Introduction
IgA nephropathy is the most important factor that
leads to end-stage kidney disease progression (1). It is well known that IgA nephropathy
is a progressive disease. The diagnosis of IgA nephropathy depends
on the pathological diagnosis of renal biopsy; the most prominent
characteristic of IgA nephropathy is IgA1-based immune complexes
deposited in the glomerular mesangial region (2), which induce the proliferation and
secretion of glomerular mesangial cells. Inflammatory factors and
chemokines then mediate glomerular inflammatory damage (3,4).
Since the pathogenesis is currently unclear, there is no specific
treatment for IgA nephropathy. In addition, as IgA nephropathy is a
progressive disease, its progressive mechanism should be studied,
and interventional measures that delay or reverse the progress of
IgA nephropathy should be identified.
Previous studies have reported that IgA1 is mainly
derived from activated B lymphocytes. B cell-activating factor
(BAFF) is a novel member of the tumor necrosis factor (TNF)
superfamily (5–8). It has been reported that BAFF, as an
important co-stimulatory factor, is critical in mediating B-cell
proliferation, maturation and antibody secretion. Numerous studies
have suggested that BAFF may be involved in the pathogenesis of IgA
nephropathy (9–14). TNF receptor-associated factor
(TRAF) is an important linker molecule in the TNF superfamily;
TRAF6 is involved in inflammation and immune responses by mediating
inflammatory and apoptotic signaling pathways (15). In addition, TRAF may serve a key
role by activating the NF-κB signaling pathway (16–23).
A previous study reported that BAFF activates B cells through the
NF-κB signaling pathway to secrete excess IgA1, which leads to IgA
nephropathy-like alterations in mouse kidneys (14). Previous studies indicated that
B-cell activation and elevated BAFF levels were present in patients
with IgA nephropathy (10,11). In addition, it was suggested that
overexpression of BAFF may be involved in the pathogenesis of IgA
nephropathy by inducing white blood cells to secrete abnormal
glycosylated IgA1 (14). However,
it is unclear whether overexpression of BAFF results in damage to
the renal parenchyma.
In the present study, primary glomerular mesangial
cells were cultured and BAFF was added to detect possible signaling
pathways. Rat models of IgA nephropathy were also generated to
examine the effect of BAFF on TRAF6/NF-κB signaling pathways.
Briefly, the aim of the present study was to investigate whether
BAFF directly damaged the renal parenchyma. These studies may help
to further explore the pathogenesis of IgA nephropathy and may aid
development of targeted therapeutic strategies.
Materials and methods
Study cases
The present study recruited 58 patients diagnosed
with primary IgA nephropathy by renal biopsy between February 2016
and December 2017 from The Affiliated Hospital of Nantong
University (Nantong, China). The recruited patients included 23
women and 35 men, with an average age of 39.21±9.3 years. In
addition, 20 patients with renal necrosis caused by renal tumors
were recruited as the control group from the Department of Urology,
The Affiliated Hospital of Nantong University between February 2016
and December 2017. The patients in the control group comprised 12
women and 8 men, with an average age of 49.32±11.27 years. The
study protocol was approved by the medical ethics committee of The
Affiliated Hospital of Nantong University, and all subjects
provided written informed consent.
This study excluded patients with secondary kidney
disease, such as systemic lupus erythematosus, Henoch-Schonlein
purpura, ankylosing spondylitis, liver disease and tumors. Patients
were not systematically treated and did not use immunosuppressive
agents prior to the start of the study.
A total of 8 ml anticoagulated blood was collected
from the upper arms of patients that were fasted for 1 night. Each
collected sample was centrifuged at 1,200 × g for 10 min at room
temperature, and the plasma was collected and maintained in an
Eppendorf tube at −80°C for storage. For examination, the frozen
plasma was rapidly thawed at 37°C. Urine was collected at 8:00 AM
and urine samples were subsequently taken for 24 h; toluene (4–5
ml) was added to prevent corrosion. The kidney tissue specimens of
patients with IgA nephropathy were obtained by ultrasound-guided
percutaneous nephrolithotomy. The kidney tissue specimens of
patients with renal tumors were collected from normal kidney
tissues away from the tumor site following removal of the kidney.
The obtained kidney tissues were partially fixed for 2 h at room
temperature in 10% formalin solution post-surgery, and partial
kidney tissues were placed in liquid nitrogen and stored at
−80°C.
Detection of urinary protein quantity
and endogenous creatinine clearance rate
The protein in urine was added to 1.5% sodium
tungstate and centrifuged at 3,000 × g for 5 min at room
temperature, and was then quantitatively determined by the biuret
method (24). The concentration of
creatinine in urine and plasma was measured using the Modular P800
Automatic Biochemistry Analyzer (Roche Diagnostics), and urine
volume was measured for 24 h. The Cockcroft formula (25) was used to calculate the clearance
rate of endogenous creatinine for 24 h.
Detection of plasma BAFF
An ELISA kit (cat. no. ab188391; Abcam) was used to
detect BAFF levels in plasma samples, according to the
manufacturer's protocol. The absorbance was measured at 450 nm,
according to the manufacturer's protocol. A standard curve was
generated according to the dilution concentration, in order to
calculate the concentration of BAFF in each specimen.
