data, MS was able to
decrease the Bax/Bcl2 apoptotic index in brain cells following CO
poisoning.
TBARS assay
The present study demonstrated that oxidative stress
was increased following CO poisoning compared to the control
(P<0.001). MS treatment (75, 150 and 300 mg/kg) dose-dependently
decreased the oxidative stress levels in rats in comparison to the
control group (P<0.05, P<0.01 and P<0.001, respectively)
(Fig. 4).
Discussion
The neuro-protective properties of MS make it a
potential candidate for the alleviation of the deleterious effects
of cerebral I/R injury (17).
Since CO induces damage by inducing hypoxia, tissues with a greater
O2 consumption, including the heart and brain, are more
vulnerable to the effects of CO poisoning (9,42).
CO poisoning intensity depends on a number of factors, including CO
levels during exposure and the exposure period (9). Furthermore, CO poisoning has acute
and delayed consequences (1,39,43).
In the current study, we demonstrated that MS decreased brain cell
necrosis, apoptosis and oxidative stress, while it increased
pro-survival Akt protein levels in a dose-dependent manner.
Previously, we reported several neuroprotective and
cardioprotective substances, which may be used to decrease CO
poisoning consequences, in animal models (2,33,36,37,42,44–46).
More specifically, in a recent study from our group, MS
administration was found to exert positive effects against the
cardiotoxicity of CO in rats (33). Magnesium dilates blood vessels and
lowers the heart rate. However, the early administration of
magnesium in high-risk patients has been shown to have no effect on
mortality (47).
Moreover, CO poisoning induces cerebral hypoxia that
may lead to infarction and necrosis (9). In previous studies, the potent
effects of MS in decreasing the infarct size in animal models were
observed (14,17). Marinov et al demonstrated
that the intra-arterial administration of a single dose of MS 90
mg/kg reduced the cerebral infarct size in animals submitted to
reversible middle cerebral artery occlusion (17). Consistent with this, the results of
this study revealed that MS (at 75, 150 and 300 mg/kg) decreased
the number and intensity of cerebral insults in a dose-dependent
manner in an animal model of CO poisoning.
It is known that Akt protein plays a key role in
cell survival by inhibiting apoptosis (48). Mechanistically, PI3kinase/Akt (also
known as protein kinase B) pathway activation is considered
neuroprotective in the case of cerebral I/R (48–50).
In the present study, it was found that MS increased brain Akt
protein expression levels and decreased apoptosis in the context of
CO poisoning. This finding is consistent with the observations of
Yu et al, indicating that the PI3kinase/Akt pathway
activation is critically important for brain cell survival in case
of cerebral ischemia through decreasing apoptosis (50).
In addition, the present study demonstrated that MS
treatment decreased the Bax/Bcl2 ratio, an apoptotic index, in
brain tissues post-CO poisoning. Bax is a mitochondrial
pro-apoptotic protein, the expression of which increases during the
activation of the intrinsic apoptotic pathway and leads to
mitochondrial injury (51). At the
same time, Bcl2 is an anti-apoptotic protein which is produced in
order to counteract pro-apoptotic signals and protect from
mitochondrial injuries (52). The
Bax/Bcl2 ratio is considered a measure of cell susceptibility to
apoptosis (53) and in the current
study, this ratio was found to be significantly decreased following
MS administration in a dose-dependent manner. Ravishankar et
al demonstrated that cerebral ischemia increased apoptosis via
increasing pro-apoptotic Bax and decreasing anti-apoptotic Bcl2
expression in an animal model of cerebral hypoxia (54). The results of this study are in
agreement with the data reported by several previous studies on MS
effects in animal models of cerebral I/R injury and clinical
studies on global cerebral ischemia associated with cardiac arrest
and cardiac surgery which showed MS neuro-protective and cerebral
anti-apoptotic properties (15,21,55).
