Introduction
Microglial cells are the major immune cell in the
central nervous system (CNS), responding against types of
endogenous and exogenous stimuli, including infection by bacteria,
viruses, prions and β-amyloid plaques (1). Microglia are activated upon exposure
to different stimuli and, depending on the environmental context,
this may be beneficial or detrimental to the functionality and
physiology of the CNS (2). The
effects of microglial phagocytosis are primarily beneficial, as it
eliminates dead cells and induces cellular anti-inflammatory
response. Removing dead or decaying cellular components may also
prevent proinflammatory intracellular responses, thus contributing
to a reduction in inflammatory burden (3). However, microglial activation and
subsequent phagocytosis may exacerbate cell cytotoxicity and
neuroinflammation (4). This may in
turn cause a respiratory burst, producing toxic reactive
oxygen/nitrogen species (ROS/RNS), which lead to oxidative damage
(5). Cellular systems are
constantly exposed to various toxic and mutagenic substances that
cause the generation of reactive metabolites. These free radicals
collectively induce oxidative stress, which may cause damage to the
cellular components or the cells themselves, thus disturbing
physiological functions. The protective strategies against
oxidative stress primarily include the initiation of cellular
detoxification systems, which, in the CNS, involves the recruitment
of antioxidant enzymes in microglial cells.
Volatile anesthetics like isoflurane have been
reported to reduce lipopolysaccharide (LPS) and interferon (IFN)-γ
induced neuronal inflammation and apoptosis (6,7).
However, the effect of volatile anesthetics on microglial
phagocytosis/engulfment requires further elucidation, particularly
regarding its potential detrimental consequences. The anesthetic
propofol has been reported to reduce arterial blood flow in the
brain, reducing intracranial pressure and maintaining the metabolic
blood-oxygen supply ratio even during hypoxia, suggesting that it
may have protective effects against hypoxic brain damage (8–10).
Propofol may also serve a protective role via the modulation of
Ca2+ and the reduction of free radicals in a
γ-aminobutyric acid (GABA) and N-methyl-D-aspartate (NMDA) receptor
dependent manner (11–14). All volatile anesthetics differ in
potency, function and adverse effects when used in different
physiological conditions (15,16).
Isoflurane (2-chloro-2-(difluoromethoxy)-1,1, 1-trifluoro-ethane;
CHF2-O-CHCl-CF3) is a halogenated ether used
as inhalational anesthetic, and has been reported to exhibit
preventative roles in neonatal hypoxia-ischemia induced brain
injury (17) and neurodegenerative
diseases (18). Isoflurane has
also been reported to have beneficial effects in animal models of
various other diseases, including LPS-induced acute lung
inflammation and injury (19,20),
glucose-induced oxidative stress (21), renal ischemia/reperfusion injury
(22) and cardiac injury models
(23).
Anthocyanins, glycosides of anthocyanidins, are the
compounds responsible for colors observed in plants (24). Anthocyanins have recently attracted
consideration due to their wide range of potential
bio-pharmacological benefits, including anti-inflammatory,
antioxidant, antiproliferative, antitumor and cardioprotective
properties (24–28). Anthocyanin-rich extract
administration to vitamin E-depleted rats has been demonstrated to
have protective effects against tert-butyl hydroperoxide-induced
hepatic toxicity by decreasing lipid peroxidation and DNA damage
(29,30). Anthocyanins, being glucosides, have
high water solubility, which allows them to be directly absorbed
and dissolved in the blood (31),
and may be incorporated into cellular membranous compartments,
including the cytosol (32).
Anthocyanins have also been reported to have free radical
scavenging and antioxidant activity (33). Therefore, these glycosides may aid
in the prevention of chronic pathophysiological conditions,
particularly considering that oxidative stress is a key factor in
the development of numerous pathologies, including
neurodegenerative diseases (34,35).
For instance, anthocyanin metabolites have been reported to protect
PC12 cells and SH-SY5Y neuroblastoma cells from oxidative stress
induced by hydrogen peroxide (36–38);
and treatment of retinal ganglion cells with anthocyanidin
components from bilberries has been reported to rescue cells from
oxidative stress induced by peroxynitrite and to protect cells from
excitotoxicity (39). Callistephin
(pelargonidin-3-O-glucoside) is a glucoside consisting about 60–80%
of the total anthocyanin content in strawberries (40). Callistephin is found in various
berries, and has been prospectively used in a number of preventive
studies (40). Callistephin has
been reported to have high activity equivalence to Trolox,
suggesting that it may be a potent intrinsic antioxidant and may
suppress free radical generation (41).
Therefore, the present study was designed to
evaluate the effects of callistephin and isoflurane on microglial
engulfment induced by LPS and IFN-γ in mouse microglial cells. The
study evaluated the association of callistephin with isoflurane in
exerting anti-inflammatory and antioxidant effects in cells exposed
to LPS.
Materials and methods
Cell culture
Mouse microglial cloned C8-B4 cells (CRL-2540™),
isolated from the cerebellum of an 8-day old mouse, were procured
from the American Type Culture Collection (Manassas, VA, USA). The
C8-B4 microglial cells were cultured in Dulbecco's modified Eagle's
medium (DMEM; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
containing 4 mM L-glutamine, 4,500 mg/l glucose, 1 mM sodium
pyruvate, 1,500 mg/l sodium bicarbonate, and supplemented with 10%
heat-inactivated fetal bovine serum (Thermo Fisher Scientific,
Inc.), penicillin and streptomycin (100 U/ml), in an incubator (New
Brunswick; Eppendorf, Hamburg, Germany) with a humidified
atmosphere with 5% CO2, at 37°C. The culture medium was
changed every 48–72 h to maintain the cell cultures.
LPS/IFN-γ treatment and isoflurane
exposure
Mouse microglial C8-B4 cells were challenged with
LPS and IFN-γ at 100 and 1 ng/µl concentrations, respectively, for
24 h at 37°C, as previously reported (41). Cells were exposed to isoflurane
after 2 h of LPS/INF-γ administration, in an airtight chamber at
the aforementioned temperature and atmospheric conditions.
Isoflurane was supplemented via a vaporizer for 1 h. The isoflurane
concentration was measured from the outlet of the chambers using an
Infrared Gas Analyzer (GE Healthcare, Chicago, IL, USA). This was
performed to ensure that the isoflurane was kept at a constant
concentration for the duration of the treatment. The
isoflurane-exposed cells were transferred from the airtight sealed
chamber to normal cell culture conditions for further incubation at
37°C for 24 h. Following optimization and the establishment of
anesthetic effect, a concentration of 2% isoflurane was used for
further experimental procedures. Callistephin (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) was dissolved in dimethyl sulfoxide
(DMSO), ensuring a final concentration of 0.1% DMSO in the cell
cultures.
Cell viability analysis
Mouse microglial C8-B4 cells were plated at a
density of 5,000 cells/well in 96-well plates for 24 h. The medium
was removed and replaced with fresh medium. Cells were treated with
LPS and INF-γ, followed by exposure to isoflurane as described
earlier. Isoflurane was tested at concentrations of 1, 2, 4, 6, 8
and 10% in an independent viability optimization assay.
Callistephin was tested at 50, 100, 150, 200, 300 and 400 µM
concentrations for 24 h in an independent viability optimization
assay. Following the previous tests for optimization of cell
viability, 2% isoflurane and 200 µM callistephin were chosen for
further experimental procedures. Microglial cell viability was
determined by the Cell Titer Glo (CTG) 2.0 assay kit (Promega
Corporation, Madison, WI, USA), according to a modified version of
the manufacturer's protocol, since luminescence was stabilized for
15 min instead of 10 min. The CTG 2.0 assay is a consistent method
of determining the number of viable cells in cultures. This is
achieved via quantification of the amount of ATP present, which
serves as an indicator of metabolically active cells. This method
is a luminescent cell viability assay system ideal for automated
high-throughput screening of cell proliferation and cytotoxicity
assays (42). C8-B4 cells in
96-well plates were treated with CTG reagent, and luminescence was
measured using an ELISA microplate spectrophotometer at 490 nm.
