Introduction
The alarmin protein, high mobility group box-1
(HMGB1), exhibits varying biological activities depending on its
location and state (1). In the
nucleus, it acts as a DNA chaperone and is involved in various
physiological functions, including repair, replication and
transcription (2); under various
stress conditions, HMGB1 is transported to the cytoplasm to
contribute to immune responses and mediate autophagy (3). When released into the extracellular
environment, HMGB1 exerts varying functions depending on the
receptors and complexes with which it interacts (4–6).
Generally, the biological activity of HMGB1 in the extracellular
matrix depends on the redox state of its three cysteines (7). When the cysteines at positions C23,
C45 and C106 are all reduced (in the thiol state), fully reduced
HMGB1 (fr-HMGB1) is formed. In disulfide HMGB1 (ds-HMGB1), C23 and
C45 form a disulfide bond with an A box in the first HMGB1 HMG-box
domain, whereas C106 in the B box remains in the thiol state. The
final variant, fully oxidized HMGB1 (ox-HMGB1), is reportedly
non-active, with all three cysteines terminally oxidized (2,8).
Previously, Frank et al (9) investigated the role of the HMGB1
redox state in inflammation, and reported that ds-HMGB1, but not
fr-HMGB1 contributed to inflammatory responses. Notably, as
reported in our previous study, fr-HMGB1 upregulated TNF-α and
induced depressive-like behavior, similar to ds-HMGB1 (4). The experimental conditions varied
between the two reports; however, the mechanisms underlying the
abilities of the two states, in particular fr-HMGB1, to induce
depressive-like behavior merited further investigation.
The serotonin hypothesis, which suggests that low
serotonin levels cause depression, was proposed in the 1960s
(10–12); in subsequent decades, the role of
serotonin in the pathogenesis of depression has been extensively
studied. Lapin and Oxenkrug (13)
hypothesized that serotonin deficiency in depression is a result of
the switch of tryptophan (Trp) metabolism from serotonin synthesis
to kynurenine (KYN) production. Then, the KYN pathway was revealed
to be a process that starts with Trp metabolism and ends with
NAD+ production (14,15).
Trp is metabolized to KYN by the rate-limiting enzyme
indoleamine-2,3-dioxygenase (IDO), which is stimulated by
proinflammatory cytokines, or tryptophan-2,3-dioxygenase (TDO;
Table I) (15). Subsequently, kynurenine
monooxygenase (KMO) synthesizes 3-hydroxykynurenine (3-HK), which
is converted into 3-hydroxyanthranilic acid (3-HANA) by
kynureninase (KYNU) (14). Then,
3-HANA is oxidized by 3-hydroxyanthranilate 3,4-dioxygenase
(3-HAO), resulting in quinolinic acid (QUIN) production and
ultimately NAD+ synthesis (14). Alternatively, KYN is directly
converted into kynurenic acid (KYNA) by the enzyme kynurenine
aminotransferase 2 (KAT2) (14).
 | Table I.Target genes analyzed via reverse
transcription-quantitative PCR. |
Table I.
Target genes analyzed via reverse
transcription-quantitative PCR.
| Gene name | Primer sequence
(5′→3′) | Function |
|---|
| IDO | F:
GCTTTGCTCTACCACATCCAC | Oxidation,
Trp→KYN |
|
| R:
CAGGCGCTGTAACCTGTGT |
|
| KAT2 | F:
ATGAATTACTCACGGTTCCTCAC | Cleavage,
KYN→KYNA |
|
| R:
AACATGCTCGGGTTTGGAGAT |
|
| KMO | F:
ATGGCATCGTCTGATACTCAGG | Oxidation,
KYN→3-HK |
|
| R:
CCCTAGCTTCGTACACATCAACT |
|
| KYNU | F:
AGTGGGCTGCACTTTTATACTG | Conversion,
3-HK→3-HANA |
|
| R:
TGCAAACAGGTTGCCTTTCAG |
|
| 3-HAO | F:
GAACGCCGTGTGAGAGTGAA | Oxidation,
3-HANA→QUIN |
|
| R:
CCAACGAACATGATTTTGAGCTG |
|
| β-actin | F:
TTCTTGGGTATGGAATCCTGT | Cytoskeleton |
|
| R:
AGCACTGTGTTGGCATAGAG |
|
The present study aimed to investigate the
mechanisms by which HMGB1 may directly induce depressive-like
behavior and determine the state in which it induces its
effects.
Materials and methods
Animals and treatments
In the present study, a total of 20 8-week-old male
mice (BALB/c, 22–25 g) were obtained from the Animal Center of the
Second Military Medical University (Shanghai, China). Prior to
experiments, mice were adapted to housing conditions (temperature,
20±1°C; humidity, 52±2%; 12:12-h light/dark cycle; access to water
and food ad libitum) for 2 weeks. Then, the animals were
randomly assigned to the vehicle control, ds-HMGB1, fr-HMGB1 or
non-oxidizable chemokine (nonoxid)-HMGB1 groups (n=5 mice/group).
All procedures were conducted in accordance with the guidelines
issued by the Second Military Medical University, and was approved
by the Committee on Ethics of Biomedicine Research, Second Military
Medical University.
