Introduction
Cervical cancer (CC) is a common gynecological
malignant tumor that may be caused by various factors, including
human papillomavirus infection, and genetic and epigenetic
alterations (1). Recently, there
has been an increase in the tendency of CC, particularly in younger
patients (2). Despite advances in
the prevention and diagnosis of CC due to widespread screening, a
large number of patients are still diagnosed with invasive CC
(3). Therefore, the identification
of novel biomarkers and elucidation of the molecular mechanisms
underlying CC are required for improving the early diagnosis and
treatment of CC.
Circular RNAs (circRNAs) derive from back-spliced
exons that form a covalently closed loop without 5′-3′ polarity or
a polyadenylated tail (4).
Advances in high-throughput DNA sequencing techniques have
facilitated identification of the functions of various circRNAs.
Accumulating evidence has demonstrated that circRNAs may be
involved in cell cycle progression, cell aging and apoptosis by
regulating gene expression and directly binding multiple proteins,
thus affecting their activity (5,6).
Additionally, circRNAs serve important roles in tumorigenesis and
have been identified as novel molecular biomarkers for the
diagnosis of various diseases. In addition, the ability of circRNAs
to serve as microRNA (miRNA/miR) sponges has been reported
(7). circRNAs may compete with
miRNAs for binding the 3′ untranslated regions (3′-UTRs) of mRNAs,
thus regulating gene expression. circRNA_0084043 has been
identified to promote melanoma cell proliferation and migration via
the miR-153-3p/snail family transcriptional repressor pathway
(8). Li et al (9) demonstrated that the circRNA isoform
of fibroblast growth factor receptor 4 promotes myoblast
differentiation by regulating the expression levels of miR-107 and
Wnt family member 3A. In addition, hsa_circ_0008039 has been
reported to modulate the malignant characteristics of breast cancer
by regulating the miR-432-5p/E2F transcription factor 3 axis
(10). However, the role and the
molecular mechanisms of circRNAs in CC progression remain
unknown.
In the present study, circRNA isoform of eukaryotic
translation initiation factor 4γ2 (circEIF4G2; circbase ID:
hsa_circ_0021254; www.circbase.org) expression was revealed to be
increased in CC tissues, and upregulation of circEIF4G2 was
associated with poor prognosis in patients with CC. Knockdown of
circEIF4G2 suppressed proliferation and malignant features of CC
cells. Mechanistically, the present findings suggested that
circEIF4G2 may promote CC cell growth and migration by sponging
miR-218, which decreased the expression levels of homeobox A1
(HOXA1).
Patients and methods
Patient samples
A total of 20 pairs of CC tissues and adjacent
normal tissues were used in the present study. The samples were
collected from patients with CC at the Second People's Hospital of
Wuhu (Wuhu, China) from January 2015 to June 2017. Patients were
recruited according to the following criteria: i) Patients were
diagnosed and confirmed by histopathological examination; ii)
patients did not receive systemic chemotherapy or radiotherapy in
the pelvic cavity prior to surgery; and iii) follow-up data could
be obtained from all patients. Patients with serious cardiovascular
disease or other malignancies were excluded. Tumors were graded
according to the tumor-node-metastasis (TNM) system (7th edition)
maintained by the American Joint Committee on Cancer and the
International Union for Cancer Control (11). Patients with CC were divided into
high and low circEIF4G2 expression groups according to the median
level of circEIF4G2 (cut-off value, 2.79). All patients provided
written informed consent, and the present study was approved by the
Clinical Research Ethics Committee of the Second People's Hospital
of Wuhu. Following surgical resection, all tissues were snap-frozen
in liquid nitrogen and stored at −80°C until further analysis.
Cell lines and transfection
Human CC cell lines, including HeLa, CasKi, C33A and
SiHa cells, were obtained from the American Type Culture Collection
(Manassas, VA, USA). Control cells were derived from normal
cervical tissues from patients that underwent hysterectomy.
Briefly, normal cervical tissues were digested by 20% collagenase
type I at 37°C for 40 min to obtain cervical epithelial cells.
Dulbecco's Modified Eagle's medium (DMEM) supplemented with 10%
fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), L-glutamine (2 mM), penicillin (100 U/ml) and
streptomycin (100 mg/ml; Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) were used to culture CC cells and the control cells at
37°C with 5% CO2.