Cell viability is presented as the percentage of viable cells as
compared with the vehicle control (100%).
Cell engulfment analysis
Cell engulfment was assessed using Nile red
polystyrene microspheres (1.0 µm; Sigma-Aldrich; Merck KGaA) at a
concentration of 10,000 particles/ml cell culture. Microglial C8-B4
cells were exposed to different treatment conditions in 6-well
culture plates for 24 h. Subsequently, Nile red polystyrene
microspheres were added to the culture wells and incubated for 30
min at 37°C in a CO2 incubator with 5% CO2.
Engulfment was facilitated by bovine serum albumin (BSA; Thermo
Fisher Scientific, Inc.) supplementation as an opsonizing agent.
Cells were washed with PBS and fixed with 100% methanol for 15 min
at 4°C. Cells were permeabilized with 0.1% Triton X-100 diluted in
PBS for 15 min, and blocked with 1% BSA for 30 min at room
temperature. Following blocking, cells were incubated with rabbit
monoclonal anti-Iba1 antibody (cat. no. ab178847; Abcam, Cambridge,
UK; dilution, 1:1,000) in the dark at 4°C overnight, and incubated
with horseradish peroxidase-conjugated goat anti-rabbit IgG
secondary antibody (cat. no. ab6702; Abcam; dilution, 1:5,000) for
1 h at room temperature the following day. The cells were washed,
and the nuclei were counterstained with Hoechst 33342 staining
reagent for 10 min at room temperature. Fixed and stained cells
were imaged under a fluorescence microscope (Zeiss AG, Oberkochen,
Germany; magnification, ×40) equipped with a digital microscopic
camera system. The percentage of engulfed microglial cells was
calculated as: No. of microglial cells with fluorescent
microspheres/total no. microglial cells ×100, and compared with the
control.
Measurement of ROS in microglial
cells
The antioxidant activity of callistephin in C8-B4
cells was determined by measuring the production of ROS using a
fluorescent indicator kit, namely the dichlorofluorescein diacetate
(DCFDA) assay kit (Thermo Fisher Scientific, Inc.), according to
the manufacturer's instructions. Briefly, mouse microglial C8-B4
cells were plated at a density of 5,000 cells/well in 96-well
plates for 24 h. The medium was removed and replaced with fresh
medium. Cells were treated with LPS and IFN-γ, followed by exposure
to isoflurane as described earlier. Cells were treated with 100 µM
callistephin for 24 h. Subsequently, medium from the culture wells
was removed and replaced with fresh medium containing DCFDA (20
µM). The plates were incubated at 37°C for 30 min, the
DCFDA-containing medium was removed and the cells were washed. The
amount of ROS production in cells was measured using a fluorescence
ELISA microplate spectrophotometer at excitation and emission
wavelengths of 495 and 529 nm, respectively. The relative
fluorescence units (RFU) were counted for each treatment
groups.
Measurement of total nitric oxide (NO)
generation
The production of total NO in microglial C8-B4 cells
was determined via the Greiss reagent assay kit (Invitrogen; Thermo
Fisher Scientific, Inc.), according to the manufacturer's
instructions. Briefly, mouse microglial C8-B4 cells were plated at
a density of 5,000 cells/well in 96-well plates for 24 h. Following
the addition of fresh medium, cells were treated with LPS and
IFN-γ, exposed to isoflurane and treated with 100 µM callistephin
for 24 h. Following incubation, culture medium from the treated
plates was transferred to a 96-well plate for determination of
total NO. The Griess reagent mixture was prepared by mixing equal
volumes of Component A [N-(1-naphthyl)ethylenediamine] and
Component B (sulfanilic acid). A total volume of 20 µl freshly
prepared Griess reagent was added to each well of the 96-well
plate, and incubated at room temperature for 30 min. The absorbance
in each well was measured using an ELISA microplate
spectrophotometer at 548 nm.
Measurement of caspase-3/7 activity in
microglial cells
The apoptosis of microglial cells was further
elucidated by analyzing the activation of caspases. Upon activation
of apoptotic processes, executioner caspases-3 and 7 are frequently
activated. Therefore, the levels of caspase-3/7 activity were
verified using a Caspase-Glo 3/7 assay kit (Promega Corporation).
The Caspase-Glo 3/7 assay is a pro-luminescent caspase-3/7 with the
tetrapeptide DEVD bound to aminoluciferin. When caspase-3/7 are
active, the DEVD-aminoluciferin is cleaved, releasing
aminoluciferin, which has thermostable luciferase activity
(43). The mouse microglial C8-B4
cells were plated at a density of 1×106 cells/well in
6-well plates. After 24 h, the medium was removed and replaced with
fresh medium containing LPS and INF-γ, followed by exposure to
isoflurane, as described earlier, and treatment with 100 µM
callistephin for 24 h. Following incubation, caspase-Glo 3/7 assay
was performed according to the manufacturer's instructions. The
assay kit results in cell lysis which, upon the addition of caspase
3/7 assay reagent, produces a luminescent signal which is
considered proportional to caspase 3/7 activity. The relative
luminescence units (RLU) were counted in different treatment groups
of cells using a spectrophotometer at 490 nm.
RNA isolation, cDNA synthesis, and
semi-quantitative polymerase chain reaction (sqPCR)
C8-B4 cells were treated with LPS (100 ng/µl) and
IFN-γ (1 ng/µl), isoflurane (2%), callistephin (100 µM) or a
combination of the two for 24 h. Cells were collected, and RNA was
isolated using TRIzol® reagent (ThermoFisher Scientific,
Inc.), according to the manufacturer's instructions. RNA was
quantified, and a total of 1 µg RNA per sample was subjected to
cDNA synthesis using the Reverse Transcriptase SuperScript III kit
(Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. The reaction was performed at 65°C for 5 min and then
cooled on ice. Subsequently, the mixture containing the SuperScript
III reverse transcriptase was incubated at 50°C for 1 h and at 70°C
for 15 min. The obtained cDNA was subjected to PCR amplification
with the iTaq DNA Polymerase (Bio-Rad Laboratories, Inc., Hercules,
CA, USA) and dNTPs mix (Thermo Fisher Scientific, Inc.), using a
Veriti™ Thermal Cycler (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The specific primers and thermocycling
conditions used in the analysis were: Inducible nitric oxide
synthase (iNOS) forward, 5′-GCAAACCCAAGGTCTACGTT-3′; iNOS reverse,
5′-GGAAAAGACTGCACCGAAGA-3′ (initial denaturation at 95°C for 5 min,
followed by 35 cycles of 95°C for 1 min, 58°C for 1 min and 72°C
for 1 min, with a final extension at 72°C for 10 min); tumor
necrosis factor-α (TNF-α) forward, 5′-AGCACAGAAAGCATGATCCG-3′;
TNF-α reverse, 5′-CTGATGAGAGGGAGGCCATT-3′ (initial denaturation at
95°C for 5 min, followed by 35 cycles of 95°C for 1 min, 59°C for 1
min and 72°C for 1 min, with a final extension at 72°C for 10 min);
cytochrome c oxidase subunit 2 (COX-2) forward,
5′CCTGTGCCTGATGATTGC-3′; COX-2 reverse, 5′-CGGTGAAACTCTGGCTAG-3′
(initial denaturation at 95°C for 5 min, followed by 35 cycles of
95°C for 1 min, 58°C for 1 min and 72°C for 1 min, with a final
extension at 72°C for 10 min); nuclear factor-κ B subunit/p65
(NF-κB-p65) forward, 5′-CCTCTGGCGAATGGCTTTAC-3′; NF-κB-p65 reverse,
5′-GCTATGGATACTGCGGTCTGG-3′ (initial denaturation at 95°C for 5
min, followed by 35 cycles of 95°C for 1 min, 60°C for 1 min and
72°C for 1 min, with a final extension at 72°C for 10 min); β-actin
forward, 5′-ATCACTATTGGCAACGAGCG-3′; β-actin reverse
5′-TCAGCAATGCCTGGGTACAT-3′ (initial denaturation at 95°C for 5 min,
followed by 35 cycles of 95°C for 1 min, 54°C for 30 sec and 72°C
for 1 min, with a final extension at 72°C for 10 min). PCR products
were separated via agarose gel electrophoresis (1.5%), and
visualized using ethidium bromide. The agarose gels were scanned
under UV light, and images were captured. The agarose gel bands
were analyzed for band densitometry using Image J Software (version
1.5; National Institutes of Health, Bethesda, MD, USA).