Immunohistochemical analysis of kidney
tissues
The obtained kidney tissues were fixed for 2 h at
room temperature with 4% formalin, dehydrated in an ascending
series of ethanol, and permeabilized by xylene. Sections were then
immersed in wax, embedded in paraffin, sliced into 2-µm sections,
dried, dewaxed and subjected to antigen retrieval with 0.01 M
citrate buffer for 40 min at 92°C. The slides were then incubated
with 3% H2O2 for 10 min at room temperature
to quench endogenous peroxidase activity, placed in 0.01 mmol/l
citrate buffer (pH 6.0) and rinsed three times with 0.01 mol/l PBS
for 3 min. Anti-human BAFF antibody (1:50; cat. no. AF124; R&D
Systems, Inc.), anti-p-NF-κBP65 (1:1,000; cat. no. ab86299; Abcam)
or rabbit anti-TRAF6 antibody (1:100; cat. no. sc-8409; Santa Cruz
Biotechnology, Inc.) were used to incubate slides overnight at 4°C.
PBS was used to rinse slides following primary antibody incubation.
Horseradish peroxidase (HRP)-labeled secondary antibody (1:500;
cat. nos. A0181 and A0208; Beyotime Institute of Biotechnology) was
then added for 30 min at room temperature, followed by washing with
PBS. PBS was used instead of primary antibodies as a negative
control. 3,3′-Diaminobenzidine was used to stain the slides for 20
min, and hematoxylin was used for counterstaining for 1 min at room
temperature. The slides were then dehydrated and mounted. Light
microscopy was employed for observations and image capturing.
Brown-yellow particles were regarded as positive cells and six
fields were randomly selected from each slice. The integrated
optical density values were determined using Image-ProPlus 6.0
image analysis processing software (Media Cybernetics, Inc.), and
the average value was used to determine the expression level.
Histopathological scores of kidney
tissues
According to the Katafuchi score (26) of IgA nephropathy, the score of
glomerular lesions was classified as 0–12 points, including: i) The
degree of glomerular mesangial cell and mesangial matrix
hyperplasia (0–4 points); ii) segmental glomerular lesions, such as
crescent formation, glomerular adhesion, segmental sclerosis and
segmental capillary fibrinous necrosis (0–4 points); and iii)
glomerular sclerosis (0–4 points). The integral evaluation criteria
were: 1, none, 0 points; 2, <25%, 1 point; 3, 25–50%, 2 points;
4, 51–75%, 3 points; and 5, >75%, 4 points. A total of 58
patients with IgA nephropathy were divided into four groups
according to the scores: 0–3 points (group I), 4–6 points (group
II), 7–9 points (group III), and 10–12 points (group IV).
Glomerular mesangial cell culture
The animal protocol in the present study was
approved by the Medical Ethics Committee of The Nantong University
Affiliated Hospital. Two male Sprague-Dawley rats (age, 8 weeks;
weight, 220–260 g), supplied by the laboratory animal center of
Nantong University, were housed under the following conditions:
Temperature, 18–26°C; relative humidity, 40–70%; noise, <85 dB;
ammonia concentration, <20 ppm; air exchange, 8–12 times/h; 15 g
food added every 24 h; drinking water, pH 2.5–2.8 acidified water,
changed 2–3 times/week. Animal health and behavior were monitored
every 12 h. The rats were intraperitoneally anesthetized with 50
mg/kg sodium pentobarbital after weighing. After disinfection, the
abdominal cavity was opened, saline was fully perfused into the
apex cordis of the heart; once the kidneys turned pale, they were
quickly moved onto ice. Rats were euthanized with an
intraperitoneal injection of 150 mg/kg sodium pentobarbital
following surgery. Subsequently, the renal capsule was discarded
from the kidneys and the renal cortex along the boundary of the
medulla was obtained to generate a homogenate. The tissue was then
consecutively filtered through 60-, 100- and 200-mesh screens,
after which, the tissue was collected and the separated glomeruli
were obtained. Subsequently, 0.1% collagenase IV (cat. no.
17104019; Thermo Fisher Scientific, Inc.) was added for 10 min at
37°C. The glomeruli (2×104) were transferred to a
culture flask with DMEM (cat. no. 31600-091; Thermo Fisher
Scientific, Inc.) containing 20% fetal bovine serum (FBS; cat. no.
M6546; Macklin, Inc.). After the glomeruli were adherent to the
culture flask wall, the cells were cultured for 4 weeks, and the
cells were digested with trypsin, subcultured and purified. The
third generation of cells was used in experiments.
Cells were cultured at 37°C in an atmosphere
containing 5% CO2, in saturated humidity. The cells were
cultured to the 8th-10th generation. When the proliferating cells
reached 80–90% confluence, 0.25% trypsin was used to digest cells.