Oxidative stress is able to damage cell components
(e.g., proteins, DNA and lipids) and organelles (56) and has been shown to be related with
various pathological conditions (57,58);
increased oxidative stress leads to neuronal death by damaging
brain cellular lipids, proteins and nucleic acids and induces
apoptosis through the transcription of the pro-apoptotic BID and
BAD factors (59). Yavuz et
al demonstrated that a single dose of MS reduced brain
oxidative stress following CO poisoning and that the
intraperitoneal administration of MS at 100 mg/kg was sufficient to
significantly decrease lipid peroxidation (60). The findings of this study (for a
brief summary, see Fig. 5) are in
agreement with that study as MS treatment reduced lipid
peroxidation at all 3 doses (75, 150 and 300 mg/kg) and proved that
lipid peroxidation co-exists with apoptotic induction in CO
poisoning and that brain cell Akt pathway activation favorably
modulates the Bax/Bcl2 apoptotic index downstream (60).
In conclusion, the current study demonstrated that
MS administration decreased the deleterious effects of CO poisoning
on the brain by decreasing neuronal necrosis and apoptosis and
reducing oxidative stress, while increasing Akt expression in brain
cells.
Acknowledgements
The authors would like to thank the Pathology
Laboratories of Amiralmomenin Hospital (Zabol University of Medical
Sciences) for their kind assistance.
Funding
The present study was part of a Pharm. D. funded by
Zabol University of Medical Sciences and Students Research
Committee of Zabol University of Medical Sciences.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
GB and RR and MH conceived and designed, and
supervised the study. JS, MS, KTa and SB collected and analyzed the
data. HJ performed the histopothological analysis. KTs, AOD, AAM,
MFW, AT and DAS interpreted the data and prepared the manuscript.
All authors have read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Zabol University of Medical Sciences, Zabol, Iran
(approval no. ZBMU.1.REC.1394.112). All the animals were treated in
accordance with the guidelines for care and use of laboratory
animals prepared by the Animal Research Ethics Committee of Zabol
University of Medical Sciences and in conformity with EU Directive
2010/63/EU for animal experiments.
Patient consent for publication
Not applicable.
Competing interests
DAS is the Editor-in-Chief for the journal, but had
no personal involvement in the reviewing process, or any influence
in terms of adjudicating on the final decision, for this article.
All the remaining authors have no competing interests to
disclose.
References
|
1
|
Weaver LK, Hopkins RO, Chan KJ, Churchill
S, Elliott CG, Clemmer TP, Orme JF Jr, Thomas FO and Morris AH:
Hyperbaric oxygen for acute carbon monoxide poisoning. N Engl J
Med. 347:1057–1067. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tabrizian K, Shahraki J, Bazzi M, Rezaee
R, Jahantigh H and Hashemzaei M: Neuro-Protective Effects of
Resveratrol on Carbon Monoxide-Induced Toxicity in Male Rats.
Phytother Res. 31:1310–1315. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rose JJ, Wang L, Xu Q, McTiernan CF, Shiva
S, Tejero J and Gladwin MT: Carbon monoxide poisoning:
Pathogenesis, management, and future directions of therapy. Am J
Respir Crit Care Med. 195:596–606. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shahsavand S, Mohammadpour AH, Rezaee R,
Behravan E, Sakhtianchi R and Moallem SA: Effect of erythropoietin
on serum brain-derived biomarkers after carbon monoxide poisoning
in rats. Iran J Basic Med Sci. 15:752–758. 2012.PubMed/NCBI
|
|
5
|
Hampson NB: Cost of accidental carbon
monoxide poisoning: A preventable expense. Prev Med Rep. 3:21–24.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Khadem-Rezaiyan M and Afshari R: Carbon
monoxide poisoning in Northeast of Iran. J Forensic Leg Med.
41:1–4. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ghorani-Azam A, Riahi-Zanjani B and
Balali-Mood M: Effects of air pollution on human health and
practical measures for prevention in Iran. J Res Med Sci.
