Introduction
Myocardial infarction (MI) is one of the leading
causes of cardiac-associated mortality globally, and is accompanied
by cardiomyocyte apoptosis, inflammation and cardiac fibrosis,
leading to an increased risk of adverse cardiac events and eventual
heart failure (1–3). Clinical management of MI has notably
improved; however, MI and the associated complications remain major
causes of morbidity and mortality (4). Therefore, the identification of novel
therapeutic strategies is important for improving cardiac function
following MI.
Isoproterenol (ISO), a synthetic catecholamine and
β-adrenergic agonist, is frequently used in preclinical studies to
induce MI in rats (5). Treatment
with ISO induces severe oxidative stress in the myocardium and
subsequent infarct-like necrosis of the heart muscles in rats,
which is accompanied by decreased cardiac function and the
increased apoptosis of cardiomyocytes (6,7). The
ISO-induced rat model of MI has been widely validated and exhibits
the greatest similarity to the symptoms of MI in clinical settings
(8). This model of MI has been
extensively used to investigate potential cardioprotective drugs
(9,10).
Identified in 1998, apelin is the endogenous ligand
of the G-protein-coupled apelin receptor (APJ) and is expressed in
various organs, including the heart, lung, liver and brain
(11). In clinical settings, the
plasma levels of apelin have been reported to decrease in patients
with cardiac dysfunction (12,13);
however, patients with a ventricular assist device exhibited marked
increases in apelin levels in the left ventricle (14). A previous study demonstrated that
the apelin/APJ signaling pathway was involved in the maintenance of
cardiac function; apelin treatment protected the heart in a rat
model of ischemia/reperfusion injury (15). Therefore, the apelin/APJ signaling
pathway may be a novel target in the treatment of heart
failure.
There has been a notable increase in the use of
herbs and their extracts to treat diseases in previous decades
(16,17). A novel compound isolated from
Alpinia katsumadai Hayata,
(3R)-5,6,7-trihydroxy-3-isopropyl-3-methylisochroman-1-one (TIM;
Fig. 1A) exhibited potent
cardioprotective effects in a recent study, reducing lipoteichoic
acid-induced damage in rat cardiomyoblast cells via the inhibition
of oxidative stress (18). Further
investigation into the cardioprotective efficacy of TIM may reveal
the compound to be a potential therapy in the treatment of
cardiovascular diseases. The aim of the present study was to
determine the effects of TIM on ISO-induced cardiac dysfunction in
rats and the underlying mechanisms.
Materials and methods
Materials
A total of 50 male Wistar rats (3–4 months old,
180–220 g) were purchased from Beijing Vital River Laboratory
Animal Technology, Co., Ltd. (Beijing, China), provided with ad
libitum access to food and water, and housed at 21±2°C with
60±5% humidity under a standard 12-h light/dark cycle. All animal
experiments were performed in accordance with the Chinese
Legislation on the Use and Care of Laboratory Animals (19), and approved by the Ethical
Committee on Animal Care and Use of Jilin University (Changchun,
China). Lactate dehydrogenase (LDH; cat. no. A020-2),
malondialdehyde (MDA; cat. no. A003-1), glutathione (GSH; cat. no.
A006-2) and superoxide dismutase (SOD; cat. no. A001-3) assay kits
were purchased from Jiancheng Bioengineering Institute (Nanjing,
China). Caspase-3/9 activity assay kits [cat. no. CASP3C
(Caspase-3); cat. no. APT173 (Caspase-9)] and ISO (cat. no.
1351005) were purchased from Sigma-Aldrich (Merck KGaA, Darmstadt,
Germany). A Cell Death Detection ELISAplus kit (cat. no.
11544675001) was purchased from Roche Applied Science (Penzberg,
Germany) to determine DNA fragmentation. A Cytochrome-c assay kit
(cat. no. MCTC0) was purchased from R&D Systems, Inc.
(Minneapolis, MN, USA). An apelin-12 immunoassay kit (cat. no.
EK-057-23) was obtained from Phoenix Pharmaceuticals Inc. (Belmont,
CA, USA). Reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) Power SYBR® Green Master Mix (cat.
no. 4367659) was purchased from Thermo Fisher Scientific, Inc.
(Waltham, MA, USA). TIM was isolated and identified by Professor
Lin from Shantou University Medical College (Shantou, China), and
kindly provided by Professor Lin for use in the present study
(20). Sodium carboxymethyl
cellulose (CMC-Na) is widely used in the food and pharmaceutical
industries due to its high viscosity and minimal toxicity (21); TIM was suspended in CMC-Na
(Changshu Wealthy Science and Technology Co., Ltd, Changshu, China)
prior to treatment. The doses of TIM used in the present study were
determined based on a preliminary study. In the preliminary study,
the protective activities of TIM were investigated using five doses
(0.5, 1, 2, 5 and 10 mg/kg; n=3/group); it was revealed that 0.5
mg/kg TIM possessed no protective effects, whereas 5 and 10 mg/kg
TIM induced weight loss in addition to improving cardiac function
in the ISO-induced MI model (data not shown). TIM (1 or 2 mg/kg)
effectively protected against ISO-induced heart dysfunction without
effects on body or heart weight. Therefore, 1 and 2 mg/kg were
selected for subsequent experiments. All other chemicals used in
the present study were of analytical grade and purchased from
Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China).
Experimental procedures
Acute MI was induced via daily intraperitoneal
injection of ISO (100 mg/kg) into rats for 2 consecutive days. Rats
were randomly assigned to four groups (n=10/group) and treated for
12 days: The normal control group was treated with saline orally
for 12 days and by intraperitoneal injection for the final 2 days;
the ISO group was treated with saline orally for 12 days and
injected with ISO on the final 2 days; and the TIM (low) and TIM
(high) groups, were treated daily with TIM (1 and 2 mg/kg,
respectively) orally for 12 days and then injected with ISO on the
final 2 days. Body weight was measured every 2 days.