Protein isolation and western
blotting
Microglial C8-B4 cells, exposed to different
treatment conditions in 6-well plates, were harvested by scraping,
washed with cold PBS and pelleted by centrifugation at 5,000 × g
for 10 min at 4°C. Cells were lysed in 0.5 ml cold cell lysis
buffer [20 mM Tris pH 7.5, 1 mM EDTA, 150 mM NaCl, 2.5 mM sodium
pyrophosphate, 1% Triton X-100, 1% sodium vanadate, 1 mM
phenylmethylsulfonyl fluoride and a protease inhibitor cocktail
(Sigma-Aldrich; Merck KGaA)] for 1 h in ice with intermittent
vortex mixing. The cell lysate was centrifuged at 12,000 × g for 20
min at 4°C. The supernatant containing the total cellular protein
was collected and the cell pellet was discarded. Protein estimation
was performed from protein isolates using the Pierce bicinchoninic
acid protein assay kit (Thermo Fisher Scientific, Inc.), according
to the manufacturer's instructions. A total of 20 µg protein per
sample was separated via 12% SDS-PAGE. Separated proteins were
transferred onto polyvinylidene difluoride membranes using a
western blot wet transfer system. Membranes with transferred
proteins were blocked with 5% fat-free dried skimmed milk prepared
in PBS with 0.1% Tween-20 (PBST) for 2 h at 4°C. Membranes were
washed with PBST buffer and incubated with primary antibodies for
different proteins overnight at 4°C. The following day, the
membranes were washed and incubated with corresponding secondary
antibodies for 1 h at room temperature. Protein bands were
visualized using Pierce enhanced chemiluminescence detection
reagent (Thermo Fisher Scientific, Inc.) on photographic film. The
primary antibodies and respective dilutions used in the western
blotting were: Anti-iNOS antibody (cat. no. ab3523; Abcam), 1:500
dilution; anti-Cox-2 antibody (cat. no. sc-1747; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), 1:500 dilution; anti-NF-κB
p65 antibody (cat. no. NB100-2176; Novus Biologicals, LLC,
Littleton, CO, USA), 1:500 dilution; anti-GAPDH antibody (cat. no.
MAB374; Merck KGaA), 1:500 dilution; anti-B-cell lymphoma 2 like 1
(Bcl-xL) antibody (cat. no. 2762; Cell Signaling Technology Inc.,
Danvers, MA, USA), 1:1,000 dilution; and poly (ADP-ribose)
polymerase 1 (PARP) antibody (cat. no. 9542; Cell Signaling
Technology Inc.), 1:1,000 dilution. The secondary antibodies used
were: Horseradish peroxidase (HRP)-conjugated anti-rabbit (cat. no.
ab6702; Abcam; dilution, 1:5,000), and HRP-conjugated anti-mouse
(cat. no. ab97046; Abcam; dilution, 1:5,000).
Statistical analysis
Data are presented as the mean ± standard deviation
of at least three independent experimental repeats. Data were
analyzed using SPSS version 17 software (SPSS, Inc., Chicago, IL,
USA) and the Systat SigmaPlot 11.0 statistical program (Systat
Software Inc., San Jose, CA, USA). Data were statistically compared
between groups using a one-way analysis of variance and Tukey's
post-hoc test for multiple comparisons. P<0.05 was considered to
indicate a statistically significant difference.
Results
Effects of isoflurane and callistephin
on C8-B4 microglial cell viability
The neuroprotective effects of callistephin and
isoflurane were evaluated in C8-B4 microglia exposed to
inflammatory LPS/IFN-γ, using the CTG 2.0 method. Firstly, the
effect of callistephin and isoflurane alone were assessed on C8-B4
cells in order to determine the effective cytotoxic concentration.
The results presented in Fig. 1A
indicated that callistephin did not affect cell viability at 50,
100, 150, 200 and 300 µM concentrations. The viability of cultured
cells was decreased by 9% at 200 µM and 12% at 300 µM, but not
significantly. However, callistephin at higher concentrations (400
µM) reduced cell viability by 18%, with statistical significance.
This demonstrated that callistephin did not affect cell viability
at moderate concentrations, but was slightly cytotoxic at
concentrations >300 µM. Likewise, the effect of isoflurane
exposure was also assessed on C8-4B cells. The results presented in
Fig. 1B indicated that cell
viability was unaffected with 1, 2, 4, 6 and 8% isoflurane.
However, isoflurane was cytotoxic at 10% with statistical
significance. At 10% isoflurane, 78% of cells were viable, which is
indicative of the notable cytotoxicity of isoflurane at higher
concentrations. Therefore, 100 µM callistephin and 2% isoflurane
were used in the subsequent experiments, and similar concentrations
have also been used in other studies (18,19,21,22).
Subsequently, the effect of an immune challenge with
100 ng/µl LPS and 1 ng/µl IFN-γ on C8-B4 cells was assessed in the
context of cell viability. Furthermore, the effect of treatment
with isoflurane, callistephin or their combination on cell
viability following the challenging step was also tested (Fig. 1C). The LPS/IFN-γ challenge to C8-B4
cells caused significant cytotoxicity, with 52±5% survival. The
treatment of LPS/IFN-γ-challenged C8-B4 cells with 2% isoflurane
had a preventative effect, with 68±5% survival (P<0.05 vs.
control and vs. LPS/IFN-γ). Likewise, LPS/IFN-γ-challenged C8-B4
cells treated with 100 µM callistephin resulted in 74±6% survival
(P<0.05 vs. control and vs. LPS/IFN-γ), meaning that
callistephin exhibited a potent preventative effect. The combined
treatment of LPS/IFN-γ-challenged C8-B4 cells with isoflurane and
callistephin resulted in a strong synergistic effect. This
co-treatment maintained survival at 91±6% (P<0.05 vs. LPS/IFN-γ;
not significant vs. control). Therefore, these results indicated
that the combination of callistephin and isoflurane maintained the
viability of C8-B4 cells following an immune challenge with
LPS/IFN-γ.