21:652016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mirahmadizadeh A, Faramarzi H, Hadizadeh
E, Moghadami M, Fardid M and Seifi A: A yearlong epidemiologic
study on unintentional acute carbon monoxide poisoning in Fars
province, Southwest Iran. Asia Pac J Med Toxicol. 5:15–19.
2016.
|
|
9
|
Winter PM and Miller JN: Carbon monoxide
poisoning. JAMA. 236:1502–1504. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ghosh A, Banerjee S, Mitra A, Muralidharan
M, Roy B, Banerjee R, Mandal AK and Chatterjee IB: Interaction of
p-benzoquinone with hemoglobin in smoker's blood causes alteration
of structure and loss of oxygen binding capacity. Toxicol Rep.
3:295–305. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Smollin C and Olson K: Carbon monoxide
poisoning (acute). BMJ Clin Evid. 12–Oct;(pii): 21032010.PubMed/NCBI
|
|
12
|
Vargas R and Ponce-Canchihuamán J:
Emerging various environmental threats to brain and overview of
surveillance system with zebrafish model. Toxicol Rep. 4:467–473.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hashemzaei AM, Keykha BS, Shahraki CF,
Mohammadpour DA and Tabrizian EK: Effects of magnesium sulfate on
the acquisition and reinstatement of morphine-induced conditioned
place preference in mice. J Fundam Appl Sci. 8:112–123. 2016.
View Article : Google Scholar
|
|
14
|
Lin JY, Chung SY, Lin MC and Cheng FC:
Effects of magnesium sulfate on energy metabolites and glutamate in
the cortex during focal cerebral ischemia and reperfusion in the
gerbil monitored by a dual-probe microdialysis technique. Life Sci.
71:803–811. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pearce A, Lockwood C, van den Heuvel C and
Pearce J: The use of therapeutic magnesium for neuroprotection
during global cerebral ischemia associated with cardiac arrest and
cardiac surgery in adults: A systematic review. JBI Database Syst
Rev Implement Reports. 15:86–118. 2017. View Article : Google Scholar
|
|
16
|
Zhao L, Wang W, Zhong J, Li Y, Cheng Y, Su
Z, Zheng W and Guan XD: The effects of magnesium sulfate therapy
after severe diffuse axonal injury. Ther Clin Risk Manag.
12:1481–1486. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Marinov MB, Harbaugh KS, Hoopes PJ, Pikus
HJ and Harbaugh RE: Neuroprotective effects of preischemia
intraarterial magnesium sulfate in reversible focal cerebral
ischemia. J Neurosurg. 85:117–124. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Muir KW: Magnesium for neuroprotection in
ischaemic stroke: Rationale for use and evidence of effectiveness.
CNS Drugs. 15:921–930. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
O'Collins VE, Macleod MR, Donnan GA, Horky
LL, van der Worp BH and Howells DW: 1,026 experimental treatments
in acute stroke. Ann Neurol. 59:467–477. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang Y, Li Q, Ahmad F and Shuaib A:
Survival and histological evaluation of therapeutic window of
post-ischemia treatment with magnesium sulfate in embolic stroke
model of rat. Neurosci Lett. 285:119–122. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou H, Ma Y, Zhou Y, Liu Z, Wang K and
Chen G: Effects of magnesium sulfate on neuron apoptosis and
expression of caspase-3, bax and bcl-2 after cerebral
ischemia-reperfusion injury. Chin Med J (Engl). 116:1532–1534.
2003.PubMed/NCBI
|
|
22
|
Dubé L and Granry JC: The therapeutic use
of magnesium in anesthesiology, intensive care and emergency
medicine: a review. Can J Anaesth. 50:732–746. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Karimani A, Mohammadpour AH, Zirak MR,
Rezaee R, Megarbane B, Tsatsakis A and Karimi G: Antidotes for
aluminum phosphide poisoning - An update. Toxicol Rep. 5:1053–1059.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu X, Zhang H, Wang K, Tu T and Jiang Y:
Protective effect of edaravone against carbon monoxide induced
apoptosis in rat primary cultured astrocytes. Biochem Res Intern.