A single cell suspension was prepared from DMEM containing 20%
FBS). After supplementing cells with serum-free low glucose DMEM
for 24 h, the glomerular mesangial cells were randomly divided into
five groups: Control (con)-short hairpin RNA (shRNA) group;
con-shRNA + BAFF (20 ng/ml; cat. no. PHC1674; Thermo Fisher
Scientific, Inc.) group, in which BAFF was added to transfected
cells for 48 h at 37°C; con-shRNA + BAFF + BAFF-receptor (R)-Fc
chimera protein (500 µg/ml; cat. no. 1162-BR-050; R&D Systems,
Inc.) group, in which BAFF-R-Fc was added to transfected cells and
after 30 min BAFF was added for 48 h at 37°C; TRAF6-shRNA group;
and TRAF6-shRNA + BAFF (20 ng/ml) group, in which BAFF was added to
transfected cells for 48 h at 37°C.
Transfection of glomerular mesangial
cells
Glomerular mesangial cells (106/ml) were
seeded into 24-well plates at 500 µl per well. Cells were
transfected after reaching 40% confluence. A total of 100 µl 6 pmol
TRAF6-shRNA (cat. no. sc-156004-SH; Santa Cruz Biotechnology,
Inc.), 2 µl Rfect (cat. no. 11011; Changzhou Baidai Biotechnology
Co., Ltd.) and serum-free medium were added to each well and mixed;
the cells and transfection agents were cultured at 37°C for 48 h.
After transfection, the cells were further cultured in RPMI-1640
medium (containing 200 U/ml penicillin G, 10 mg/l gentamicin and
10% FBS) overnight at 37°C. Cells in the control group were
transfected with the Con-shRNA (5′-AAGAAACCATGCAAAGTAAGGTT-3′;
Santa Cruz Biotechnology, Inc.).
Establishment of IgA nephropathy model
and transfection with rats
A total of 60 Sprague-Dawley male rats (age, 8
weeks; weight, 220–260 g), supplied by the laboratory animal center
of Nantong University, were randomly divided into four groups:
Con-small interfering RNA (siRNA; 0.2 µM), con-siRNA (0.2 µM) +
IgA, BAFF-RFc chimera protein (2 µg/ml) + IgA, and TRAF6-siRNA (0.2
µM) + IgA. Rats were housed under the following conditions:
Temperature, 18–26°C; relative humidity, 40–70%; noise, <85 dB;
ammonia concentration, <20 ppm; air exchange, 8–12 times every
hour; 15 g food was added every 24 h; drinking water, pH 2.5–2.8
acidified water, changed 2–3 times every week. Animal health and
behavior were monitored every 12 h.
TRAF6-siRNA (cat. no. sc-156004; Santa Cruz
Biotechnology, Inc.) was mixed with Entranster™ in vivo
(cat. no. 4000-3; Engreen Biosystem) at a ratio of 1:2. A total of
1 ml solution was injected through the tail vein in rats, and the
relevant experiments were performed after 24 h. Rats in the control
group were injected with Con-siRNA (cat. no. A06001; Shanghai
GenePharma Co., Ltd.). Rats were injected with siRNAs 24 h prior to
IgA nephropathy model generation (27).
To establish the IgA nephropathy model,
Sprague-Dawley rats were acclimated for 1 week. Subsequently, the
rats were anesthetized by intraperitoneal injection of 1% sodium
pentobarbital (40 mg/kg) and the left kidney was removed. After 1
week, 3 mg BSA (cat. no. Abs9157; Shanghai Absin Biotechnology)
mixed with complete Freund's adjuvant medium was injected into both
hind footpads of the mice, followed by repeated subcutaneous
multi-site injections of the same solution every 2 weeks. A total
of 2 weeks after the injection of BSA into the footpads, 6 mmol/l
hydrochloric acid-acidified water containing 0.1% BSA was
administered every other day. Blood was drawn after three
immunization injections of BSA, and the serum anti-BSA antibody
titer was measured using the double immunodiffusion method
(28). When the antibody titer
reached 1:16, 3 mg BSA was intraperitoneally injected daily. After
3 weeks, 100 µg lipopolysaccharide (cat. no. L2880; Sigma-Aldrich;
Merck KGaA) was intraperitoneally injected. The model was
established after 4 weeks (28).
After fasting for 12 h, the rats were placed in a metabolic cage,
urine was collected over 24 h, urine volume was recorded and the
24-h urine protein quantity was measured, as aforementioned. All
rats were euthanized via an intraperitoneal injection of 150 mg/kg
sodium pentobarbital. Once the heartbeat stopped and pupils
dilated, serum was obtained, and Scr and BAFF were detected. The
methods of measurement were the same as those aforementioned for
analysis of human specimens. In addition, the left kidney was
removed and fixed in 10% neutralized formaldehyde solution for 2 h
at room temperature. The samples were stored at −80°C for further
testing.
Nucleoplasm separation of cells and
kidney tissues
Cells were treated according to the aforementioned
grouping and dosing methods. Subsequently, the cells were
harvested, and the nuclei were extracted using the BestBio
Nucleus/Cytoplasmic Isolation kit (Bestbio). Subsequently,
5–10×106 cells were centrifuged at 500 × g for 3 min at
4°C to collect cells. Cells were washed twice with PBS, and a
mixture consisting of cold Buffer A and protease inhibitor was
added. After shaking twice, the solution containing cells was
centrifuged at 16,000 × g for 5 min at 4°C. The mixture of cold
Buffer B and protease inhibitor was then added to the pellet. After
shaking, the cell suspension was centrifuged at 16,000 × g for 10
min at 4°C, and the supernatant was quickly transferred into a
precooled, clean centrifuge tube to obtain nuclear proteins.
