Introduction
Lipopolysaccharide (LPS) is a main ingredient of the
outer membrane of Gram-negative bacteria. It is one of the major
factors that induce inflammation (1). In previous studies, LPS has been
revealed to stimulate a wide variety of cells (including
osteoblasts, intestinal epithelial cells, and vascular endothelial
cells) and activate a series of signaling pathways with
pathological consequences (2–4). In
cell and animal models, LPS can induce inflammatory response by
stimulating cells to produce inflammatory factors [interleukin
(IL)-2, IL-6, IL-8, tumor necrosis factor (TNF)-α], cell adhesion
molecules [intercellular adhesion molecule 1 (ICAM-1), vascular
cell adhesion molecule 1 (VCAM-1)] and reactive oxygen species
(ROS) through the nuclear factor-kappa B (NF-κB) signaling pathway
(5–9). Vascular endothelial injury is
associated with excessive secretion of inflammatory mediators under
LPS stimulation (10). Therefore,
inhibiting the expression of inflammatory mediators in vascular
endothelial cells can alleviate the extent of damage. Studies have
revealed that pharmacological inhibition of cytokine overproduction
is a useful strategy to control vascular inflammation (11,12).
Moreover, use of drugs to protect vascular endothelial cells and
endothelial cell-cell recanalization also promotes the suppression
of inflammation (13). Therefore,
protection of vascular endothelial cells is an effective strategy
for the prevention and treatment of inflammatory diseases.
N-acetylcysteine (NAC) is a prerequisite for the
synthesis of glutathione (GSH) in the body (14). NAC is commonly used as an
antioxidant and free-radical scavenger, which also detoxifies
active neutrophils and free radicals (by binding or reduction) and
reduces the ROS activity, thereby protecting cells against
oxidative damage (15,16). NAC has been used to treat a wide
range of diseases such as chronic bronchitis, ulcerative colitis,
liver cancer, and asthma. It is a safe and well tolerated
supplement with no obvious side effects (17). NAC was revealed to downregulate the
expression of LPS-mediated pro-inflammatory factors and attenuate
LPS-induced inflammation in osteoblasts, macrophage, neutrophils
and animal models (2,18–21).
In addition, NAC inhibited the activation of NF-κB signaling
pathway induced by LPS and TNF-α (22,23).
However, the protective effect of NAC on LPS-mediated human
umbilical vein endothelial cells (HUVECs) and whether its mechanism
is related to the NF-κB pathway has been rarely reported.
In the present study, the protective effect of NAC
on LPS-mediated HUVECs was assessed by determining the secretions
of four factors, i.e., IL-8, TNF-α, ICAM-1, and nitric oxide (NO).
Furthermore, it was assessed whether the protective effect of NAC
on HUVECs is related to the NF-κB signaling pathway.
Characterization of the effects of NAC on vascular endothelial
cells and identification of the underlying protective mechanisms
may widen the application of NAC, such as for treatment of
periodontitis and peri-implantitis. Clinically, it is our hope that
this research will provide a theoretical foundation to further the
prevention and treatment of inflammatory diseases.
Materials and methods
Instruments and chemical reagents
NAC and Escherichia coli LPS were obtained
from Sigma-Aldrich; Merck KGaA. BAY11-7082 was obtained from Biomol
GmbH. Cell Counting Kit-8 (CCK-8) reagent was purchased from
Dojindo Molecular Technologies. IL-8 and TNF-α ELISA kits were
manufactured by BioLegend, Inc. Antibodies against ICAM-1 (cat. no.
ab53013), NF-κB (p65; cat. no. ab32536) and phosphorylated NF-κB
(p-p65; cat. no. ab76302) were obtained from Cell Signaling
Technology, Inc. NO and inducible nitric oxide synthase (iNOS)
assay kits were purchased from the Nanjing Institute of Jiancheng
Bioengineering. Dulbecco's Modified Eagle's Medium (DMEM) and fetal
bovine serum (FBS) were purchased from HyClone; GE Healthcare Life
Sciences. All other supplies used in the experiments were reagent
grade.
Cell culture and preparation of NAC,
LPS, and BAY11-7082
HUVECs were purchased from the American Type Culture
Collection. These were cultured in high glucose DMEM with the
addition of 10% FBS, 100 mg/ml streptomycin, and 100 units/ml
penicillin in a 5% CO2 environment at 37°C. The entire
medium was replaced once every 48 h. HUVECs were used in cell
culture at passages 2–5 as previously described (1). NAC and LPS were dissolved in pure
medium and BAY11-7082 was dissolved in dimethyl sulfoxide (DMSO,
200 µl).
The experiment consisted of seven groups: The
control group, the LPS group (only LPS), the NAC group (only NAC),
the LPS+NAC group (cells pre-treated with NAC for 1 h before the
addition of LPS, L+N), the BAY11-7082 group (only BAY11-7082; 5
µmol/l) (24), the LPS+BAY11-7082
group (cells pretreated with BAY11-7082 for 1 h before the addition
of LPS, L+B), the DMSO group (the final concentration was 0.05%).
The groups are presented in Table
I.
 | Table I.The seven experimental groups. |
Table I.
The seven experimental groups.
| Groups | Control | LPS | NAC | BAY11-7082 | LPS+NAC | LPS+BAY11-7082 | DMSO |
|---|
| LPS | − | + | − | − | + | + | − |
| NAC | − | − | + | − | + | − | − |
| BAY11-7082 | − | − | − | + | − | + | − |
| DMSO | − | − | − | − | − | − | + |
Cell viability assay and cell
morphology observation
The effect of NAC on cell viability in the HUVECs
model was first investigated by CCK-8 assay. In this process,
HUVECs were treated with different concentrations of NAC (0.1,
0.25, 0.5, 1, 5 and 10 mM) for 24 h. Subsequently, the effect of
NAC was evaluated on LPS-induced inflammation of HUVECs by
pre-treatment of cells with different concentrations of NAC (0.1,
0.25, 0.5, 1, 5 mM) for 1 h before LPS (100 ng/ml) treatment of
cells. HUVECs (density 1×105/ml) were cultivated in
96-well plates at 37°C in 5% CO2 for 24 h followed by
their incubation with CCK-8 reagent for 2 h. An MCC 340 microplate
reader (Thermo Fisher Scientific, Inc.), wavelength set at 450 nm,
was used to detect the absorbance value representing cell
proliferation. In the seven treatment groups, the morphology of
HUVECs was observed using a phase contrast microscope. The
experiments were repeated three times.
