Introduction
Drosophila is an excellent model organism for
the study of male fertility and spermatogenesis (1–3).
Drosophila has a short life cycle, distinct development
stages, and a streamlined genome with high levels of gene
conservation, with certain genes similar to those in humans
(3). Furthermore, various genetic
tools and reagent resources for complex biological processes are
available in Drosophila (4,5).
The process of spermatogenesis is conserved between
Drosophila and humans (6,7). A
number of genes including Boule homolog, RNA binding protein and
Deleted in azoospermia have been identified to be involved in male
fertility, sharing similar phenotypes in Drosophila and
humans (8,9). Spermatogenesis in the adult
Drosophila testis involves germline stem cells,
spermatogonia, spermatocytes, spermatids and sperm (10). At the tip of the Drosophila
testis, a stem cell niche controls the maintenance and
differentiation of germline stem cells (GSCs) and cyst stem cells
(CySCs) by somatic hub cells. GSCs divide to generate two cell
types: A new stem cell and a gonialblast that proliferates and
differentiates into spermatocytes (7,11).
Cyst cells provide a microenvironment for germ cell proliferation,
growth and differentiation (6).
Adenosine 5′-triphosphate (ATP) synthase is an
enzyme complex that creates the energy storage molecule ATP, which
is commonly used to provide energy for the majority of tissues and
organisms (12,13). Mitochondrial ATP synthase has been
identified in the mitochondrial inner membrane and is involved in
oxidative phosphorylation, catalyzing ATP synthesis via a proton
(H+) gradient (14,15). Mitochondrial defects due to
dysfunctional ATP synthase complexes in humans have been
demonstrated to cause neuromuscular disorders (16). The ATP synthase complex typically
contains at least 15 subunits [a, A6L, b, c, d, e, f, g, oligomycin
sensitivity-conferring protein (OSCP), f6, α, β, γ, δ and ε] that
are divided among two regions, F1 and F0. The F1 portion extends
into the mitochondrial matrix, physically attaching in two ways to
the F0 portion, which is embedded in the inner membrane, via the F1
central stalk that allows rotation, and via the peripheral or
stator stalk, which stabilizes the complex (17).
In Drosophila, ATP is generated by two main
approaches: Glycolysis; and mitochondrial respiration.
Mitochondrial respiration is the most efficient source of ATP;
therefore, the ATP required for the growth of germ cells is
primarily obtained through mitochondrial respiration. Notably,
certain studies indicated that ATP synthase subunits were required
for mitochondrial morphogenesis and germ cell development (18,19).
An additional study suggested that inhibiting ATP synthase activity
suppresses boar sperm motility (20). Mitochondrial ferritin (FtMt), which
is a highly expressed protein in the testis, is a functional
ferritin binding to mitochondria. FtMt contributes to sperm
epididymis maturation and to male fertility, and the cauda
epididymides of FtMt−/− mice have been identified
to exhibit decreased fertility (21). A previous study on
Drosophila ovaries demonstrated that ATP synthase promoted
the maturation of mitochondrial cristae during differentiation
through dimerization and specific upregulation of the ATP synthase
complex (22). However, the
regulatory function of other ATP synthase subunits in
spermatogenesis in the Drosophila testis remains
unclear.
The Upstream Activation Sequence/Gal4 transcription
factor (UAS/Gal4) system-based RNA interference (RNAi) silencing
method has previously been used to analyze the male biological
reproductive process in Drosophila (23). Different Gal4 drivers exhibit
different expression levels and patterns. The present study
examined the role of ATP synthase subunits in mediating the
maturation and cell fate of late-stage germ cells within the
Drosophila testes. A total of 2 Gal4 stocks were used to
drive UAS-ATPsyn RNAi expression in Drosophila testis.
Nanos-Gal4 (Nos-Gal4) has germ cell-specific expression, primarily
in early stage germ cells (24,25).
The expression of bag of marbles-Gal4 (bam-Gal4) is restricted to
transit-amplifying spermatogonia (TA-spermatogonia) (25,26).
The results suggested that ATP synthase is required for male
fertility and serves important roles in maintaining germ cell
differentiation and growth in Drosophila testes; these data
may provide novel insights for the etiological diagnosis of male
infertility.
Materials and methods
Fly strains
All flies were fed with standard corn meal food at
25°C. Information about the alleles and transgenes used in the
present study are available either in FlyBase (www.flybase.org) or as stated: Nos-Gal4 (4937;
Bloomington Drosophila Stock Center, Dept Biology, Indiana
University, Bloomington, IN, USA), bam-Gal4; Δ86/+ was a gift from
Professor Dahua Chen (Institute of Zoology, Chinese Academy of
Sciences, Beijing, China) and has been described in a previous
study (11). All UAS-RNAi
transgenic fly lines were obtained from The TsingHua Fly Center
(THFC; Beijing, China). Drosophila melanogaster
Canton-Special flies were used as the wild type (WT) strain.
Fly crosses and male fertility
test
Fly crosses and male fertility tests were performed
as described in our previous study (3). Transgenic UAS-RNAi males were crossed
with virgin females carrying nos-Gal4 or bam-Gal4 drivers at 25°C.
The testis phenotype was analyzed by using F1 RNAi adult male flies
for immunofluorescence staining. Single male fertility tests were
performed using a single F1 RNAi adult male flies enclosed for 3
days with three WT virgin females at room temperature. All F1 RNAi
adult male flies were analyzed at 48 h after hatching.
Immunofluorescence and antibodies
Fly testes were dissected in 1X PBS and fixed at
room temperature for 30 min in 4% paraformaldehyde. Following
washing three times in 1X PBS with 0.1% Triton X-100 (PBST) and
blocking at room temperature for 1 h in 5% bovine serum albumin
(Sangon Biotech, Shanghai, China) dissolved in 1XPBS, the samples
were incubated with primary antibodies overnight at 4°C. Following
washing three times for 10 min in 0.1% PBST, the samples were then
incubated for 1 h with secondary antibodies (Alexa
Fluor® 488 or Cy™ 3) at room temperature
followed by three washes in 0.1% PBST. Testes were then stained
with Hoechst 33342 (1.0 mg/ml; Invitrogen; Thermo Fisher
Scientific, Inc, Waltham, MA, USA) at room temperature for 5 min
prior to mounting.
The antibodies used were as follows: Mouse anti-EYA
transcriptional coactivator and phosphatase 1 [Eya; 1:20; eya10H6;
Developmental Studies Hybridoma Bank (DSHB), Iowa City, IA, USA];
rat anti-Drosophila E-cadherin homolog (DE-cadherin; 1:20; DCAD2;
DSHB); rabbit anti-DEAD-Box helicase 4 (Vasa; 1:1000; cat. no.
sc-30210; Santa Cruz Biotechnology, Inc., Dallas, TX, USA).
Secondary antibodies conjugated to Alexa Fluor®
488-rabbit (cat. no. 711-545-152; Jackson ImmunoResearch
Laboratories, Inc., West Grove, PA, USA) or Cy™ 3-mouse
(cat. no. 715-165-150; Jackson ImmunoResearch Laboratories, Inc.)
were diluted at 1:1,000.
Bioinformatics analysis
Diagrams indicating the oxidative phosphorylation
process and distribution of the subunits of ATP synthase were
adopted from the Kyoto Encyclopedia of Genes and Genomes Database
(http://www.kegg.jp). Gene ontology (GO) analysis was
performed, and clusters were analyzed using the Database For
Annotation, Visualization, and Integrated Discovery (DAVID)
Bioinformatics Database (https://david.ncifcrf.gov/) (27,28).