Intracerebroventricular injection and
sample preparation
Intracerebroventricular injections were performed as
previously described (Fig. 1)
(4). At 20 h after injection of
HMGB1 (4 mg/ml) or vehicle (0.9% normal saline), behavioral tests
were performed to evaluate depressive-like behavior. Then, mice
were anesthetized with 4% chloral hydrate (400 mg/kg,
intraperitoneal) and hippocampi were dissected immediately
following decapitation, flash frozen in liquid nitrogen, and stored
at −80°C until subsequent use.
Behavioral tests
All behavioral tests were performed during the dark
phase (07:00 p.m.-09:00 p.m.). The sucrose preference test (SPT),
tail suspension test (TST) and open field test (OFT) were used as
behavioral parameters to evaluate depression-like behavior as
previously described (4).
Reagents
Ds-HMGB1 (cat. no. HM-122), fr-HMGB1 (cat. no.
HM-116) and nonoxid-HMGB1 (cat. no. HM-132) were purchased from
HMGBiotech. DMEM containing 4.5 g/l D-glucose and L-glutamine (cat.
no. 11965-092) and heat inactivated horse serum (cat. no.
26050-070) were obtained from Gibco (Thermo Fisher Scientific,
Inc.). Hank's balanced salt solution (HBSS; cat. no. B410) was
purchased from BasalMedia.
Organotypic hippocampal slice cultures
(OHSCs)
Hippocampi from 7-day-old BALB/c pups (20 mice;
Animal Center of the Second Military Medical University) were
obtained for OHSCs as previously described (16,17).
In brief, mice were sacrificed immediately upon arrival, and brains
were extracted and sectioned into 400-µm transverse slices using a
vibratome (ZQP-86; Shanghai Zhisun Equipment Co., Ltd.). The
hippocampal slices were placed onto 0.45-µm porous membrane inserts
(cat. no. FHLC 02500; EMD Millipore) and cultured in 6-well culture
plates containing 1.25 ml medium (25% horse serum, 25% HBSS and 50%
DMEM). Slices were maintained in an incubator (37°C, 5%
CO2) for 7 days, with the medium replaced every 2 days.
OHSCs were rinsed three times with serum-free DMEM and incubated
for 2 h prior to addition of serum-free medium and treatments [80
nM HMGB1, 1 mM H2O2 (18)]. Fr-HMGB1 was exposed to 1 mM
H2O2 for 1 h and dialyzed prior to treatment.
Tissue and supernatant samples were collected and frozen at −80°C
for subsequent analysis following 6 h of treatment. Additionally,
lactate dehydrogenase released by damaged cells was measured as a
surrogate marker of tissue damage (data not shown).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from mouse tissues and OHSCs
using TRNzol-A+ reagent (cat. no. DP421; Tiangen Biotech Co., Ltd.)
and reverse transcribed into cDNA using a PrimeScript™ RT Master
Mix (Perfect Real Time) kit (cat. no. RR036A; Takara Bio, Inc.). RT
solution was prepared on ice, and RT was performed at 37°C for 15
min and 85°C for 5 sec, and cDNA was stored at 4°C. Forward and
reverse primer sequences are presented in Table I. qPCR was performed using an
SYBR® Premix Ex Taq™ (Tli RNaseH Plus) kit (cat. no.
RR420A; Takara Bio, Inc.). The conditions of reverse transcription
reaction were run in this sequence: 37°C for 15 min, 85°C for 5
sec, 4°C for stored. qPCR was conducted under the following
conditions: 95°C for 30 sec, followed by 40 cycles of 95°C for 5
sec and 60°C for 34 sec. Experiments were performed in triplicate,
and β-actin was used to normalize target gene expression following
quantification of expression using the 2−∆∆Cq method
(19).
Western blot analysis
The proteins extracted from mouse hippocampi were
blended in microfuge tubes with 25 mg tissue/0.25 ml RIPA buffer
containing 1 mM PMSF protease inhibitor (Beyotime Institute of
Biotechnology). The samples were kept on ice for 30 min and
centrifuged at 10,000 × g for 5 min at 4°C before the lysate
supernatants were collected. The protein concentration was
determined using a BCA Protein Assay kit (cat. no. P0010, Beyotime
Institue of Biotechnology). Samples containing equal quantities of
protein (20 µg) were separated via SDS-PAGE on 10% gels and
transferred onto polyvinylidene fluoride membranes. The membranes
were blocked by 5% nonfat dried milk in TBS-0.1% Tween-20 at room
temperature for 1 h and incubated overnight at 4°C with the
following primary antibodies from ProteinTech Group, Inc.:
Anti-GAPDH (1:2,000; cat. no. 10494-1-AP); anti-IDO (1:200; cat.
no. 66528-1-lg); anti-KMO (1:500; cat. no. 10698-1-AP); anti-KYNU
(1:1,000; cat. no. 11796-1-AP) and anti-KAT2 (1:800; cat. no.
14983-1-AP). Following incubation with the secondary antibody
[IRDye-conjugated anti-rabbit (cat. no. 926-32211) and anti-mouse
(cat. no. 926-68070) immunoglobulin G; 1:5,000; LI-COR
Biosciences)] for 1 h at room temperature, the membranes were
scanned. The integrated optical density (IOD) was calculated by use
of an Odyssey Infrared Imaging System (LI-COR Biosciences) and
ImageJ software (1.48v; National Institutes of Health).