Real time semi-quantitative polymerase
chain reaction (RT-qPCR) for mRNA expression of TNF-α, IL-8, iNOS,
and ICAM-1
HUVECs were seeded in 6-well plates, treated with
drugs corresponding to their group for 24 h. Total RNA was
extracted from HUVECs using TRIzol reagent (Invitrogen Life
Technologies; Thermo Fisher Scientific, Inc.) according to the
manufacturer's recommendations. The cDNA was then reverse
transcribed from total RNA using the PrimeScript™ RT reagent kit
(Takara Bio, Inc.) and maintained at 42.8°C for 1 h under the use
of oligo (dT) primers. The prepared cDNA was subjected to PCR
amplification according to the manufacturer's instructions. The ABI
Prism 7300 Sequence Detection PCR System (Applied Biosystems;
Thermo Fisher Scientific, Inc.) was used to monitor the
fluorescence signal intensity during PCR reaction in real time. The
thermocycling conditions were as follows: Initial denaturation at
95°C for 5 min, followed by 40 cycles of 95°C for 10 sec and 60°C
for 30 sec, with a final extension at 75°C for 10 min. The
following primer sequences were used: IL-8 forward,
5′-AACTGAGAGTGATTGAGAGT-3′ and reverse, 5′-ATGAATTCTCAGCCCTCTTC-3′;
TNF-α forward, 5′-AGCTGGTGGTGCCATCAGAG-3′ and reverse,
5′-TGGTAGGAGACGGCGATGCG-3′; iNOS forward,
5′-AGGCCACCTCGGATATCTCT-3′ and reverse, 5′-GCTTGTCTCTGGGTCCTCTG-3′;
ICAM-1 forward, 5′-CGAAGGTTCTTCTGAGC-3′ and reverse,
5′-TCTGCTGAGACCCCTCTTG-3′. The housekeeping gene
glyceraldehyde-phosphate dehydrogenase (GAPDH) served as the
control.
Enzyme-linked immunosorbent assay
(ELISA) for protein expression of TNF-α and IL-8
HUVECs (1.0×105 cells/well) were seeded
into a 24-well plate, randomly divided into seven groups and
treated with the corresponding agents (Control group, LPS group,
NAC group, LPS+NAC group, BAY group, LPS+NAY group, DMSO group) and
cultured for 24 h. The supernatant was collected to detect TNF-α
and IL-8 protein concentrations using ELISA kits (BioLegend, Inc.)
according to the manufacturer's recommended protocol. All
experiments were conducted in triplicate.
Assessment of NO and iNOS
The activity of iNOS in HUVECs was detected using
the nitric reductase method. After treatment of cells for 24 h (as
described in the section Real time semi-quantitative polymerase
chain reaction (RT-qPCR) for mRNA expression of TNF-α, IL-8, iNOS,
and ICAM-1), these were digested with 0.25% trypsin and stopped
with 10% FBS high glucose DMEM medium. Cell pellets were obtained
after centrifugation for 5 min at 200 × g. The cells were rinsed
with an appropriate amount of Hank's solution; subsequently, these
were centrifuged again for 5 min at 200 × g, and the supernatant
was removed to obtain cell pellets. The washing steps were repeated
twice. Then 500 µl Hank's solution was added to each group of
cells, mixed well, and pulverized ultrasonically. The supernatant
was obtained for protein concentration measurement and iNOS
detection. After cell treatment for 24 h, the media supernatant was
collected from each group, followed by the addition of reagents 1
and 2, according to the manufacturer's recommended protocol for the
NO assay kit. Finally, the microplate reader, wavelength set at 550
nm, was used to detect the absorbance values after mixing. All
experiments were repeated three times.
Western blot analysis for NF-κB p65,
phosphorylated p65, ICAM-1 protein expression
After seven groups of cells were treated with the
corresponding drugs [detection of NF-κB protein expression after
drug treatment for 2 h (25);
detection of ICAM-1 protein expression after drug treatment for 24
h], the cells were rinsed with an appropriate amount of
phosphate-buffered saline (PBS). Then 200 µl lysate was added to
each group of cells, mixed well, and pulverized ultrasonically for
5 min. Then the samples were lysed on ice at 4°C for 20 min,
followed by centrifugation for 15 min at 12,000 × g. Then, the
protein expression in the supernatant was determined using the
Bicinchoninic Acid (BCA) method. Depending on the sample loading,
5X loading buffer was added and boiled for 10 min. Denatured
protein sample (80 µg) was electrophoresed at 100 V [100 mg/l
sodium dodecyl sulfate-polyacrylamide (SDS-PAGE)] and transferred
to a polyvinylidene fluoride (PVDF) film (350 mA, 2 h). Next 50 g/l
bovine serum albumin (BSA) was added at normal temperature for 2 h
and incubated overnight with p65 (cat. no. ab32536; 1:1,000
dilution; Abcam), phosphorylated p65 (cat. no. ab76302; 1:1,000
dilution; Abcam), ICAM-1 (cat. no. ab53013; 1:2,000 dilution;
Abcam), and β-actin (cat. no. bs-0061R; 1:1,000 dilution; Beijing
Boaosen Biological Co., Ltd.) at 4°C. On the subsequent day, the
solution was thoroughly washed with TBST. Next, the horseradish
peroxidase-conjugated goat anti-rabbit IgG secondary antibody (cat.
no. bs-0295G-HRP; 1:3,000 dilution; Beijing Boaosen Biological Co.,
Ltd.) was added with the solution and incubated together for 2 h at
room temperature, which was added with TBST to wash again. Then the
developer was added for development, and the exposed negative was
developed, fixed, baked and photographed in a dark room. The gray
values of the NF-κB pathway protein, the ICAM-1 protein, and the
internal reference protein β-actin expression band were analyzed
using the Quantity One gel imaging scanning system. Representation
of the NF-κB signaling pathway protein expression level was the
gray scale ratio of phosphorylated p65 and p65; the protein
expression level of ICAM-1 is represented by the gray scale ratio
of ICAM-1 and the internal reference β-actin.
Statistical analysis
Experimental data are expressed as the mean ±
standard deviation (SD) values from three experiments. One-way
ANOVA followed by LSD post-hoc tests were used to assess
between-group differences; P-values <0.05 were considered
indicative of statistical significance.
Results
Effects of different concentrations of
NAC on HUVECs and the morphology of HUVECs in all seven treatment
groups
When the cells were treated with different
concentrations of NAC (0.1, 0.25, 0.5, 1, 5 and 10 mM), there was
no significant change in cell viability up to 5 mM relative to the
control. However, cytoxicity was observed at NAC concentration of
10 mM (Fig. 1A). In the
experiments to assess the effects of NAC on LPS-mediated
inflammation, the LPS group (1 µg/ml) exhibited significantly
reduced cell viability compared with the control group. However,
pretreatment with NAC (0.1, 0.25, 0.5, 1, 5 mM) significantly
alleviated the LPS-induced inhibition of cell viability, especially
at NAC concentration of 1 mM (Fig.