Effects of callistephin and isoflurane
on microglial engulfment
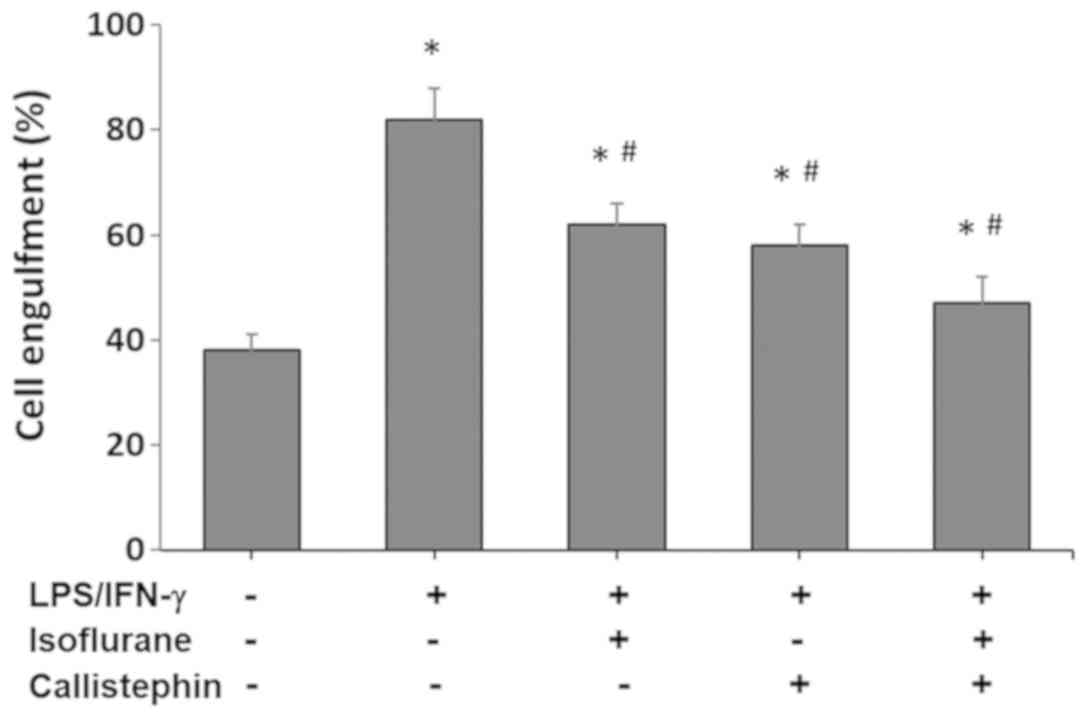
Microglial phagocytosis involves engulfment of
cellular material or entire cells. The process is followed by
digestion of the material through lysosomal degradation (3,4).
Cell engulfment was assessed using Nile red polystyrene
microspheres, and the percentage of engulfed microglial cells is
presented and compared with the control in Fig. 2. The control levels of microglial
engulfment were 38±3% in the vehicle control. The challenge with
LPS and IFN-γ increased the percentage of C8-B4 microglia
engulfment to 82±6% (P<0.05 vs. control). Treatment with
isoflurane, applied 2 h after the challenge with LPS and IFN-γ
stimulation, caused a decrease in microglial engulfment to 62±4%
(P<0.05 vs. LPS/IFN-γ and control). Similarly, treatment with
callistephin decreased microglial engulfment to 58±4% (P<0.05
vs. LPS/IFN-γ and control). Lastly, co-treatment of
LPS/IFN-γ-challenged C8-B4 microglial cells with isoflurane and
callistephin decreased microglial engulfment to 47±5% (P<0.05
vs. LPS/IFN-γ and control). These results suggested that the
combined effect of callistephin and isoflurane reduced the
phagocytic responses of cultured microglia following an immune
challenge with LPS/IFN-γ, which was expected considering the
results of the cytotoxicity assay.
Callistephin prevents oxidative stress
in microglia and enhances the effect of isoflurane
Metabolism of anthocyanins in the gut by microflora
results in the production of phenolic acids and aldehydes. The
metabolized anthocyanins have unique chemical structures derived
from the parent compounds, and may be metabolized into
phloroglucinol aldehyde (44,45).
The possible beneficial health effects of anthocyanins may be
therefore due to their metabolites. These have reported
neuroprotective capabilities as they are thought to regulate the
levels of oxidative stress mediators (44,46).
Consequentially, the antioxidant potential of callistephin and the
combination of callistephin and isoflurane were evaluated in C8-B4
microglia challenged with LPS and IFN-γ. A DCFDA assay was
performed to assess the levels of ROS generated across the
different treatment combinations (Fig.
3), and it demonstrated that ROS generation, (as indicated by
RFU levels) drastically increased in LPS/IFN-γ-challenged C8-B4
cells by 1.82±0.12-fold compared with the control.
LPS/IFN-γ-exposed C8-B4 cells treated with isoflurane alone
produced 1.44-fold more ROS compared with control cells, while
treatment with callistephin resulted in a 1.31-fold increase. The
co-treatment of LPS/IFN-γ-challenged C8-B4 cells with isoflurane
and callistephin prevented the production of ROS, as the RFU levels
of the co-treatment group were similar to the control (P>0.05
vs. control). These results collectively indicated that
callistephin may have been significantly effective in combination
with isoflurane in reducing the oxidative stress generated
following exposure to LPS/IFN-γ.
Anti-inflammatory effects of
callistephin and isoflurane on microglia
Nitrosative stress, caused by the generation and
accumulation of toxic RNS, including NO and peroxynitrite, is
capable of causing damage to vital cellular components. The damage
may be generated, for instance, by modifications to
S-nitrosylation, involved in protein homeostasis and the
mitochondrial respiratory chain system (47). The generation of RNS and ROS is
associated with diseases, including amyotrophic lateral sclerosis
and Parkinson's disease (48).
Neuroinflammatory processes may generate excess NO and
peroxynitrite via activation of microglia, which is involved in the
etiology and progression of certain neurodegenerative diseases
(47–49). Therefore, compounds that
effectively scavenge RNS or prevent NO generation may markedly
suppress inflammatory responses in neuronal cells. This may be of
therapeutic significance as it may ameliorate the symptoms of
certain neurodegenerative diseases. Thus, the capabilities of
callistephin and isoflurane in reducing the inflammatory responses
in microglia challenged with LPS/INF-γ were evaluated. Common
indicators of the inflammatory response in microglia include
enhanced production of the inflammatory cytokine TNF-α (50) and the production of the
proinflammatory proteins Cox-2 (51) and iNOS, the latter being
responsible for the production of NO (52).
Firstly, NO levels in control and
LPS/IFN-γ-challenged cells, treated with callistephin, isoflurane
or both, were evaluated (Fig. 4).
The results demonstrated that LPS/INF-γ induced a considerable
increase in NO (32.6±2.3 µM) compared with the control (4.8±0.3
µM). The treatment of LPS-challenged C8-B4 cells with isoflurane
led to a significant reduction in NO levels compared with the
LPS/IFN-γ treatment (22.4±2.4 µM). Treatment with callistephin also
resulted in a significant decrease in the NO levels (18.7±1.9 µM)
compared with the LPS/IFN-γ treatment. The combination of
callistephin and isoflurane prevented an increase in the NO levels
following the immune challenge with LPS/IFN-γ, (9.2±2.2 µM;
P>0.05 vs. control). Therefore, treatment with callistephin may
have enhanced the protective effects of isoflurane in C8-B4 cells
against nitrosative stress.
 | Figure 4.Effect of callistephin and isoflurane
on NO concentration. The levels of NO were measured in control
C8-B4 cells, cells challenged with LPS (100 ng/µl) and IFN-γ (1
ng/µl), and challenged cells treated with isoflurane, callistephin
or both. NO concentrations were determined using the Griess assay
system, and absorbance was measured at 548 nm, with the NO
concentration represented in µM. Overall, isoflurane, callistephin
or co-treatment with both compounds resulted in a decrease in NO
production. *P<0.05 vs. control; #P<0.05 vs.
LPS/IFN-γ. NO, nitric oxide; LPS, lipopolysaccharide; IFN-γ,
interferon-γ. |
Secondly, the expression pattern of genes involved
in inflammatory pathways were evaluated in the same treatment
conditions. Thus, a semi-quantitative PCR analysis of iNOS, TNF-α,
COX-2, and the p65 subunit of NF-κB was performed (Fig. 5A). The results revealed that
LPS/IFN-γ exposure increased the expression of each gene compared
with the control. LPS/IFN-γ-challenged C8-B4 cells treated with
isoflurane exhibited lower expression levels for each gene, with
the exception of NF-κB, whose expression was slightly increased.
Challenged cells treated with callistephin exhibited lower
expression levels of iNOS, TNF-α and COX-2, and slight increase in
NF-κB levels. Lastly, the combination of callistephin with
isoflurane reduced the expression level of iNOS, TNF-α and COX-2,
while NF-κB expression was markedly reduced (Fig. 5A). The combined treatment was most
effective on regulating the expression of iNOS gene, which is has
been reported to be upregulated in certain neurodegenerative
diseases (52,53). PCR also demonstrated that TNF-α and
COX-2 expression were approximately equally suppressed by the
combination of callistephin and isoflurane. However, NF-κB
expression was only moderately reduced (Fig. 5A).
