Introduction
Quorum-sensing (QS) mechanisms allow microorganisms
to adapt different biological functions to the environment
according to their population density via the secretion of
self-inducing signalling molecules. These quorum-sensing molecules
(QSMs) are able to suppress or activate gene expression (1–3).
QSMs have been described in bacteria and in yeast such as
Candida, a well-known contaminant for dentures.
Candida-secreted QSMs induce phenotypic adaptations that
include morphological changes (4),
secretion of virulence factors (5)
and biofilm formation (6). The
following four types of QSMs have been identified in Candida
albicans: i) 2-phenylethanol and tryptophol derived from
phenylalanine and tryptophan, respectively (7); ii) the MARS molecule of unknown
structure (8); iii) farnesol
(9) and its derivative farnesoic
acid (10); and iv) tyrosol
(11). Farnesol and tyrosol are
the two molecules that have been the most widely studied to
date.
Farnesol is a metabolite of the mevalonate/sterol
synthesis pathway in C. albicans. In suspension,
Candida blastoconidia produce farnesol up to a maximal
concentration of 10–50 µM in the stationary phase (9). Candida growth in liquid
culture medium is correlated with an increase in resistance to
oxidative stress by the expression of superoxide dismutase and
catalase, suggesting a link with QSMs (12). Furthermore, farnesol accumulation
blocks the yeast-hyphal transition of C. albicans at high
cell densities without blocking the elongation of pre-existing
hyphae (13) or influencing cell
growth rates (9). A previous study
suggested that exogenous farnesol, which was tested on a C.
albicans strain that does not produce endogenous farnesol,
suppresses hyphal formation by inducing morphological changes in
the yeast cell wall and by suppression of the expression of
aspartyl proteinases (14).
Farnesol prevents C. albicans biofilm formation (15). Numerous stages of biofilm
development are influenced by farnesol, including cell adhesion to
substrates, mature biofilm architecture, and cell dispersion from
the biofilm. Due to the effect of farnesol on morphology, and the
importance of morphology in biofilm formation, it has been
suggested that exogenous farnesol affects biofilm development by
repressing hyphal formation and the expression of genes specific to
filamentation (6). A number of
studies have expressed an interest in using this molecule in
therapeutics or hygiene (3,15–17).
Combining farnesol with fluconazole reduces the thickness of C.
albicans biofilm and the minimal inhibitory concentration of
fluconazole, indicating that farnesol inhibits the development of
fluconazole resistance in C. albicans strains known to be
resistant to this antifungal (18). A similar effect was also
demonstrated on C. dubliniensis (19). The significant synergy between
farnesol and three different antifungals (micafungin, fluconazole
and amphotericin B) has beneficial effects against C.
albicans biofilm (20).
Tyrosol, derived from tyrosine, accelerates the
formation of germ tubes without compensating for the effect of
farnesol in blocking germination (21). This molecule is considered a minor
QSM, whose influence is observed only when the concentration of
farnesol is low or absent in the environment (5). At micromolar concentrations, tyrosol
stimulates hyphal production during the early stages of C.
albicans biofilm formation (22). In the millimolar range, exogenous
tyrosol has been reported to inhibit the formation of biofilms
(23). The combination of tyrosol
with other antifungals (amphotericin B, itraconazole and
fluconazole) has a synergistic effect on C. albicans and
C. tropicalis biofilms (23).
An improved understanding of the action of these two
QSMs may lead to the design of novel antifungal strategies that
target Candida biofilm formation and development,
particularly for prophylactic approaches in oral hygiene. Current
antifungals should be reserved for the treatment of patients with
infections, as these drugs are less active against yeasts organised
in biofilms (17–20). Furthermore, antifungal use on
yeast-colonised dentures in the oral environment promotes the
emergence of resistant strains (17–20).
From this perspective, the present study aimed to compare the
effects of both QSMs (farnesol and tyrosol) individually on C.
albicans biofilm.
Materials and methods
Preparation of QSMs
The two QSMs (tyrosol and farnesol) were purchased
from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). Farnesol is
insoluble in water, unlike tyrosol (solubility threshold, 25.3
mg/ml); therefore, a stock solution of farnesol was produced by
transferring 50 µl reagent (trans,
trans−3,7,11-trimethyl-2,6,10-dodecatriene-1-ol,
C15H26O, PM: 222.37 g/mol, density: 0.879
g/ml) to 6.275 ml 1% (v/v) DMSO (24 mM). Subsequent dilutions were
made in 1% DMSO and incorporated into the reaction medium (final
concentrations, 0.001–3 mM farnesol in 0.1% DMSO). The farnesol
controls contained DMSO at a similar concentration to that present
in the assays (0.1%). Tyrosol [4-(2-hydroxyethyl)phenol,
C8H10O2, PM: 138.16 g/mol] was
brought to final concentrations of 1–20 mM from a 22-mM stock
solution in Sabouraud broth.