1B). Therefore, this was selected as the optimal concentration
of NAC. When HUVECs were treated with the appropriate concentration
of the corresponding drug, the morphology of the seven groups of
HUVECs was not significantly altered under phase contrast
microscope (Fig. 1C).
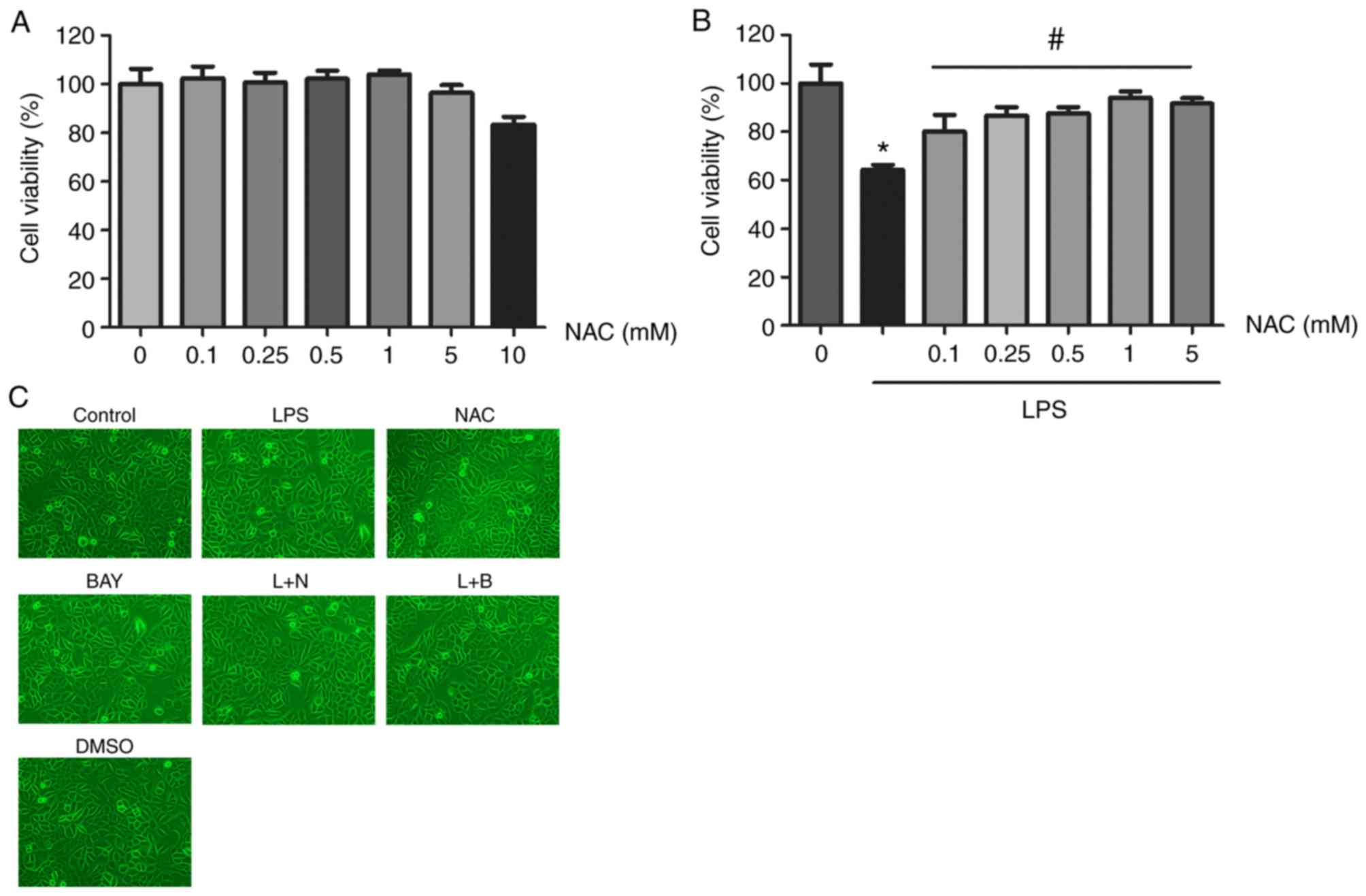 | Figure 1.Effects of NAC and/or LPS on
viability and toxicity of HUVECs. (A) Cell viability was measured
by the CCK-8 assay. HUVECs were incubated with gradient
concentrations of NAC (0.1, 0.25, 0.5, 1, 5, 10 mmol/l) for 24 h.
(B) LPS inhibited the viability of HUVECs, but treatment with NAC
at concentrations (0.1, 0.25, 0.5, 1, 5 mmol/l) restored cell
viability. Values were expressed as the mean ± SD of three
independent experiments. *P<0.05 vs. the control group;
#P<0.05 vs. the LPS group. (C) Morphology of HUVECs
with treatment under phase contrast microscope (magnification,
×200). NAC, N-acetylcysteine; LPS, lipopolysaccharide; HUVECs,
human umbilical vein epithelial cells. |
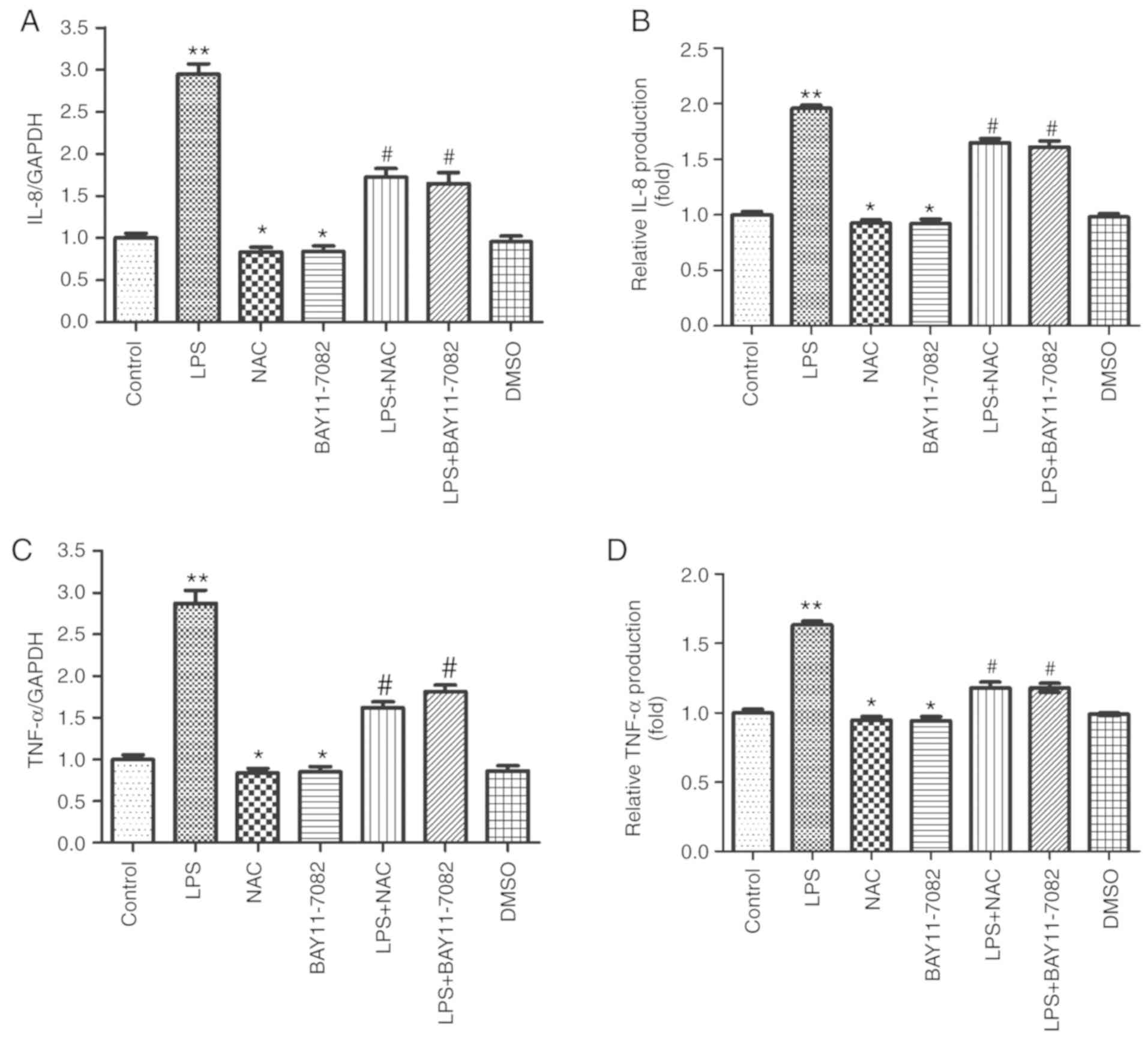
NAC attenuates LPS-induced production
of IL-8 and TNF-α in HUVECs
The mRNA and protein expression of IL-8 and TNF-α
were examined in LPS-stimulated HUVECs using RT-qPCR and ELISA. It