Lastly, the protein levels of iNOS, COX-2 and NF-κB
were evaluated (Fig. 5B). The
results indicated that C8-B4 cells challenged with LPS/IFN-γ
exhibited higher levels of iNOS, COX-2 and NF-κB. The treatment of
challenged C8-B4 cells with isoflurane or callistephin caused a
reduction in the level of iNOS and COX-2 compared with the
challenged cells. The combination of callistephin with isoflurane
further reduced the expression levels of iNOS, COX-2 and NF-κB
protein compared with the LPS/IFN-γ-challenged cells (Fig. 5B). The observations from gene and
protein expression analysis suggested and strengthened the
hypothesis that callistephin may synergistically interact with
isoflurane to prevent microglial inflammation.
Callistephin and isoflurane protect
against microglial inflammation via p38
Cell death induction in the context of inflammation
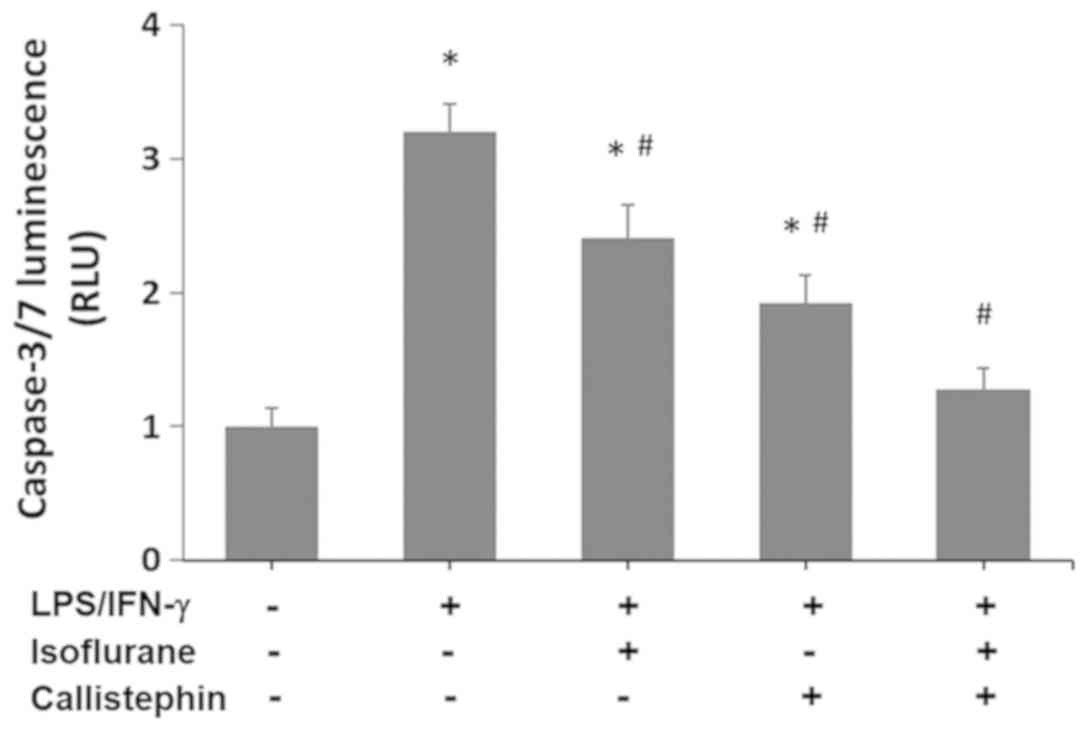
is characterized by activation of caspase-3/7 (35), and this was also evaluated in the
present study using a Caspase-Glo 3/7 assay. The results (Fig. 6) indicated that the activity of
caspase-3/7 in C8-B4 cells challenged with LPS/IFN-γ increased
3.2-fold compared with the control (1-fold RFU). Treatment with
isoflurane significantly decreased the caspase-3/7 activity levels
(2.41-fold RFU) compared with LPS/IFN-γ challenged cells
(P<0.05), yet they remained significantly higher than the
control. Similarly, treatment with callistephin further decreased
the caspase-3/7 activity (1.92-fold RFU) compared with LPS/IFN-γ
challenge alone (P<0.05). Lastly, the combination of
callistephin with isoflurane prevented an increase in caspase-3/7
activity (1.28-fold RFU) following the immune challenge with
LPS/IFN-γ. The level of caspase activity observed with the
co-treatment was significantly decreased compared with LPS/IFN-γ
(P<0.05) and not significantly different compared with the
control. These observations demonstrate that while isoflurane and
callistephin may have suppressed the apoptosis in microglial cells,
with callistephin being slightly more efficient, their combination
may have synergistically prevented apoptosis in microglial cells
challenged with LPS/IFN-γ.
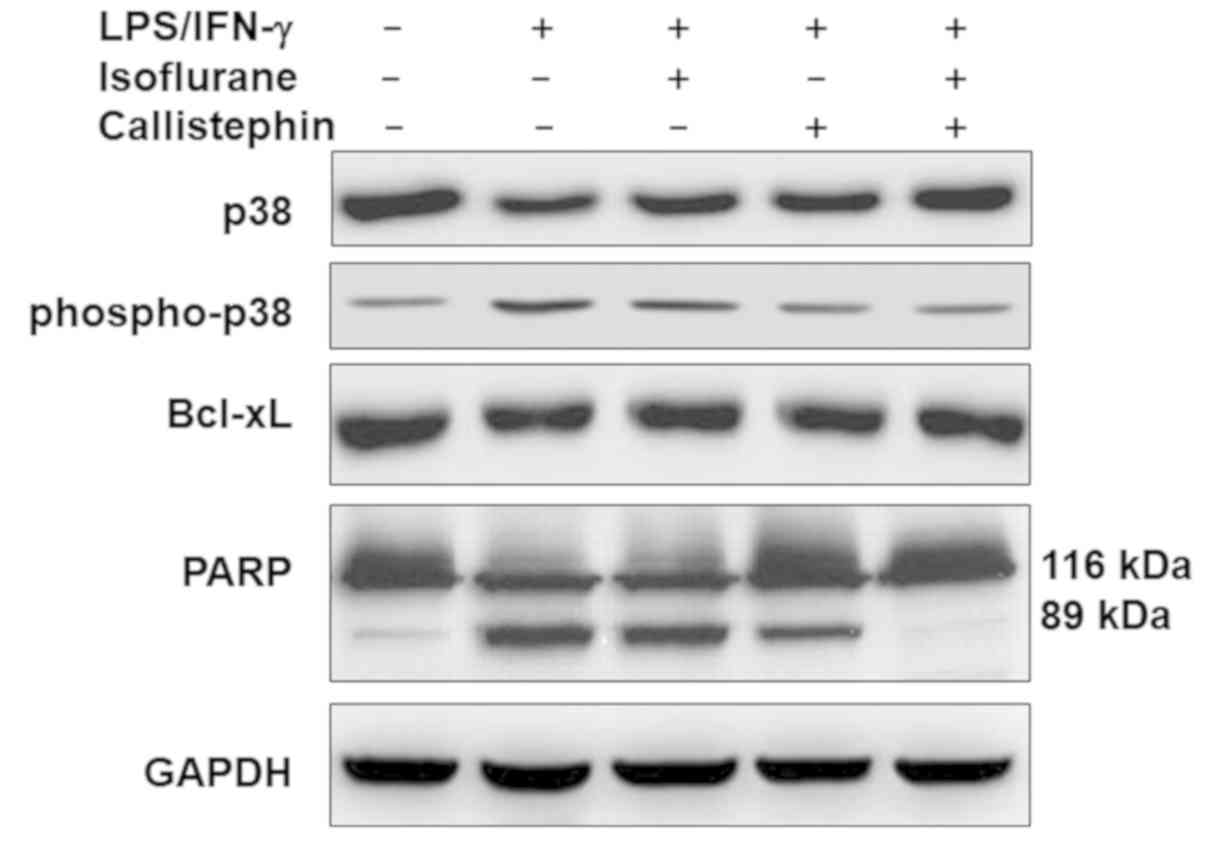
Microglial activation following CNS injury promotes
phagocytosis, a process regulated by the mitogen-activated protein
kinase (MAPK) p38 (54). p38 is a
group of important intracellular signaling proteins that respond to
cellular stress and inflammatory cytokines, including LPS challenge
(54). Thus, to understand the
probable mechanism underlying the observed effects of callistephin,
its effect on the expression of proteins associated with cell
growth signaling was evaluated (Fig.