ELISA
The concentrations of tumor necrosis factor-α
(TNF-α; cat. no. F11630) and interleukin-1β (IL-1β; cat. no.
F10770) in the OHSC supernatant were detected using corresponding
ELISA kits according to the manufacturer's protocols (Westang,
Inc.). Briefly, 100 µl of each sample was added to ELISA plates in
duplicate. The lower limits of detection were 4 pg/ml for TNF-α and
8 pg/ml for IL-1β. The absorbance was measured on a microplate
reader (Synergy™ H1; BioTek Instruments, Inc.).
Total reactive oxygen species (ROS)
measurement
Hippocampal ROS levels were measured in hippocampal
lysates using the OxiSelect™ in vitro ROS/RNS Assay kit from
Cell Biolabs, Inc. (cat. no. STA-347). The samples and standards
(hydrogen peroxide) were mixed with fluorogenic stabilized
dichlorodihydrofluorescein (DCF) and incubated at room temperature
for 30 min. In the presence of ROS, DCF was oxidized and the
fluorescence was detected (excitation 480 nm/emission 530 nm).
Following the completion of all behavioral tests,
mice were intraperitoneally anesthetized using 4% chloral hydrate
(400 mg/kg, intraperitoneal administration) and perfused
transcardially with 0.9% saline followed by ice-cold 4%
paraformaldehyde (PFA; cat. no. G1101; Wuhan Servicebio Technology
Co., Ltd.). Following fixation for 4–8 h fix in 4% PFA, brains were
dehydrated in 20% sucrose for 2–3 days. The brains were then
embedded in paraffin and sectioned (4 µm). Prior to assays,
sections were deparaffinized in xylene and rehydrated in a graded
ethanol series. In brief, slices were incubated with 20 µM
dihydroethidium (DHE; cat. no. D7008; Sigma-Aldrich; Merck KGaA)
for 30 min at 37°C, followed by incubation with DAPI (1:1,000; cat.
no. D9542; Sigma-Aldrich; Merck KGaA) for 10 min at room
temperature. Images were obtained using a fluorescence microscope
(magnification, ×200; Carl Zeiss AG) at an excitation wavelength of
370 nm and emission wavelength of 420 nm.
Statistical analysis
GraphPad Prism 6.01 (GraphPad Software, Inc.) was
used for data analysis. Two-way ANOVA with post hoc Tukey's
multiple comparison test was performed to evaluate the main effects
and interactions of fr-HMGB1 and H2O2 in
vivo. One-way ANOVA followed by Tukey's multiple comparison
test was used to analyze the effects of fr-HMGB1 and ds-HMGB1 in
vivo and in vitro. The Mann-Whitney test was performed
to compare ROS levels in the control and fr-HMGB1 groups. Data are
presented as the mean ± standard error of the mean. Each experiment
was repeated three times. P<0.05 was considered to indicate a
statistically significant difference.
Results
Central administration of fr- and
ds-HMGB1, but not nonoxid-HMGB1, induces depressive-like
behavior
Several behavioral tests were conducted to evaluate
the depressive-like behavior of mice following administration of
recombinant HMGB1, including fr-HMGB1, ds-HMGB1 and nonoxid-HMGB1.
Nonoxid-HMGB1, in which all cysteines are replaced by serine
residues, is a mutant analogue of fr-HMGB1 (18). The TST was used to evaluate
antidepressant activity (20) in
experimental animals. In the SPT, low intake of sucrose solution is
hypothesized to indicate anhedonia and impaired sensitivity to
reward (21). The total distance
travelled during the can be affected by a number of factors,
including an animal's sickness behavior (22), and its anxiety status can be
assayed from the central distance covered (22).
Sucrose preference was significantly decreased
following injection of fr- (P<0.05) and ds-HMGB1 (P<0.01)
compared with control treatment (F=8.665, P=0.0012; Fig. 2A). Additionally, the duration of
immobility in the TST (F=9.749, P=0.0007; Fig. 2B) was strongly significantly
increased following ds-HMGB1 (P<0.001) administration, and
significantly increased in fr-HMGB1-treated (P<0.05) mice
compared with the control group. Conversely, nonoxid-HMGB1 did not
significantly affect sucrose preference (Fig. 2A) or increase immobility duration
(Fig. 2B). In addition, there were
no significant differences in total (Fig. 2C) and central distance travelled in
the OFT (Fig. 2D) between groups.
The results indicated that the fr- and ds-HMGB1-treated animals
exhibited depressive-like behavior without a notable anxiety or
sickness phenotype, consistent with our previous study (4). Based on these findings, the molecular
mechanisms and role of HMGB1 in mouse models were further
studied.
Administration of fr-HMGB1 and
ds-HMGB1 upregulates the expression of the enzymes of the KYN
pathway in vivo, whereas nonoxid-HMGB1 induces no effects
As described, ds-HMGB1 and fr-HMGB1 contributed to
depressive-like behavior, whereas nonoxid-HMGB1 did not. The mRNA
expression and relative protein levels of essential KYN pathway
enzymes in the hippocampus were analyzed following
intracerebroventricular administration of various HMGB1 forms.