Measurement of heart function
Blood pressure was recorded 48 h following the first
ISO injection using a computerized, non-invasive tail-cuff system.
Rats were subsequently anesthetized using a mixture of ketamine (40
mg/kg), xylazine (8 mg/kg) and acepromazine (1 mg/kg), and left
ventricular function was measured by inserting a heparin-filled
catheter (500 U/ml) into the left ventricle. Left ventricular
systolic pressure (LVSP), left ventricular end-diastolic pressure
(LVEDP) and maximum left ventricular contraction/relaxation
velocity (±LV dp/dtmax) were recorded using a BL-420E
monitor system.
Sample collection
Following measurement of cardiac function, blood
samples (500 µl) were collected from the hearts of anesthetized
rats. Rats were subsequently sacrificed via inhalation of
CO2 for a minimum of 5 min using a flow rate of 2 l/min
in a 10 l chamber. Rats were kept in the chamber until a heartbeat
could no longer be felt. Mortality was confirmed by removal of the
heart. Following sacrifice, the heart weight was recorded, and
myocardial tissues from the injured areas of the hearts were
collected and washed with ice-cold physiological saline for further
analysis. The heart index was defined as the heart weight/body
weight ratio.
Immunoassay measurement
The myocardial tissues were homogenized on ice. The
homogenate was centrifuged at 6,000 × g for 20 min at 4°C,
the supernatant was collected and the protein concentration was
quantified using the Bradford assay. Apelin, cytochrome-c, LDH,
MDA, SOD and GSH were measured with the corresponding assay kits
according to the manufacturers' protocols.
DNA fragmentation assay
DNA fragmentation was determined using a Cell Death
Detection ELISAplus kit. The myocardial tissues were lysed for 30
min at room temperature, and then the homogenate was centrifuged
for 10 min at 2,000 × g at 4°C. Supernatant (20 µl) was incubated
with a mixture of anti-DNA-peroxidase and anti-histone-biotin for
30 min at room temperature. Following the addition of
2,2′-azino-bis (3-ethylbenzthiazoline-6-sulfonic acid) as the
substrate for 20 min at room temperature, the levels of peroxidase
in the immunocomplex were quantified. The absorbance at 405 nm was
detected using a microplate reader.
Caspase activity measurement
The homogenate from myocardial tissues was analyzed
for caspase-3 and caspase-9 activity using assay kits, according to
the manufacturer's protocols.
RT-qPCR
Total RNA was extracted from myocardial tissues
using TRIzol® (Thermo Fisher Scientific, Inc.). mRNA was
then reverse transcribed into cDNA using the iScript™
Reverse Transcription Supermix kit (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). RT was conducted as follows: 25°C for 5 min;
46°C for 20 min; and 95°C for 1 min). qPCR was performed using
SYBR® Green Supermix (Bio-Rad Laboratories, Inc) as
follows: 95°C for 10 min, and 40 cycles of 95°C for 15 sec and 60°C
for 60 sec. The expression levels of target genes were determined
using the 2−ΔΔCq method (22). mRNA expression was normalized to
the housekeeping gene β-actin. The gene-specific primer sequences
were as follows: Apelin, forward, 5′-GTGAAGCCCAGAACTTCGAG-3′ and
reverse, 3′-CAGCGATAACAGGTGCAAGA-5′; APJ, forward,
5′-TGTACGCCAGTGTCTTTTGC-3′ and reverse, 3′-CTGTTTTCCGGGATGTCAGT-5′;
and β-actin, forward, 5′-AGCCATGTACGTAGCCATCC-3′ and reverse,
3′-CTCTCAGCTGTGGTGGTGAA-5′. The experiment was repeated three
times.
Western blotting
Total cellular and nuclear protein was extracted
from the myocardial tissues using NE-PER™ Nuclear and
Cytoplasmic Extraction Reagents (Thermo Fisher Scientific, Inc.).
Protein concentration was determined using the Bradford method.
Following boiling, protein (50 µg/lane) was separated via 4–12%
SDS-PAGE and then transferred onto polyvinylidene difluoride
membranes. The membranes were blocked with 1% bovine serum albumin
(cat. no. A9306; Sigma-Aldrich; Merck KGaA) for 1 h at room
temperature, and then incubated overnight at 4°C with the following
primary antibodies (Abs): Anti-cleaved caspase-3 rabbit monoclonal
(m)Ab (1:1,000; cat. no. #9664; Cell Signaling Technology, Inc.,
Danvers, MA, USA); anti-cleaved caspase-9 rabbit polyclonal (p)Ab
(1:500; cat. no. C7729; Sigma-Aldrich; Merck KGaA); anti-B-cell
lymphoma 2 (Bcl-2) rabbit pAb (1:1,000; cat. no. ab196495; Abcam,
Cambridge, UK); anti-Bcl-2-associated X protein (Bax) rabbit mAb
(1:2,000; cat. no. ab182733; Abcam); anti-nuclear factor-like 2
(Nrf2) rabbit pAb (1:1,000; cat. no. ab92946; Abcam); anti-APJ
rabbit pAb (1:500; cat. no. ab214369; Abcam); anti-apelin rabbit
pAb (1:1,000; cat. no. ab125213; Abcam); anti-nicotinamide adenine
dinucleotide phosphate (NADPH) oxidase 4 rabbit mAb (1:3,000; cat.
no. ab133303; Abcam); anti-Lamin B1 rabbit mAb (1:3,000; cat. no.
ab133741; Abcam) and anti-β-actin rabbit pAb (1:3,000; cat. no.
ab8227; Abcam). Following washing with PBS-0.1% Tween-20 (PBS-T),
membranes were incubated with horseradish peroxidase-conjugated
goat anti-rabbit secondary antibody (1:8,000; cat. no. ab6721;
Abcam) for 1 h at room temperature. Membranes were then washed
three times with PBS-T and visualized using an enhanced
chemiluminescence system (Bio-Rad Laboratories, Inc.).