Microorganisms
The investigation was conducted in C.
albicans ATCC 10231 (Culti-loops™; Thermo Fisher Scientific,
Inc., Waltham, MA, USA), a reference strain often used to test
antifungals (24), and six
clinical strains of C. albicans (provided by Oral Biology
Unit, Laboratory of Physiology and Pharmacology, Université Libre
de Bruxelles, Brussels, Belgium), which were isolated from dentures
using sterile cotton swabs (Eurotubo®; Deltalab,
Barcelona, Spain). Yeast were aerobically grown at 37°C for 24 h in
Sabouraud broth (CM147; Oxoid; Thermo Fisher Scientific, Inc.) or
on solid Sabouraud agar (BD Diagnostics, Erembodegem, Belgium).
Clinical isolates were identified according to the appearance of
their colonies on CHROMagar™ medium (BD Diagnostics), by
chlamydoconidia formation on rice extract agar containing
polysorbate 80 (BD Diagnostics) and using the API yeast
identification system (bioMérieux, Marcy-l'Etoile, France). All
in-vitro studies were conducted in a third subculture on
Sabouraud agar, from which the yeasts were transferred into
Sabouraud broth. Candida suspensions were adjusted to
107 yeast cells (blastoconidia) per millilitre by
dilution following counting in a Thoma cell counting chamber
(Marienfeld, Lauda-Königshofen, Germany). A number of the
experiments were conducted under anaerobic conditions that were
generated by Anaerogen™ sachets placed in anaerobic 3.5-l jars
(Oxoid; Thermo Fisher Scientific, Inc.).
Biofilm production and
quantitation
Candida biofilm formation was achieved in
flat-bottomed 96-well polystyrene plates (12×8-well columns;
CellStar®; Greiner Bio-One International GmbH, Neuburg,
Germany) for both the reference and clinical strains. Each
experimental condition included eight replicates distributed in one
column of the microplate. In addition to the different
concentrations tested, each experiment always included controls
without QSMs and sterility controls without cell suspension to
attest to the absence of accidental contamination during handling.
The minimal inhibitory concentration of both investigated QSMs
(farnesol and tyrosol) was estimated by the evaluation of
turbidimetric growth in broth and by quantitation of attached
biomass in the reactive wells. Yeast growth and their capacity to
form a biofilm were assessed after 24 h at 37°C in liquid Sabouraud
medium with increasing concentrations of farnesol (0.001–3 mM) and
tyrosol (1–20 mM). Each well (except those for the sterility
controls) initially contained 0.25×106 blastoconidia
suspended in 25 µl Sabouraud broth (final volume in each well, 250
µl). The contents of the plate were mixed for 5 min at room
temperature using a plate shaker (IKA™, Boutersem, Belgium) and
then incubated at 37°C for 24 h. The following day, yeast growth
was photometrically assessed at 600 nm using a microplate reader
(Packard SpectraCount™; PerkinElmer, Inc., Waltham, MA, USA). This
turbidimetric evaluation did not differentially evaluate free cells
and those attached to the bottom of the wells. The assay data were
compared to the controls. Following the opacimetric reading at 24
h, the biofilms were quantitated by crystal violet staining
according to a method adapted from a previously published study
(25). Briefly, the Sabouraud
broth was carefully aspirated using a multi-channel pipette (VWR
International, Leuven, Belgium) and the microplate wells were
gently (considering the fragility of biofilm structures) washed
three times with 250 µl saline to remove the non-adherent cells.
The attached biomass was fixed with 250 µl methanol (Sigma-Aldrich;
Merck KGaA) for 15 min at room temperature and then stained with
250 µl 2% Hucker crystal violet solution (8 g crystal violet in 80
ml 95% ethanol, plus 3.2 g ammonium oxalate in 320 ml distilled
water) at room temperature for a further 5 min. After gentle
rinsing with running tap water and drying with a hair dryer, the
coloured biomass attached to the walls of the microplate wells was
solubilised with 250 µl 2 M acetic acid for 30 min, and the
absorbance was measured at 600 nm using a Packard SpectraCount™
microplate reader. All samples with an absorbance value >2.000
were diluted 10-fold in 2 M acetic acid. The coefficient of
variation for the Packard SpectraCount™ microplate reader is
<5%.
In a number of the experiments, the culture medium
was diluted 100- and 1,000-fold in saline following the 24-h
incubation and prior to biofilm quantitation. A 20-µl aliquot of
each dilution was seeded onto Sabouraud agar, containing 0.4 g/l
chloramphenicol and 0.04 g/l gentamycin, in order to count the
colony forming units (CFUs) after 48 h at 37°C using an e-Count™
colony counter (Heathrow Scientific, Vernon Hills, IL, USA).