Small interfering RNAs (siRNAs) targeting circEIF4G2
and HOXA1, siRNA-negative control (si-NC), miR-218 mimic, miR-NC,
miR-218 inhibitor and miR-218 inhibitor-NC were purchased from
Guangzhou RiboBio Co., Ltd. (Guangzhou, China). The sequences were
as follows: si-circEIF4G2, 5′-AUGCUCCCAGCUUUUGGAAAA-3′; si-HOXA1,
5′-GGAUGUCUGUAAUAAAUAAAU-3′; si-NC, 5′-UUCUCCGAACGUGUCACGU-3′;
miR-218 mimic, 5′-UUGUGCUUGAUCUAACCAUGU-3′; miR-NC,
5′-UUCUCCGAACGUGUCACGU-3′; miR-218 inhibitor,
5′-ACAUGGUUAGAUCAAGCACAA-3′; and miR-218 inhibitor-NC,
5′-CAGUACUUUUGUGUAGUACAA-3′. The cDNA sequence of circEIF4G2 was
synthesized and cloned into the lentiviral expression vector
pLVXIRES-neo (Clontech Laboratories, Inc., Mountainview, CA, USA)
by Guangzhou RiboBio Co., Ltd., and the resulting p-circEIF4G2
vector (1×109 PFU) was used to overexpress circEIF4G2.
293T cells (Sangon Biotech Co., Ltd., Shanghai, China) were used
for packaging the lentivirus. HeLa or C33A cells (2×105)
were transfected with a combination of nucleic acids (50 nM) using
Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol.
Following incubation for 48 h, cells were harvested for further
experimentation.
Cell Counting kit-8 (CCK-8) assay
HeLa or C33A cells in the logarithmic growth phase
were cultured in 96-well plates (1×104 cells/well)
overnight. Once cells reached 60% confluence they were transfected
and were then cultured for 24, 48, 72 or 96 h. At each time point,
10 µl CCK-8 solution (Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan) was added for 4 h. Cell proliferation was measured
based on the absorbance detected at 450 nm using a microplate
reader (Thermo Fisher Scientific, Inc.).
Colony formation assay
HeLa or C33A cells transfected with si-circEIF4G2 or
si-NC were digested into single cells using trypsin. Cells
(5×102 cells/well) were then mixed with 0.35% agarose
and DMEM, and plated onto a solid layer of agarose/DMEM. Following
solidification of the upper agarose layer, the plates were
incubated at 37°C with 5% CO2 for 2 weeks. Then, the
colonies were fixed with 4% paraformaldehyde for 15 min and stained
with 0.05% crystal violet for 30 min at room temperature. The
number of colonies was assessed using a light microscope
(magnification, ×100).
Wound healing assay
HeLa or C33A cells were seeded in 6-well plates at a
density of 5×105 cells/well. The cells were then
scratched in a straight line using a 10-µl sterile pipette tip and
the cellular debris was washed using PBS. Subsequently, the cells
were incubated at 37°C for 48 h and images of the wounds were
captured using an IX71 microscope (magnification, ×100; Olympus
Corporation, Tokyo, Japan).
Matrigel invasion assay
Transfected HeLa or C33A cells (2.5×103
cells/well) in serum-free DMEM were plated in the upper chamber of
a Transwell system with 8-µm pores, which was pre-coated with
Matrigel (BD Biosciences, Franklin Lakes, NJ, USA). DMEM containing
10% FBS was added to the lower chamber. After a 24-h incubation,
the invasive cells were fixed with methanol at 37°C for 30 min and
stained with 0.1% crystal violet at 37°C for 30 min. Subsequently,
images of the invasive cells were captured and the cells were
counted using an IX71 microscope (magnification, ×100).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from tissues or cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). Following quantification using a NanoDrop™ spectrophotometer
(NanoDrop Technologies; Thermo Fisher Scientific, Inc., Wilmington,
DE, USA), RNA underwent cDNA synthesis using a High Capacity cDNA
RT kit (Applied Biosystems; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) according to the manufacturer's protocol. qPCR
was performed using the SYBR Select Master Mix (Applied Biosystems;
Thermo Fisher Scientific, Inc.) on an ABI 7900 system (Applied
Biosystems; Thermo Fisher Scientific, Inc.), with GAPDH or U6 as an
internal control. The PCR conditions were as follows: 95°C for 3
min, followed by 40 cycles of 95°C for 10 sec, 60°C for 30 sec and
72°C for 30 sec. Primer sequences are listed in Table I. The 2−ΔΔCq method was
used to determine the relative gene expression level (12).
 | Table I.Primers used for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primers used for reverse
transcription-quantitative polymerase chain reaction.