Statistical analysis
All data were presented as the mean ± standard
deviation (n=3). SPSS version 13.0 software (SPSS, Inc., Chicago,
IL, USA) was used for data analysis. The normality of data was
determined using a Kolmogorov-Smirnov test. Differences between two
groups were analyzed using t-tests; differences between >2
groups were analyzed using one-way analyses of variance followed by
a Dunnett's test. P<0.05 was considered to indicate a
statistically significant difference.
Results
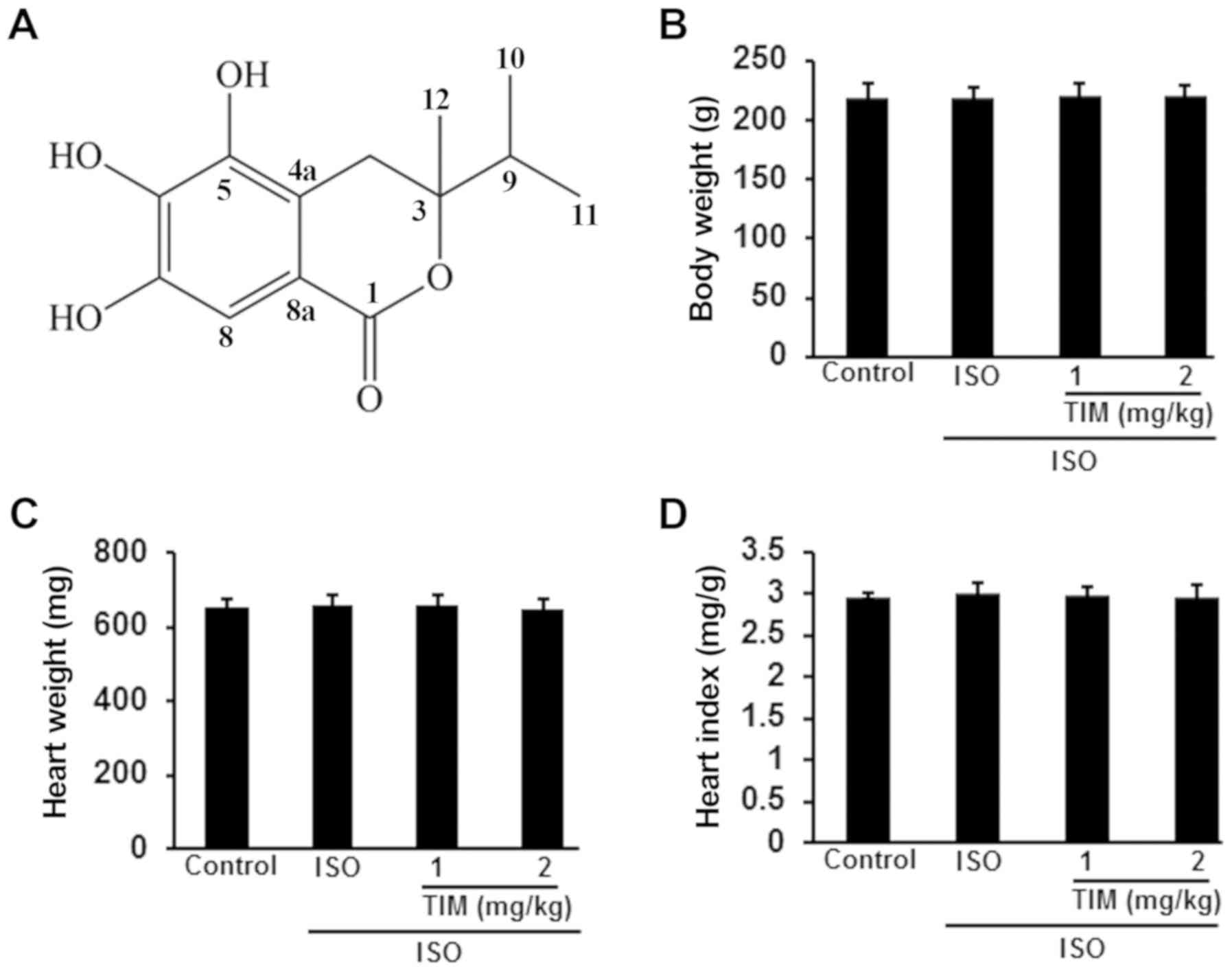
Effects of TIM on the body and heart
weights of rats
Rats with ISO-induced MI were treated with low and
high doses of TIM. Compared with the control, there were no
significant differences in the body weight, heart weight or heart
index of rats following ISO or TIM treatment (Fig. 1).
TIM enhances cardiac function
following MI in rats
ISO treatment significantly altered cardiac
function; the blood pressure, ±LV dP/dtmax and LVSP of
ISO rats were significantly decreased compared with the control,
whereas the LVEDP was significantly increased (Fig. 2). Conversely, treatment with 1 and
2 mg/kg TIM significantly increased blood pressure, ±LV
dp/dtmax and LVSP, and decreased the LVEDP of rats
compared with ISO treatment alone (Fig. 2).
 | Figure 2.TIM enhances cardiac function
following ISO-induced MI in rats. Effects of ISO-induced MI and
treatment with TIM on (A) blood pressure, (B) ±LV
dP/dtmax, (C) LVSP and (D) LVEDP. Data are presented as
the mean ± standard deviation. Samples were measured in triplicate.
Data distributions were analyzed using a Kolmogorov-Smirnov test.
##P<0.01 vs. control; *P<0.05 and **P<0.01 vs.