Subsequently, the effect of the QSMs on Candida biofilm was
also studied over a longer period of 48–72 h, with the addition of
the molecules prior to biofilm formation or following a 24-h
incubation at 37°C. Under this last condition, the culture medium
was renewed after 24 h.
Fluorescent staining
Fresh solutions of fluorescein diacetate (5 mg/ml in
acetone) and ethidium bromide (5 mg/ml in phosphate-buffered
saline, pH 7.5) (Sigma-Aldrich; Merck KGaA) were separately diluted
100-fold in phosphate-buffered saline and then mixed at a 1:1
ratio. The fluorescent reagent and Candida biofilm suspended
in saline washing liquid were mixed at a 1:1 ratio, and
subsequently incubated for 15 min at 37°C prior to microscopic
examination (Leica DM2000; Leica Microsystems, Inc., Buffalo Grove,
IL, USA). Green fluorescence was considered as living cells and
orange staining as non-viable cells.
Statistical analysis
The data are presented as a percentage following
division of each experimental value by the arithmetic mean of the
paired control values obtained under the same conditions.
Considering the intra-assay control as 100% eliminated the bias of
inter-assay variability. The data are presented as the mean ±
standard error of the mean (SEM), unless otherwise indicated. Data
management and statistical tests (mean, standard deviation, SEM,
Kolmogorov-Smirnov test, unpaired Student's t-test, and ANOVA
supplemented with a Dunnett's multiple comparison post-hoc test)
were performed using GraphPad Prism software, version 7.01 for
Windows (GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Effect of farnesol and tyrosol on
Candida growth
DMSO used to solubilize farnesol did not inhibit the
growth of C. albicans ATCC 10231 at final concentrations of
0.1–2.5% (data not shown). Neither farnesol molecule nor that of
tyrosol, despite the concentration, modified the pH of the reaction
medium (data not shown). Figs. 1
and 2 present the effect of
different concentrations of farnesol (0.001–3 mM) and tyrosol (1–20
mM) on the growth of C. albicans ATCC 10231 and six clinical
strains isolated from dentures, respectively. Following a 24-h
incubation at 37°C, the turbidity of the culture medium in all
control wells (n=96 in six independent experiments) represented an
absorbance at 600 nm of 0.735±0.010 for the reference strain and
0.885±0.024 for the clinical strains. The replicates in each series
of 8 control wells exhibited a coefficient of variation of <9%
for turbidimetric measurements. Farnesol (Fig. 1) did not modify the observed
turbidity (Dunnett's, NS) at concentrations of 1 µM-0.3 mM (n=6).
The absorbance at 600 nm following a 24-h incubation at 37°C varied
from 103.0±2.9 to 112.1±4.5% of that observed in the paired
controls for the ATCC strain and from 96.0±2.0 to 103.9±2.0% for
the isolates. A 1 mM concentration of farnesol slightly reduced the