| Gene symbol | Forward primer
(5′→3′) | Reverse primer
(5′→3′) |
|---|
| circEIF4G2 |
TTTTTCAACAAAGCAAGGTCAA |
TCTAGGTCCCACTGTCCTCA |
| miR-218 |
CGCGCGCGTTGTGCTTGATCTAA |
AGTGCAGGGTCCGAGGTATT |
| HOXA1 |
GGGTGTCCTACTCCCACTCA |
GGACCATGGGAGATGAGAGA |
| GAPDH |
GTGTTTCCTCGTCCCGTAGA |
GAATTTGCCGTGAGTGGAGT |
| U6 |
GCTTCGGCAGCACATATACTAAAAT |
TACTGTGCGTTTAAGCACTTCGC |
Western blot analysis
Total protein was extracted from transfected HeLa or
C33A cells lysed using the Pierce cell lysis buffer (Pierce; Thermo
Fisher Scientific, Inc., Waltham, MA, USA). Bicinchoninic acid
protein assay kit (Takara Biotechnology Co., Ltd., Dalian, China)
was used to quantify protein concentration. Equal amounts of
protein (30 µg/lane) were separated by 10% SDS-PAGE and were
subsequently transferred onto polyvinylidene difluoride membranes
(EMD Millipore, Billerica, MA, USA). The membranes were blocked in
5% skim milk for 2 h at 37°C, and then incubated with primary
antibodies overnight at 4°C. The primary antibodies used were
anti-HOXA1 (1:1,000; cat. no. ab230513) and anti-β-actin (1:2,000;
cat. no. ab8227; Abcam, Cambridge, UK). Subsequently, membranes
were incubated for 2 h at room temperature with horseradish
peroxidase-conjugated secondary antibody (1:2,000; cat. no.
ab97051; Abcam). Bands were visualized using an enhanced
chemiluminescence detection kit (EMD Millipore).
Target prediction
The potential miRNAs interacting with circEIF4G2
were predicted using Starbase 2.0 (http://starbase.sysu.edu.cn/). Putative targets of
miR-218 were predicted using TargetScan (release 6.2; http://www.targetscan.org), microRNA.org (version 3.3a; http://www.microrna.org/microrna/home.do) and miRDB
(http://www.mirdb.org).
Dual-luciferase reporter assay
To construct the recombinant luciferase vectors,
wild type (WT) and mutant (MUT) circEIF4G2 sequences were
synthesized by Shanghai GenePharma Co., Ltd. (Shanghai, China) and
separately cloned into pmirGLO luciferase vectors (Promega
Corporation, Madison, WI, USA) between the NheI and XbaI sites. In
addition, WT and MUT 3′-UTRs of HOXA1 were synthesized by Shanghai
GenePharma Co., Ltd. and separately cloned into pmirGLO luciferase
vectors between the SacI and XbaI sites. HeLa or C33A cells were
cotransfected with 50 nM miR-218 mimic or miR-NC, and the
recombinant luciferase vectors using Lipofectamine® 3000
(Invitrogen; Thermo Fisher Scientific, Inc.). After a 48-h
incubation, luciferase activity was detected using a
dual-luciferase reporter assay system (Promega Corporation)
according to the manufacturer's protocol. Renilla luciferase
activity was detected and used as the internal control.
RNA immunoprecipitation (RIP)
assay
RNA-binding protein immunoprecipitation kit (EMD
Millipore) was used to perform the RIP assay according to the
manufacturer's protocol. HeLa or C33A cells at 80–90% confluency
were incubated with the RIP buffer containing magnetic beads coated
with anti-argonaute RNA-induced silencing complex (RISC) catalytic
component 2 (AGO2) antibodies (cat. no. MABE253, EMD Millipore) or
anti-immunoglobulin G (IgG; cat. no. AB22-K, EMD Millipore)
antibodies. RT-qPCR was performed to detect the expression levels
of immunoprecipitated RNA of circEIF4G2 and miR-218.
Statistical analysis
Data were analyzed using SPSS 17.0 software (SPSS,
Inc., Chicago, IL, USA). Data are presented as the means ± standard
deviation from three experiments. Comparisons between two groups
were analyzed using Student's t-test, whereas multiple groups were
compared using one-way analysis of variance followed by Tukey's
post hoc test. Kaplan-Meier analysis was performed to calculate the
overall survival rate, and a log-rank test was conducted to compare
the survival distributions between two groups. Pearson correlation
analysis was performed between the expression levels of circEIF4G2
and miR-218 in CC tissues. P<0.05 was considered to indicate a
statistically significant difference.