The data were evaluated for statistical differences using the
Benjamini-Hochberg method.
Statistical analysis
Experiments were repeated at least three times. The
fertility rate was evaluated for statistical differences using
one-way analysis of variance and Least Significance Difference
post-hoc test by SPSS software (v22; IBM Corp., Armonk, NY, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Structure of the Drosophila
testis
Drosophila has been demonstrated to be an
excellent model organism in which to screen genes essential for
male fertility. Adult Drosophila testes contain
spermatogonia, spermatocytes, spermatids and sperm. In the present
study, a whole mount of WT testis was captured by light microscope
(Fig. 1A). At the apical tip of
the Drosophila testis (Fig.
1B), the stem cell niche in the Drosophila testis
maintained the self-renewal and differentiation capabilities of
GSCs and CySCs. The results demonstrated that spermatogonial cells,
but not spermatocytes, were extensively stained by Hoechst 33342
DNA dye (Fig. 1C).
Immunofluorescence staining with Vasa, Eya and DE-cad was able to
identify germ cells, cyst cells, and somatic hub and cyst cells,
respectively (Fig. 1D-F).
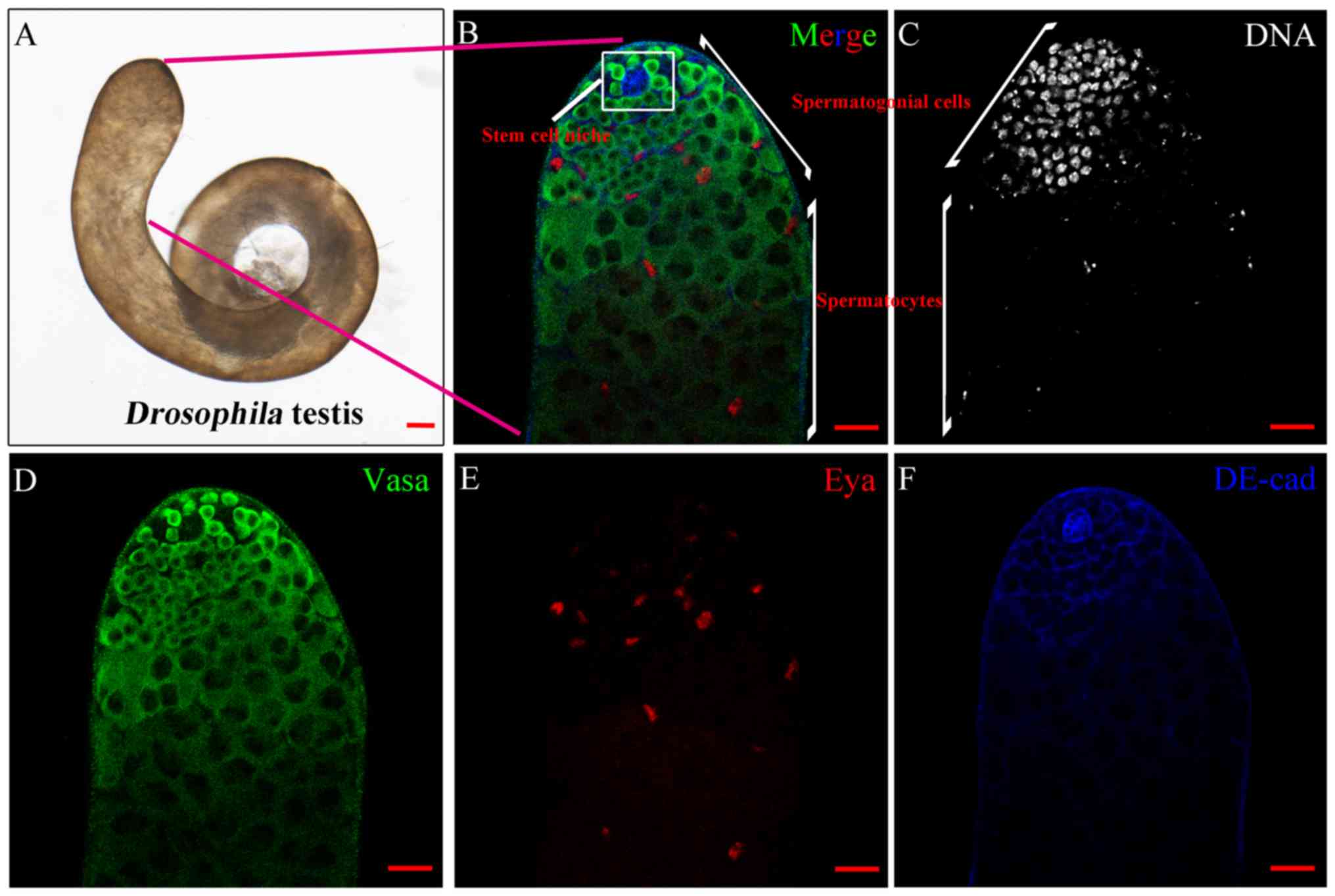 | Figure 1.Structure of the WT testis in
Drosophila. (A) Whole mount of the wild type testis by light
microscope. Scale bar, 100 µm. (B) Complete immunofluorescence
image of the tip of the testis with stem cell niche, spermatogonial
cells and spermatocytes, as labelled. Scale bar, 20 µm. (C) DNA
(grey) staining highlighted the area of spermatogonial cells. Scale
bar, 20 µm. (D) Vasa (green) and (E) Eya (red) stained cells
represented germ and cyst cells, respectively. Scale bars, 20 µm.
(F) Hub cells and cyst cells were stained by DE-cadherin (blue).