As presented in Fig.
3A, IDO (F=8.630, P=0.0008) was upregulated in
ds-HMGB1-(P=0.0132) and fr-HMGB1-treated (P<0.01) hippocampi
compared with the control group. Similar results were obtained for
KMO (F=5.955, P=0.0049; Fig. 3A)
and KYNU (F=7.114, P=0.0019; Fig.
3A) after ds-HMGB1 administration. Notably, fr-HMGB1 treatment
significantly increased KMO (P<0.05; Fig. 3A) and KYNU (P=0.0161; Fig. 3A) gene expression levels compared
with the vehicle group, suggesting that ds-HMGB1 and fr-HMGB1 each
activated the KYN pathway in vivo. Conversely, central
administration of nonoxid-HMGB1 did not significantly affect IDO,
KMO and KYNU mRNA levels (Fig.
3A). Treatment with the three HMGB1 did not significantly alter
3-HAO and KAT2 expression levels compared with the control group
(Fig. 3A).
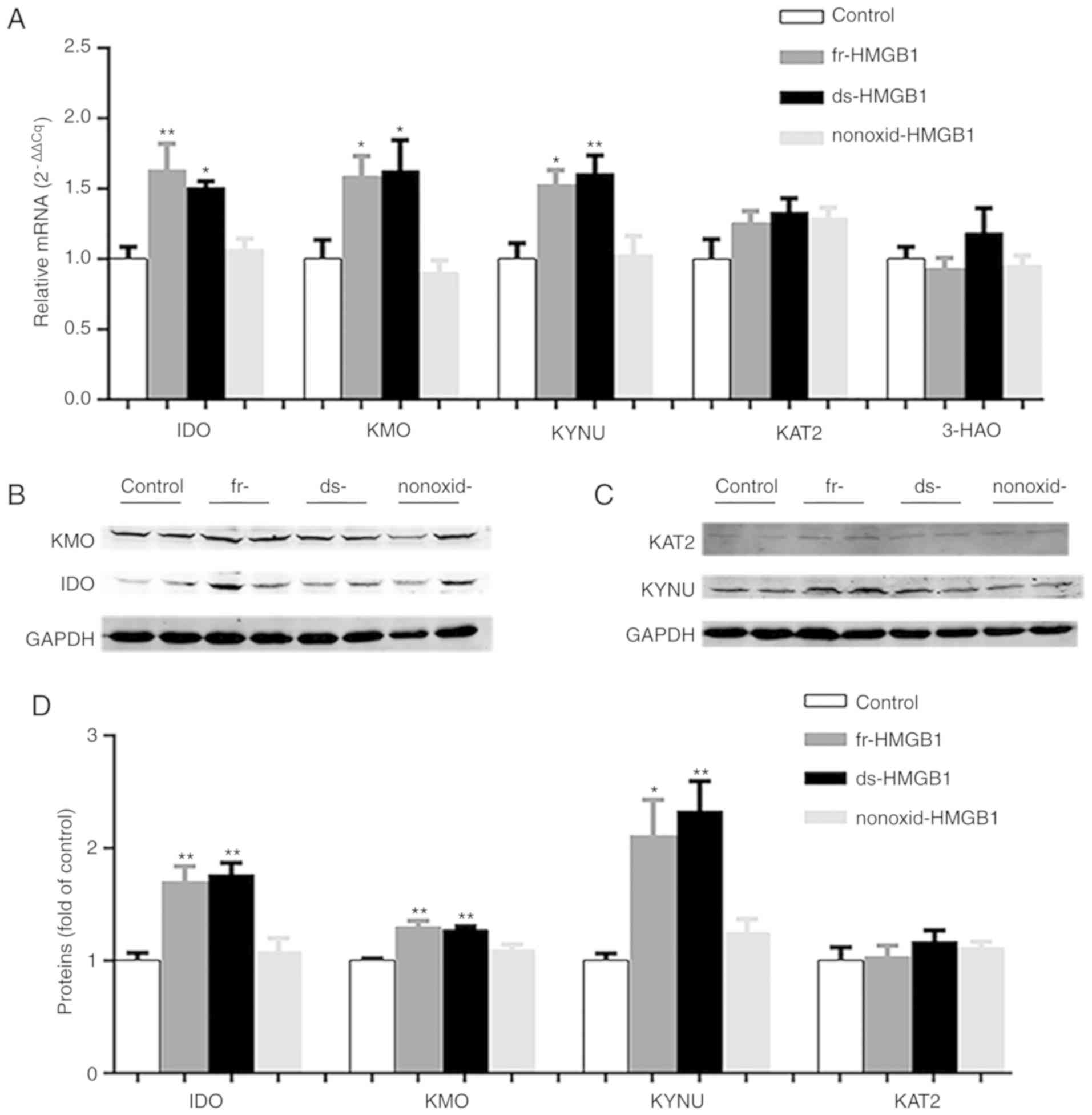 | Figure 3.Expression levels of enzymes in the
kynurenine pathway are increased in fr-HMGB1 and ds-HMGB1-treated
mice. (A) Gene expression levels of IDO, KMO, KYNU, KAT2 and 3-HAO
relative to control samples, as determined via reverse
transcription-quantitative PCR analysis. Representative western
blots of the protein levels of (B) IDO and KMO, (C) KAT2 and KYNU
in hippocampi. (D) Semi-quantification of protein expression. Data
are presented as the mean ± SEM (n=5/group). *P<0.05,
**P<0.01. ds-, disulfide; fr-, fully reduced; HMGB1, high
mobility group box-1; IDO, indoleamine-2,3-dioxygenase; KAT2,
kynurenine aminotransferase 2; KMO, kynurenine monooxygenase; KYNU,
kynureninase; nonoxid-, non-oxidizable chemokine; 3-HAO,
3-hydroxyanthranilate 3,4-dioxygenase. |
In addition to detecting the mRNA expression of KYN
pathway-associated enzymes, their relative protein expression was
also determined. As presented in Fig.