Rat kidney samples were cut into small pieces. After
adding PBS, the tissues were homogenized using a tissue
homogenizer, until bulky solids were not visible after settling on
ice for 5 min. The supernatant was carefully transferred into a
precooled clean centrifuge tube, followed by centrifugation at 500
× g for 2–3 min at 4°C. The supernatant was then discarded and the
same procedure as aforementioned was performed to extract nuclear
proteins.
Western blotting and flow cytometry
for the detection of BAFF-R expression in mesangial cells
The cells were collected and lyzed on ice for 30 min
with RIPA lysis buffer (cat. no. P0013B; Beyotime Institute of
Biotechnology). Centrifugation was then conducted at 8,000 × g for
5 min at 4°C. Total proteins were extracted from cells and the
protein concentration was determined using the bicinchoninic acid
(BCA) assay. After the proteins were mixed with loading buffer for
5 min, 30 µg protein was separated by SDS-PAGE on 8% gels. Proteins
were then transferred to a nitrocellulose membrane and blocked with
50 g/l skim milk powder in TBS-0.05% Tween (TBST). Membranes were
then incubated with anti-BAFF-R antibody (1:1,000; cat. no. ab5965;
Abcam) overnight at 4°C. The corresponding HRP-labeled goat
anti-rabbit secondary antibody (1:4,000; cat. no. ab205718; Abcam)
was added to the membrane at 4°C for 2 h. Proteins were detected
using an enhanced chemiluminescence (ECL) system (cat. no. PE0010;
Beijing Solarbio Science & Technology Co., Ltd.). An
Odyssey® CLx Imaging system (LI-COR Biosciences) was
used to capture images and densitometric analysis was conducted
using Image Lab 3.0 software (Bio-Rad Laboratories, Inc.). β-actin
(1:5,000; cat. no. ab95437; Abcam) was used to incubate membranes
at 4°C overnight and was employed as a loading control.
For flow cytometric detection of BAFF-R, glomerular
mesangial cells (107/ml) were seeded into 24-well plates
at 500 µl/well. Cells were incubated with primary mouse anti-BAFF-R
antibody (1:1,000; cat. no. ab233775; Abcam) for 30 min in PBS
supplemented with 3% FBS at 4°C. Subsequently, cells were incubated
with FITC-conjugated anti-mouse secondary antibody (1:5,000; cat.
no. ab6785; Abcam) for 30 min at 4°C. Rat Ig (cat. no. AS10-923;
Agrisera) at the same concentration was used as a negative control.
BAFF-R expression on the mesangial cell membrane was detected using
an Attune NxT Flow Cytometer (Thermo Fisher Scientific, Inc.).
Attune™ NxT software version 2.1 (Thermo Fisher Scientific, Inc.)
was used to analyze the results.
Reverse transcription-quantitative PCR
(RT-qPCR) analysis of TRAF6, fibronectin (FN), connective tissue
growth factor (CTGF) and NF-κBP65 mRNA expression in glomerular
mesangial cells
After cells were treated with the aforementioned
reagents, they were harvested. RNA extraction was performed using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). A total of 1 ml TRIzol® was added to every
1×106 cells. The integrity of the extracted product was
evaluated by 1% TBE-agarose electrophoresis, and the concentration
was measured using a spectrophotometer. cDNA was synthesized using
a RT kit (RevertAid First Strand cDNA synthesis kit; cat. no.
K1621; Thermo Fisher Scientific, Inc.). The reactions were carried
in a 12-µl volume: 3 µg RNA template, 11 µl primer and RNase-free
ddH2O. The reaction was maintained at 70°C for 5 min and was placed
in an ice bath for 30 sec. After centrifugation (8,000 × g for 3
min at room temperature), the following reagents were added: 4 µl
5X Reaction Buffer, 1 µl RNase Inhibitor (20 U/µl), 2 µl dNTP Mix
(10 mmol/l), and the sample was incubated at 37°C for 5 min.