ISO. DBP, diastolic blood pressure; ISO, isoproterenol; ±LV
dP/dtmax, maximum left ventricular
contraction/relaxation velocity; LVEDP, left ventricular
end-diastolic pressure; LVSP, left ventricular systolic pressure;
MI, myocardial infarction; SBP, systolic blood pressure; TIM,
(3R)-5,6,7-trihydroxy-3-isopropyl-3-methylisochroman-1-one. |
TIM treatment protects cardiomyocytes
against ISO-induced MI
ISO treatment induced severe damage to
cardiomyocytes, as determined by the significant increases in LDH
levels, cytochrome-c release and DNA damage compared with the
control; however, TIM treatment significantly ameliorated these
effects (Fig. 3A-C). Furthermore,
ISO treatment significantly increased the activity of caspases,
upregulated the expression of cleaved caspase-3, cleaved caspase-9
and Bax, and downregulated the expression of Bcl-2; treatment with
TIM induced the opposite effect (Fig.
3D and E).
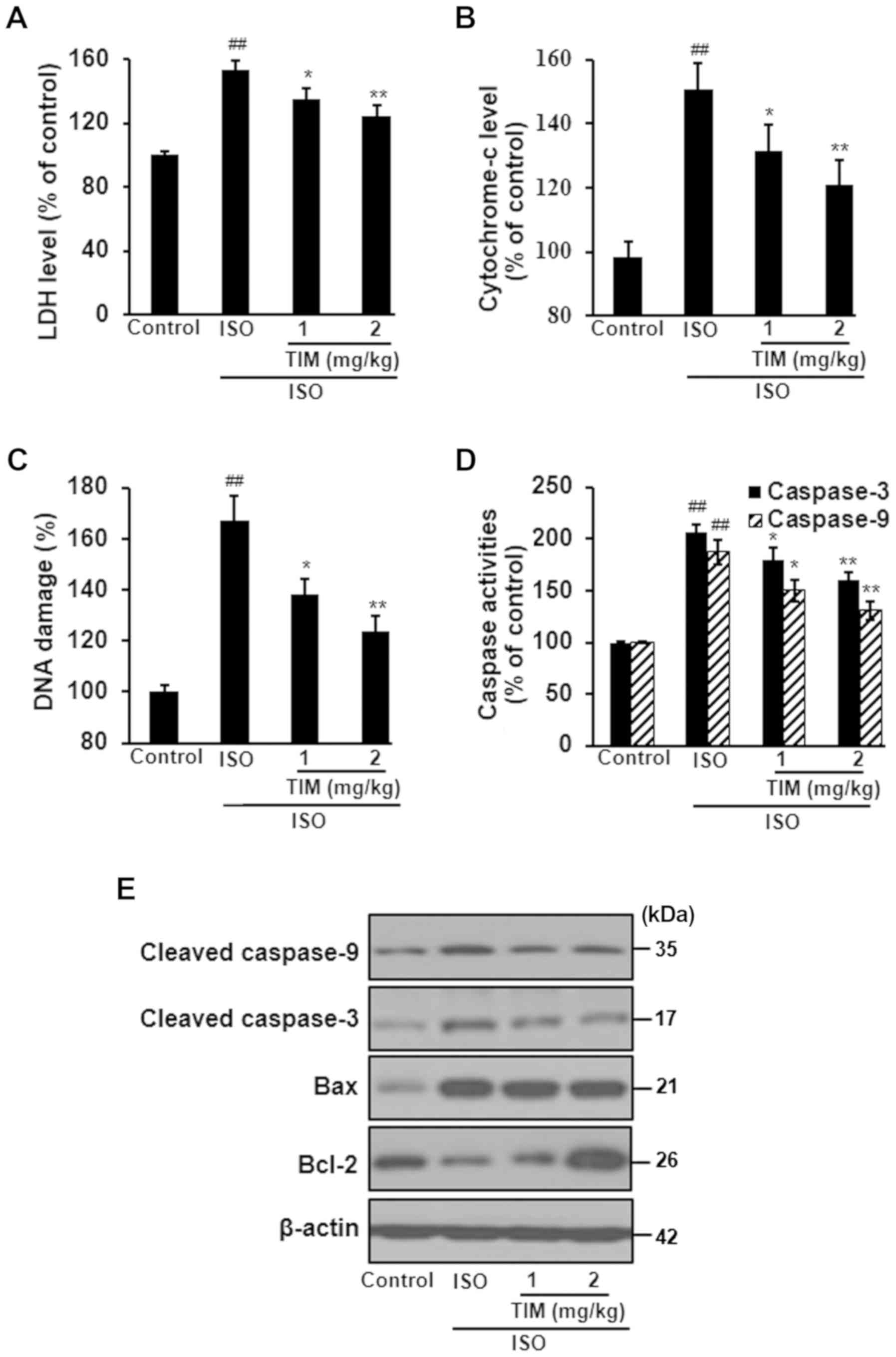 | Figure 3.TIM treatment protects cardiomyocytes
against ISO-induced MI. Levels of (A) LDH, (B) cytochrome-c, (C)
DNA damage and (D) caspase-3/9 activity following ISO-induced MI
and treatment with TIM. (E) Representative western blot of cleaved
caspases-3 and −9, Bax and Bcl-2 following ISO-induced MI and
treatment with TIM. Data are presented as the mean ± standard
deviation. Samples were measured in triplicate. Data distributions
were analyzed using a Kolmogorov-Smirnov test.
##P<0.01 vs. control; *P<0.05 and **P<0.01 vs.