was revealed that 100 ng/ml of LPS enhanced the mRNA expression of
IL-8 (Fig. 2A) and TNF-α (Fig. 2C). However, pretreatment of cells
with 1 mM NAC inhibited the mRNA expression of IL-8 and TNF-α. The
effect of NAC was similar to that observed after pretreatment of
cells with BAY11-7082 (a specific NF-κB inhibitor). Moreover, a
similar phenomenon was observed with respect to the protein
expression of IL-8 and TNF-α (Fig. 2B
and D). This result indicated that 1 mM of NAC could
significantly reduce the production of inflammatory mediators (IL-8
and TNF-α) in LPS-stimulated HUVECs.
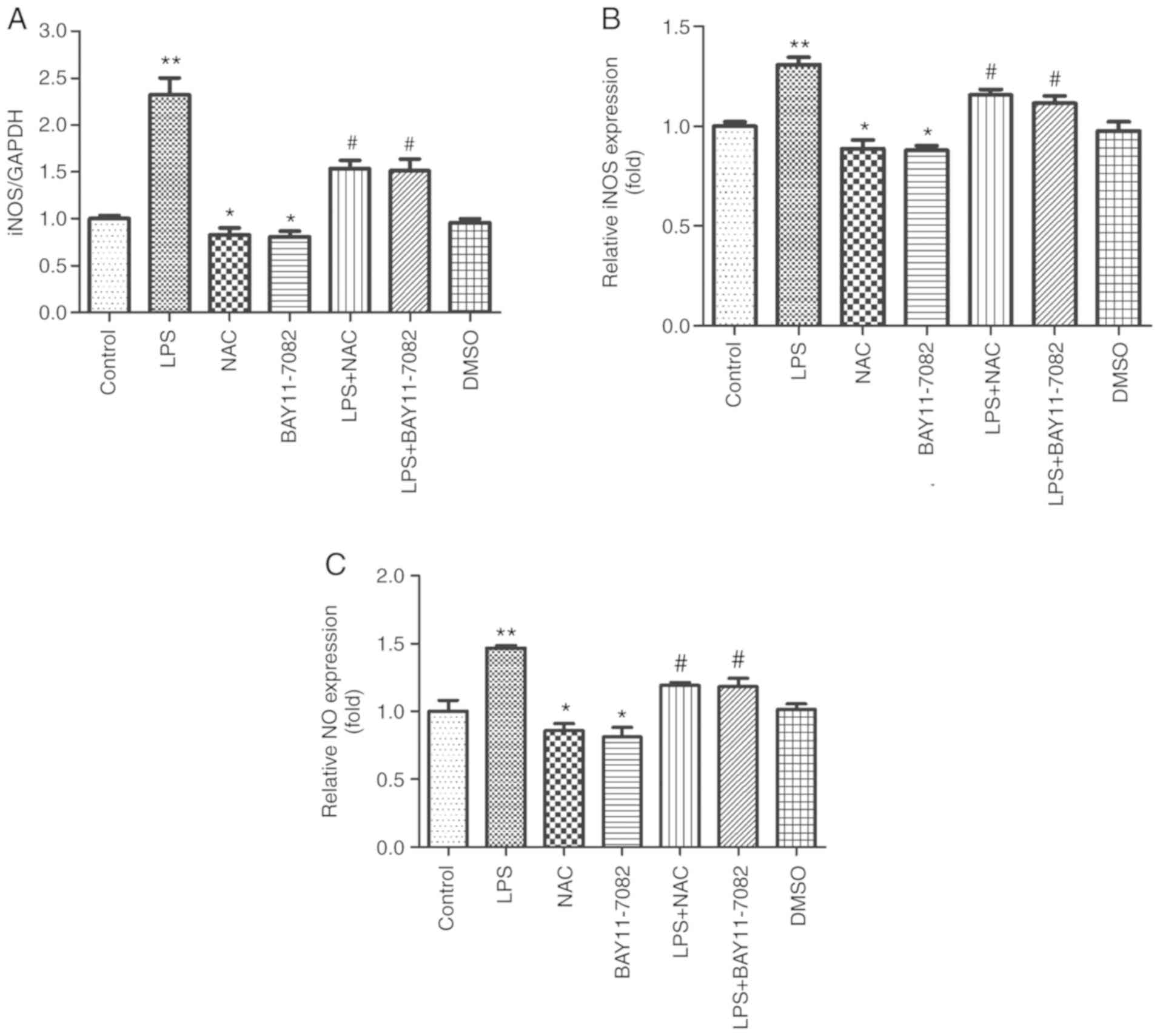
Effects of NAC on the production of NO
and iNOS in HUVECs stimulated by LPS
iNOS mRNA was detected by RT-qPCR and the NO content
and the viability of iNOS were determined in LPS-stimulated HUVECs
using the nitric reductase method. As revealed in Fig. 3A, it was revealed that LPS induced
an increase in the iNOS mRNA expression (P<0.01), while
pretreatment with NAC and BAY11-7082 significantly reduced the iNOS
mRNA expression. Fig. 3B and C
revealed that NAC significantly reduced the activity of iNOS and
the NO content (P<0.05). This indicated that NAC can decrease
the viability of iNOS and the production of NO in HUVECs stimulated
by LPS.