Subsequently, 1 µl M-MLV RT (20 U/µl) was added, and the sample was
incubated at 37°C for 60 min and 70°C for 10 min. SYBR Green II
qPCR reagent (SYBR premix Ex Taq II; cat. no. DRR820A; Takara Bio,
Inc.) was used for qPCR with an ABI 7300 cycler (Applied
Biosystems; Thermo Fisher Scientific, Inc.). For consistent
amplification efficiency, each specimen was tested for TRAF6, CTGF,
FN, NF-κBP65 and GAPDH gene expression. The total reaction system
comprised 20 µl, which included 10 µl 2X Real Master Mix, 0.4 µl
ROX, 0.8 µl each of the upstream and downstream primers, and 2 µg
cDNA as a reaction template. The thermocycling conditions were as
follows: Initial denaturation at 95°C for 3 min, followed by 40
cycles of denaturation at 95°C for 15 sec, primer annealing at 62°C
for 30 sec and elongation at 72°C for 30 sec, and a final
elongation step at 72°C for 7 min. The fluorescence signal was
recorded after reading the plate to obtain a melting curve; the
melting curve was then analyzed. Samples were randomly selected for
2% agarose gel electrophoresis to determine whether the amplified
products were the desired fragments. Quantum CX5 (Vilber Lourmat)
was used to capture images and densitometric analysis was conducted
using AlphaImager 2000 image analyzer (Alpha Innotech). With GAPDH
as the housekeeping gene, 2−∆∆Cq ×100% indicated the
relative expression of each gene:
∆Cq(test)=Cq(target,test)-Cq(ref,test);
∆Cq(calibrator)=Cq(target,calibrator)-Cq(ref,calibrator);
∆∆Cq=∆Cq(test)-ΔCq (calibrator). Calibrator refers to the control
group; test refers to the experimental group(s); ref refers to the
housekeeping gene; target refers to the gene of interest. Three
replicate wells were set for each reaction to calculate the mean
value for each ΔCq (29). Primers
were designed by Shanghai Bioengineering, as follows: TRAF6,
forward (F) 5′-TGATAGTGTGGGTGGAACTGC-3′, reverse (R)
5′-CAGATGGGGCATTCATACTTG-3′; FN, F 5′-CCGGTGGCTGTCAGTCAAAG-3′, R
5′-AAACCTCGGCTTCCTCCATAA-3′; CTGF, F 5′-CCCTGACCCAACTATGATGC-3′, R
5′-CCTTACTCCCTGGCTTTACG-3′; NF-κBP65, F 5′-TTCCTGGCGAGAGAAGCAC-3′,
R 5′-AAGCTATGGATACTGCCCTCT-3′; and GAPDH, F
5′-ACGGATTTGCTCGTATTG-3′ and R 5′-GGAAGATGGTGATGGGATT-3′
Western blot analysis of the protein
expression levels of TRAF6, FN, CTGF and p-NF-κBP65 in glomerular
mesangial cells
Glomerular mesangial cells were treated according to
cell groups for 48 h, after which, total protein was extracted by
lysing cells on ice with 150 µl RIPA lysis solution in each well of
a 6-well plate. The protein concentration was determined using a
BCA protein quantitative kit (Beyotime Institute of Biotechnology).
Total and nuclear proteins (60 µg/well) were separated by SDS-PAGE
on 8% gels and were transferred to a nitrocellulose membrane. The
membranes were blocked with TBST containing 5% skim milk powder at
room temperature for 1 h, and were then incubated with anti-TRAF6
(1:1,000; cat. no. ab33915; Abcam), anti-FN (1:1,000; cat. no.
MA517075; Thermo Fisher Scientific, Inc.), anti-CTGF (1:1,000; cat.
no. ab6992; Abcam), anti-p-NF-κBP65 (1:1,000; cat. no. ab86299;
Abcam), anti-NF-κBP65 (1:1,000; cat. no. ab16502; Abcam),
anti-β-actin (1:5,000; cat. no. ab95437; Abcam) and anti-Histone H3
(1:5,000; cat. no. ab176842; Abcam) overnight at 4°C. After
sufficient washing with TBST (5 min/three times), HRP-conjugated
secondary antibodies (1:5,000; cat. no. ab205718; Abcam; and cat.
no. 31430; Thermo Fisher Scientific, Inc.) was added at room
temperature for 1 h. Proteins were detected using an ECL system. An
Odyssey® CLx Imaging system (LI-COR Biosciences) was
used to capture images and densitometric analysis was conducted
using Image Lab 3.0 software (Bio-Rad Laboratories, Inc.). β-actin
was used as a cytoplasmic loading control; Histone H3 was used as
nuclear-specific loading control.
Western blotting to detect protein
expression levels of BAFF, TRAF6, FN, CTGF and p-NF-κBP65 in rat
kidney tissues
A total of 200 µl RIPA lysis solution (Beyotime
Institute of Biotechnology) was added per 20 mg kidney tissue
samples to extract proteins. The extracted total and nuclear
protein samples (60 µg/well) were subjected to SDS-PAGE on 8% gels.
After electrophoresis, the samples were transferred to
polyvinylidene difluoride membranes, which were blocked with TBST
containing 5% skim milk powder at 4°C overnight. Membranes were
then washed twice with TBST, and incubated with anti-BAFF (1:1,000;
cat. no. ab8396; Abcam), anti-FN (1:1,000), anti-TRAF6 (1:1,000),
anti-CTGF (1:1,000) anti-β-actin (1:5,000), anti-Histone H3
(1:5,000), anti-NF-κBP65 (1:1,000) and anti-p-NF-κBP65 (1:1,000) at
37°C for 2 h, and were washed twice with TBST for 10 min. The
membranes were then incubated with the aforementioned secondary
antibodies (1:2,000) at 37°C for 2 h, and washed with TBST four
times (10 min/wash). The chemiluminescence method (GE Healthcare)
was used for visualization. The Odyssey imaging system was used to
develop images. ImageJ software (National Institutes of Health) was
used for densitometric analysis; grayscale values of target
proteins were normalized to the loading proteins. β-actin was used
as a cytoplasmic loading control; Histone H3 was used as
nuclear-specific loading control.