doi.org/10.1155/2017/5839762.
|
|
25
|
Song S, Jacobson KN, McDermott KM, Reddy
SP, Cress AE, Tang H, Dudek SM, Black SM, Garcia JG, Makino A and
Yuan JXJ: ATP promotes cell survival via regulation of cytosolic
[Ca2+] and Bcl-2/Bax ratio in lung cancer cells. Am J
Physiol Cell Physiol. 310:C99–C114. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang B, Liu Y, Li Y, Zhe X, Zhang S and
Zhang L: Neuroglobin promotes the proliferation and suppresses the
apoptosis of glioma cells by activating the PI3K/AKT pathway. Mol
Med Rep. 17:2757–2763. 2018.PubMed/NCBI
|
|
27
|
Wang F, Li H and Qiao JO: 1 O
acetylbritannilactone combined with gemcitabine elicits growth
inhibition and apoptosis in A549 human non small cell lung cancer
cells. Mol Med Rep. 12:5568–5572. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hashemzaei M, Delarami Far A, Yari A,
Heravi RE, Tabrizian K, Taghdisi SM, Sadegh SE, Tsarouhas K,
Kouretas D, Tzanakakis G, et al: Anticancer and apoptosis inducing
effects of quercetin in vitro in vivo. Oncol Rep.
38:819–828. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rezaee MA, Mohammadpour AH, Imenshahidi M,
Mahmoudi M, Sankian M, Tsarouhas K, Tsakalof A, Tsatsakis AM and
Moallem SA: Protective effect of erythropoietin on myocardial
apoptosis in rats exposed to carbon monoxide. Life Sci.
148:118–124. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang Y, Li Y, Song L, Li Y, Jiang S and
Zhang S: The transplantation of Akt-overexpressing amniotic
fluid-derived mesenchymal stem cells protects the heart against
ischemia-reperfusion injury in rabbits. Mol Med Rep. 14:234–242.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang P, Zhao J, Hou L, Yang L, Wu K and
Zhang L: Vitamin E succinate induces apoptosis via the PI3K/AKT
signaling pathways in EC109 esophageal cancer cells. Mol Med Rep.
14:1531–1537. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Luan Q, Pan L, He D, Gong X and Zhou H:
SC79, the AKT Activator Protects Cerebral Ischemia in a Rat Model
of Ischemia/Reperfusion Injury. Med Sci Monit. 24:5391–5397. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tabrizian K, Khodayari H, Rezaee R,
Jahantigh H, Bagheri G, Tsarouhas K and Hashemzaei M: Magnesium
sulfate protects the heart against carbon monoxide-induced
cardiotoxicity in rats. Res Pharm Sci. 13:65–72. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Huang C, Guo X, Zhao H, An R, Lian K,
Zhang X, Zhang J, Yan F, Xie H, Wang S and Tao L: Nicotine induces
H9C2 cell apoptosis via Akt protein degradation. Mol Med Rep.
16:6269–6275. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kashafi E, Moradzadeh M, Mohamadkhani A
and Erfanian S: Kaempferol increases apoptosis in human cervical
cancer HeLa cells via PI3K/AKT and telomerase pathways. Biomed
Pharmacother. 89:573–577. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hashemzaei M, Barani AK, Iranshahi M,
Rezaee R, Tsarouhas K, Tsatsakis AM, Wilks MF and Tabrizian K:
Effects of resveratrol on carbon monoxide-induced cardiotoxicity in
rats. Environ Toxicol Pharmacol. 46:110–115. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mohamadpour AH, Moallem SA, Hashemzaei M,
Abnous K, Tabatabaee Yazdi SA and Imenshahidi M: Effects of
granulocyte colony-stimulating factor on electrocardiogram changes
after carbon monoxide poisoning in rats. Drug Chem Toxicol.