ISO. Bcl-2, B-cell lymphoma 2; Bax, Bcl-2-associated X protein;
ISO, isoproterenol; LDH, lactate dehydrogenase; MI, myocardial
infarction; TIM,
(3R)-5,6,7-trihydroxy-3-isopropyl-3-methylisochroman-1-one. |
Treatment with TIM reduces oxidative
stress
Oxidative stress in myocardial tissue was determined
by the levels of MDA and GSH, the activity of SOD, and the protein
expression of NADPH oxidase 4 and Nrf2. Compared with normal
control rats, oxidative stress was significantly induced following
ISO treatment, with increased levels of MDA, reduced activity of
SOD and decreased levels of GSH (Fig.
4A-C). Conversely, treatment with TIM significantly reduced MDA
levels, and increased the levels of GSH and the activity of SOD,
when compared with ISO treatment alone. Furthermore, TIM treatment
markedly downregulated NADPH oxidase 4, and increased total and
nuclear Nrf2 protein expression (Fig.
4D and E).
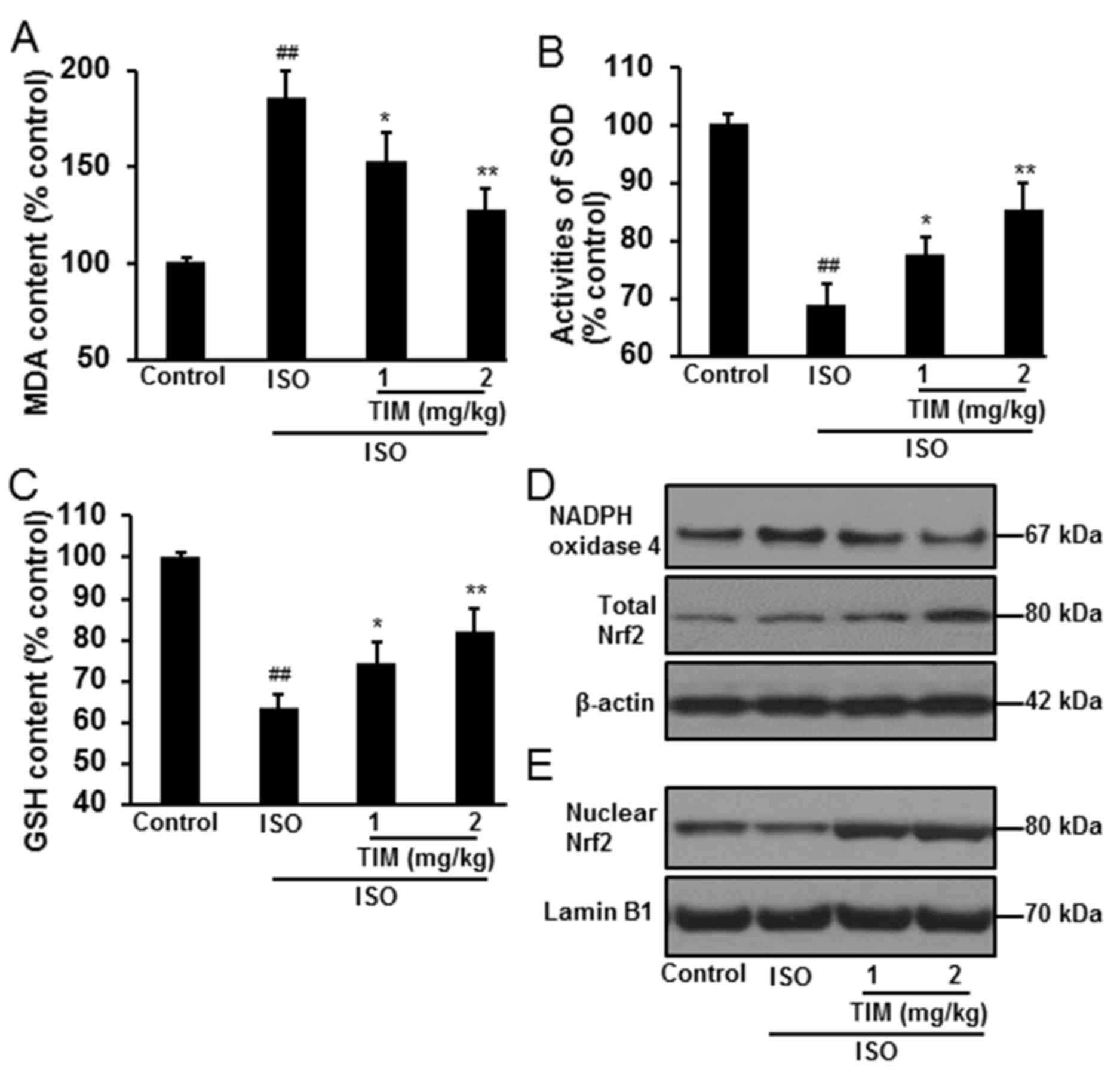 | Figure 4.TIM treatment reduces oxidative
stress in rat cardiomyocytes following ISO-induced MI. Levels of
(A) MDA, (B) SOD activity and (C) GSH following ISO-induced MI and
treatment with TIM. (D and E) Representative western blot of NADPH
oxidase 4, and total and nuclear Nrf2 protein expression following
ISO-induced MI and treatment with TIM. Data are presented as the
mean ± standard deviation. Samples were measured in triplicate.
Data distributions were analyzed using a Kolmogorov-Smirnov test.
##P<0.01 vs. control; *P<0.05 and **P<0.01 vs.
ISO. GSH, glutathione; ISO, isoproterenol; MDA, malondialdehyde;
MI, myocardial infarction; NADPH, nicotinamide adenine dinucleotide
phosphate; Nrf2, nuclear factor-like 2; SOD, superoxide dismutase;
TIM,
(3R)-5,6,7-trihydroxy-3-isopropyl-3-methylisochroman-1-one. |
TIM increased apelin and APJ
Compared with the control, the plasma and myocardial
levels of apelin were significantly decreased following ISO
treatment; however, treatment with TIM increased apelin levels when
compared with ISO treatment alone (Fig. 5A and B). Furthermore, treatment
with TIM eliminated the ISO-induced decreases in the expression of
apelin and APJ mRNA and protein (Fig.