3B-D, ds- and fr-HMGB1 treatment significantly upregulated the
hippocampal expression of IDO (F=13.16, P=0.0004), KMO (F=12.1,
P=0.0006) and KYNU (F=8.9, P=0.0022) proteins compared with the
control group; conversely, there was no significant difference in
the protein levels of KAT2 (Fig. 3C
and D) between four groups. In addition, no significant effects
were identified for nonoxid-HMGB1 treatment in those four enzymes
(Fig. 3B-D). The altered
expression of KYN pathway enzymes in mice following administration
of different forms of HMGB1, combined with HMGB1 central
administration-induced depressive-like behavior (Fig. 2A-D), further indicated the
molecular mechanisms underlying the effects of different forms of
HMGB1 in relation to depression.
Following oxidation by
H2O2, fr-HMGB1 activates the enzymes of the
KYN pathway in cultured hippocampus slices
To further investigate the varied effects of fr- and
ds-HMGB1 on the KYN pathway, essential proteins of the KYN pathway
were evaluated following treatment of OHSCs with fr- and ds-HMGB1
in vitro. There were no significant differences in IDO, KMO,
KYNU and KAT2 expression levels between the control and fr-HMGB1
groups (Fig. 4A). Consistent with
the results of central ds-HMGB1 administration, the expression
levels of IDO (F=10.07, P=0.0033), KMO (F=17.88, P=0.0003) and KYNU
(F=4.779, P=0.0321) were increased in tissue slices following
ds-HMGB1 administration compared with the control group (Fig. 4A). Notably, KAT2 mRNA gene
expression was decreased in the ds-HMGB1 treatment group (F=5.082,
P=0.0273; Fig. 4A), in contrast to
the in vivo experiments. In addition, ds- and fr-HMGB1 did
not significantly alter 3-HAO expression compared with the control
group (Fig. 4A). These results
indicated that ds-HMGB1 administration, but not fr-HMGB1, activated
the KYN pathway in vitro.
 | Figure 4.Oxidized fr-HMGB1 induces the
kynurenine pathway in cultured hippocampus slices. Gene expression
levels of IDO, KMO, KYNU, KAT2 and 3-HAO in OHSCs treated with (A)
fr- and ds-HMGB1, and (B) fr-HMGB1 and H2O2,
as determined via reverse transcription-quantitative PCR analysis.
Data are presented as the mean ± SEM (n=3-5/group). *P<0.05,
**P<0.01, ***P<0.001 vs. Control. ds-, disulfide; fr-, fully
reduced; HMGB1, high mobility group box-1; IDO,
indoleamine-2,3-dioxygenase; KAT2, kynurenine aminotransferase 2;
KMO, kynurenine monooxygenase; KYNU, kynureninase; 3-HAO,
3-hydroxyanthranilate 3,4-dioxygenase. |
To determine the mechanism by which fr-HMGB1 induces
the KYN pathway in vivo but not in vitro, the
expression of KYN pathway effectors in OHSCs was evaluated
following treatment with various combinations of fr-HMGB1 and
H2O2. As presented in Fig. 4B, one-way ANOVA demonstrated
significant differences between the three groups (P=0.0153,
F=9.078). Multiple comparisons indicated that exposure for 6 h to
fr-HMGB1 + H2O2 resulted in significantly
increased IDO expression (P<0.05). In addition to IDO, other
essential enzymes (KMO, KYNU, 3-HAO and KAT2) involved in the
metabolism of KYN along the KYN pathway were analyzed via RT-qPCR.
No effects on KMO, KYNU, KAT2 and 3-HAO expression levels were
observed following fr-HMGB1 treatment compared with the control
group (Fig. 4B); however,
following oxidation by H2O2, fr-HMGB1
significantly increased the gene expression levels of KMO (F=7.841,
P=0.0212; Fig. 4B) and KYNU
(F=11.99, P=0.008; Fig. 4B) in
tissue slices. Combined with the aforementioned results, these
findings suggested that fr-HMGB1 activated the KYN pathway
indirectly following oxidation.
Effects of fr-HMGB1 on
neuroinflammatory cytokines in vitro and ROS production in the
brain
It has been reported that central inflammatory
cytokine levels are increased during the development of
depressive-like behavior (23,24).