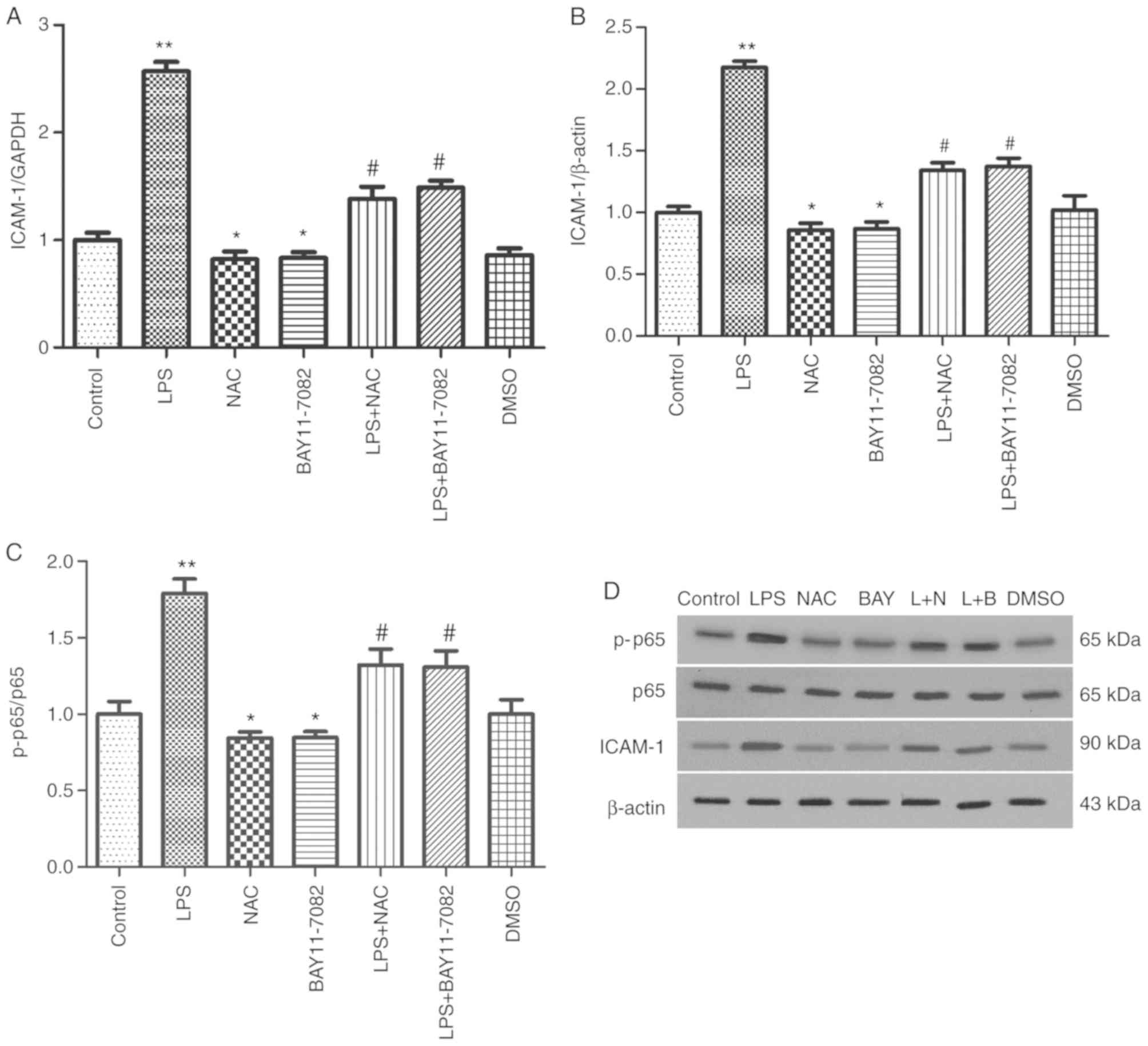
NAC decreases the expression of ICAM-1
and inhibits the activation of the NF-κB signaling pathway in
HUVECs stimulated by LPS
ICAM-1 is a major proinflammatory chemokine involved
in vascular response and migration of neutrophils to inflammatory
foci (10). RT-qPCR and western
blotting were performed to assess the effects of NAC on LPS-induced
expression of ICAM-1. LPS stimulation significantly increased the
mRNA expression of ICAM-1. However, pretreatment with NAC and
BAY11-7082 significantly attenuated the increase (P<0.05;
Fig. 4A). Consistent with the
results of RT-qPCR, western blot results revealed that LPS
stimulation significantly increased ICAM-1 secretion compared with
the control group (P<0.01; Fig. 4B
and D). However, pre-treatment of cells with NAC and BAY11-7082
significantly reduced the expression of ICAM-1 protein. These
results revealed that NAC significantly attenuated the LPS-induced
increased expression of ICAM-1 in HUVECs, which supported the
protective effect of NAC on HUVECs.
The protein expression levels of p65 and
phosphorylated p65, which represent excitation of the NF-κB
pathway, were examined by western blot analysis. The protein
expression of p-p65/p65 in the LPS group was significantly higher
than that in the control group (P<0.01). However, the expression
of p-p65/p65 appeared to decrease in the NAC and BAY11-7082 groups,
compared with the control group. Similarly, upon comparison of the
LPS group with the LPS+NAC and LPS+BAY11-7082 groups, the p-p65/p65
expression in the latter two groups was significantly lower
(P<0.05; Fig. 4C and D). These
findings indicated that LPS can activate the NF-κB signaling
pathway in HUVECs, whereas NAC restrained the LPS-mediated
activation of NF-κB. The effect of NAC was similar to that of
BAY-117082.
Discussion
The inflammatory process plays a part in gingivitis,
periodontitis and peri-implantitis (26–28),
which are characterized by cardinal signs of inflammation (redness,
swelling, heat, and pain). Thus, inflammation is mainly caused by
the reaction of the vascular system. The vascular endothelial cell
monolayer forms a selective semi-permeable barrier, which plays an
important role in regulating tissue fluid homeostasis and vascular
cell migration (29). In addition,
vascular endothelial cells have the ability to activate and
regulate inflammatory processes (30–32).