Results
circEIF4G2 is upregulated in CC
tissues and cells
To investigate the expression levels of circEIF4G2
in CC, 20 pairs of CC tissues and corresponding adjacent normal
tissues were collected and RT-qPCR was performed to detect
circEIF4G2 expression. The clinical characteristics of patients
with CC are presented in Table
II. High expression of circEIF4G2 was significantly associated
with tumor size and lymph node metastasis, but not with age or
tumor-node-metastasis stage (Table
II). circEIF4G2 was significantly upregulated in CC tissues
compared with in adjacent normal tissues (Fig. 1A). Increased expression of
circEIF4G2 was also detected in various CC cell lines, including
HeLa, CasKi, C33A and SiHa cells, compared with in control cells
derived from tissues from patients that underwent hysterectomy
(Fig. 1B). To examine the
possibility of using circEIF4G2 expression as a prognostic marker
in patients with CC, Kaplan-Meier curve analysis was performed, and
CC samples exhibiting an increased expression of circEIF4G2 were
compared with CC samples exhibiting a decreased expression level of
circEIF4G2. The results suggested that increased expression levels
of circEIF4G2 were associated with poor prognosis in patients with
CC (Fig. 1C).
 | Table II.Clinical characteristics of patients
with cervical cancer. |
Table II.
Clinical characteristics of patients
with cervical cancer.
|
| Expression level of
circEIF4G2 relative to the median |
|
|---|
|
|
|
|
|---|
| Clinicopathological
characteristic | Low, n=10 | High, n=10 | P-value |
|---|
| Age |
|
| 0.370 |
| ≤45
years | 4 | 7 |
|
| >45
years | 6 | 3 |
|
| Tumor size |
|
| 0.003a |
| ≤4
cm | 7 | 0 |
|
| >4
cm | 3 | 10 |
|
| Lymph node
metastasis |
|
| 0.011a |
|
Negative | 6 | 0 |
|
|
Positive | 4 | 10 |
|
| TNM stage |
|
| 0.057 |
|
I/II | 9 | 4 |
|
|
III/IV | 1 | 6 |
|
circEIF4G2 knockdown suppresses the
proliferation and malignant features of CC cells
To investigate the role of circEIF4G2 in CC cells,
HeLa and C33A cells were selected for further analyses, due to the
increased expression level of circEIF4G2. A siRNA targeting
circEIF4G2 was designed and transfected into HeLa and C33A cells.
The RT-qPCR analysis revealed that following transfection with
si-circEIF4G2, the expression levels of circEIF4G2 were decreased
by 62.3% in HeLa cells and by 61.3% in C33A cells compared with the
corresponding control (Fig. 2A).
Subsequently, CCK-8 assay and colony formation assay were performed
to examine the effects of circEIF4G2 knockdown on cell
proliferation. circEIF4G2 knockdown significantly suppressed cell
proliferation and colony formation in HeLa and C33A cells (Fig. 2B-D). Wound healing and Matrigel
invasion assays were performed to investigate the role of
circEIF4G2 in the malignant features of HeLa and C33A cells. The
results suggested that circEIF4G2 knockdown significantly inhibited
cell migration and invasion (Fig. 2E
and F).
circEIF4G2 inhibits the expression of
miR-218
To investigate the mechanism underlying circEIF4G2
function, the potential miRNAs interacting with circEIF4G2 were
predicted using Starbase 2.0, as circRNAs may serve as sponges of
miRNAs, thus regulating gene expression. The in silico results
suggested that a possible miR-218 binding site was present in the
sequence of circEIF4G2 (Fig. 3A).
The expression levels of miR-218 were upregulated following
transfection with miR-218 mimic, conversely, transfection with
miR-218 inhibitor downregulated the expression levels of miR-218 in
HeLa and C33A cells (Fig. 3B).
Furthermore, dual-luciferase reporter assay results suggested that
miR-218 overexpression significantly suppressed the luciferase
activity of WT-circEIF4G2 luciferase plasmid; however, the
luciferase activity of the plasmid containing the MUT-circEIF4G2
sequence was not affected in HeLa or C33A cells (Fig. 3C). To examine the role of
circEIF4G2 on miR-218 expression, RT-qPCR was performed following
circEIF4G2 knockdown. The results suggested that the expression
levels of miR-218 in HeLa and C33A cells transfected with
si-circEIF4G2 exhibited a 4.97- and 3.70-fold increase compared
with HeLa and C33A cells transfected with si-NC, respectively
(Fig. 3D). The expression levels
of circEIF4G2 were significantly increased in HeLa and C3AA cells
transfected with p-circEIF4G2 (Fig.