turbidimetric growth of the clinical strains, while it did not
modify growth of the ATCC strain [88.9±3.7 (Dunnett's, P<0.01)
and 101.7±4.0% (Dunnett's, NS), respectively]. Similarly, 3 mM
farnesol reduced the turbidimetric growth of all strains, but only
significantly for the clinical strains [83.0±3.3 (Dunnett's,
P<0.001) in the isolates and 92.4±5.0% (Dunnett's, NS) in the
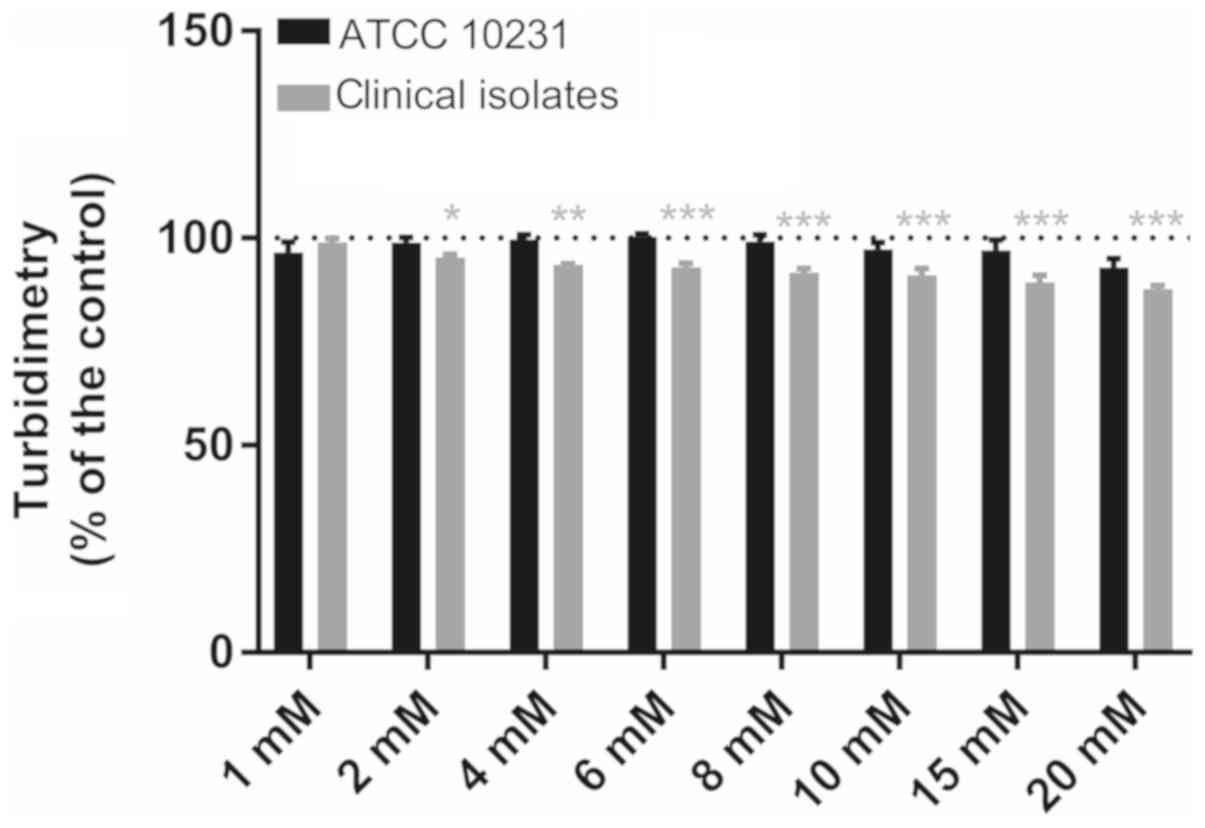
reference strain]. Tyrosol (Fig.
2) at concentrations of 1–20 mM did not affect the growth of
the ATCC 10231 strain (Dunnett's, NS), despite a non-significant
reduction of 8.1±2.2% at 20 mM (n=6). Thus, the absorbance at 600
nm following a 24-h incubation at 37°C varied from 92.9±2.2 to
100.4±4.5% of that observed in the paired controls. By contrast,
for the clinical strains, 2–20 mM tyrosol slightly and gradually
reduced the turbidimetric growth from 4.6±0.7 (Dunnett's,
P<0.05) to 12.3±0.9% (Dunnett's, P<0.01). However, in the
ATCC 10231 strain culture supernatants diluted 50,000 times
following a 24-h incubation at 37°C in the presence of QSMs, the
CFU count was not significantly different with either farnesol
(ANOVA, P=0.4921) or tyrosol (P=0.8202) relative to their paired
control. The number of CFUs expressed as logarithms is presented in
Fig. 3.
Effect of farnesol and tyrosol on
Candida biofilm formation
Figs. 4 and
5 present the results of the
evaluation of the biomass attached to the inner wall of the
microplate wells following a 24-h incubation at 37°C in the
presence of each investigated QSM. Following crystal violet
staining and 10-fold dilution, the controls exhibited an absorbance
at 600 nm of 0.539±0.048 (n=6 independent experiments) for the
reference strain and 0.668±0.072 (n=6 independent experiments) for
the clinical strains. The replicates in each series of 8 control
wells exhibited a coefficient of variation of <30% (10.9–29.5%)
for biofilm quantitation. Fig. 4
demonstrates a statistically significant inhibitory effect of
farnesol at 1 and 3 mM on the formation of biofilms by both the
reference strain and the clinical strains (Dunnett's, P<0.001).
At a concentration of 3 mM, the attached biomass averaged only
10.5±4.6 (n=6) and 27.8±4.5% (n=6) of the paired controls for the
ATCC strain and the clinical isolates, respectively. Fig. 5 presents the effect of different
tyrosol concentrations between 1 and 20 mM on biofilm formation by
both C. albicans ATCC 10231 and the clinical strains. The
data obtained in the presence of tyrosol reveal a fixed biomass
that was inversely proportional to the tyrosol concentration when
it was ≥4 mM, and became significant at 6 mM in the reference
strain (Dunnett's, P<0.001) and at 8 mM in the clinical strains
(Dunnett's, P<0.05). At 20 mM tyrosol, the amount of biofilm was
only 9.9±2.8 (n=6) and 10.0±2.9% (n=6), respectively, of the mean
value of the paired controls.