35:353–360. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Rodkey FL, Hill TA, Pitts LL and Robertson
RF: Spectrophotometric measurement of carboxyhemoglobin and
methemoglobin in blood. Clin Chem. 25:1388–1393. 1979.PubMed/NCBI
|
|
39
|
Moallem SA, Mohamadpour AH, Abnous K,
Sankian M, Sadeghnia HR, Tsatsakis A and Shahsavand S:
Erythropoietin in the treatment of carbon monoxide neurotoxicity in
rat. Food Chem Toxicol. 86:56–64. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Draper HH and Hadley M: Malondialdehyde
determination as index of lipid peroxidation. Methods Enzymol.
186:421–431. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hasanzadeh D, Mahdavi M, Dehghan G and
Charoudeh HN: Farnesiferol C induces cell cycle arrest and
apoptosis mediated by oxidative stress in MCF-7 cell line. Toxicol
Rep. 4:420–426. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ghorbani M, Mohammadpour AH, Abnous K,
Movassaghi AR, Sarshoori JR, Shahsavand S, Hashemzaei M and Moallem
SA: G-CSF administration attenuates brain injury in rats following
carbon monoxide poisoning via different mechanisms. Environ
Toxicol. 32:37–47. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Guzman JA: Carbon monoxide poisoning. Crit
Care Clin. 28:537–548. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hashemzaei M, Imen Shahidi M, Moallem SA,
Abnous K, Ghorbani M and Mohamadpour AH: Modulation of JAK2, STAT3
and Akt1 proteins by granulocyte colony stimulating factor
following carbon monoxide poisoning in male rat. Drug Chem Toxicol.
39:375–379. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Tabrizian K, Shahriari Z, Rezaee R,
Jahantigh H, Bagheri G, Tsarouhas K, Docea AO, Tsatsakis A and
Hashemzaei M: Cardioprotective effects of insulin on carbon
monoxide-induced toxicity in male rats. Hum Exp Toxicol. Jan
1–2018.doi:10.1177/0960327118788134. PubMed/NCBI
|
|
46
|
Hashemzaei M, Mohammadpour AH, Imenshahidi
M, Rezaee R and Moallem SA: Does granulocyte colony stimulating
factor have protective effects against carbon monoxide-induced
apoptosis? Biologia. 73:1153–1157. 2018. View Article : Google Scholar
|
|
47
|
Antman EM: Early administration of
intravenous magnesium to high-risk patients with acute myocardial
infarction in the Magnesium in Coronaries (MAGIC) Trial: A
randomised controlled trial. The Lancet. 360:1189–1196. 2002.
View Article : Google Scholar
|
|
48
|
Zhang H, Xiong X, Liu J, Gu L, Li F, Wan Y
and Xu S: Emulsified Isoflurane Protects Against Transient Focal
Cerebral Ischemia Injury in Rats via the PI3K/Akt Signaling
Pathway. Anesth Analg. 122:1377–1384. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wen X-R, Fu Y-Y, Liu H-Z, Wu J, Shao XP,
Zhang XB, Tang M, Shi Y, Ma K, Zhang F, et al: Neuroprotection of
sevoflurane against Ischemia/Reperfusion-induced brain injury
through inhibiting JNK3/Caspase-3 by enhancing Akt signaling
pathway. Mol Neurobiol. 53:1661–1671. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yu Z-H, Cai M, Xiang J, Zhang ZN, Zhang
JS, Song XL, Zhang W, Bao J, Li WW and Cai DF: PI3K/Akt pathway
contributes to neuroprotective effect of Tongxinluo against focal
cerebral ischemia and reperfusion injury in rats. J Ethnopharmacol.
181:8–19. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Scorrano L, Oakes SA, Opferman JT, Cheng
EH, Sorcinelli MD, Pozzan T and Korsmeyer SJ: BAX and BAK
regulation of endoplasmic reticulum Ca2+ A control point
for apoptosis. Science. 300:135–139. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Edlich F and Martinou JC: Bcl-2 protein
interplay on the outer mitochondrial membrane. Mitochondria and
Cell Death. Hockenbery DM: Springer Sci Bus Med; New York, NY: pp.