3E). Additionally, miR-218 expression levels were significantly
downregulated in HeLa and C33A cells transfected with p-circEIF4G2
(Fig. 3F). RIP assay was performed
in HeLa and C33A cells using anti-AGO2 antibodies, and the
expression levels of circEIF4G2 and miR-218 were detected using
RT-qPCR. The RIP results suggested that circEIF4G2 and miR-218 were
significantly enriched following immunoprecipitation of AGO2
compared with IgG (Fig. 3G and H).
Furthermore, the correlation analysis of the expression levels of
circEIF4G2 and miR-218 suggested that the expression levels of
miR-218 and circEIF4G2 were negatively correlated in CC tissues
(Fig. 3I).
 | Figure 3.circEIF4G2 inhibits the expression of
miR-218. (A) Predicted interaction between circEIF4G2 and miR-218.
(B) Expression levels of miR-218 in HeLa and C33A cells transfected
with miR-218 mimic or miR-218 inhibitor. (C) Interaction between
circEIF4G2 and miR-218 was examined using dual-luciferase reporter
assay in HeLa cells and C33A cells. (D) Expression levels of
miR-218 in HeLa and C33A cells transfected with si-circEIF4G2 or
si-NC. (E) Expression levels of circEIF4G2 in HeLa and C33A cells
transfected with p-circEIF4G2 or p-ctrl. (F) Expression levels of
miR-218 in HeLa and C33A cells transfected with p-circEIF4G2 or
p-ctrl. RNA immunoprecipitation assay was performed in (G) HeLa and
(H) C33A cells, and the increase in the expression levels of
circEIF4G2 and miR-218 were detected by reverse
transcription-quantitative polymerase chain reaction. (I)
Correlation analysis of the expression levels of circEIF4G2 and
miR-218 in CC tissues. *P<0.05 vs. corresponding control. AGO2,
argonaute RISC catalytic component 2; circEIF4G2, circular RNA
isoform of eukaryotic translation initiation factor 4γ2; ctrl,
control; IgG, immunoglobulin G; miR, microRNA; MUT, mutant; p-,
plasmid; si-, small interfering RNA; WT, wild type. |
circEIF4G2 induces the expression of
HOXA1 by sponging miR-218
The downstream mechanism of the circEIF4G2/miR-218
axis was further investigated. Following in silico prediction
analysis, HOXA1 was identified to be a potential target of miR-218
(Fig. 4A). To examine the
association between miR-218 and HOXA1, dual-luciferase reporter
assay was performed and the data suggested that transfection with
miR-218 mimic significantly suppressed the luciferase activity of
HOXA1-WT plasmid compared with the negative control (Fig. 4B); however, the luciferase activity
of HOXA1-MUT plasmid was not affected. To further investigate the
effects of miR-218 and circEIF4G2 on HOXA1 expression, HeLa and
C33A cells were transfected with si-circEIF4G2 and/or miR-218
inhibitor. Knockdown of circEIF4G2 significantly decreased the
expression levels of HOXA1, whereas transfection with miR-218
inhibitor increased the expression levels of HOXA1 (Fig. 4C and D). The results revealed that,
compared with cells transfected with miR-218 inhibitor, the
concomitant knockdown of circEIF4G2 and miR-218 decreased the
expression levels of HOXA1 by 68.5 and 71.1% in HeLa cells and C33A
cells, respectively (Fig. 4C and
D). The protein expression levels of HOXA1 were consistent with
the RT-qPCR results (Fig. 4E). The
mRNA expression levels of HOXA1 in CC tissues were analyzed and
HOXA1 was identified to be upregulated in CC tissues compared with
in normal tissues (Fig. 4F). In
addition, the expression levels of HOXA1 were significantly
decreased following transfection with si-HOXA1 in HeLa or C33A
cells (Fig. 4G). Therefore,
si-HOXA1 was used to knockdown HOXA1 in the further
experiments.