The effects of farnesol and tyrosol on biofilm
formation were evaluated simultaneously under both aerobic and
anaerobic conditions (Figs. 6 and
7). Notably, there was a
significant reduction in biofilm production (63.2%) in anaerobiosis
compared with that observed under aerobic conditions (Fig. 6) (unpaired Student's t-test,
P<0.0001); while the reduction observed in turbidimetric growth
in anaerobiosis was only 8.2% (non-Gaussian distribution,
Mann-Whitney test, P<0.0001). The data reported in Fig. 7 confirms the effects of QSMs on the
production of biofilm both aerobically and anaerobically. A 3 mM
concentration of farnesol exerted similar effects despite the
O2 level; the residual attached biomass was 44.6±3.0% in
aerobiosis, whereas in anaerobiosis it was 37.8±3.8%. Statistical
analysis revealed a Gaussian distribution of the data, and the
result of the Student's t-test was non-significant (P=0.1720). A 20
mM concentration of tyrosol reduced the attached biomass under
aerobic conditions (fixed residual biomass, 8.2±0.8% of the matched
control) more than in anaerobiosis (17.5±2.0%). Statistical
analysis demonstrated a non-Gaussian distribution of the data in
anaerobiosis, and a significant non-parametric Mann-Whitney test
(P=0.0002) indicated a greater inhibitory effect of 20 mM tyrosol
in aerobiosis than in anaerobiosis. Lower concentrations of
farnesol (0.003 mM) or tyrosol (1 mM) resulted in no significant
changes in attached biomass (ANOVA and Kruskal-Wallis test, NS)
under anaerobic compared to aerobic conditions.
The viability of the fungal cells in the presence of
farnesol or tyrosol, already demonstrated by culturing the
planktonic cells, was confirmed by fluorescent microscopic
examination of the biofilms following staining with fluorescein
diacetate, which stains the living cells, and with ethidium
bromide, which stains the dead cells. A similar assay conducted on
C. albicans ATCC 10231 blastoconidia prior to and following
a 30-min incubation at 80°C revealed 100% live and 100% dead cells,
respectively. Fig. 8A and B
demonstrate that there was >95% living planktonic Candida
cells following incubation at 37°C for 30 min, and 100% dead
planktonic cells (C. albicans ATCC 10231) following
incubation at 85°C for 30 min, assuring the reliability of the
reagents. Fig. 8C-F presents the
characteristics of the C. albicans ATCC 10231 biofilm formed
in the presence of 3 mM farnesol (Fig.
8D) or 20 mM tyrosol (Fig. 8F)
in comparison to their respective controls (Fig. 8C and E). The microscopic
observation revealed that >95% of the fungal cells
(blastoconidia and filaments) were alive following contact with
QSMs as compared to their respective controls. Notably, there was a
scarcity of filaments in the biofilms following incubation with 3
mM farnesol or 20 mM tyrosol.
Effect of farnesol and tyrosol on
Candida pre-formed biofilms
To investigate the effects of QSMs in contact with
pre-formed biofilms, the incubation time was extended to 72 h, with
renewal of the culture medium at 24 h. Under these conditions and
in the absence of QSMs, the attached biomass increased over time,
as shown in Fig. 9. In a set of
six experiments on C. albicans ATCC 10231, the increase in
attached biomass at 24 and 48 h following renewal of the culture
medium averaged 170.6±25.0 and 236.7±46.6% of the paired values,
respectively, which was observed prior to the medium change.
Following the addition of QSMs at the beginning of biofilm
formation (Fig. 10), the biomass
attached after 24 h (expressed as a percentage of that obtained
with the paired control) was reduced significantly (Dunnett's,
P<0.001) in the presence of 3 mM farnesol (44.6±3.0%, n=6), but
remained unchanged in the presence of 0.003 mM farnesol (90.5±5.2%,
n=6). Similarly, tyrosol at 20 mM significantly reduced (Dunnett's,
P<0.001) the formation of biofilm (8.2±0.8%, n=6), but had no
effect at 1 mM (102.1±2.9%, n=6). Fig. 10 presents the amount of attached
biomass observed when the QSMs were added to pre-formed biofilms.
When adding 0.003 mM vs. 3 mM farnesol to the pre-formed biofilms,
the biomass attached after 24 h was 106.1±8.4 (NS) and 96.3±10.3%,
respectively, of the paired controls, and the biomass attached
after 48 h was 98.5±5.2 (Dunnett's, NS) and 92.7±9.5% (Dunnett's,
NS), respectively. Similarly, when adding 1 mM vs. 20 mM tyrosol to
the pre-formed biofilms, the amount of biomass attached after 24 h
was 104.3±1.7 (Dunnett's, NS) and 76.7±4.3% (Dunnett's,
P<0.001), respectively, of the paired controls, and the biomass
attached after 48 h was 98.5±5.2 (Dunnett's, NS) and 66.8±5.1%
(Dunnett's, P<0.001), respectively. The small reduction in
biomass observed following the addition of 20 mM tyrosol to the
pre-formed biofilms was significant.