Scale bar, 20 µm. Vasa, DEAD-Box helicase 4; Eya, EYA
transcriptional coactivator and phosphatase 1. |
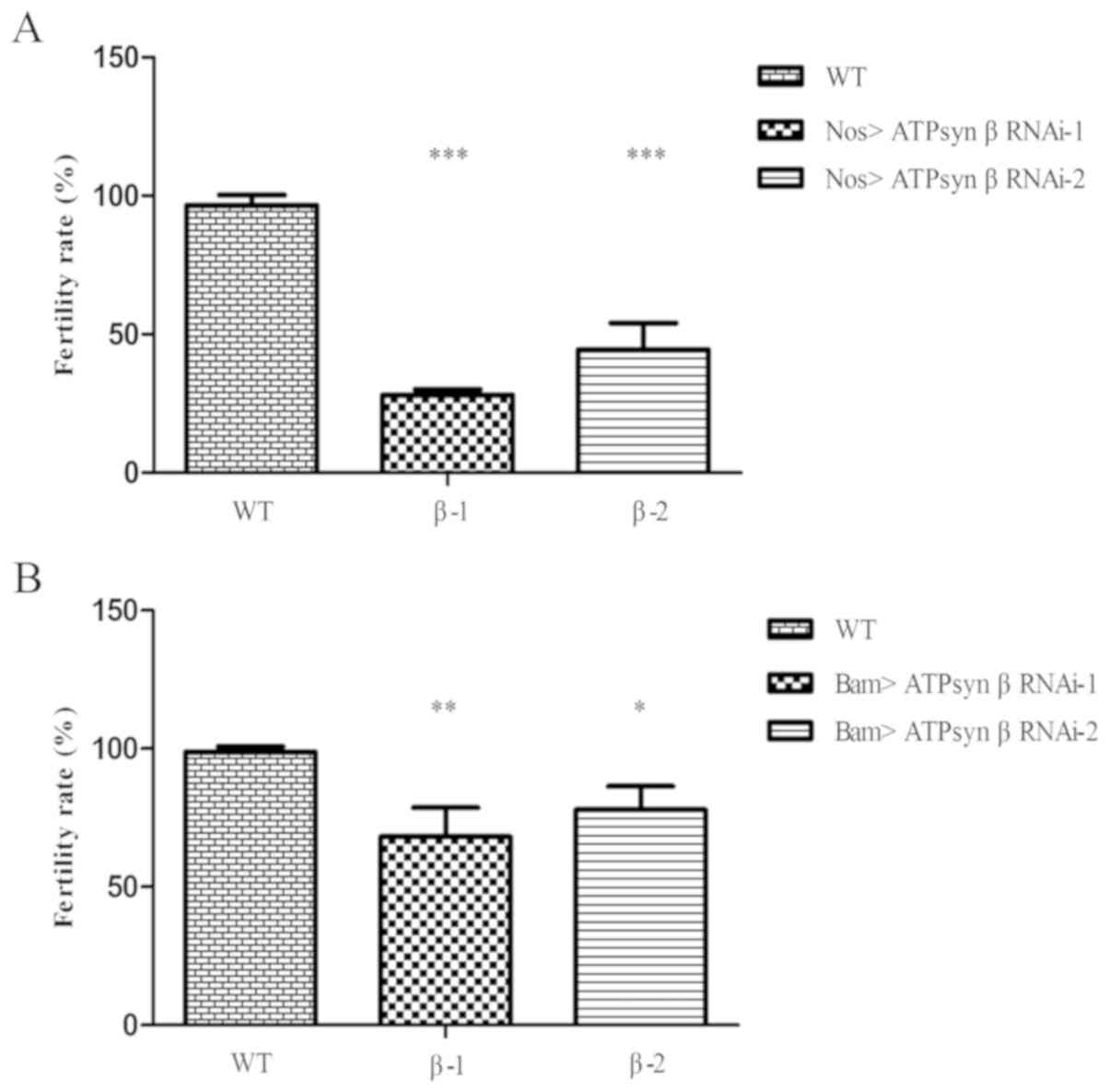
ATP synthase β subunit is required for
male fertility
To examine the function of the ATP synthase β
subunit in Drosophila testes, two independent ATP synthase β
RNAi lines (ATPsyn β RNAi-1 and ATPsyn β RNAi-2) were selected to
evaluate the male fertility rate. In the nos>UAS-ATPsyn β RNAi
flies (Fig. 2A), numerous males
[69.66% (n=89) for ATPsyn β RNAi-1; 53.85% (n=78) for ATPsyn β
RNAi-2] were infertile compared with WT flies (3.30%; n=91). When
bam-Gal4 was used to knock down ATPsyn β expression in 2- to
16-cell spermatogonia (Fig. 2B),
it was identified that a proportion of the resulting males [32.32%
(n=99) for ATPsyn β RNAi-1; 22.86% (n=105) for ATPsyn β RNAi-2]
were infertile. The results suggested that ATP synthase β was
essential for male fertility.
ATP synthase β subunit is essential
for germ cell maturation
To additionally explore the role of the ATP synthase
β subunit, the structure of the testis and changes in the
expression pattern were detected at the molecular level by
immunofluorescence. The Eya gene is required for the development of
the Drosophila compound eyes and organ morphogenesis
(29,30). A previous study has indicated that
Eya is a protein with nuclear localization that is expressed in
mature cyst cells in Drosophila testes (31). Eya was used as a positive marker
for cyst cells in the present study. Vasa usually functions as a
common marker label for germ cells.
Knockdown of the ATP synthase β subunit in early
germ cells resulted in a significant decrease in Vasa-positive germ
cells in spermatocytes in nos>ATPsyn β RNAi testes [59.10% of
testes with defects in ATPsyn β RNAi-1 (n=22); 61.90% of testes
with defects in ATPsyn β RNAi-2 (n=21)] compared with WT testes
(n=32), but there was no significant difference in the number of
GSCs and spermatogonia between the knockdown and WT testes
(Fig. 3A-C). Despite the
dysfunction in germ cell maturation, hub and cyst cells were not
affected. These data suggest that loss of the ATP synthase β
subunit may cause defects in the later stages of germ cell
maturation, but not affect the early stages of spermatogenesis and
somatic cells.
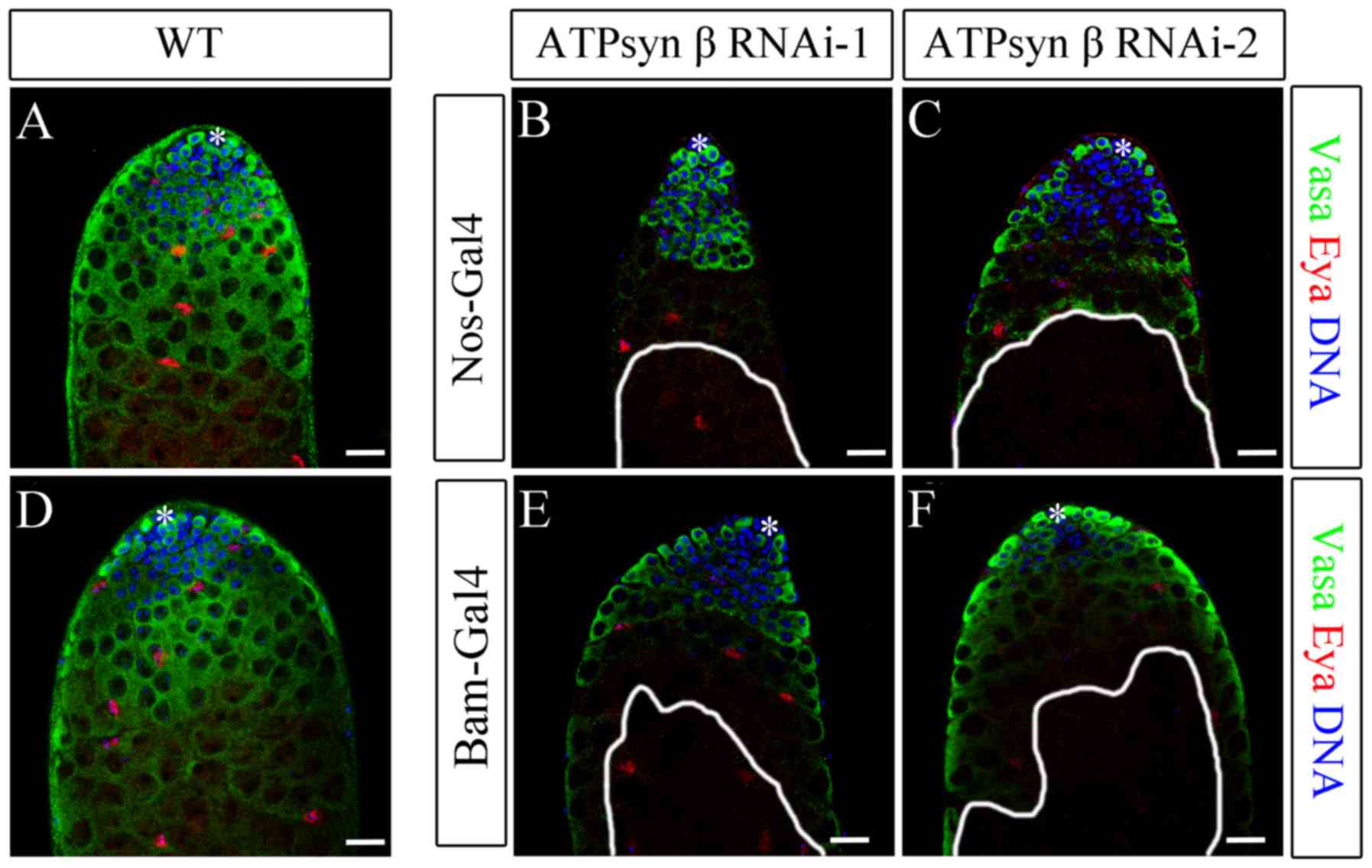 | Figure 3.Knockdown of ATP synthase β subunit
with nos-Gal4 and bam-Gal4. (A-C) Immunofluorescence of (A) WT, (B)
nos>ATPsyn β RNAi-1 and (C) nos>ATPsyn β RNAi-2 testes. (D-F)
Immunofluorescence of (D) WT, (E) bam>ATPsyn β RNAi-1 and (F)
bam>ATPsyn β RNAi-2 testes. Representative images of WT testes
illustrate the tip of the testis with hub cells, GSCs, CySCs,
differentiated germ cells and mature cyst cells. Germ, cyst, and
undifferentiated germ cells were stained to detect Vasa (green
stain), Eya (red stain), and DNA (blue stain). Areas enclosed by
white lines represent regions of germ cells with maintenance
defects. Scale bars, 20 µm WT, wild type; ATP, adenosine
5′-triphosphate; ATPsyn, ATP synthase; Nos, Nanos; Bam, bag of
marbles; RNAi, RNA interference; *, hub cells; Vasa, DEAD-Box
helicase 4; Eya, EYA transcriptional coactivator and phosphatase
1. |
To additionally evaluate the biological function of
the ATP synthase β subunit, ATPsyn β was knocked down using
bam-Gal4. The Bam protein is a key differentiation factor in early
germ cells, and it determines the differentiation fate of
spermatogonia and triggers spermatocyte development (32,33).