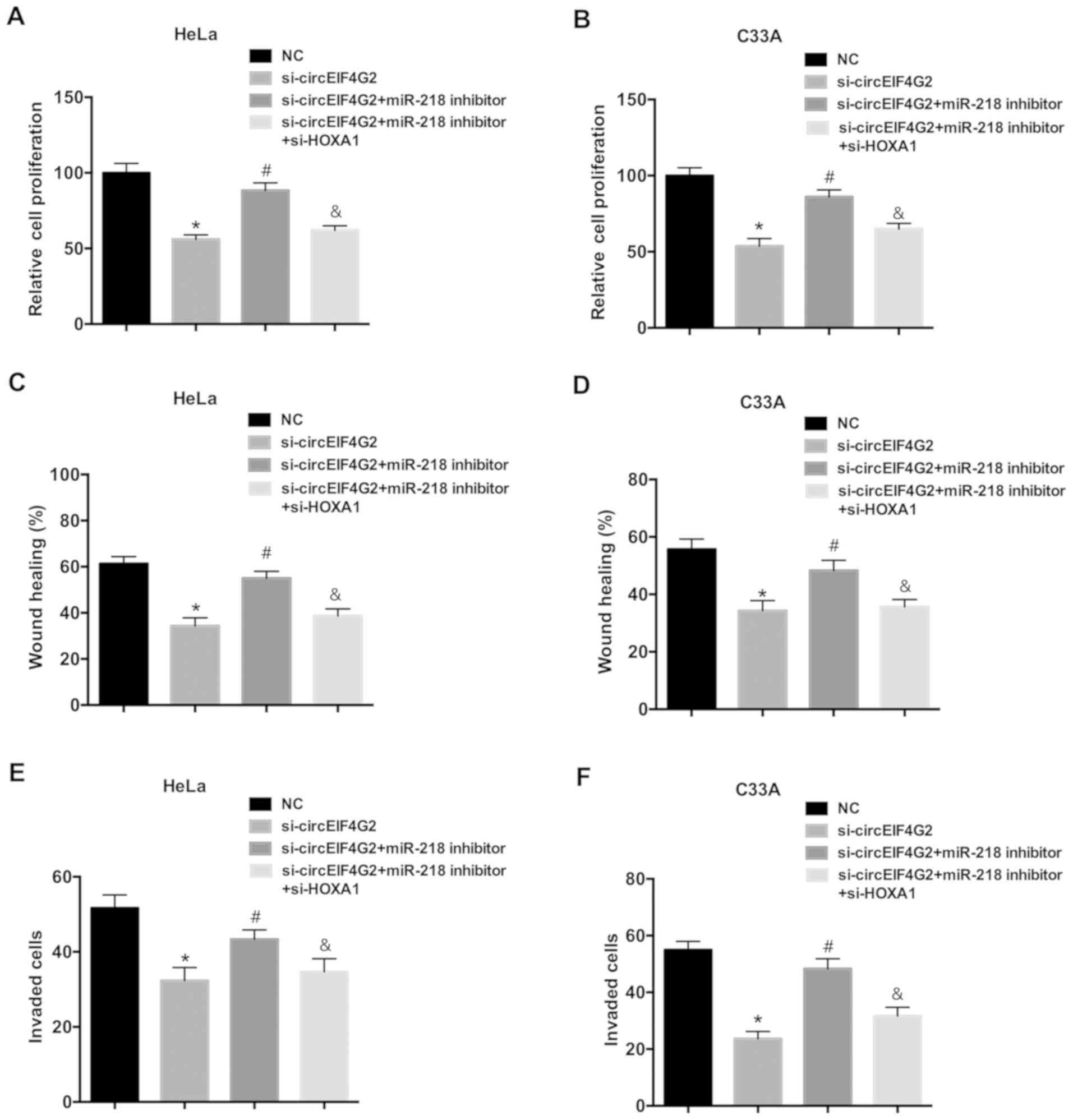
circEIF4G2 promotes cell proliferation
and migration via the miR-218/HOXA1 pathway
To further investigate the role of the miR-218/HOXA1
axis in the oncogenic function of circEIF4G2, rescue experiments
were performed in HeLa and C33A cells. Transfection with the
miR-218 inhibitor attenuated the inhibitory effects of circEIF4G2
knockdown on proliferation, migration and invasion of HeLa and C33A
cells (Fig. 5). Furthermore, HOXA1
knockdown reversed the effects of the miR-218 inhibitor.
Collectively, the present data suggested that circEIF4G2 may
promote cell proliferation and migration via the miR-218/HOXA1
pathway.