Discussion
The present study aimed to document the anti-biofilm
effect of two QSMs (farnesol and tyrosol) at the beginning of
Candida biofilm formation or to a pre-formed Candida
biofilm. The effects on biofilm formation must be distinguished
from those on pre-formed biofilm for considering separately the
preventive and curative applications of these molecules. A recent
study (26) considered the
anti-biofilm effect of farnesol and tyrosol on pre-formed
Candida biofilms: The authors found a significant reduction
in biofilm metabolism with no effect on the biomass by using
farnesol or a tyrosol/farnesol combination.
In the present study, farnesol at 3 mM exerted a
greater effect when added at the beginning of biofilm formation
than when added to pre-formed biofilm. Similarly, tyrosol at 20 mM
exhibited a stronger action when added at the beginning of biofilm
formation than when added to pre-formed biofilm. Despite these
significant reductions in attached biomass, the yeast growth, as
evaluated by turbidimetry, varied little in the presence of the two
investigated molecules. Therefore, there is an anti-biofilm effect
distinct from any fungicidal or fungistatic action. The absence of
fungicidal or fungistatic action observed by turbidimetry was
corroborated by culturing the supernatants on solid agar followed
by counting of the CFUs and by viability tests using fluorescent
microscopy. These molecules, at the highest tested concentrations,
had a greater effect in the initial phases of biofilm formation;
however, the data do not differentiate an anti-adhesion effect from
a reduction in biofilm growth. The effect of farnesol in
anaerobiosis was not significantly different from that observed in
aerobiosis. However, in anaerobiosis, the effect of tyrosol
slightly, but significantly, decreased in comparison with that
observed in aerobiosis. Notably, in the absence of both farnesol
and tyrosol, there was a marked reduction in biofilm production in
anaerobiosis compared to aerobiosis.
The QSM doses observed as inhibitory in vitro
may not be reached in the oral environment. In culture media, the
QSM concentrations quoted in the literature are in the micromolar
range or lower for both farnesol (27) and tyrosol (11); however, they cannot be
representative of those present in biofilms, which may concentrate
these molecules. Intra-biofilm concentrations of QSMs have been
scarcely investigated to date. Only one study, to the best of our
knowledge, mentions a lower secretion of farnesol in Candida
biofilm than in the culture medium of planktonic cells (28), and another mentions 50% higher
tyrosol secretion by Candida biofilm than by planktonic
cells (22). It is difficult to
evaluate QSM concentrations during biofilm production as this
requires harvesting of the experimental biofilm from the culture
medium without modifying the intra-biofilm composition, while the
biofilm itself can also behave as an open compartment with exchange
between intra- and extra-biofilm areas. The concentrations of QSMs
found to have an anti-biofilm effect were exogenously added in the
millimolar range in the present study, which is higher than the
doses generated in culture. It is therefore questionable whether
the observed anti-biofilm effect is due to toxicity rather than a
biological effect (27). Further
studies should clarify and differentiate the metabolic pathways and
cellular mechanisms involved in QS from those involved in biofilm
toxicity. In this context, it would be appropriate to investigate
the consequences of farnesol conversion to farnesoic acid with
respect to biofilm formation, as previously described in C.
albicans ATCC 10231 (29).
Prior to suggesting the use of these molecules for
preventive purposes in an oral hygiene setting, future studies
should further clarify the repercussions of these QSMs on the
homeostasis of the oral microbiome. To date, few studies have
addressed the effects of QSMs on oral ecology. Tyrosol suspended in
saliva at a concentration above the solubility threshold has been
reported to reduce the adherence of C. albicans and C.
glabrata to acrylic resin, a material used in the manufacture
of dentures (30). In the same
study, tyrosol significantly reduced the biomass of C.
albicans single biofilm and C. albicans/glabrata
mixed biofilm. Farnesol at a concentration of 3.12 mM (31) and tyrosol at concentrations of
>20 mM (32) have been reported
to inhibit the development of single and mixed biofilms formed by
C. albicans and the cariogenic oral bacteria
Streptococcus mutans. These studies suggest the development
of novel oral strategies to prevent oral diseases caused by
pathogenic biofilms, such as denture stomatitis and dental caries;
however, tyrosol exerted no significant reduction in acid
production by Candida and S. mutans (26). Some notable data have been produced
concerning minor bacterial species in the oral cavity. On one hand,
both tyrosol and farnesol affect certain virulence factors of the
bacteria Pseudomonas aeruginosa, suggesting a possible
effect of yeast QSMs in mixed biofilms when P. aeruginosa is
associated with C. albicans (33). On the other hand, farnesol at a
concentration of ≥100 µM inhibits Staphylococcus aureus
biofilm formation (19).