Therefore, TNF-α and IL-1β levels in OHSC supernatants following
treatment with fr-HMGB1, in presence or absence of its oxidant,
were detected by ELISA. As presented in Fig. 5C, there were significant fr-HMGB1 ×
H2O2 interactions for secreted TNF-α
(F=4.793, P=0.039) and IL-1β (F=16.45, P=0.0006) protein levels.
Fr-HMGB1 treatment did not increase the levels of TNF-α and IL-1β
in control slices; however, combination with
H2O2 treatment increased cytokine levels
(TNF-α, P<0.001; IL-1β, P<0.001; Fig. 5C). These findings indicated that
fr-HMGB1 activated TNF-α and IL-1β signaling only following
oxidation. In addition, hippocampal ROS levels were significantly
increased in the fr-HMGB1 group compared with the control group
(P<0.05; Fig. 5A and B), as
measured by DHE and DCF fluorescence.
Discussion
Consistent with our previous study (4), the findings of the present study
demonstrated that i) both states of HMGB1 induced the KYN pathway,
resulting in depressive-like behavior; ii) KYN pathway activation
occurred via similar mechanisms, with ds-HMGB1 directly
upregulating KYN pathway enzymes (IDO, KMO and KYNU), and fr-HMGB1
upregulating expression only following oxidation to ds-HMGB1.
As aforementioned, HMGB1 exists in three different
forms. Its biological activities rely on these forms, which vary
based on the oxidation states of cysteine residues at C23, C45 and
C106 (18,25). Ds-HMGB1 acts as a proinflammatory
cytokine by binding to toll-like receptors (7,25),
whereas fr-HMGB1 mediates chemotaxis via interactions with receptor
for advanced glycation end products (18). Conversely, ox-HMGB1 has no known
active function. Consistent with our previous study (4), the present study demonstrated that
ds-HMGB1 induced the activation of the KYN pathway and other
proinflammatory cytokines in vivo and ex vivo, thus
verifying the possible etiology of ds-HMGB1-induced depression.
Of note, fr-HMGB1 also induced the KYN pathway,
upregulating cytokines such as TNF-α in vivo (4), and contributing to the development of
depressive behavior. Conversely, it did not upregulate the KYN
pathway or increase TNF-α and IL-1β levels ex vivo unless in
presence of the oxidant H2O2. Furthermore,
the mutant analogue of fr-HMGB1, nonoxid-HMGB1, which exhibits
chemoattractant activity but lacks oxidizable activity (18), did not induce depressive-like
behavior or KYN pathway activation. This difference between the two
states of HMGB1 is proposed to result from the fact that
nonoxid-HMGB1 cannot be oxidized into ds-HMGB1, whereas
extracellular fr-HMGB1 is able to alter its state from a reduced to
oxidized form in vivo (25). Overall, combined with ex
vivo findings, it is proposed that fr-HMGB1 may contribute to
depression following oxidation in vivo. Additionally, the
levels of ROS were increased following central administration of
fr-HMGB1, supporting the aforementioned hypothesis. Increased ROS
levels suggested that the extracellular milieu may be more
oxidizing, enabling the formation of disulfide bridges (26).
The present study revealed that ds-HMGB1 and
fr-HMGB1 activated the KYN pathway. As aforementioned, Trp
metabolism by IDO or TDO2 is the first step of the KYN pathway;
when Trp is converted into KYN, it can be metabolized by KMO, KYUN
and 3-HAO to yield 3-HK, QUIN and NAD+; alternatively,
it can be converted into KYNA by KAT2. Of note, KYN derivatives in
the Trp-NAD+ pathway include neurotoxic compounds such
as 3-HK and QUIN (14), unlike the
protective product KYNA synthesized by KAT2. The increased
expression levels of IDO, KMO and KYNU, but not KAT2 suggested that
fr-HMGN1 and ds-HMGB1 induced the Trp-NAD+ pathway, but
not the Trp-KYNA axis (Fig. 6).
KYNU is detected in microglia (27), whereas KAT2 is produced by
astrocytes (28,29). These findings suggested the
involvement of microglial (but not astrocytic) activation.
Subsequently, activated microglia may secrete abundant glutamate
(Fig. 6), which is also considered
to be neurotoxic (30), and uptake
KYN (14). Thus, the
Trp-NAD+ pathway in microglia requires further
investigation. Of note, differences in KAT2 expression in response
to HMBG1 treatment were observed between whole hippocampi and slice
cultures; KAT2 exhibited a trend towards upregulated expression
in vivo but was downregulated in vitro, suggesting
that extrahippocampal regions may serve protective functions;
however, this requires further investigation.
 | Figure 6.Model of fr- and ds-HMGB1-mediated
neurotoxicity. Ds-HMGB1 upregulated the expression of certain
enzymes associated with the kynurenine pathway mainly located in
microglia but not in astrocytes; fr-HMGB1 induced similar effects
following oxidation. It was hypothesized that the disbalance of the
two kynurenine pathway arms in the brain reinforced neurotoxic
metabolite neurotransmission, inhibited the production of
neuroprotective compounds and contributed to the occurrence of
depression. ds-, disulfide; fr-, fully reduced; HMGB1, high
mobility group box-1; IDO, indoleamine-2,3-dioxygenase; KAT2,
kynurenine aminotransferase 2; KMO, kynurenine monooxygenase; KYN,
kynurenine; KYNA, kynurenic acid; KYNU, kynureninase; 3-HAO,
3-hydroxyanthranilate 3,4-dioxygenase; Trp, tryptophan. |
The effects of HMGB1 have been reported in numerous
diseases, including sepsis, arthritis and ischemia-reperfusion,
with the application of HMGB1-blocking therapies improving symptoms
in rodent models of these disorders (31–33);
certain studies focused on ds-HMGB1 (6,9,34).