Discussion
Accumulating evidence has demonstrated that the
dysregulation of circRNAs is associated with the occurrence and
development of cancer and other diseases (13,14).
circRNAs have been identified to exhibit an increased stability
compared with linear RNAs due to their closed-loop structure and
resistance to RNase R (15).
circRNAs are expressed in various human cells and certain circRNAs
exhibit tissue- and disease-specificity. The expression levels of
circRNAs have been reported to be increased in brain tissues,
particularly in synapses. Due to the advances in circRNA microarray
and bioinformatics analyses, the identity and function of numerous
novel circRNAs have been identified. For example, Chen et al
(16) demonstrated, using circRNA
microarray analysis, that the expression levels of hsa_circ_0128298
are increased in hepatocellular carcinoma (HCC) tissues, and that
hsa_circ_0128298 may be used as a novel diagnostic and prognostic
biomarker for patients with HCC. Wang et al (17) demonstrated that circ_0067934 serves
as an oncogene and its expression levels are associated with poor
prognosis in patients with non-small cell lung cancer. In the
present study, circEIF4G2 was identified to be upregulated in CC
tissues and cells compared with in adjacent normal tissues and
cells. Furthermore, an increase in the expression levels of
circEIF4G2 was associated with poor prognosis in patients with CC.
In CC cells, loss-of-function experiments suggested that circEIF4G2
knockdown suppressed cell proliferation, colony formation,
migration and invasion, indicating that circEIF4G2 may serve
oncogenic roles.
It has been demonstrated that circRNAs may serve as
miRNA sponges. To investigate the molecular mechanism of circEIF4G2
in CC, the potential targets of circEIF4G2 were predicted via
bioinformatics analysis and the results suggested that miR-218 may
be a target of circEIF4G2. To validate the in silico
prediction results, luciferase activity and RIP assays were
performed; the in vitro results suggested that circEIF4G2
may interact with miR-218 in CC cells. The RIP results suggested
that circEIF4G2 was present in AGO2-containing RISCs via physical
associations with miR-218. It has been reported that AGO2 is a core
component of the RISC, the formation of which is a necessary step
in RNA silencing; miRNAs were present in the cytoplasm in the form
of miRNA-ribonucleoprotein complexes that also contained AGO2
(18). RT-qPCR results suggested
that circEIF4G2 knockdown increased the expression levels of
miR-218 in CC cells. Additionally, the expression levels of miR-218
and circEIF4G2 were negatively correlated in CC tissues. Our
previous study demonstrated that miR-218 suppresses cell
progression by targeting APC regulator of WNT signaling pathway in
CC (19). miR-218 has previously
been demonstrated to serve as a tumor suppressor in various types
of cancer, including ovarian, bladder and prostate cancer (20–22).
In the present study, transfection with the miR-218 inhibitor
reversed the inhibitory effects of circEIF4G2 knockdown, and
restored the malignant features of HeLa and C33A cells.
The present study further investigated the coding
gene downstream to the circEIF4G2/miR-218 axis. HOXA1 was
identified as a target of miR-218 in CC cells. Furthermore, it was
suggested that circEIF4G2 increased the expression levels of HOXA1
by sponging miR-218. HOXA1 is a member of the HOXA gene family,
which has previously been demonstrated to serve roles in
organogenesis and in the regulation of cell fate (23). Numerous studies have reported that
the dysregulation of HOXA1 is associated with human cancer
progression, including colon cancer and esophageal cancer (24,25).
These previous studies observed that HOXA1 serves as an oncogene,
which is able to promote cell growth, invasion and metastasis. A
recent study by Eoh et al (26) demonstrated that upregulation of
HOXA1 is associated with poor survival rate in patients with in CC.
In the present study, HOXA1 knockdown was able to reverse the
effects of the miR-218 inhibitor on CC cells, and circEIF4G2 was
able to promote cell proliferation and migration via the
miR-218/HOXA1 pathway.
Collectively, the present results suggested that
circEIF4G2 may be a novel oncogene in CC. Furthermore, circEIF4G2
may promote CC cell growth and migration by sponging miR-218, thus
increasing the expression levels of HOXA1.
Acknowledgements
Not applicable.
Funding
The present study was supported by The National
Science Foundation of China (grant no. 8742543).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YM and YL designed and conceived the present study,
and performed the experiments. LZ analyzed the data.
Ethics approval and consent to
participate
The present study was approved by the Clinical
Research Ethics Committee of The Second People's Hospital of Wuhu.