The present study demonstrates a specific
anti-biofilm effect, independent of fungicidal or fungistatic
action, of both farnesol and tyrosol, as tested in C.
albicans ATCC 10231 and six strains isolated from dentures.
This inhibition of biofilm formation by the exogenous addition of
QSMs may result from a disturbance in the QSM mechanisms or from a
limitation of yeast adhesion on the support; however, another toxic
action on the metabolic pathways involved in biofilm formation
cannot be excluded. Prior to suggesting the use of these molecules
for preventive purposes in an oral hygiene setting, further studies
are required to clarify the metabolic pathways, gene expression
regulation and cellular mechanisms involved in QS, as well as other
repercussions on the oral microbiome.
Acknowledgements
The authors would like to thank Ms. Ilhame Dardour
and Mr. Hadrien Kerkhofs of the Medical Biology Department, Haute
Ecole Francisco Ferrer (Brussels, Belgium) and Ms. Latifa Manouach
of the Dentistry Department, Université de Bruxelles (Brussels,
Belgium) for their enthusiastic participation in the study in the
context of their final year dissertations. Furthermore, the authors
thank Mr. G. Vegh (Laboratory of Physiology and Pharmacology,
Université Libre de Bruxelles (Brussels, Belgium) for his technical
help in microscope management.
Funding
The present study was supported by a grant
(2013–2014) from the Xenophilia Funds (Université Libre de
Bruxelles).
Availability of data
The data sets used and analysed in the current study
are available from the corresponding author on reasonable
request.
Authors' contributions
SS contributed to the acquisition, management and
interpretation of data. ZBO validated the identification of
Candida strains, collaborated in the interpretation of the
data and critically revised the manuscript. PC contributed to the
conception and design of this investigation; and was involved in
drafting the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Miller MB and Bassler BL: Quorum sensing
in bacteria. Annu Rev Microbiol. 55:165–199. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hogan DA: Talking to themselves:
Autoregulation and quorum sensing in fungi. Eukaryot Cell.
5:613–619. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Albuquerque P and Casadevall A: Quorum
sensing in fungi-A review. Med Mycol. 50:337–345. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cho T, Nagao JI, Imayoshi R, Kaminishi H,
Aoyama T and Nakayama H: Quorum sensing and morphological
regulation in the pathogenic fungus Candida albicans. J Oral
Biosci. 52:233–239. 2010. View Article : Google Scholar
|
|
5
|
Kruppa M: Quorum sensing and Candida
albicans. Mycoses. 52:1–10. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Deveau A and Hogan DA: Linking quorum
sensing regulation and biofilm formation by Candida
albicans. Methods Mol Biol. 692:219–233. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lingappa BT, Prasad M, Lingappa Y, Hunt DF
and Biemann K: Phenethyl alcohol and tryptophol: Autoantibiotics
produced by the fungus Candida albicans. Science.
163:192–194. 1969. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hazen KC and Cutler JE: Autoregulation of
germ tube formation by Candida albicans. Infect Immun.
24:661–666. 1979.PubMed/NCBI
|
|
9
|
Hornby JM, Jensen EC, Lisec AD, Tasto JJ,
Jahnke B, Shoemaker R, Dussault P and Nickerson KW: Quorum sensing
in the dimorphic fungus Candida albicans is mediated by
farnesol. Appl Environ Microbiol. 67:2982–2992. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Oh KB, Miyazawa H, Naito T and Matsuoka H:
Purification and characterization of an autoregulatory substance
capable of regulating the morphological transition in Candida
albicans. Proc Natl Acad Sci USA. 98:4664–4668. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen H, Fujita M, Feng Q, Clardy J and
Fink GR: Tyrosol is a quorum-sensing molecule in Candida
albicans. Proc Natl Acad Sci USA. 101:5048–5052. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Westwater C, Balish E and Schofield DA:
Candida albicans-conditioned medium protects yeast cells
from oxidative stress: A possible link between quorum sensing and
oxidative stress resistance. Eukaryot Cell. 4:1654–1661. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mosel DD, Dumitru R, Hornby JM, Atkin AL
and Nickerson KW: Farnesol concentrations required to block germ
tube formation in Candida albicans in the presence and
absence of serum. Appl Environ Microbiol. 71:4938–4940. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Décanis N, Tazi N, Correia A, Vilanova M
and Rouabhia M: Farnesol, a fungal quorum-sensing molecule triggers
Candida albicans morphological changes by downregulating the
expression of different secreted aspartyl proteinase genes. Open
Microbiol J. 5:119–126. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ramage G, Saville SP, Wickes BL and
López-Ribot JL: Inhibition of Candida albicans biofilm
formation by farnesol, a quorum-sensing molecule. Appl Environ
Microbiol. 68:5459–5463. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shchepin R, Hornby JM, Burger E, Niessen
T, Dussault P and Nickerson KW: Quorum sensing in Candida
albicans: Probing farnesol's mode of action with 40 natural and
synthetic farnesol analogs. Chem Biol. 10:743–750. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shareck J and Belhumeur P: Modulation of
morphogenesis in Candida albicans by various small
molecules. Eukaryot Cell. 10:1004–1012. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu LH, Wei X, Ma M, Chen XJ and Xu SB:
Possible inhibitory molecular mechanism of farnesol on the
development of fluconazole resistance in Candida albicans
biofilm. Antimicrob Agents Chemother. 56:770–775. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jabra-Rizk MA, Shirtliff M, James C and
Meiller T: Effect of farnesol on Candida dubliniensis
biofilm formation and fluconazole resistance. FEMS Yeast Res.