69–83. 2016, View Article : Google Scholar
|
|
53
|
Raisova M, Hossini AM, Eberle J, Riebeling
C, Wieder T, Sturm I, Daniel PT, Orfanos CE and Geilen CC: The
Bax/Bcl-2 ratio determines the susceptibility of human melanoma
cells to CD95/Fas-mediated apoptosis. J Invest Dermatol.
117:333–340. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ravishankar S, Ashraf QM, Fritz K, Mishra
OP and Delivoria-Papadopoulos M: Expression of Bax and Bcl-2
proteins during hypoxia in cerebral cortical neuronal nuclei of
newborn piglets: Effect of administration of magnesium sulfate.
Brain Res. 901:23–29. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Chamorro Á, Dirnagl U, Urra X and Planas
AM: Neuroprotection in acute stroke: Targeting excitotoxicity,
oxidative and nitrosative stress, and inflammation. Lancet Neurol.
15:869–881. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Rezaee R, Behravan E, Behravan J, Soltani
F, Naderi Y, Emami B and Iranshahi M: Antigenotoxic activities of
the natural dietary coumarins umbelliferone, herniarin and
7-isopentenyloxy coumarin on human lymphocytes exposed to oxidative
stress. Drug Chem Toxicol. 37:144–148. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Yaribeygi H, Mohammadi MT, Rezaee R and
Sahebkar A: Crocin improves renal function by declining Nox-4,
IL-18, and p53 expression levels in an experimental model of
diabetic nephropathy. J Cell Biochem. 119:6080–6093. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Yaribeygi H, Mohammadi MT, Rezaee R and
Sahebkar A: Fenofibrate improves renal function by amelioration of
NOX-4, IL-18, and p53 expression in an experimental model of
diabetic nephropathy. J Cell Biochem. 119:7458–7469. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Mattson MP: Apoptosis in neurodegenerative
disorders. Nat Rev Mol Cell Biol. 1:120–129. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Yavuz Y, Mollaoglu H, Yürümez Y, Ucok K,
Duran L, Tünay K and Akgün L: Therapeutic effect of magnesium
sulphate on carbon monoxide toxicity-mediated brain lipid
peroxidation. Eur Rev Med Pharmacol Sci. 17 Suppl 1:28–33.
2013.PubMed/NCBI
|
|
61
|
Koning G, Leverin A-L, Nair S,
Schwendimann L, Ek J, Carlsson Y, Gressens P, Thornton C, Wang X,
Mallard C and Hagberg H: Magnesium induces preconditioning of the
neonatal brain via profound mitochondrial protection. J Cereb Blood
Flow Metab. 1–Jan;2017.(Epub ahead of print). https://doi.org/10.1177/0271678X17746132
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Kaya M, Gulturk S, Elmas I, Kalayci R,
Arican N, Kocyildiz ZC, Kucuk M, Yorulmaz H and Sivas A: The
effects of magnesium sulfate on blood-brain barrier disruption
caused by intracarotid injection of hyperosmolar mannitol in rats.
Life Sci. 76:201–212. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Ravn HB, Vissinger H, Kristensen SD,
Wennmalm A, Thygesen K and Husted SE: Magnesium inhibits platelet
activity--an infusion study in healthy volunteers. Thromb Haemost.
75:939–944. 1996.PubMed/NCBI
|
|
64
|
Watson KV, Moldow CF, Ogburn PL and Jacob
HS: Magnesium sulfate: Rationale for its use in preeclampsia. Proc
Natl Acad Sci USA. 83:1075–1078. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Altura BM, Altura BT, Carella A, Gebrewold
A, Murakawa T and Nishio A: Mg2+-Ca2+
interaction in contractility of vascular smooth muscle:
Mg2+ versus organic calcium channel blockers on myogenic
tone and agonist-induced responsiveness of blood vessels. Can J
Physiol Pharmacol. 65:729–745. 1987. View Article : Google Scholar : PubMed/NCBI
|