Statistical analysis
Statistical analysis was performed using SPSS
statistical software for Windows, version 17.0 (SPSS, Inc.). The
experiments were conducted three times. Data are expressed as the
mean ± standard deviation. Data were analyzed by one-way ANOVA
followed by a Tukey post hoc test for multiple groups, and by
Student's t-test for two groups. The correlation between two
variables was analyzed using Pearson's correlation. P<0.05 was
considered to indicate a statistically significant difference.
Results
Plasma BAFF levels in patients with
IgA nephropathy are positively correlated with the Katafuchi score
of renal damage
With the aggravation of pathological damage, the
proteinuria level of patients with IgA nephropathy increased. In
addition, plasma BAFF levels were significantly increased and the
endogenous creatinine clearance rate was decreased. Pairwise
comparisons were made between the groups, and these differences
were statistically significant (P<0.05; Fig. 1). Proteinuria (r=0.45; P<0.05)
and BAFF levels (r=0.62; P<0.05) were positively correlated with
the Katafuchi score of renal damage (data not shown). The
endogenous creatinine clearance rate was negatively correlated with
the Katafuchi score of renal damage (r=−0.72; P<0.05) (data not
shown). The expression levels of BAFF, TRAF6, FN, p-NF-κBp65 and
CTGF were markedly higher in the kidneys of patients with IgA
nephropathy than those in the control group. The overexpression of
proteins was mainly concentrated in the glomerular mesangial area
(Fig. 2).
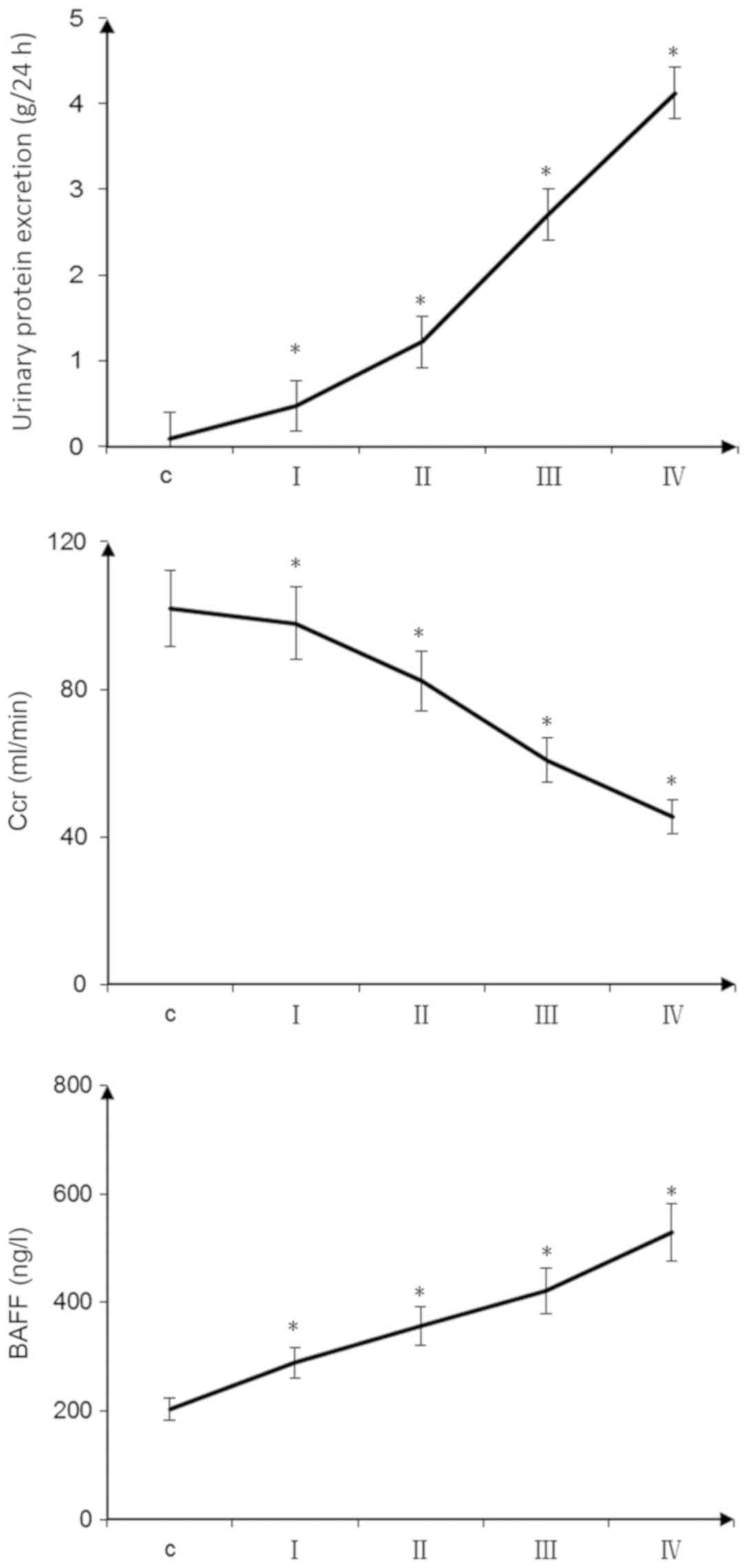 | Figure 1.Clinical indicators of patients with
IgA nephropathy. The 24-h urine protein content and plasma BAFF
concentrations of patients with IgA nephropathy were increased with
increasing glomerular pathological score. Endogenous Ccr decreased
with increasing glomerular pathological score (*P<0.05, pairwise
comparisons between groups: I vs. C, II vs. I, III vs. II, IV vs.