Several studies have revealed that LPS can induce vascular
endothelial cells to produce excess inflammatory factors (such as
IL-8 and TNF-α), increase the release of ICAM-1, and increase the
amount of NO and iNOS. All these changes may lead to endothelial
damage and hyperpermeability of the endothelial monolayer (33–37).
It has been reported in the literature (38,39)
that although monocytes and macrophages are the main source of
IL-8, neutrophils, endothelial cells and epithelial cells can also
be stimulated to produce IL-8. Such production is induced by
pro-inflammatory agents, such as other cytokines: IL-1, IL-17 and
TNF-α. IL-8 is an important mediator of inflammation and plays a
crucial role in the aggregation of monocytes at the site of
inflammation (35,40). TNF-α is an inflammatory mediator
that activates endothelial cells, increases secretion of other
inflammatory cytokines, and impairs vascular integrity (33). Adhesion molecules are the key to
leukocyte adhesion and migration during the inflammatory response
(10). On one hand, a pre-adherent
surface is presented to the leukocytes by activated endothelial
cells. On the other hand, ICAM-1 is also associated with the
process of extravasation of leukocytes at the site of inflammation.
Therefore, increased secretion of ICAM-1 leads to endothelial
functional disorder, increased capillary permeability, and tissue
injury, which cause an inflammatory response (10,41).
iNOS, a cellular enzyme, is involved in the oxidation of L-arginine
to NO. In endothelial cells, in vivo the activation and
secretion of iNOS is usually associated with inflammatory diseases
(42,43). Excessive NO caused by iNOS
activation can bring about vasodilation, congestion and
microvascular injury, thereby participating in the inflammatory
process (44). These inflammatory
mediators and vascular endothelial cell injury would induce
inflammatory reactions.
NAC has been revealed to affect other immune cell
types, such as leukocytes, neutrophils and monocytes (19,45,46).
Therefore, whether NAC has a protective effect on vascular
endothelial cells and inhibits inflammation were examined; in
addition, the underlying mechanism of the protective effect of NAC
was assessed. In the present study, the mRNA expression of the four
factors determined by RT-qPCR were consistent with the protein
expression of IL-8 and TNF-α detected by ELISA, and those of iNOS
and NO detected by nitric reductase method, and ICAM-1 protein
detected by western blotting. Thus, LPS significantly upregulated
the mRNA and protein expression of IL-8, TNF-α, NO, and ICAM-1;
however, pretreatment of cells with NAC and BAY11-7082
significantly inhibited the LPS-induced upregulation. The results
indicated that the inhibitory effect of NAC on the expression of
inflammatory mediators was similar to that of BAY11-7082, an
inhibitor of the NF-κB signaling pathway. These findings inidcated
that the LPS-induced secretion of IL-8, TNF-α, NO, and ICAM-1 by
HUVECs was mediated via the NF-κB signaling pathway. In addition,
the inhibitory effect of NAC on inflammatory mediators was likely
mediated via inhibition of the NF-κB pathway.
NF-κB, as a crucial transcription factor, plays a
role in inflammation (5).
Typically, NF-κB remains in the cytoplasm bound to the NF-κB
protein (IκB) inhibitors in cells that are not activated. After
exposure to LPS, the IκB kinase (IκK) complex is activated,
inducing phosphorylation, followed by disintegration of IκB; this
induces the translocation of NF-κB p65 from the cytoplasm to the
nucleus. In the nucleus, NF-κB p65 binds to the DNA sites linked to
inflammatory factor expression (47,48),
including cell factors, chemotactic factors, and cell adhesion
molecules (49,50). Moreover, NF-κB is also related to
leukocyte penetrability, transference, and conglutination (51,52).
However, whether the NF-κB signaling pathway mediates the
inhibitory effect of NAC on LPS-induced secretion of IL-8, TNF-α,
NO, and ICAM-1 by HUVECs remains unclear. The present findings
suggest that NF-κB pathway is involved in mediating the protective
effects of NAC. The expression of phosphorylated p65 and p65 in the
NF-κB signaling pathway (Fig. 4C and
D) was examined. After treatment of HUVECs with LPS, NAC and
BAY11-7082, the activation of the NF-κB signaling pathway was
indicated by p-p65/p65. The results revealed that pretreatment of
cells with NAC and BAY11-7082 attenuated the activation of NF-κB
signaling pathway by LPS.
Collectively, the present results indicated that NAC
inhibited LPS-mediated production of IL-8, TNF-α, NO, and ICAM-1 in
HUVECs, thereby reducing IL-8-induced monocyte aggregation and
downregulating the release of other inflammatory mediators caused
by TNF-α. In addition, it protected vascular integrity, attenuated
the increase in vascular permeability caused by ICAM-1, and the
oxidative damage of NO on HUVECs. Overall this prevented vascular
endothelial cell injury and attenuated inflammation. In addition,
the present study also revealed that the protective effect of NAC
on HUVECs was related to inhibition of the activated NF-κB pathway;
the underlying mechanism of this effect may be linked to the
antioxidant properties of NAC. NAC can scavenge free radicals,
possibly preventing the oxidation of L-spermine to NO and
inhibiting IκB protein degradation and the process of IκK
phosphorylation. The present study still has some limitations,
thus, further experiments are required to clarify the specific
mechanisms and more functional assays are required to assess cell
adhesion or tube formation in the future. Nonetheless, to the best
of the authors' knowledge, the present study is the first to
demonstrate that NAC inhibits the activation of the NF-κB signaling
pathway and the production of inflammatory mediators in HUVECs.
Therefore, NAC is expected to be used in more fields. The potential
applications may include the prevention and treatment of
peri-implantitis and periodontitis. The present study, provides
preliminary evidence to prevent and treat inflammatory diseases.
Further research and clinical trials are required prior to clinical
use of NAC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Health
Commission of Sichuan Province (grant no. 16PJ174), Southwest
Medical University (grant no. 2016013 and 2015-YJ023) and Luzhou
Science and Technology Bureau [grant no. 2010-s-16 (1/3)].
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
ZZ was mainly responsible for the execution of the
experiments, data analysis and completion of the first draft of the
paper. TX contributed to performing the experiments and revising of
the paper. RZ and JH analyzed data and revised the manuscript. LG
was mainly responsible for the design of the experiment and the
final revision of the article. All authors have read and approved
the final article and agree to be accountable for all aspects of
the research in ensuring that the accuracy or integrity of any part
of the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Affiliated Hospital of Southwest Medical
University (Luzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ku SK, Zhou W, Lee W, Han MS, Na M and Bae
JS: Anti-inflammatory effects of hyperoside in human endothelial
cells and in mice. Inflammation. 38:784–799. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Guo L, Zhang H, Li W, Zhan D and Wang M:
N-acetyl cysteine inhibits lipopolysaccharide-mediated induction of
interleukin-6 synthesis in MC3T3-E1 cells through the NF-kB
signaling pathway. Arch Oral Boil. 93:149–154. 2018. View Article : Google Scholar
|
|
3
|
Kanmani P and Kim H: Functional
capabilities of probiotic strains on attenuation of intestinal
epithelial cell inflammatory response induced by TLR4 stimuli.