5C and D).
Discussion
Numerous medicinal products have been derived from
herbal plants (23). TIM is a
novel compound isolated from Alpinia katsumadai Hayata that
exhibited cardioprotective effects against lipoteichoic
acid-induced damage in rat cardiomyoblast cells via the inhibition
of oxidative stress (18). In the
present study, it was demonstrated that TIM protected
cardiomyocytes against ISO-induced MI, potentially via the
apelin/APJ signaling pathway. Injection with ISO induced severe
cardiac dysfunction in rats; however, treatment with TIM
ameliorated left ventricular contractile dysfunction, as determined
by increased blood pressure, ±LV dp/dtmax and LVSP, and
decreased LVEDP. These findings suggested that TIM improved cardiac
function in ISO-treated rats.
Cardiomyocyte apoptosis serves an important role in
the progression of cardiac dysfunction in acute and long-term
settings following MI; apoptosis reduces the number of normal
contractile cardiomyocytes, leading to adverse ventricular
remodeling (24,25). Drugs that prevent cardiomyocyte
apoptosis, including angiotensin II receptor antagonists and
β-blockers, have been reported to be effective in the treatment of
heart failure, providing a potential target in the prevention of
pathological progression (26,27).
As a principal cytotoxic lesion, DNA double-strand breaks are
frequently investigated to determine cytotoxicity (28,29).
The levels of caspase-3 and caspase-9 activity have been used to
evaluate apoptosis (30).
Additionally, two important members of the Bcl-2 family, Bcl-2 and
Bax, are directly associated with the regulation of apoptosis;
Bcl-2 inhibits cell apoptosis, whereas Bax promotes apoptosis, and
the Bcl-2/Bax ratio of cells determines their fate following
apoptotic stimulation (31–33).
In the present study, injecting rats with ISO induced cardiomyocyte
damage, characterized by DNA damage, increased levels of
caspase-3/9 activity, marked downregulation of Bcl-2 expression and
upregulation of Bax expression. By contrast, these ISO-induced
effects were ameliorated by TIM treatment, indicating that TIM may
protect myocardial cells against apoptosis in vivo.
Increased oxidative stress was observed in the
myocardium following treatment with ISO. Oxidative stress affects
various biological macromolecules and suppresses cellular functions
(34,35). NADPH oxidase 4 is expressed
primarily in the mitochondria of cardiac myocytes (36). It was reported that cardiac
hypertrophy and apoptosis were attenuated, and improved cardiac
function was observed in NADPH oxidase 4-deficient mice compared
with wild-type mice in a pressure overload model (37). Conversely, overexpression of NADPH
oxidase 4 in mouse heart tissue exacerbated cardiac dysfunction,
fibrosis and apoptosis in response to pressure overload, indicating
that NADPH oxidase 4 was a major source of oxidative stress in the
failing heart, thereby mediating mitochondrial and cardiac
dysfunction (37). Oxidative
stress-induced damage has been hypothesized to be a major
pathogenic mechanism underlying numerous disorders, and previous
studies have reported that supplementation of external antioxidants
may be an effective strategy to maintain the balance between
antioxidative and intracellular oxidative systems (38,39).
Nrf2 is important in cell defense against oxidative stress; it is
inactive in the cytoplasm when bound to Kelch-like ECH-associated
protein 1 (Keap1), but is released from Keap1 upon activation and
moves into the cell nucleus (40).
Nrf2 then binds with antioxidant response elements and induces the
expression of cytoprotective targets, including antioxidant
proteins, phase II detoxifying enzymes and molecular
proteasome/chaperones (41). In
the present study, treatment with TIM upregulated cytoplasmic and
nuclear Nrf2 expression in cardiac tissue, which was accompanied by
reductions in MDA levels and the protein expression of NADPH
oxidase 4, and increased SOD activity and GSH levels. These results
indicated that TIM may induce antioxidative gene expression to
restore oxidative homeostasis.
Identified as an endogenous ligand of the
G-protein-coupled receptor APJ, apelin is expressed in various
tissues, including the heart, where it exhibits potent hypotensive
and positive inotropic properties, inducing endothelium- and nitric
oxide-dependent vasodilatation (42,43).
It was previously revealed that apelin-deficient mice developed
progressive heart failure; however, exogenous administration of
apelin exerted inotropic effects on animals (44). These findings were consistent with
previous clinical observations that revealed that a disturbance in
the endogenous apelin/APJ signaling pathway is associated with
cardiac dysfunction in humans (45), indicating that the apelin/APJ
pathway serves an important role in regulating cardiovascular
homeostasis. An increasing body of evidence has indicated that
apelin/APJ signaling functions as a critical mediator of
cardiovascular homeostasis and is involved in the pathophysiology
of cardiovascular diseases; targeting the apelin/APJ axis promotes
cardioprotection against cardiovascular diseases (46,47).
The results of the present study demonstrated that apelin levels
were significantly decreased in the plasma and myocardium following
ISO treatment; however, TIM treatment produced the opposite
effects, and increased the mRNA and protein expression of apelin
and APJ. These findings indicated that TIM may improve cardiac
function via activation of the apelin/APJ signing pathway.
In conclusion, it was demonstrated that TIM exerted
cardioprotective effects in a rat model of ISO-induced MI,
ameliorating cardiac dysfunction and inhibiting cardiomyocyte
apoptosis. These effects may have been mediated at least partially
via the apelin/APJ signaling pathway. These findings provide
evidence for the development of TIM as a therapeutic agent in the
treatment of MI.