All patients provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cordeiro MN, De Lima RCP, Paolini F, Melo
ARDS, Campos APF, Venuti A and De Freitas AC: Current research into
novel therapeutic vaccines against cervical cancer. Expert Rev
Anticancer Ther. 18:365–376. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Castanon A and Sasieni P: Is the recent
increase in cervical cancer in women aged 20–24 years in England a
cause for concern? Prev Med. 107:21–28. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Origoni M, Prendiville W and Paraskevaidis
E: Cervical cancer prevention: New frontiers of diagnostic
strategies. Biomed Res Int. 2015:2509172015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen LL and Yang L: Regulation of circRNA
biogenesis. RNA Biol. 12:381–388. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Salzman J: Circular RNA expression: Its
potential regulation and function. Trends Genet. 32:309–316. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ebbesen KK, Kjems J and Hansen TB:
Circular RNAs: Identification, biogenesis and function. Biochim
Biophys Acta. 1859:163–168. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Han B, Chao J and Yao H: Circular RNA and
its mechanisms in disease: From the bench to the clinic. Pharmacol
Ther. 187:31–44. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Luan W, Shi Y, Zhou Z, Xia Y and Wang J:
circRNA_0084043 promote malignant melanoma progression via
miR-153-3p/Snail axis. Biochem Biophys Res Commun. 502:22–29. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li H, Wei X, Yang J, Dong D, Hao D, Huang
Y, Lan X, Plath M, Lei C, Ma Y, et al: circFGFR4 promotes
differentiation of myoblasts via binding miR-107 to relieve its
inhibition of Wnt3a. Mol Ther Nucleic Acids. 11:272–283. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu Y, Lu C, Zhou Y, Zhang Z and Sun L:
Circular RNA hsa_circ_0008039 promotes breast cancer cell
proliferation and migration by regulating miR-432-5p/E2F3 axis.
Biochem Biophys Res Commun. 502:358–363. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dragomir M and Calin GA: Circular RNAs in
cancer-lessons learned from microRNAs. Front Oncol. 8:1792018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li M, Ding W, Sun T, Tariq MA, Xu T, Li P
and Wang J: Biogenesis of circular RNAs and their roles in
cardiovascular development and pathology. FEBS J. 285:220–232.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Qu S, Yang X, Li X, Wang J, Gao Y, Shang
R, Sun W, Dou K and Li H: Circular RNA: A new star of noncoding
RNAs. Cancer Lett. 365:141–148. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen D, Zhang C, Lin J, Song X and Wang H:
Screening differential circular RNA expression profiles reveal that
hsa_circ_0128298 is a biomarker in the diagnosis and prognosis of
hepatocellular carcinoma. Cancer Manag Res. 10:1275–1283. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang J and Li H: CircRNA circ_0067934
silencing inhibits the proliferation, migration and invasion of
NSCLC cells and correlates with unfavorable prognosis in NSCLC. Eur
Rev Med Pharmacol Sci. 22:3053–3060. 2018.PubMed/NCBI
|
|
18
|
Filipowicz W, Bhattacharyya SN and
Sonenberg N: Mechanisms of post-transcriptional regulation by
microRNAs: Are the answers in sight? Nat Rev Genet. 9:102–114.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mao Y, Zhang L, Li Y, Yan M and He L:
MiR-218 suppresses cell progression by targeting APC in cervical
cancer. Int J Clin Exp Pathol. 10:2259–2269. 2017.
|
|
20
|
Zhu W, Shao Y and Peng Y: MicroRNA-218
inhibits tumor growth and increases chemosensitivity to CDDP
treatment by targeting BCAT1 in prostate cancer. Mol Carcinog.
56:1570–1577. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li P, Yang X, Cheng Y, Zhang X, Yang C,
Deng X, Li P, Tao J, Yang H, Wei J, et al: MicroRNA-218 increases
the sensitivity of bladder cancer to cisplatin by targeting Glut1.
Cell Physiol Biochem. 41:921–932. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li N, Wang L, Tan G, Guo Z, Liu L, Yang M
and He J: MicroRNA-218 inhibits proliferation and invasion in
ovarian cancer by targeting Runx2. Oncotarget. 8:91530–91541.
2017.PubMed/NCBI
|
|
23
|
De Kumar B, Parker HJ, Paulson A, Parrish
ME, Zeitlinger J and Krumlauf R: Hoxa1 targets signaling pathways
during neural differentiation of ES cells and mouse embryogenesis.
Dev Biol. 432:151–164. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li H, Li J, Yang T, Lin S and Li H:
MicroRNA-433 represses proliferation and invasion of colon cancer
cells by targeting homeobox A1. Oncol Res. 26:315–322. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li Q, Zhang X, Li N, Liu Q and Chen D:
miR-30b inhibits cancer cell growth, migration, and invasion by
targeting homeobox A1 in esophageal cancer. Biochem Biophys Res
Commun. 485:506–512. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Eoh KJ, Kim HJ, Lee JY, Nam EJ, Kim S, Kim
SW and Kim YT: Upregulation of homeobox gene is correlated with
poor survival outcomes in cervical cancer. Oncotarget.
8:84396–84402. 2017. View Article : Google Scholar : PubMed/NCBI
|