III). Scoring groups: I, 0–3 points; II, 4–6 points; III, 7–9
points; and IV, 10–12 points. BAFF, B cell-activating factor; C,
control; Ccr, creatinine clearance rate. |
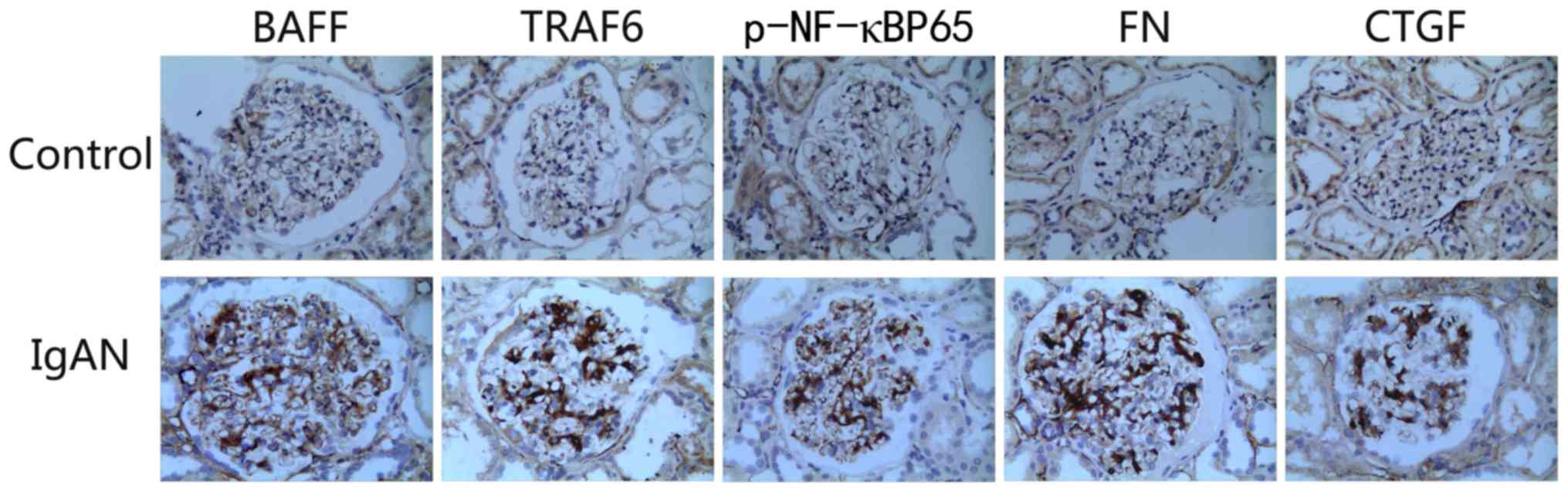 | Figure 2.Immunohistochemistry to detect the
expression of various factors in the kidneys of patients with IgAN
(magnification, ×400). In comparison with the healthy control
group, the expression levels of BAFF, TRAF6, p-NF-κBp65, FN and
CTGF were markedly increased in the kidneys of patients with IgAN.
BAFF, B cell-activating factor; CTGF, connective tissue growth
factor; FN, fibronectin; IgAN, IgA nephropathy; p-, phosphorylated;
TRAF, tumor necrosis factor receptor-associated factor. |
BAFF-R is expressed on the surface of
glomerular mesangial cells
Western blotting was used to detect the expression
of BAFF-R protein. The results revealed that there was a small
amount of BAFF-R protein expression in mesangial cells. Following
the addition of 20 ng/ml BAFF for 48 h, the protein expression
levels of BAFF-R were significantly increased in mesangial cells
(P<0.05). The presence of BAFF-R on the surface of the mesangial
cell membrane was confirmed by flow cytometry (Fig. 3).
BAFF induces the mRNA expression
levels of TRAF6, FN, CTGF and NF-κBP65 in glomerular mesangial
cells
Following the addition of 20 ng/ml BAFF to
glomerular mesangial cells for 48 h, the mRNA expression levels of
FN, TRAF6, CTGF and NF-κBP65 were significantly increased in the
glomerular mesangial cells (P<0.05 vs. the control group).
Glomerular mesangial cells transfected with the shRNA-TRAF6 plasmid
exhibited reduced expression of TRAF6 mRNA (P<0.05 vs. the
control group), and following treatment with 20 ng/ml BAFF for 48
h, the mRNA expression levels of NF-κBP65 were significantly lower
than those observed in the con-shRNA + BAFF group, and the mRNA
expression levevls of FN and CTGF were also decreased (P<0.05
vs. the con-shRNA + BAFF group; Fig.