In bam>ATPsyn β RNAi testes [47.83% of testes with defects in
ATPsyn β RNAi-1 (n=23); 55.17% of testes with defects in ATPsyn β
RNAi-2 (n=29)], the spermatocytes partially disappeared, whereas
the hub and cyst cells remained (Fig.
3D-F). Knockdown of ATPsyn β driven by bam-Gal4 was identified
to exhibit a similar phenotype of germ cell maturation defects.
Knockdown of major ATP synthase
subunits causes partial male infertility in Drosophila testes
In the present study, the function of ATP synthase
in Drosophila testis was systematically analyzed. A total of
2 independent ATPsyn β RNAi lines were used and defects in germ
cell maturation in testes whose RNAi knockdown was driven by the
combination of nos-Gal4 and bam-Gal4 were also identified,
indicating that the ATPsyn β subunit serves a key role in germ cell
fate. However, the function of other major ATP synthase subunits
remained uncharacterized.
To detect whether other ATP synthase subunits also
served significant roles in male fertility and germ cell mature in
testes, an in vivo RNAi screening assay was conducted using
a USA-Gal4 system. To additionally explore the function of ATP
synthase in testes, RNAi lines of ATP synthase subunits from THFC
were selected (Table I). The RNAi
lines from the THFC were from the same RNAi collection as the
Transgenic RNAi Project (34).
Finally, the function of 8 of the 15 (53.3%) major ATP synthase
subunits, namely, ATPsyn b, ATPsyn c, ATPsyn d, ATPsyn OSCP, ATPsyn
f6, ATPsyn α, ATPsyn β, and ATPsyn γ were screened for.
 | Table I.ATPsyn RNA interference strains used
in the screening process. |
Table I.
ATPsyn RNA interference strains used
in the screening process.
| ATPsyn | THFC no. | BDSC no. | TRiP no. | Annotation
symbol | Gene symbol | Hairpin ID |
|---|
| b | THU2903 | 28062 | JF02899 | CG8189 | ATPsynB | TR02373P.1 |
| c | THU0360 | 35464 | GL00390 | CG1746 | ATPsynC | SH01517.N2 |
| d | THU1424 | 33740 | HMS01078 | CG6030 | ATPsynD | SH01691.N |
| OSCP | TH01379.N2 | – | – | CG4307 | ATPsynO | SH03719.N2 |
| f6 | TH01666.N | – | – | CG4412 | ATPsynCF6 | SH04247.N |
| α | THU2900 | 28059 | JF02896 | CG3612 | ATPsyn-α | TR02362P.1 |
| β-1 | THU2798 | 27712 | JF02792 | CG11154 | ATPsyn-beta | TR02374P.1 |
| β-2 | THU2896 | 28056 | JF02892 | CG11154 | ATPsyn-beta | TR02347P.1 |
| γ | THU3092 | 28723 | JF03150 | CG7610 | ATPsyn-gamma | TR02371P.1 |
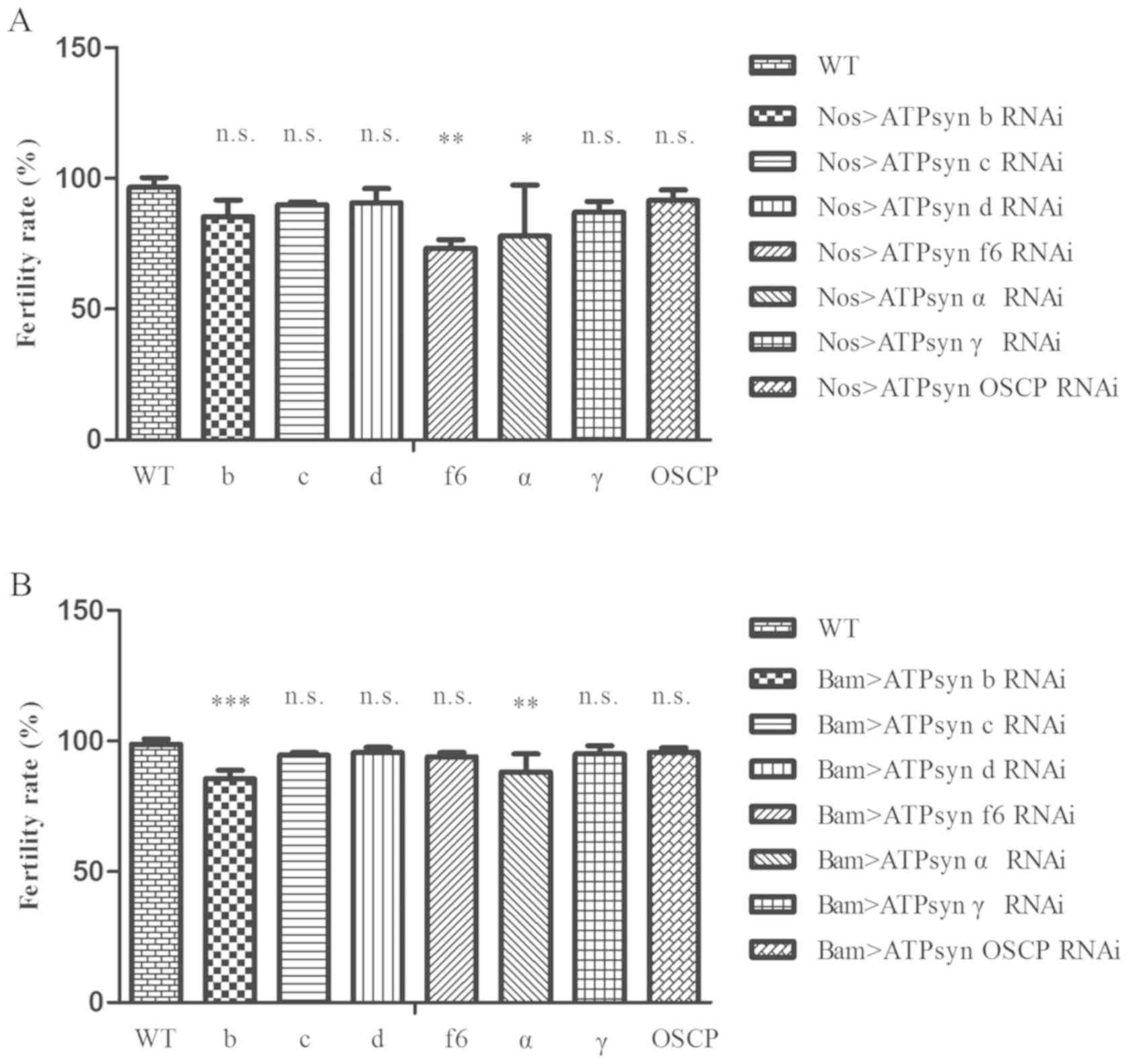
For the male fertility test, it was identified that
the fertility levels of the ATPsyn f6 RNAi and ATPsyn α RNAi
strains, driven by nos-Gal4, were partially affected (Fig. 4A; P<0.05). In addition, males
with ATPsyn b RNAi and ATPsyn α RNAi, driven by bam-Gal4. exhibited
a decrease in the fertility rate (Fig.