In the present study, it was demonstrated that an oxidative
environment in vivo and ex vivo may oxidize fr-HMGB1,
conferring ds-HMGB1 characteristics and mediating depression via
the KYN pathway.
The present study investigated the role of fr-HMGB1
in the induction KYN pathway; however, the role of other mechanisms
cannot be excluded. Additionally, the effects of systemic ROS
depletion using antioxidant compounds or vitamins supplements, and
the measurement of antioxidant enzyme levels or KYN pathway
metabolites in vivo or in vitro require
investigation. Overall, further study is required to determine the
precise mechanisms and effects of fr-HMGB1.
Acknowledgements
The authors would like to thank Dr Huang Xiao
(Institute of Neuroscience and Key Laboratory of Molecular
Neurobiology of Ministry of Education, Second Military Medical
University, Shanghai, China) for technical assistance and editing
of the manuscript.
Funding
The present study was financially supported by the
National Natural Science Foundation of China (grant nos. 81171124
and 81771301).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BW drafted the manuscript and contributed to all
aspects of the experimental design and research procedure,
including western blotting and qPCR assays. YJL was involved in
conducting the behavioral measurements. LLL and JML contributed to
conducting the experiments. WJS and CLJ made substantial
contributions to the conception of the study and the editing of the
manuscript. YXW was involved in designing the study and
interpreting the data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Committee on
Ethics of Biomedicine Research, Second Military Medical University
and conducted in accordance with the guidelines of Animal
Experimentation set by the committee.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Magna M and Pisetsky DS: The role of HMGB1
in the pathogenesis of inflammatory and autoimmune diseases. Mol
Med. 20:138–146. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kang R, Chen R, Zhang Q, Hou W, Wu S, Cao
L, Huang J, Yu Y, Fan XG, Yan Z, et al: HMGB1 in health and
disease. Mol Aspects Med. 40:1–116. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Avgousti DC, Herrmann C, Kulej K, Pancholi
NJ, Sekulic N, Petrescu J, Molden RC, Blumenthal D, Paris AJ, Reyes
ED, et al: A core viral protein binds host nucleosomes to sequester
immune danger signals. Nature. 535:173–177. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lian YJ, Gong H, Wu TY, Su WJ, Zhang Y,
Yang YY, Peng W, Zhang T, Zhou JR, Jiang CL and Wang YX: Ds-HMGB1
and fr-HMGB induce depressive behavior through neuroinflammation in
contrast to nonoxid-HMGB1. Brain Behav Immun. 59:322–332. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Balosso S, Liu J, Bianchi ME and Vezzani
A: Disulfide-containing high mobility group box-1 promotes
N-methyl-D-aspartate receptor function and excitotoxicity by
activating Toll-like receptor 4-dependent signaling in hippocampal
neurons. Antioxid Redox Signal. 21:1726–1740. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mazarati A, Maroso M, Iori V, Vezzani A
and Carli M: High-mobility group box-1 impairs memory in mice
through both toll-like receptor 4 and receptor for advanced
glycation end products. Exp Neurol. 232:143–148. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Frank MG, Weber MD, Watkins LR and Maier
SF: Stress sounds the alarmin: The role of the danger-associated
molecular pattern HMGB1 in stress-induced neuroinflammatory
priming. Brain Behav Immun. 48:1–7. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang H, Antoine DJ, Andersson U and Tracey
KJ: The many faces of HMGB1: Molecular structure-functional
activity in inflammation, apoptosis, and chemotaxis. J Leukoc Biol.
93:865–873. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Frank MG, Weber MD, Fonken LK, Hershman
SA, Watkins LR and Maier SF: The redox state of the alarmin HMGB1
is a pivotal factor in neuroinflammatory and microglial priming: A
role for the NLRP3 inflammasome. Brain Behav Immun. 55:215–224.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Coppen AJ: Depressed states and
indolealkylamines. Adv Pharmacol. 6:283–291. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Carlsson A, Corrodi H, Fuxe K and Hökfelt
T: Effect of antidepressant drugs on the depletion of intraneuronal
brain 5-hydroxytryptamine stores caused by
4-methyl-alpha-ethyl-meta-tyramine. Eur J Pharmacol. 5:357–366.
1969. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schildkraut JJ: The catecholamine
hypothesis of affective disorders: A review of supporting evidence.
Am J Psychiatry. 122:509–522. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lapin IP and Oxenkrug GF: Intensification
of the central serotoninergic processes as a possible determinant
of the thymoleptic effect. Lancet. 1:132–136. 1969. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Maddison DC and Giorgini F: The kynurenine
pathway and neurodegenerative disease. Semin Cell Dev Biol.