7). The western blot analysis revealed that challenging C8-B4
cells with LPS/IFN-γ slightly downregulated the level of total p38,
which was further recovered following treatment with isoflurane,
callistephin or with their combination. The levels of
phosphorylated p38 were upregulated in LPS/IFN-γ-challenged C8-B4
cells compared with the control group. This upregulation was
decreased following treatment with isoflurane or callistephin. The
combination of callistephin and isoflurane may have further reduced
the challenge-induced increase in phosphorylation levels of
p38.
The relative levels of apoptosis-associated proteins
Bcl-xL and PARP were further evaluated. The results (Fig. 7) suggested that
LPS/IFN-γ-challenged C8-B4 cells may have exhibited reduced levels
of Bcl-xL. This was further confirmed by analyzing PARP, as the
presence of its cleaved form (89 kDa fragment) confirmed higher
caspase activity following LPS/IFN-γ-challenge. Isoflurane or
callistephin treatment may have elevated the levels of Bcl-xL and
the activity of caspase-3 in LPS/IFN-γ-challenged C8-B4 cells.
Likewise, compared with isoflurane, callistephin further reduced
the level of cleaved-PARP, elevated following LPS/IFN-γ challenge.
Furthermore, the combination of callistephin may have prevented
alterations to the levels of both proteins compared with the
control. These results suggest that LPS/IFN-γ-challenged microglial
cells may have undergone inflammatory apoptotic cell death via p38
phosphorylation, and that the combination of callistephin and
isoflurane may have prevented this apoptotic effect.
Discussion
Anthocyanins are a unique family of compounds with
natural antioxidant properties that have attracted considerable
attention for their therapeutic potential (24–30).
Various types of berries, including blueberries, cranberries,
chokeberries and lingonberries, contain a variety of polyphenolics
with protective properties (24,32,33).
Anthocyanin rich fractions have been reported to reduce oxidative
stress, and to protect neurons from inflammatory response in the
CNS (30). Anthocyanins may
protect neurons from various damaging chemicals and processes,
including carbon tetrachloride, psychological stress, d-galactose,
accelerated senescence, ischemia/reperfusion or middle cerebral
artery occlusion (55). Isoflurane
is a volatile substance commonly used for neurosurgical procedures.
Isoflurane, at clinically relevant concentrations, has been
reported to reduce the inflammation, oxidative stress and NO
production in microglia following exposure to LPS and IFN-γ in
microglia (6,7). However, microglial overactivity may
have detrimental consequences (2–4), as
inflammation and phagocytosis may cause lasting harm to healthy
neural tissue.
Thus, the present study aimed to understand the
effects of isoflurane and callistephin following LPS/IFN-γ-induced
inflammatory responses in mouse C8-4B microglial cells, and
assessed the impact of callistephin in combination with isoflurane,
a previously reported neuroprotective anesthetic agent (17–19).
The results indicated that exposure to LPS/IFN-γ increased
microglial engulfment, which is required for the removal of cell
debris or damaging agents from the neural tissue (9). LPS/IFN-γ induced a strong immune
response in microglia, which was verified by the increase in the
expression inflammatory mediators like iNOS, TNF-α and COX-2,
typical inflammation markers (47–49).
The present results further suggested that treatment with
isoflurane callistephin may have reduced microglial apoptosis by
reducing p38 phosphorylation. Callistephin and isoflurane may have
also reduced microglial engulfment responses and oxidative stress
following an immune challenge. Notably, it is possible that
callistephin may have enhanced the protective effects of isoflurane
by suppressing inflammation and the consequent production of ROS
and RNS.
The possible beneficial health effects of
anthocyanin may be due to its metabolites, phenolic acids and
poly-galacturonic acid, which have been shown to exhibit
neuroprotective capabilities as they may regulate the levels of
mediators of oxidative stress (44,45).
Callistephin and kuromanin, anthocyanins derived from strawberries
and black rice, were reported to exhibit neuroprotective effects
against mitochondrial oxidative stress-induced death of cultured
cerebellar granule neurons by reducing B-cell lymphoma-2 expression
and reducing mitochondrial glutathione, and were also reported to
reduce nitrosative stress (56,57).
Two common phenolic acids, 4-hydroxybenzoic acid and protocatechuic
acid, were observed to mitigate oxidative stress induced by
hydrogen peroxide and may have suppressed neuronal cell death in
cerebellar granule neurons (58).
These reports seem to suggest that anthocyanins, including
callistephin, may have neuroprotective and anti-inflammatory
characteristics that may be of use in the treatment of certain
aspects of neurodegenerative diseases.
Inflammatory immune responses in the CNS are
primarily generated by microglia activation (4–6). The
common markers of inflammatory responses in microglia include
enhanced production of proinflammatory cytokines, including TNF-α
(50), induction of
proinflammatory proteins, including COX-2 (50) and promoting the activity of iNOS,
which leads to the production of NO (52). In addition to oxidative stress,
nitrosative stress, caused by the generation and accumulation of
toxic RNS like NO and peroxynitrite, may damage various vital
cellular components. The present study demonstrated that
LPS/IFN-γ-challenged microglia overproduced ROS and NO, which may
have potentiated apoptosis. The ROS generated by the immune
challenge were subsequently suppressed by independent treatment
with isoflurane and callistephin, while the combination of both
caused a synergistic suppression of ROS generation. Similarly,
LPS/IFN-γ exposure was also observed to increase NO, and treatment
with isoflurane and callistephin reduced these levels, while the
co-treatment further reduced these NO levels. This may have been
achieved via reducing/suppressing the expression of TNF-α iNOS,
COX-2 and NF-κB, which were seemingly overexpressed following
exposure to LPS/IFN-γ. Notably, the combination of callistephin
with isoflurane caused the greatest reduction in the expression
levels of these inflammation indicators. In addition, protein
levels were also suggestive of the same results.
LPS and IFN-γ are associated with injury in the CNS,
and therefore promote microglial engulfment, phagocytosis and
systemic inflammation (50–52).
Injuries to the CNS cause activation of microglial cells, which
migrate towards the injury site and eliminate decaying cells and
other debris (59). Among various
molecular mechanisms, MAPK p38 is an intracellular signaling
pathway involved in cellular stress and inflammation responses
(54). LPS is known to activate
p38 by binding to Toll-like receptors, which are a class of
pattern-recognition receptors in the innate immune system that
induce inflammatory responses (54). A report has also indicated that p38
is involved in LPS-induced microglial phagocytosis of axonal debris
(60). The present study further
suggested that individual treatment with isoflurane and
callistephin may have suppressed the phosphorylation of p38 caused
by LPS/IFN-γ exposure. Furthermore, these treatments may have also
reduced capspase-3 activation and decreased the expression of the
apoptosis markers Bcl-xL and cleaved PARP. Just as for the
inflammation markers, callistephin may have also enhanced the
protective effects of isoflurane by synergistically modulating the
protein levels of these apoptosis associated proteins and the
phosphorylation levels of p38. Therefore, the modulation of p38
phosphorylation may be the underlying mechanism by which
callistephin and isoflurane exert neuroprotective effects.