4B; P<0.05). These results demonstrated that defects in
sections of the ATP synthase subunits may affect male fertility in
Drosophila testes.
Knockdown of major ATP synthase
subunits results in germ cell maturation defects in Drosophila
testes
When nos-Gal4 was used for screening, all the lines
exhibited germ cell maturation defects. These testes were stained
with the germ cell marker Vasa. In the majority of the ATPsyn RNAi
testes, early germ cells, including GSCs and TA-spermatogonia, were
Vasa-positive. In addition, certain spermatocytes in a number of
ATPsyn RNAi testes were Vasa-negative (8/15 in ATPsyn b RNAi; 9/17
in ATPsyn c RNAi; 6/19 in ATPsyn d RNAi, 10/25 in ATPsyn OSCP RNAi;
11/19 in ATPsyn f6 RNAi; 15/25 in ATPsyn α RNAi; and 13/27 in
ATPsyn γ RNAi). ATPsyn RNAi testes were also stained with the
somatic cyst cell marker Eya, and all cells were identified to be
positive for Eya (Fig. 5A-G). The
results suggest that ATP synthase does not affect the survival of
GSCs and TA-spermatogonia. However, ATP synthase may be vital for
germ cell maturation.
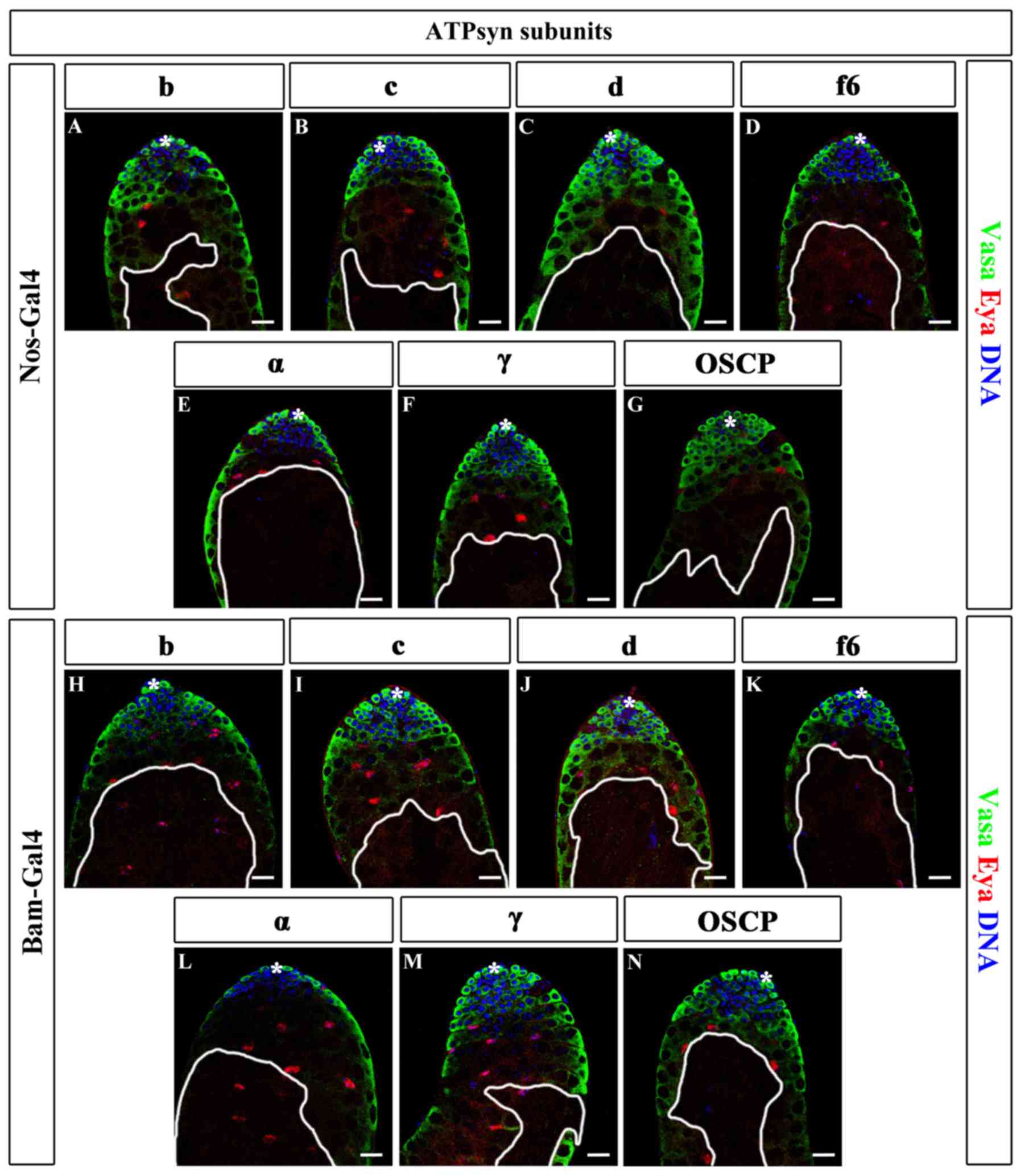 | Figure 5.Knockdown of major ATP synthase
subunits in germ cells. (A-G) Immunofluorescence of ATPsyn RNAi
testes driven by nos-Gal4, including (A) b, (B) c, (C) d, (D) f6,
(E) α, (F) γ and (G) OSCP. (H-N) Immunofluorescence of ATPsyn
subunits in RNAi testes driven by bam-Gal4, including (H) b, (I) c,
(J) d, (K) f6, (L) α, (M) γ and (N) OSCP. Areas enclosed by white
lines represent regions of germ cells with maintenance defects.