Acknowledgements
The authors thank Professor Lin from Shantou
University Medical College (Shantou, China) for providing TIM in
this study.
Funding
The present study was financially supported by the
Department of Science and Technology of Jilin Province (grant no.
20170622012JC).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
PY and HY made substantial contributions to the
conception and design of the study. Experiments were performed and
analyzed by MD, LG and CZ. The manuscript was drafted by MD, PY and
HY.
Ethics approval and consent to
participate
All animal experiments were performed in accordance
with the Chinese Legislation on the Use and Care of Laboratory
Animals, and approved by the Ethical Committee on Animal Care and
Use of Jilin University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bogomolov AN, Kozlov KL, Kurochkina ON and
Olesiuk IB: Coronary stenting in elderly patients with acute
myocardial infarction (review). Adv Gerontol. 26:151–160. 2013.(In
Russian). PubMed/NCBI
|
|
2
|
Wartenberg KE: Malignant middle cerebral
artery infarction. Curr Opin Crit Care. 18:152–163. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang G, Min D, Yan J, Yang M and Lin G:
Protective role and mechanism of snakegourd peel against myocardial
infarction in rats. Phytomedicine. 42:18–24. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bakhta O, Blanchard S, Guihot AL,
Tamareille S, Mirebeau-Prunier D, Jeannin P and Prunier F:
Cardioprotective role of colchicine against inflammatory injury in
a rat model of acute myocardial infarction. J Cardiovasc Pharmacol
Ther. 23:446–455. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Senthil S, Chandramohan G and Pugalendi
KV: Isomers (oleanolic and ursolic acids) differ in their
protective effect against isoproterenol-induced myocardial ischemia
in rats. Int J Cardiol. 119:131–133. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Grimm D, Elsner D, Schunkert H, Pfeifer M,
Griese D, Bruckschlegel G, Muders F, Riegger GA and Kromer EP:
Development of heart failure following isoproterenol administration
in the rat: Role of the renin-angiotensin system. Cardiovasc Res.
37:91–100. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang GX, Kimura S, Nishiyama A, Shokoji
T, Rahman M, Yao L, Nagai Y, Fujisawa Y, Miyatake A and Abe Y:
Cardiac oxidative stress in acute and chronic isoproterenol-infused
rats. Cardiovasc Res. 65:230–238. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang SB, Tian S, Yang F, Yang HG, Yang XY
and Du GH: Cardioprotective effect of salvianolic acid A on
isoproterenol-induced myocardial infarction in rats. Eur J
Pharmacol. 615:125–132. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhou R, He LF, Li YJ, Shen Y, Chao RB and
Du JR: Cardioprotective effect of water and ethanol extract of
Salvia miltiorrhiza in an experimental model of myocardial
infarction. J Ethnopharmacol. 139:440–446. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Padmanabhan M and Prince PSM: Effects of
pharmacological amounts of S-allylcysteine on lipids in normal and
isoproterenol-induced myocardial infarction in rats. J Sci Food
Agric. 86:772–777. 2006. View Article : Google Scholar
|
|
11
|
Cheng J, Luo X, Huang Z and Chen L:
Apelin/APJ system: A potential therapeutic target for endothelial
dysfunction-related diseases. J Cell Physiol. Dec 26–2018.(Epub
ahead of print).
|
|
12
|
Tatemoto K, Hosoya M, Habata Y, Fujii R,
Kakegawa T, Zou MX, Kawamata Y, Fukusumi S, Hinuma S, Kitada C, et
al: Isolation and characterization of a novel endogenous peptide
ligand for the human APJ receptor. Biochem Biophys Res Commun.
251:471–476. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chong KS, Gardner RS, Morton JJ, Ashley EA
and McDonagh TA: Plasma concentrations of the novel peptide apelin
are decreased in patients with chronic heart failure. Eur J Heart
Fail. 8:355–360. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen MM, Ashley EA, Deng DX, Tsalenko A,
Deng A, Tabibiazar R, Ben-Dor A, Fenster B, Yang E, King JY, et al:
Novel role for the potent endogenous inotrope apelin in human
cardiac dysfunction. Circulation. 108:1432–1439. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tao J, Zhu W, Li Y, Xin P, Li P, Liu M, Li
J, Redington AN and Wei M: Apelin-13 protects the heart against
ischemia-reperfusion injury through inhibition of ER-dependent
apoptotic pathways in a time-dependent fashion. Am J Physiol Heart
Circ Physiol. 301:H1471–H1486. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kang K, Tarchick MJ, Yu X, Beight C, Bu P
and Yu M: Carnosic acid slows photoreceptor degeneration in the
Pde6b(rd10) mouse model of retinitis pigmentosa. Sci Rep.
6:226322016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu YZ, Qiao F, Xu GW, Zhao J, Teng JF, Li
C and Deng WJ: Neuroprotective metabolites from the endophytic
fungus Penicillium citrinum of the mangrove Bruguiera gymnorrhiza.
Phytochem Lett. 12:148–152. 2015. View Article : Google Scholar
|
|
18
|
Liu Z, Xie L, Bian T, Qi G and Wang Z:
(3R)-5,6,7-trihydroxy-3-isopropyl-3-methylisochroman-1-one reduces
lipoteichoic acid-induced damage in rat cardiomyoblast cells.
Anatol J Cardiol. 19:198–204. 2018.PubMed/NCBI
|
|
19
|
Ministry of Science and Technology, .