As discussed, isoflurane has been reported to have
numerous neuroprotective effects: The reduction of neuronal
inflammation and apoptosis (6,7);
suppression of neonatal hypoxia-ischemia induced neuronal injury
(15); suppression of oxidative
stress; and reduction of the levels of pro-inflammatory mediators
(19–21). Phytochemicals, namely callistephin,
have also been reported to exhibit anti-inflammatory, antioxidant,
antiproliferative and antitumoral properties (24–30),
and also to ameliorate certain aspects of neurodegenerative
diseases (34–39). The present study suggested that the
combined use of isoflurane and callistephin may therefore further
help prevent neuronal apoptosis and degeneration.
In conclusion, the present study suggested that
callistephin may have maintained the viability of C8-B4 cells
against an inflammatory shock through downregulation of caspase-3/7
activity. These results further support previous studies reporting
on the neuroprotective effects of phenols by reducing oxidative
stress-induced cell death in neuronal cells (24–30).
The observations from gene and protein expression analyses
indicated that callistephin, like isoflurane, may have interacted
with signaling pathways and regulated gene expression and protein
levels. Moreover, the present study also revealed that callistephin
may have strengthened the neuroprotective effects of isoflurane in
maintaining the viability of microglia and reducing inflammatory
responses. Finally, single treatment or co-treatment with
callistephin and isoflurane may have attenuated the immune response
and improved cell viability via phosphorylation of p38. Overall the
present study suggested that the use of callistephin may be of help
in the treatment of aspects of neurodegenerative diseases
associated with microglial activation.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
LZ conceived and supervised the present study,
analyzed data and wrote the manuscript. SC, TL and XW performed
experiments, analyzed data and assisted in the preparation of the
manuscript. HH and WL analyzed data, performed literature search
and assisted in the preparation of the manuscript. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hanisch UK and Kettenmann H: Microglia:
Active sensor and versatile effector cells in the normal and
pathologic brain. Nat Neurosci. 10:1387–1394. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Butovsky O, Landa G, Kunis G, Ziv Y,
Avidan H, Greenberg N, Schwartz A, Smirnov I, Pollack A, Jung S and
Schwartz M: Induction and blockage of oligodendrogenesis by
differently activated microglia in an animal model of multiple
sclerosis. J Clin Invest. 116:905–915. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Poon IK, Hulett MD and Parish CR:
Molecular mechanisms of late apoptotic/necrotic cell clearance.
Cell Death Differ. 17:381–397. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lucin KM and Wyss-Coray T: Immune
activation in brain aging and neurodegeneration: Too much or too
little? Neuron. 64:110–122. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sierra A, Abiega O, Shahraz A and Neumann
H: Janus-faced microglia: Beneficial and detrimental consequences
of microglial phagocytosis. Front Cell Neurosci. 7:62013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim JA, Li L and Zuo Z: Delayed treatment
with isoflurane attenuates lipopolysaccharide and interferon
gamma-induced activation and injury of mouse microglial cells.
Anesthesiology. 111:566–573. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu X, Kim JA and Zuo Z: Isoflurane
preconditioning reduces mouse microglial activation and injury
induced by lipopolysaccharide and interferon-gamma. Neuroscience.
154:1002–1008. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hara M, Kai Y and Ikemoto Y: Propofol
activates GABAA receptor-chloride ionophore complex in dissociated
hippocampal pyramidal neurons of the rat. Anesthesiology.
79:781–788. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kochs E, Hoffman WE, Werner C, Thomas C,
Albrecht RF and Schulte am Esch J: The effects of propofol on brain
electrical activity, neurologic outcome, and neuronal damage
following incomplete ischemia in rats. Anesthesiology. 76:245–252.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hans P, Bonhomme V, Collette J, Albert A
and Moonen G: Propofol protects cultured rat hippocampal neurons
against N-methyl-D-aspartate receptor-mediated glutamate toxicity.
J Neurosurg Anesthesiol. 6:249–253. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Daskalopoulos R, Korcok J, Farhangkhgoee
P, Karmazyn M, Gelb AW and Wilson JX: Propofol protection of
sodium-hydrogen exchange activity sustains glutamate uptake during
oxidative stress. Anesth Analg. 93:1199–1204. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Grasshoff C and Gillessen T: The effect of
propofol on increased superoxide concentration in cultured rat
cerebrocortical neurons after stimulation of N-methyl-d-aspartate
receptors. Anesth Analg. 95:920–922. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
O'Shea SM, Wong LC and Harrison NL:
Propofol increases agonist efficacy at the GABA(A) receptor. Brain
Res. 852:344–348. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yano T, Nakayama R and Ushijima K:
Intracerebroventricular propofol is neuroprotective against
transient global ischemia in rats: Extracellular glutamate level is
not a major determinant. Brain Res. 883:69–76. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Burchell SR, Dixon BJ, Tang J and Zhang
JH: Isoflurane provides neuroprotection in neonatal hypoxic
ischemic brain injury. J Investig Med. 61:1078–1083. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Khan KS, Hayes I and Buggy DJ:
Pharmacology of anaesthetic agents II: Inhalation anaesthetic
agents. Cont Edu Anaes Crit Care Pain. 14:601–611. 2014.
|
|
17
|
Zhou Y, Lekic T, Fathali N, Ostrowski RP,
Martin RD, Tang J and Zhang JH: Isoflurane posttreatment reduces
neonatal hypoxic-ischemic brain injury in rats by the
sphingosine-1-phosphate/phosphatidylinositol-3-kinase/Akt pathway.
Stroke. 41:1521–1527. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao DA, Bi LY, Huang Q, Zhang FM and Han
ZM: Isoflurane provides neuroprotection in neonatal hypoxic
ischemic brain injury by suppressing apoptosis. Braz J Anesthesiol.
66:613–621. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chung IS, Kim JA, Kim JA, Choi HS, Lee JJ,
Yang M, Ahn HJ and Lee SM: Reactive oxygen species by isoflurane
mediates inhibition of nuclear factor κB activation in
lipopolysaccharide-induced acute inflammation of the lung. Anesth
Analg. 116:327–335. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Harr JN, Moore EE, Stringham J, Wohlauer
MV, Fragoso M, Jones WL, Gamboni F, Silliman CC and Banerjee A:
Isoflurane prevents acute lung injury through ADP-mediated platelet
inhibition. Surgery. 152:270–276. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kinoshita H, Matsuda N, Iranami H, Ogawa
K, Hatakeyama N, Azma T, Kawahito S and Yamazaki M: Isoflurane
pretreatment preserves adenosine triphosphate-sensitive K(+)
channel function in the human artery exposed to oxidative stress
caused by high glucose levels. Anesth Analg. 115:54–61. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim M, Kim M, Kim N, D'Agati VD, Emala CW
Sr and Lee HT: Isoflurane mediates protection from renal
ischemia-reperfusion injury via sphingosine kinase and
sphingosine-1-phosphate-dependent pathways. Am J Physiol Renal
Physiol. 293:F1827–F1835. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lang XE, Wang X, Zhang KR, Lv JY, Jin JH
and Li QS: Isoflurane preconditioning confers cardioprotection by
activation of ALDH2. PLoS One. 8:e524692013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Moyer RA, Hummer KE, Finn CE, Frei B and
Wrolstad RE: Anthocyanins, phenolics, and antioxidant capacity in
diverse small fruits: Vaccinium, rubus, and ribes. J Agric Food
Chem. 50:519–525. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mishra SK and Kim MK: Vitamin A and cancer
risk. Vitamin A and Carotenoids: Chemistry, Analysis, Function and
Effects. The Royal Society of Chemistry; London: pp. 485–500. 2012,
View Article : Google Scholar
|
|
26
|
Yeh CT and Yen GC: Induction of apoptosis
by the Anthocyanidins through regulation of Bcl-2 gene and
activation of c-Jun N-terminal kinase cascade in hepatoma cells. J
Agric Food Chem. 53:1740–1749. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hou DX: Potential mechanisms of cancer
chemoprevention by anthocyanins. Curr Mol Med. 3:149–159. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Domitrovic R: The molecular basis for the
pharmacological activity of anthocyans. Curr Med Chem.