Biofactors. 45:223–235. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jung JY, Woo SM, Kim WJ, Lee BN, Nör JE,
Min KS, Choi CH, Koh JT, Lee KJ and Hwang YC: Simvastatin inhibits
the expression of inflammatory cytokines and cell adhesion
molecules induced by LPS in human dental pulp cells. Int Endod.
50:377–386. 2017. View Article : Google Scholar
|
|
5
|
Lee W and Bae JS: Anti-inflammatory
effects of aspalathin and nothofagin from rooibos (aspalathus
linearis) in vitro and in vivo. Inflammation. 38:1502–1516. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen L, Li W, Qi D and Wang D: Lycium
barbarum polysaccharide protects against LPS-induced ARDS by
inhibiting apoptosis, oxidative stress, and inflammation in
pulmonary endothelial cells. Free Radic Res. 52:480–490. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jin L, Zhang LM, Xie KQ, Ye Y and Feng L:
Paeoniflorin suppresses the expression of intercellular adhesion
molecule-1 (ICAM-1) in endotoxin-treated human monocytic cells. Br
J Pharmacol. 164:694–703. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Guha M and Mackman N: LPS induction of
gene expression in human monocytes. Cell Signal. 13:85–94. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ma MM, Li Y, Liu XY, Zhu WW, Ren X, Kong
GQ, Huang X, Wang LP, Luo LQ and Wang XZ: Cyanidin-3-O-glucoside
ameliorates lipopolysaccharide-induced injury both in vivo and in
vitro suppression of NF-kappaB and MAPK pathways. Inflammation.
38:1669–1682. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hu Y, He K and Zhu H: Chinese herbal
medicinal ingredients affect secretion of NO, IL-10, ICAM-1 and
IL-2 by endothelial cells. Immunopharmacol Immunotoxicol.
37:324–328. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yoo H, Ku SK, Lee T and Bae JS: Orientin
inhibits HMGB1-induced inflammatory responses in HUVECs and in
murine polymicrobial sepsis. Inflammation. 37:1705–1717. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim IS, Yang EJ, Shin DH, Son KH, Park HY
and Lee JS: Effect of arazyme on the lipopolysaccharideinduced
inflammatory response in human endothelial cells. Mol Med Rep.
10:1025–1029. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao YD, Huang X, Yi F, Dai Z, Qian Z,
Tiruppathi C, Tran K and Zhao YY: Endothelial FoxM1 mediates bone
marrow progenitor cell-induced vascular repair and resolution of
inflammation following inflammatory lung injury. Stem Cells.
32:1855–1864. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tsai ML, Huang HP, Hsu JD, Lai YR, Hsiao
YP, Lu FJ and Chang HR: Topical N-acetylcysteine accelerates wound
healing in vitro and in vivo via the PKC/Stat3 pathway. Int J Mol
Sci. 15:7563–7578. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pawlowska-Goral K, Kurzeja E and Stec M:
N-acetylcysteine protects against fluoride-induced oxidative damage
in primary rat hepatocytes. Toxicol In Vitro. 27:2279–2282. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dinicola S, De Grazia S, Carlomagno G and
Pintucci JP: N-acetylcysteine as powerful molecule to destroy
bacterial biofilms. A systematic review. Eur Rev Med Pharmacol Sci.
18:2942–2948. 2014.PubMed/NCBI
|
|
17
|
Mokhtari V, Afsharian P, Shahhoseini M,
Kalantar SM and Moini A: A review on various uses of N-acetyl
cysteine. Cell J. 19:11–17. 2017.PubMed/NCBI
|
|
18
|
Ohnishi T, Bandow K, Kakimoto K, Kusuyama
J and Matsuguchi T: Long-time treatment by low-dose
N-acetyl-L-cysteine enhances proinflammatory cytokine expressions
in LPS-stimulated macrophages. PLoS One. 9:e872292014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Köse SA and Nazıroğlu M: N-acetyl cysteine
reduces oxidative toxicity, apoptosis, and calcium entry through
TRPV1 channels in the neutrophils of patients with polycystic ovary
syndrome. Free Radic Res. 49:338–346. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Khatib N, Weiner Z, Ginsberg Y, Awad N and
Beloosesky R: Protective effect of N-acetyl-cysteine (NAC) in
lipopolysaccharide (LPS)-associated inflammatory response in rat
neonates. Rambam Maimonides Med J. 8:2017. View Article : Google Scholar
|
|
21
|
Beloosesky R, Ginsberg Y, Khatib N, Maravi
N, Ross MG, Itskovitz-Eldor J and Weiner Z: Prophylactic maternal
N-acetylcysteine in rats prevents maternal inflammation-induced
offspring cerebral injury shown on magnetic resonance imaging. Am J
Obstet Gynecol. 208:213.e211–216. 2013. View Article : Google Scholar
|
|
22
|
Wu XY, Luo AY, Zhou YR and Ren JH:
N-acetylcysteine reduces oxidative stress, nuclear factorkappaB
activity and cardiomyocyte apoptosis in heart failure. Mol Med Rep.
10:615–624. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Oka S, Kamata H, Kamata K, Yagisawa H and
Hirata H: N-acetylcysteine suppresses TNF-induced NF-kappaB
activation through inhibition of IkappaB kinases. FEBS Lett.
472:196–202. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wegelin O, van Melick HHE, Hooft L, Bosch
JLHR, Reitsma HB, Barentsz JO and Somford DM: Comparing three
different techniques for magnetic resonance imaging-targeted
prostate biopsies: A systematic review of in-bore versus magnetic
resonance imaging-transrectal ultrasound fusion versus cognitive
registration. Is there a preferred technique? Eur Urol. 71:517–531.
2017.
|
|
25
|
Zha L, Chen J, Sun S, Mao L, Chu X, Deng
H, Cai J, Li X, Liu Z and Cao W: Soyasaponins can blunt
inflammation by inhibiting the reactive oxygen species-mediated
activation of PI3K/Akt/NF-κB pathway. PLoS One. 9:e1076552014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Renvert S and Quirynen M: Risk indicators
for peri-implantitis. A narrative review. Clin Oral Implants Res.