Current Laboratory Animal Laws Regulations. Policies and
Administration in China. 2018.
|
|
20
|
Chen DY, Yang F and Lin YQ:
Neuroprotective constituent from the seeds of Alpinia
katsumadai Hayata. Phytochem Lett. 18:59–63. 2016. View Article : Google Scholar
|
|
21
|
Mondal MI and Yeasmin MS: Toxicity study
of food-grade carboxymethyl cellulose synthesized from maize husk
in Swiss albino mice. Int J Biol Macromol. 92:965–971. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li L, Zhou X, Li N, Sun M, Lv J and Xu Z:
Herbal drugs against cardiovascular disease: Traditional medicine
and modern development. Drug Discov Today. 20:1074–1086. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang X, Wang Q, Guo W and Zhu YZ: Hydrogen
sulfide attenuates cardiac dysfunction in a rat model of heart
failure: A mechanism through cardiac mitochondrial protection.
Biosci Rep. 31:87–98. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Abbate A, Biondi-Zoccai GG, Bussani R,
Dobrina A, Camilot D, Feroce F, Rossiello R, Baldi F, Silvestri F,
Biasucci LM and Baldi A: Increased myocardial apoptosis in patients
with unfavorable left ventricular remodeling and early symptomatic
post-infarction heart failure. J Am Coll Cardiol. 41:753–760. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ahmet I, Krawczyk M, Heller P, Moon C,
Lakatta EG and Talan MI: Beneficial effects of chronic
pharmacological manipulation of beta-adrenoreceptor subtype
signaling in rodent dilated ischemic cardiomyopathy. Circulation.
110:1083–1090. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Soga M, Kamal FA, Watanabe K, Ma M,
Palaniyandi S, Prakash P, Veeraveedu P, Mito S, Kunisaki M,
Tachikawa H, et al: Effects of angiotensin II receptor blocker
(candesartan) in daunorubicin-induced cardiomyopathic rats. Int J
Cardiol. 110:378–385. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yuan J, Adamski R and Chen J: Focus on
histone variant H2AX: To be or not to be. FEBS Lett. 584:3717–3724.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kuo LJ and Yang LX: Gamma-H2AX-a novel
biomarker for DNA double-strand breaks. In Vivo. 22:305–309.
2008.PubMed/NCBI
|
|
30
|
Xin BR, Liu JF, Kang J and Chan WP: (2R,
3S)-pinobanksin-3-cinnamate, a new flavonone from seeds of Alpinia
galanga willd., presents in vitro neuroprotective effects. Mol Cell
Toxicol. 10:165–172. 2014. View Article : Google Scholar
|
|
31
|
Kirkland RA and Franklin JL: Bax, reactive
oxygen, and cytochrome c release in neuronal apoptosis. Antioxid
Redox Signal. 5:589–596. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Brunelle JK and Letai A: Control of
mitochondrial apoptosis by the Bcl-2 family. J Cell Sci.
122:437–441. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cory S and Adams JM: The Bcl2 family:
Regulators of the cellular life-or-death switch. Nat Rev Cancer.
2:647–656. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Adibhatla RM and Hatcher JF: Lipid
oxidation and peroxidation in CNS health and disease: From
molecular mechanisms to therapeutic opportunities. Antioxid Redox
Signal. 12:125–169. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang X and Michaelis EK: Selective
neuronal vulnerability to oxidative stress in the brain. Front
Aging Neurosci. 2:122010.PubMed/NCBI
|
|
36
|
Kuroda J and Sadoshima J: NADPH oxidase
and cardiac failure. J Cardiovasc Transl Res. 3:314–320. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kuroda J, Ago T, Matsushima S, Zhai P,
Schneider MD and Sadoshima J: NADPH oxidase 4 (Nox4) is a major
source of oxidative stress in the failing heart. Proc Natl Acad Sci
USA. 107:15565–15570. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Halliwell B: Oxidative stress and
neurodegeneration: Where are we now? J Neurochem. 97:1634–1658.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Birben E, Sahiner UM, Sackesen C, Erzurum
S and Kalayci O: Oxidative stress and antioxidant defense. World
Allergy Organ J. 5:9–19. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Itoh K, Wakabayashi N, Katoh Y, Ishii T,
Igarashi K, Engel JD and Yamamoto M: Keap1 represses nuclear
activation of antioxidant responsive elements by Nrf2 through
binding to the amino-terminal Neh2 domain. Genes Dev. 13:76–86.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kobayashi M and Yamamoto M: Nrf2-Keap1
regulation of cellular defense mechanisms against electrophiles and
reactive oxygen species. Adv Enzyme Regul. 46:113–140. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Medhurst AD, Jennings CA, Robbins MJ,
Davis RP, Ellis C, Winborn KY, Lawrie KW, Hervieu G, Riley G,
Bolaky JE, et al: Pharmacological and immunohistochemical
characterization of the APJ receptor and its endogenous ligand
apelin. J Neurochem. 84:1162–1172. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Berry MF, Pirolli TJ, Jayasankar V,
Burdick J, Morine KJ, Gardner TJ and Woo YJ: Apelin has in vivo
inotropic effects on normal and failing hearts. Circulation 110 (11
Suppl 1). II187–II193. 2004.
|
|
44
|
Kuba K, Zhang L, Imai Y, Arab S, Chen M,
Maekawa Y, Leschnik M, Leibbrandt A, Markovic M, Schwaighofer J, et
al: Impaired heart contractility in Apelin gene-deficient mice
associated with aging and pressure overload. Circ Res. 101:e32–e42.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Japp AG and Newby DE: The apelin-APJ
system in heart failure: Pathophysiologic relevance and therapeutic
potential. Biochem Pharmacol. 75:1882–1892. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
He L, Xu J, Chen L and Li L: Apelin/APJ
signaling in hypoxia-related diseases. Clin Chim Acta. 451:191–198.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yu XH, Tang ZB, Liu LJ, Qian H, Tang SL,
Zhang DW, Tian GP and Tang CK: Apelin and its receptor APJ in
cardiovascular diseases. Clin Chim Acta. 428:1–8. 2014. View Article : Google Scholar : PubMed/NCBI
|