6:1063–1073. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Katragkou A, McCarthy M, Alexander EL,
Antachopoulos C, Meletiadis J, Jabra-Rizk MA, Petraitis V, Roilides
E and Walsh TJ: In vitro interactions between farnesol and
fluconazole, amphotericin B or micafungin against Candida
albicans biofilms. J Antimicrob Chemother. 70:470–478. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nickerson KW, Atkin AL and Hornby JM:
Quorum sensing in dimorphic fungi: Farnesol and beyond. Appl
Environ Microbiol. 72:3805–3813. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Alem MA, Oteef MD, Flowers TH and Douglas
LJ: Production of tyrosol by Candida albicans biofilms and
its role in quorum sensing and biofilm development. Eukaryot Cell.
5:1770–1779. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cordeiro RA, Teixeira CE, Brilhante RS,
Castelo-Branco DS, Alencar LP, de Oliveira JS, Monteiro AJ,
Bandeira TJ, Sidrim JJ, Moreira JL and Rocha MF: Exogenous tyrosol
inhibits planktonic cells and biofilms of Candida species
and enhances their susceptibility to antifungals. FEMS Yeast Res.
15:fov0122015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Blanco MT, Pérez-Giraldo C, Blanco J,
Morán FJ, Hurtado C and Gómez-García AC: In vitro studies of
activities of some antifungal agents against Candida
albicans ATCC 10231 by the turbidimetric method. Antimicrob
Agents Chemother. 36:898–901. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Stepanović S, Vuković D, Dakić I, Savić B
and Švabić-Vlahović M: A modified microtiter-plate test for
quantification of staphylococcal biofilm formation. J Microbiol
Methods. 40:175–179. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Monteiro DR, Arias LS, Fernandes RA, Deszo
da Silva LF, de Castilho MOVF, da Rosa TO, Vieira APM, Straioto FG,
Barbosa DB and Delbem ACB: Antifungal activity of tyrosol and
farnesol used in combination against Candida species in the
planktonic state or forming biofilms. J Appl Microbiol.
123:392–400. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Krom BP, Levy N, Meijler MM and Jabra-Rizk
MA: Farnesol and Candida albicans: Quorum sensing or not
quorum sensing. Isr J Chem. 56:295–301. 2016. View Article : Google Scholar
|
|
28
|
Martins M, Henriques M, Azeredo J, Rocha
SM, Coimbra MA and Oliveira R: Morphogenesis control in Candida
albicans and Candida dubliniensis through signaling
molecules produced by planktonic and biofilm cells. Eukaryot Cell.
6:2429–2436. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Riekhof WR and Nickerson KW: Quorum
sensing in Candida albicans: Farnesol versus farnesoic acid.
FEBS Lett. 591:1637–1640. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Monteiro DR, Feresin LP, Arias LS, Barão
VA, Barbosa DB and Delbem ACB: Effect of tyrosol on adhesion of
Candida albicans and Candida glabrata to acrylic
surfaces. Med Mycol. 53:656–665. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fernandes RA, Monteiro DR, Arias LS,
Fernandes GL, Delbem AC and Barbosa DB: Biofilm formation by
Candida albicans and Streptococcus mutans in the
presence of farnesol: A quantitative evaluation. Biofouling.
32:329–338. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Arias LS, Delbem AC, Fernandes RA, Barbosa
DB and Monteiro DR: Activity of tyrosol against single and
mixed-species oral biofilms. J Appl Microbiol. 120:1240–1249. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Abdel-Rhmam SH, El-Mahdy AM and El-Mowafy
M: Effect of tyrosol and farnesol on virulence and antibiotic
resistance of clinical isolates of Pseudomonas aeruginosa.
Biomed Res Int. 2015:4564632015.PubMed/NCBI
|