Scale bars, 20 µm. *, hub cells; ATP, adenosine 5′-triphosphate;
ATPsyn, ATP synthase; Nos, Nanos; Bam, bag of marbles; WT, wild
type; RNAi, RNA interference; OSCP, oligomycin
sensitivity-conferring protein; Vasa, DEAD-Box helicase 4; Eya, EYA
transcriptional coactivator and phosphatase 1. |
Next, the ability of germ cell maturation in ATP
synthase-deficient testes, driven by bam-Gal4, was examined.
Similar germ cell maturation defects were identified in
bam>ATPsyn RNAi testes: In 48.00% (12/25) ATPsyn b RNAi, 29.17%
(7/24) ATPsyn c RNAi, 29.63% (8/27) ATPsyn d RNAi, 44.00% (11/25)
ATPsyn OSCP RNAi, 42.11% (8/19) ATPsyn f6 RNAi, 31.82% (7/22)
ATPsyn α RNAi and 47.80% (11/23) ATPsyn γ RNAi testes, some of the
spermatocytes were Vasa-negative, while the early germ cells and
somatic hub and cyst cells were not affected (Fig. 5H-N). These data indicate that the
major ATP synthase subunits are key factors for germ cell
maturation.
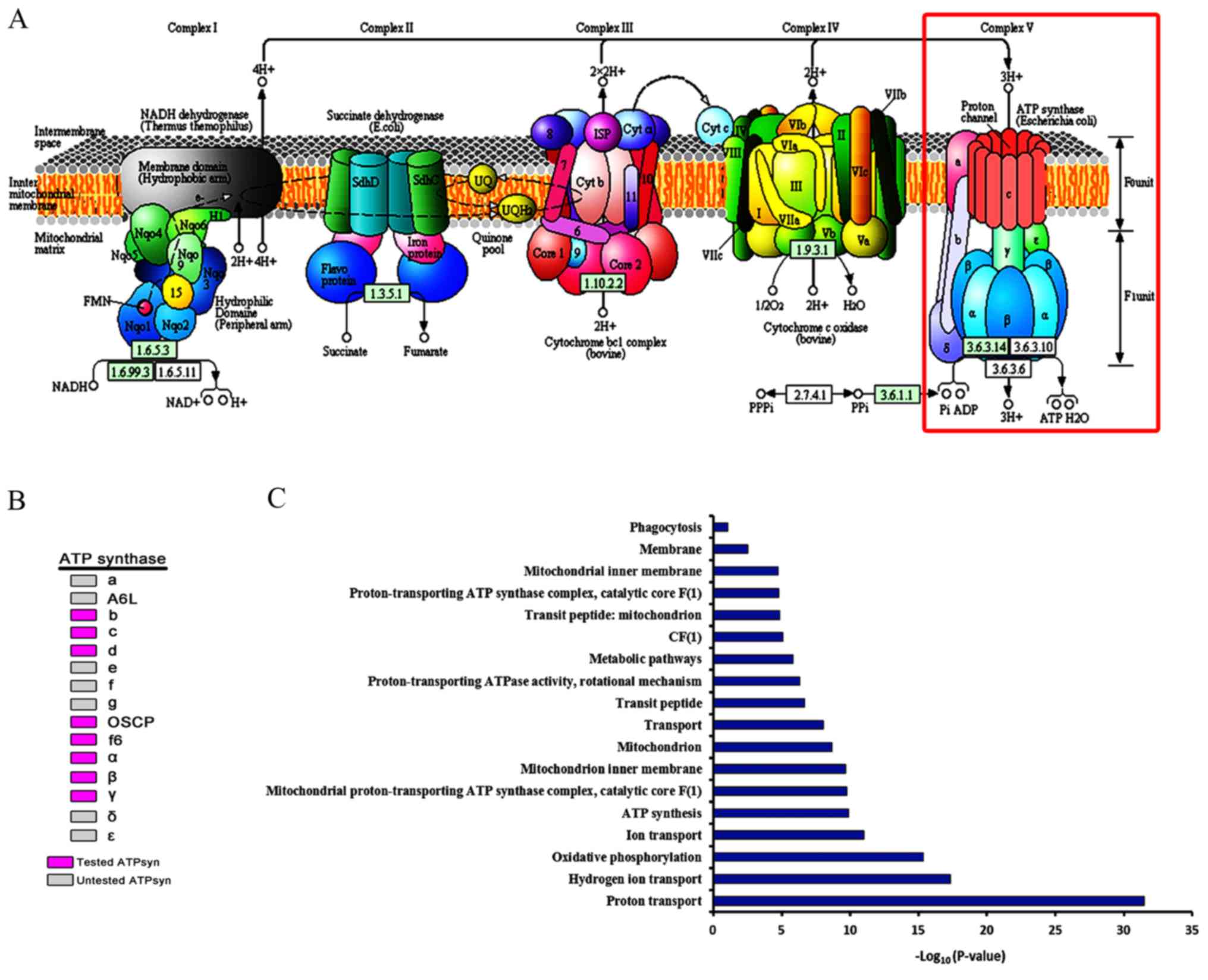
ATP synthase Assembly and GO
analysis
The roles of most of the major ATP synthase subunits
remain to be elucidated. Analysis of the major ATP synthase
subunits driven by nos-Gal4 and bam-Gal4 indicated that ATP
synthase subunits may assemble into a complex and participate in
germ cell development via oxidative phosphorylation (Fig. 6A). In the present study, 8 of the
15 major ATP synthase subunits were examined, and were identified
as being required for the maturation of germ cells during the later
stages (Fig. 6B). Cellular and
cell cycle components required for basic metabolism are generally
thought to be expressed at consistent levels throughout the
development process. The present study additionally conducted GO
analysis of major ATP synthase subunits using DAVID. The list of
the top functional annotations of ATP synthase contained oxidative
phosphorylation, proton transport, mitochondrion, and ATP synthesis
(P<0.05; Fig. 6C). The results
from the present study revealed that the mitochondrial ATP synthase
serves an important role in promoting the developmentally regulated
maturation of germ cells in Drosophila testis.
Discussion
The Drosophila testis provides an excellent
model to study spermatogenesis. Mitochondrial ATP synthase
catalyzes ATP synthesis and produces energy for germ cell
development. Mitochondria are widely expressed in germ cells and
serve key roles in male fertility and germ cell survival (35,36).
At present, only a few studies have analyzed the regulatory network
of ATP synthase in the testis (19,37,38).
The present study, using Drosophila as an in vivo
model, systematically analyzed a series of ATP synthase subunits
and explored their common regulation of germ cell maturation in the
Drosophila testis. Notably, it was identified that ATPsyn β
is a regulatory factor in germ cell maturation. Wen et al
(39) demonstrated that knockout
of hpRNA1 inhibited its target ATPsyn β in testes, and affected
spermatogenesis and male fertility. Notably, hpRNA1 mutant seminal
vesicles exhibited a lower density of sperm in the accessory gland
and mild defection of nuclear organization in the testis tail. The
present study primarily focused on male fertility and germ cell
maturation. Knockdown of ATPsyn β resulted in partial infertility
and caused germ cell maturation defects. It was also observed that
a small proportion of germ cells were able to undergo the meiosis
process and form sperm, which was consistent with the data from Wen
et al (39).