40:134–141. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dantzer R, O'Connor JC, Lawson MA and
Kelley KW: Inflammation-associated depression: From serotonin to
kynurenine. Psychoneuroendocrinology. 36:426–436. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brooks AK, Lawson MA, Smith RA, Janda TM,
Kelley KW and McCusker RH: Interactions between inflammatory
mediators and corticosteroids regulate transcription of genes
within the Kynurenine Pathway in the mouse hippocampus. J
Neuroinflammation. 13:982016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang B, Lian YJ, Su WJ, Peng W, Dong X,
Liu LL, Gong H, Zhang T, Jiang CL and Wang YX: HMGB1 mediates
depressive behavior induced by chronic stress through activating
the kynurenine pathway. Brain Behav Immun. 72:51–60. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Venereau E, Casalgrandi M, Schiraldi M,
Antoine DJ, Cattaneo A, De Marchis F, Liu J, Antonelli A, Preti A,
Raeli L, et al: Mutually exclusive redox forms of HMGB1 promote
cell recruitment or proinflammatory cytokine release. J Exp Med.
209:1519–1528. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cryan JF, Mombereau C and Vassout A: The
tail suspension test as a model for assessing antidepressant
activity: Review of pharmacological and genetic studies in mice.
Neurosci Biobehav Rev. 29:571–625. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu MY, Yin CY, Zhu LJ, Zhu XH, Xu C, Luo
CX, Chen H, Zhu DY and Zhou Q: Sucrose preference test for
measurement of stress-induced anhedonia in mice. Nat Protoc.
13:1686–1698. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Seibenhener ML and Wooten MC: Use of the
open field maze to measure locomotor and anxiety-like behavior in
mice. J Vis Exp. e524342015.PubMed/NCBI
|
|
23
|
Liu YN, Peng YL, Liu L, Wu TY, Zhang Y,
Lian YJ, Yang YY, Kelley KW, Jiang CL and Wang YX: TNFα mediates
stress-induced depression by upregulating indoleamine
2,3-dioxygenase in a mouse model of unpredictable chronic mild
stress. Eur Cytokine Netw. 26:15–25. 2015.PubMed/NCBI
|
|
24
|
Zhang Y, Liu L, Liu YZ, Shen XL, Wu TY,
Zhang T, Wang W, Wang YX and Jiang CL: NLRP3 inflammasome mediates
chronic mild stress-induced depression in mice via
neuroinflammation. Int J Neuropsychopharmacol. 18(pii): pyv0062015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang H, Lundbäck P, Ottosson L,
Erlandsson-Harris H, Venereau E, Bianchi ME, Al-Abed Y, Andersson
U, Tracey KJ and Antoine DJ: Redox modification of cysteine
residues regulates the cytokine activity of high mobility group
box-1 (HMGB1). Mol Med. 18:250–259. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tang D, Kang R, Zeh HJ III and Lotze MT:
High-mobility group box 1, oxidative stress, and disease. Antioxid
Redox Signal. 14:1315–1335. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Guillemin GJ, Smith DG, Smythe GA, Armati
PJ and Brew BJ: Expression of the kynurenine pathway enzymes in
human microglia and macrophages. Adv Exp Med Biol. 527:105–112.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schwarcz R and Pellicciari R: Manipulation
of brain kynurenines: Glial targets, neuronal effects, and clinical
opportunities. J Pharmacol Exp Ther. 303:1–10. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Du F, Schmidt W, Okuno E, Kido R, Köhler C
and Schwarcz R: Localization of kynurenine aminotransferase
immunoreactivity in the rat hippocampus. J Comp Neurol.
321:477–487. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Piani D, Spranger M, Frei K, Schaffner A
and Fontana A: Macrophage-induced cytotoxicity of
N-methyl-D-aspartate receptor positive neurons involves excitatory
amino acids rather than reactive oxygen intermediates and
cytokines. Eur J Immunol. 22:2429–2436. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Goldstein RS, Gallowitsch-Puerta M, Yang
L, Rosas-Ballina M, Huston JM, Czura CJ, Lee DC, Ward MF, Bruchfeld
AN, Wang H, et al: Elevated high-mobility group box 1 levels in
patients with cerebral and myocardial ischemia. Shock. 25:571–574.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kokkola R, Li J, Sundberg E, Aveberger AC,
Palmblad K, Yang H, Tracey KJ, Andersson U and Harris HE:
Successful treatment of collagen-induced arthritis in mice and rats
by targeting extracellular high mobility group box chromosomal
protein 1 activity. Arthritis Rheum. 48:2052–2058. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhao H, Liu Z, Shen H, Jin S and Zhang S:
Glycyrrhizic acid pretreatment prevents sepsis-induced acute kidney
injury via suppressing inflammation, apoptosis and oxidative
stress. Eur J Pharmacol. 781:92–99. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee G, Espirito Santo AI, Zwingenberger S,
Cai L, Vogl T, Feldmann M, Horwood NJ, Chan JK and Nanchahal J:
Fully reduced HMGB1 accelerates the regeneration of multiple
tissues by transitioning stem cells to GAlert-. Proc
Natl Acad Sci USA. 115:E4463–E4472. 2018. View Article : Google Scholar : PubMed/NCBI
|




