26 (Suppl 11):S15–S44. 2015. View Article : Google Scholar
|
|
27
|
Kinane DF, Stathopoulou PG and Papapanou
PN: Periodontal diseases. Nat Rev Dis Primers. 3:170382017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang HW, Tang XS, Tian ZW, Wang Y, Yang WY
and Hu JZ: Effects of
nano-hydroxyapatite/polyetheretherketone-coated, sandblasted,
large-grit, and acid-etched implants on inflammatory cytokines and
osseointegration in a peri-implantitis model in beagle dogs. Med
Sci Monit. 23:4601–4611. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cines DB, Pollak ES, Buck CA, Loscalzo J,
Zimmerman GA, McEver RP, Pober JS, Wick TM, Konkle BA, Schwartz BS,
et al: Endothelial cells in physiology and in the pathophysiology
of vascular disorders. Blood. 91:3527–3561. 1998.PubMed/NCBI
|
|
30
|
Lingen MW: Role of leukocytes and
endothelial cells in the development of angiogenesis in
inflammation and wound healing. Arch Pathol Lab Med. 125:67–71.
2001.PubMed/NCBI
|
|
31
|
Wang Y, Gao Y, Yu W, Jiang Z, Qu J and Li
K: Lycopene protects against LPS-induced proinflammatory cytokine
cascade in HUVECs. Pharmazie. 68:681–684. 2013.PubMed/NCBI
|
|
32
|
Imai Y, Dobrian AD, Weaver JR, Butcher MJ,
Cole BK, Galkina EV, Morris MA, Taylor-Fishwick DA and Nadler JL:
Interaction between cytokines and inflammatory cells in islet
dysfunction, insulin resistance and vascular disease. Diabetes Obes
Metab. 15 (Suppl 3):S117–S129. 2013. View Article : Google Scholar
|
|
33
|
Giusti L, Gabriele M, Penno G, Garofolo M,
Longo V, Del Prato S, Lucchesi D and Pucci L: A fermented whole
grain prevents lipopolysaccharides-induced dysfunction in human
endothelial progenitor cells. Oxid Med Cell Longev.
2017:10262682017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen G, Zhao J, Yin Y, Wang B, Liu Q, Li
P, Zhao L and Zhou H: C-type natriuretic peptide attenuates
LPS-induced endothelial activation: Involvement of p38, Akt, and
NF-kB pathways. Amino Acids. 46:2653–2663. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lin Q, Qin X, Shi M, Qin Z, Meng Y, Qin Z
and Guo S: Schisandrin B inhibits LPS-induced inflammatory response
in human umbilical vein endothelial cells by activating Nrf2. Int
Immunopharmacol. 49:142–147. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu XJ, Mu E, Liang YJ, Zhang ZD and Ma
XC: High glucose enhances permeability of human pulmonary
microvascular endothelial cells by lipopolysaccharide stimulation
in vitro and effect of DDAH/NOS/NO imbalance on its pathogenesis.
Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 25:140–144. 2013.(In
Chinese). PubMed/NCBI
|
|
37
|
Tamura EK, Cecon E, Monteiro AW, Silva CL
and Markus RP: Melatonin inhibits LPS-induced NO production in rat
endothelial cells. J Pineal Res. 46:268–274. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dong R and Zheng S: Interleukin-8: A
critical chemokine in biliary atresia. J Gastroenterol Hepatol.
30:970–976. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang Y, Wang W, Wang L, Wang X and Xia J:
Regulatory mechanisms of interleukin 8 production induced by tumor
necrosis factor-α in human hepatocellular carcinoma cells. J Cell
Mol Med. 16:496–506. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ghasemi H, Ghazanfari T, Yaraee R,
Faghihzadeh S and Hassan ZM: Roles of IL-8 in ocular inflammations:
A review. Ocul Immunol Inflamm. 19:401–412. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rossi B, Angiari S, Zenaro E, Budui SL and
Constantin G: Vascular inflammation in central nervous system
diseases: Adhesion receptors controlling leukocyte-endothelial
interactions. J Leukoc Boil. 89:539–556. 2011. View Article : Google Scholar
|
|
42
|
Alderton WK, Cooper CE and Knowles RG:
Nitric oxide synthases: Structure, function and inhibition. Biochem
J. 357:593–615. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gunnett CA, Lund DD, McDowell AK, Faraci
FM and Heistad DD: Mechanisms of inducible nitric oxide
synthase-mediated vascular dysfunction. Arterioscler Thromb Vasc
Biol. 25:1617–1622. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hauser B, Matejovic M and Radermacher P:
Nitric oxide, leukocytes and microvascular permeability: Causality
or bystanders? Crit Care. 12:1042008. View
Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chen QZ, Fu ZD, Zhou YB, Zhou LF, Yang CT
and Li JH: N-acetyl-L-cysteine reduces the ozone-induced lung
inflammation response in mice. Sheng Li Xue Bao. 68:767–774.
2016.(In Chinese). PubMed/NCBI
|
|
46
|
Gamage AM, Lee KO and Gan YH: Effect of
oral N-acetyl cysteine supplementation in type 2 diabetic patients
on intracellular glutathione content and innate immune responses to
Burkholderia pseudomallei. Microbes Infect. 16:661–671. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Franceschelli S, Pesce M, Ferrone A, Gatta
DM, Patruno A, Lutiis MA, Quiles JL, Grilli A, Felaco M and
Speranza L: Biological effect of licochalcone C on the regulation
of PI3K/Akt/eNOS and NF-kB/iNOS/NO signaling pathways in H9c2 cells
in response to LPS stimulation. Int J Mol Sci. 18:E6902017.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ghosh S, May MJ and Kopp EB: NF-kappa B
and Rel proteins: Evolutionarily conserved mediators of immune
responses. Annu Rev Immunol. 16:225–260. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhang H, Qin G, Liang G, Li J, Chiu I,
Barrington RA and Liu D: Suppression of complement regulatory
protein C1 inhibitor in vascular endothelial activation by
inhibiting vascular cell adhesion molecule-1 action. Biochem
Biophys Res Commun. 358:1120–1127. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
He W, Qu T, Yu Q, Wang Z, Lv H, Zhang J,
Zhao X and Wang P: LPS induces IL-8 expression through TLR4, MyD88,
NF-kappaB and MAPK pathways in human dental pulp stem cells. Int
Endod J. 46:128–136. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lockyer JM, Colladay JS, Alperin-Lea WL,
Hammond T and Buda AJ: Inhibition of nuclear factor-kappaB-mediated
adhesion molecule expression in human endothelial cells. Circ Res.
82:314–320. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yamagami H, Yamagami S, Inoki T, Amano S
and Miyata K: The effects of proinflammatory cytokines on
cytokine-chemokine gene expression profiles in the human corneal
endothelium. Invest Ophthalmol Vis Sci. 44:514–520. 2003.
View Article : Google Scholar : PubMed/NCBI
|