A previous study indicated that the ATP synthase
served to promote the maturation of mitochondrial cristae during
differentiation through dimerization and specific upregulation of
the ATP synthase complex in Drosophila ovaries (22). Proliferating cysts in ATP synthase
knockdown models failed to continue to differentiate and were
unable to progress from four- to eight-cell cysts in
Drosophila ovaries, and they demonstrated that mitochondrial
ATP synthase serves a critical role in stem cell differentiation
process (22). Despite the fact
that germline stem cells are strictly controlled by stem cell
niches in both Drosophila testes and ovaries, gametogenesis
in Drosophila testes is quite different from that in
ovaries. The results from the present study demonstrated that
knockdown of ATP synthase subunits did not result in germ cell
differentiation defects, but affected germ cell maintenance in
Drosophila testes, indicating its critical role in
spermatogenesis.
A previous study has demonstrated that the knotted
onions (knon) gene encodes a testis-specific paralog of ATP
synthase subunit d, and is required for the internal structure of
the nebenkern and its subsequent disassembly and elongation. knon
knockout mutants exhibited aberrant mitochondrial elongation during
spermatogenesis and faulty nebenkern morphology (19). Knockdown of the ATP synthase
subunits f6 and g in Drosophila testis revealed a phenotype
similar to that of knon mutants (19). In addition, ATP synthase subunit b
has been demonstrated to be essential for the growth, development
and male fertility in Caenorhabditis elegans (40). Deficiency of the ATP synthase
subunit b in testes was identified to disrupt nuclear bundles
during spermatogenesis and cause abnormal shaping and spermatid
elongation (40). Chen et
al (41) demonstrated that
ubiquitous knockdown of ATP synthase subunit b resulted in growth
defects, and knockdown of ATP synthase subunit b in testes caused
infertility and abnormal spermatogenesis, which was consistent with
the data from the present study. Nevertheless, Chen et al
(41) only used one
testis-associated Gal4 for the knockdown of ATP synthase subunit b,
and primarily focused on the staining of testicle tail in
Drosophila. The present study additionally examined the male
fertility and testicular apex staining by two different germ
cell-associated Gal4s (nos-Gal4 and bam-Gal4), and several markers
to distinguish different cell types were also used; it was
identified that spermatocytes were Vasa-negative in knockdown
testes.
The present study described evidence associating
germ cell mature and cell survival to ATP synthase. The data
demonstrated the role of a cell biological process in germ cell
development, and these results contribute to the knowledge of the
role of ATP synthase in ATP production.
In a number of cell types, dimerization of ATP
synthase complexes with their axes at a certain angle is important
for determining the sharp positive curvature of the inner
mitochondrial membrane (19,42).
It was hypothesized that deficiency of ATP synthase subunits in
Drosophila testis may alter ATP synthase dimerization or
affect the assembly and stability of the ATP synthase complex in
the inner mitochondrial membrane, blocking the delivery of energy,
which is essential for germ cell development. However, knockdown of
ATP synthase subunits in early germ cells did not affect the
survival of GSCs. It was hypothesized that GSCs in
Drosophila testes may obtain their energy through glycolysis
or the noncanonical approach.
Future studies on ATP synthase subunits and other
members of the oxidative phosphorylation system may explore their
regulatory network and mechanism underlying the clustered regularly
interspersed short palindromic repeats (CRISPR) associated protein
9/CRISPR system in Drosophila. Future studies should also
examine the mutation rate of ATP synthase subunits in patients with
oligoasthenospermia. The data from the present study may assist to
reveal the mechanisms underlying male infertility and
oligoasthenospermia.
Acknowledgements
The authors would like to thank Professor Dahua Chen
from Institute of Zoology, Chinese Academy of Sciences for sharing
reagents and stocks.
Funding
The present study was supported by National Natural
Science Foundation of China (grant nos. 31701298 and 81402100),
Natural Science Foundation of Jiangsu Province (grant no.
BK20170562), Key Research Foundation of Zhenjiang Social
Development (grant nos. SH2017013, SH2017020, SH2016028, SH2016031
and SH2014026), Key Research Foundation of Zhenjiang Health Science
and Technology (grant no. SHW2016001), Open Fund of State Key
Laboratory of Reproductive Medicine of Nanjing Medical University
(grant no. SKLRM-KA201603), the Foundation of Health and Family
Planning Commission of Jiangsu Province (grant no. Q201408), the
Foundation for Young Medical Talents of Jiangsu province (grant no.
QNRC2016840), Six Talent Peaks Project in Jiangsu Province (grant
no. WSW-007), Science Foundation of Doctorate Research of
Affiliated Hospital of Jiangsu University (grant no.
jdfyRC2016005), Suzhou Key Medical Center (grant no. SZZX201505),
Suzhou Introduced Project of Clinical Medical Expert Team (grant
no. SZYJTD201708) and Jiangsu Provincial Medical Innovation Team
(grant no. CXTDB2017013).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JY, BC and JF conceived and designed the
experiments. BZ, CQ, XC, YY and XL performed the experiments. BX,
ZH and JL analyzed the data. CS, XH and QS contributed to the fly
feeding and part of the data analysis. ML and HL contributed to the
fly crosses and male fertility test. HL, JF and JY wrote the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by Ethics Committee
for Biomedical Research at Affiliated Hospital of Jiangsu
University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hu Z, Li Z, Yu J, Tong C, Lin Y, Guo X, Lu
F, Dong J, Xia Y, Wen Y, et al: Association analysis identifies new
risk loci for non-obstructive azoospermia in Chinese men. Nat
Commun. 5:38572014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yu J, Liu Y, Lan X, Wu H, Wen Y, Zhou Z,
Hu Z, Sha J, Guo X and Tong C: CHES-1-like, the ortholog of a
non-obstructive azoospermia-associated gene, blocks germline stem
cell differentiation by upregulating Dpp expression in
Drosophila testis. Oncotarget. 7:42303–42313.
2016.PubMed/NCBI
|
|
3
|
Yu J, Wu H, Wen Y, Liu Y, Zhou T, Ni B,
Lin Y, Dong J, Zhou Z, Hu Z, et al: Identification of seven genes
essential for male fertility through a genome-wide association
study of non-obstructive azoospermia and RNA interference-mediated
large-scale functional screening in Drosophila. Hum Mol
Genet. 24:1493–1503. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu H, Sun L, Wen Y, Liu Y, Yu J, Mao F,
Wang Y, Tong C, Guo X, Hu Z, et al: Major spliceosome defects cause
male infertility and are associated with nonobstructive azoospermia
in humans. Proc Natl Acad Sci USA. 113:4134–4139. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hackstein JH, Hochstenbach R and Pearson
PL: Towards an understanding of the genetics of human male
infertility: Lessons from flies. Trends Genet. 16:565–572. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
de Cuevas M and Matunis EL: The stem cell
niche: Lessons from the Drosophila testis. Development.
138:2861–2869. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Spradling A, Fuller MT, Braun RE and
Yoshida S: Germline stem cells. Cold Spring Harb Perspect Biol.
3:a0026422011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fuller MT and Spradling AC: Male and
female Drosophila germline stem cells: Two versions of
immortality. Science. 316:402–404. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu EY, Lee DF, Klebes A, Turek PJ,
Kornberg TB and Reijo Pera RA: Human BOULE gene rescues meiotic
defects in infertile flies. Hum Mol Genet. 12:169–175. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
White-Cooper H: Studying how flies make
sperm-investigating gene function in Drosophila testes. Mol
Cell Endocrinol. 306:66–74. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu J, Lan X, Chen X, Yu C, Xu Y, Liu Y, Xu
L, Fan HY and Tong C: Protein synthesis and degradation are
essential to regulate germline stem cell homeostasis in
Drosophila testes. Development. 143:2930–2945. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
He J, Ford HC, Carroll J, Ding S, Fearnley
IM and Walker J: Persistence of the mitochondrial permeability
transition in the absence of subunit c of human ATP synthase. Proc
Natl Acad Sci USA. 114:3409–3414. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Junge W and Nelson N: ATP synthase. Annu
Rev Biochem. 84:631–657. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Walker JE: The ATP synthase: The
understood, the uncertain and the unknown. Biochem Soc Trans.