18:4454–4469. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang CJ, Wang JM, Lin WL, Chu CY, Chou FP
and Tseng TH: Protective effect of Hibiscus anthocyanins against
tert-butyl hydroperoxide-induced hepatic toxicity in rats. Food
Chem Toxicol. 38:411–416. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ramirez-Tortosa C, Andersen ØM, Cabrita L,
Gardner PT, Morrice PC, Wood SG, Duthie SJ, Collins AR and Duthie
GG: Anthocyanin-rich extract decreases indices of lipid
peroxidation and DNA damage in vitamin E-depleted rats. Free Radic
Biol Med. 31:1033–1037. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tsuda T, Horio F and Osawa T: Absorption
and metabolism of cyanidin 3-O-beta-D-glucoside in rats. FEBS Lett.
449:179–182. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Youdim KA, Martin A and Joseph JA:
Incorporation of the elderberry anthocyanins by endothelial cells
increases protection against oxidative stress. Free Radic Biol Med.
29:51–60. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tsuda T, Horio F and Osawa T: The role of
anthocyanins as an antioxidant under oxidative stress in rats.
Biofactors. 13:133–139. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lin MT and Beal MF: Mitochondrial
dysfunction and oxidative stress in neurodegenerative diseases.
Nature. 443:787–795. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Turner C and Schapira AH: Mitochondrial
dysfunction in neurodegenerative disorders and ageing. Adv Exp Med
Biol. 487:229–251. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Heo HJ and Lee CY: Strawberry and its
anthocyanins reduce oxidative stress-induced apoptosis in PC12
cells. J Agric Food Chem. 53:1984–1989. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tarozzi A, Merlicco A, Morroni F, Franco
F, Cantelli-Forti G, Teti G, Falconi M and Hrelia P: Cyanidin
3-O-glucopyranoside protects and rescues SH-SY5Y cells against
amyloid-beta peptide-induced toxicity. Neuroreport. 19:1483–1486.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tarozzi A, Morroni F, Hrelia S, Angeloni
C, Marchesi A, Cantelli-Forti G and Hrelia P: Neuroprotective
effects of anthocyanins and their in vivo metabolites in SH-SY5Y
cells. Neurosci Lett. 424:36–40. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Matsunaga N, Imai S, Inokuchi Y, Shimazawa
M, Yokota S, Araki Y and Hara H: Bilberry and its main constituents
have neuroprotective effects against retinal neuronal damage in
vitro and in vivo. Mol Nutr Food Res. 53:869–877. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rice-Evans CA, Miller NJ and Paganga G:
Structure-antioxidant activity relationships of flavonoids and
phenolic acids. Free Radic Biol Med. 20:933–956. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ma H, Johnson SL, Liu W, DaSilva NA,
Meschwitz S, Dain JA and Seeram NP: Evaluation of polyphenol
anthocyanin-enriched extracts of blackberry, black raspberry,
blueberry, cranberry, red raspberry, and strawberry for free
radical scavenging, reactive carbonyl species trapping,
anti-glycation, anti-β-amyloid aggregation, and microglial
neuroprotective effects. Int J Mol Sci. 19:E4612018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Rahman MM, Ichiyanagi T, Komiyama T,
Hatano Y and Konishi T: Superoxide radical- and
peroxynitrite-scavenging activity of anthocyanins;
structure-activity relationship and their synergism. Free Radic
Res. 40:993–1002. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Reimer TA, Anagnostopoulos I, Erdmann B,
Lehmann I, Stein H, Daniel P, Dörken B and Rehm A: Reevaluation of
the 22-1-1 antibody and its putative antigen, EBAG9/RCAS1, as a
tumor marker. BMC Cancer. 5:472005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ryu JH, Wang Z, Fan D, Han SH, Do SH and
Zuo Z: Isoflurane attenuates mouse microglial engulfment induced by
lipopolysaccharide and interferon-gamma possibly by inhibition of
p38 mitogen-activated protein kinase. Neuroreport. 27:1101–1105.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Fleschhut J, Kratzer F, Rechkemmer G and
Kulling SE: Stability and biotransformation of variousdietary
anthocyanins in vitro. Eur J Nutr. 45:7–18. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Woodward G, Kroon P, Cassidy A and Kay C:
Anthocyanin stability and recovery: Implications for the analysis
of clinical and experimental samples. J Agric Food Chem.
57:5271–5278. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Carreras MC, Franco MC, Peralta JG and
Poderoso JJ: Nitric oxide, complex I, and the modulation of
mitochondrial reactive species in biology and disease. Mol Aspects
Med. 25:125–139. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chinta SJ and Andersen JK: Nitrosylation
and nitration of mitochondrial complex I in Parkinson's disease.
Free Radic Res. 45:53–58. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Di Filippo M, Chiasserini D, Tozzi A,
Picconi B and Calabresi P: Mitochondria and the link between
neuroinflammation and neurodegeneration. J Alzheimers Dis. 20
(Suppl 2):S369–S379. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Hensley K, Fedynyshyn J, Ferrell S, Floyd
RA, Gordon B, Grammas P, Hamdheydari L, Mhatre M, Mou S, Pye QN, et
al: Message and protein-level elevation of tumor necrosis factor
alpha (TNF alpha) and TNF alpha-modulating cytokines in spinal
cords of the G93A-SOD1 mouse model for amyotrophic lateral
sclerosis. Neurobiol Dis. 14:74–80. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Almer G, Guégan C, Teismann P, Naini A,
Rosoklija G, Hays AP, Chen C and Przedborski S: Increased
expression of the pro-inflammatory enzyme cyclooxygenase-2 in
amyotrophic lateral sclerosis. Ann Neurol. 49:176–185. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Almer G, Vukosavic S, Romero N and
Przedborski S: Inducible nitric oxide synthase up-regulation in a
transgenic mouse model of familial amyotrophic lateral sclerosis. J
Neurochem. 72:2415–2425. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Brown GC and Neher JJ: Inflammatory
neurodegeneration and mechanisms of microglial killing of neurons.
Mol Neurobiol. 41:242–247. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Doyle SE, O'Connell RM, Miranda GA, Vaidya
SA, Chow EK, Liu PT, Suzuki S, Suzuki N, Modlin RL, Yeh WC, et al:
Toll-like receptors induce a phagocytic gene program through p38. J
Exp Med. 199:81–90. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Shin WH, Park SJ and Kim EJ: Protective
effect of anthocyanins in middle cerebral artery occlusion and
reperfusion model of cerebral ischemia in rats. Life Sci.
79:130–137. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Kelsey N, Hulick W, Winter A, Ross E and
Linseman D: Neuroprotective effects of anthocyanins on apoptosis
induced by mitochondrial oxidative stress. Nutr Neurosci.
14:249–259. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Winter AN, Ross EK, Khatter S, Miller K
and Linseman DA: Chemical basis for the disparate neuroprotective
effects of the anthocyanins, callistephin and kuromanin, against
nitrosative stress. Free Radic Biol Med. 103:23–34. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Winter AN, Brenner MC, Punessen N,
Snodgrass M, Byars C, Arora Y and Linseman DA: Comparison of the
neuroprotective and anti-inflammatory effects of the anthocyanin
metabolites, protocatechuic acid and 4-hydroxybenzoic acid. Oxid
Med Cell Longev. 2017:62970802017. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Brown GC and Neher JJ: Microglial
phagocytosis of live neurons. Nat Rev Neurosci. 15:209–216. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Tanaka T, Ueno M and Yamashita T:
Engulfment of axon debris by microglia requires p38 MAPK activity.
J Biol Chem. 284:21626–21636. 2009. View Article : Google Scholar : PubMed/NCBI
|