41:1–16. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mitchell P: Chemiosmotic coupling in
oxidative and photosynthetic phosphorylation. 1966. Biochim Biophys
Acta 1807. 1507–1538. 2011.
|
|
16
|
Kucharczyk R, Zick M, Bietenhader M, Rak
M, Couplan E, Blondel M, Caubet SD and di Rago JP: Mitochondrial
ATP synthase disorders: Molecular mechanisms and the quest for
curative therapeutic approaches. Biochim Biophys Acta 1793.
186–199. 2009.
|
|
17
|
Velours J, Paumard P, Soubannier V,
Spannagel C, Vaillier J, Arselin G and Graves PV: Organisation of
the yeast ATP synthase F(0): A study based on cysteine mutants,
thiol modification and cross-linking reagents. Biochim Biophys Acta
1458. 443–456. 2000.
|
|
18
|
Hendriks WK, Colleoni S, Galli C, Paris
DB, Colenbrander B, Roelen BA and Stout TA: Maternal age and in
vitro culture affect mitochondrial number and function in equine
oocytes and embryos. Reprod Fertil Dev. 27:957–968. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sawyer EM, Brunner EC, Hwang Y, Ivey LE,
Brown O, Bannon M, Akrobetu D, Sheaffer KE, Morgan O, Field CO, et
al: Testis-specific ATP synthase peripheral stalk subunits required
for tissue-specific mitochondrial morphogenesis in
Drosophila. BMC Cell Biol. 18:162017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ramió-Lluch L, Yeste M, Fernández-Novell
JM, Estrada E, Rocha L, Cebrián-Pérez JA, Muiño-Blanco T, Concha
II, Ramírez A and Rodríguez-Gil JE: Oligomycin A-induced inhibition
of mitochondrial ATP-synthase activity suppresses boar sperm
motility and in vitro capacitation achievement without modifying
overall sperm energy levels. Reprod Fertil Dev. 26:883–897. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Maccarinelli F, Regoni M, Carmona F, Poli
M, Meyron-Holtz EG and Arosio P: Mitochondrial ferritin deficiency
reduces male fertility in mice. Reprod Fertil Dev. 29:2005–2010.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Teixeira FK, Sanchez CG, Hurd TR, Seifert
JR, Czech B, Preall JB, Hannon GJ and Lehmann R: ATP synthase
promotes germ cell differentiation independent of oxidative
phosphorylation. Nat Cell Biol. 17:689–696. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ni JQ, Zhou R, Czech B, Liu LP, Holderbaum
L, Yang-Zhou D, Shim HS, Tao R, Handler D, Karpowicz P, et al: A
genome-scale shRNA resource for transgenic RNAi in
Drosophila. Nat Methods. 8:405–407. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu M, Lim TM and Cai Y: The
Drosophila female germline stem cell lineage acts to
spatially restrict DPP function within the niche. Sci Signal.
3:ra572010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
White-Cooper H: Tissue, cell type and
stage-specific ectopic gene expression and RNAi induction in the
Drosophila testis. Spermatogenesis. 2:11–22. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Singh SR, Zhen W, Zheng Z, Wang H, Oh SW,
Liu W, Zbar B, Schmidt LS and Hou SX: The Drosophila homolog
of the human tumor suppressor gene BHD interacts with the JAK-STAT
and Dpp signaling pathways in regulating male germline stem cell
maintenance. Oncogene. 25:5933–5941. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang da W, Sherman BT and Lempicki RA:
Systematic and integrative analysis of large gene lists using DAVID
bioinformatics resources. Nat Protoc. 4:44–57. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huang da W, Sherman BT and Lempicki RA:
Bioinformatics enrichment tools: Paths toward the comprehensive
functional analysis of large gene lists. Nucleic Acids Res.
37:1–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Furuya M, Qadota H, Chisholm AD and
Sugimoto A: The C. elegans eyes absent ortholog EYA-1 is required
for tissue differentiation and plays partially redundant roles with
PAX-6. Dev Biol. 286:452–463. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Karandikar UC, Jin M, Jusiak B, Kwak S,
Chen R and Mardon G: Drosophila eyes absent is required for
normal cone and pigment cell development. PLoS One. 9:e1021432014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fabrizio JJ, Boyle M and DiNardo S: A
somatic role for eyes absent (eya) and sine oculis (so) in
Drosophila spermatocyte development. Dev Biol. 258:117–128.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bunt SM and Hime GR: Ectopic activation of
Dpp signalling in the male Drosophila germline inhibits germ
cell differentiation. Genesis. 39:84–93. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shivdasani AA and Ingham PW: Regulation of
stem cell maintenance and transit amplifying cell proliferation by
tgf-beta signaling in Drosophila spermatogenesis. Curr Biol.
13:2065–2072. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yan D, Neumüller RA, Buckner M, Ayers K,
Li H, Hu Y, Yang-Zhou D, Pan L, Wang X, Kelley C, et al: A
regulatory network of Drosophila germline stem cell
self-renewal. Dev Cell. 28:459–473. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen JV and Megraw TL: Spermitin: A novel
mitochondrial protein in Drosophila spermatids. PLoS One.
9:e1088022014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wu CH, Zong Q, Du AL, Zhang W, Yao HC, Yu
XQ and Wang YF: Knockdown of Dynamitin in testes significantly
decreased male fertility in Drosophila melanogaster. Dev
Biol. 420:79–89. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Collins CM, Malacrida B, Burke C, Kiely PA
and Dunleavy EM: ATP synthase F1 subunits recruited to centromeres
by CENP-A are required for male meiosis. Nat Commun. 9:27022018.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Midzak AS, Chen H, Aon MA, Papadopoulos V
and Zirkin BR: ATP synthesis, mitochondrial function, and steroid
biosynthesis in rodent primary and tumor Leydig cells. Biol Reprod.
84:976–985. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wen J, Duan H, Bejarano F, Okamura K,
Fabian L, Brill JA, Bortolamiol-Becet D, Martin R, Ruby JG and Lai
EC: Adaptive regulation of testis gene expression and control of
male fertility by the Drosophila hairpin RNA pathway. Mol
Cell. 57:165–178. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kawasaki I, Hanazawa M, Gengyo-Ando K,
Mitani S, Maruyama I and Iino Y: ASB-1, a germline-specific isoform
of mitochondrial ATP synthase b subunit, is required to maintain
the rate of germline development in Caenorhabditis elegans.
Mech Dev. 124:237–251. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chen YN, Wu CH, Zheng Y, Li JJ, Wang JL
and Wang YF: Knockdown of ATPsyn-b caused larval growth defect and
male infertility in Drosophila. Arch Insect Biochem Physiol.
88:144–54. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Davies KM, Anselmi C, Wittig I,
Faraldo-Gómez JD and Kühlbrandt W: Structure of the yeast F1FO-ATP
synthase dimer and its role in shaping the mitochondrial cristae.
Proc Natl Acad Sci USA. 109:13602–13607. 2012. View Article : Google Scholar : PubMed/NCBI
|