4).
 | Figure 4.Reverse transcription-quantitative
PCR was performed to detect the mRNA expression levels of NF-κBp65
and TRAF6, FN and CTGF in the cytoplasm. A, con-shRNA; B, con-shRNA
+ BAFF; C, con-shRNA + BAFF + BAFF-RFc chimera protein; D,
TRAF6-shRNA; E, TRAF6-shRNA + BAFF. *P<0.05 vs. group A;
#P<0.05 vs. group B. BAFF, B cell-activating factor;
BAFF-R, BAFF receptor; CTGF, connective tissue growth factor; FN,
fibronectin; p-, phosphorylated; TRAF, tumor necrosis factor
receptor-associated factor; shRNA, short hairpin RNA; con-,
control. |
BAFF enhances expression of TRAF6, FN,
CTGF and nuclear p-NF-κBP65 proteins in glomerular mesangial
cells
Following the addition of 20 ng/ml BAFF to the
glomerular mesangial cells for 48 h, the protein expression levels
of TRAF6 in mesangial cells and p-NF-κBP65 in the nuclei of
mesangial cells were significantly increased. Notably, total NF-κB
protein expression in the cells did not change, and the proportion
of p-F-κBP65/NF-κBP65 was significantly higher (P<0.05 vs. the
control group). In addition, the expression levels of the
downstream proteins, FN and CTGF, were enhanced by BAFF treatment
(P<0.05 vs. the control group). Mesangial cells transfected with
the shRNA-TRAF6 plasmid exhibited reduced expression of TRAF6
protein (P<0.05 vs. the control group), and after 48 h of
treatment with 20 ng/ml BAFF, the protein expression levels of
p-NF-κBP65 in the nucleus were significantly lower than those
observed in the con-shRNA + BAFF group, and FN and CTGF protein
expression was also decreased (P<0.05 vs. con-shRNA + BAFF
group; Fig. 5).
 | Figure 5.Western blot analysis to detect the
protein expression levels of TRAF6, p-NF-κBP65, FN and CTGF in
glomerular mesangial cells. A, con-shRNA; B, con-shRNA + BAFF; C,
con-shRNA + BAFF + BAFF-RFc chimera protein; D, TRAF6-shRNA; E,
TRAF6-shRNA + BAFF. *P<0.05 vs. group A; #P<0.05
vs. group B. BAFF, B cell-activating factor; BAFF-R, BAFF receptor;
CTGF, connective tissue growth factor; FN, fibronectin; p-,
phosphorylated; TRAF, tumor necrosis factor receptor-associated
factor; shRNA, short hairpin RNA; con-, control. |
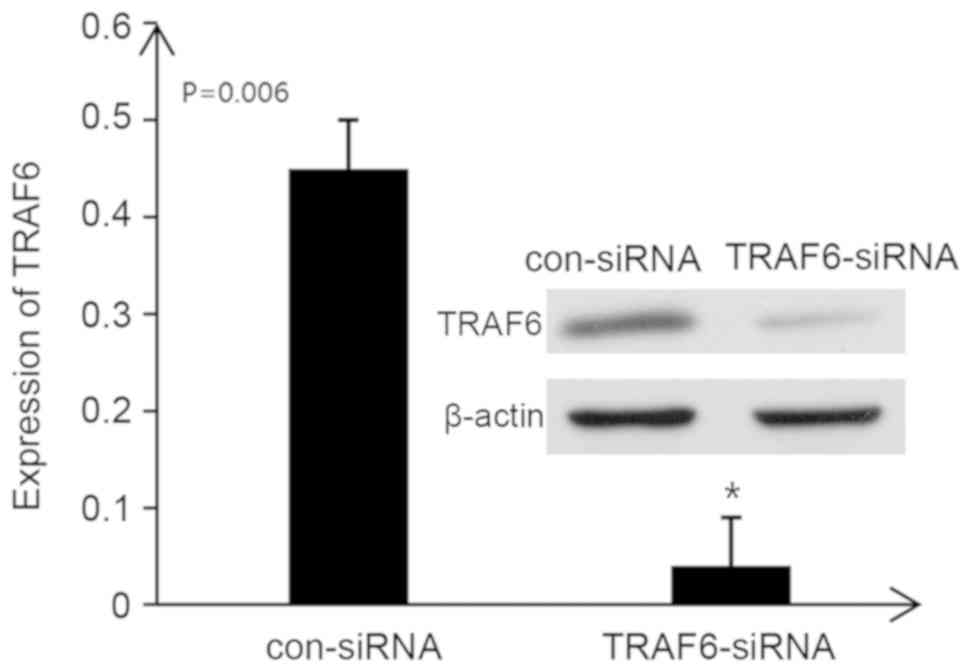
TRAF6-siRNA inhibits the expression of
TRAF6 in rat kidney tissues
When TRAF6-siRNA was administered into the tail vein
of rats, the protein expression levels of TRAF6 in the kidneys of
rats with IgA nephropathy were significantly decreased (P<0.05).
These findings suggested that TRAF6 expression in the kidneys of
rats with IgA nephropathy was successfully inhibited by TRAF6-siRNA
(Fig. 6).
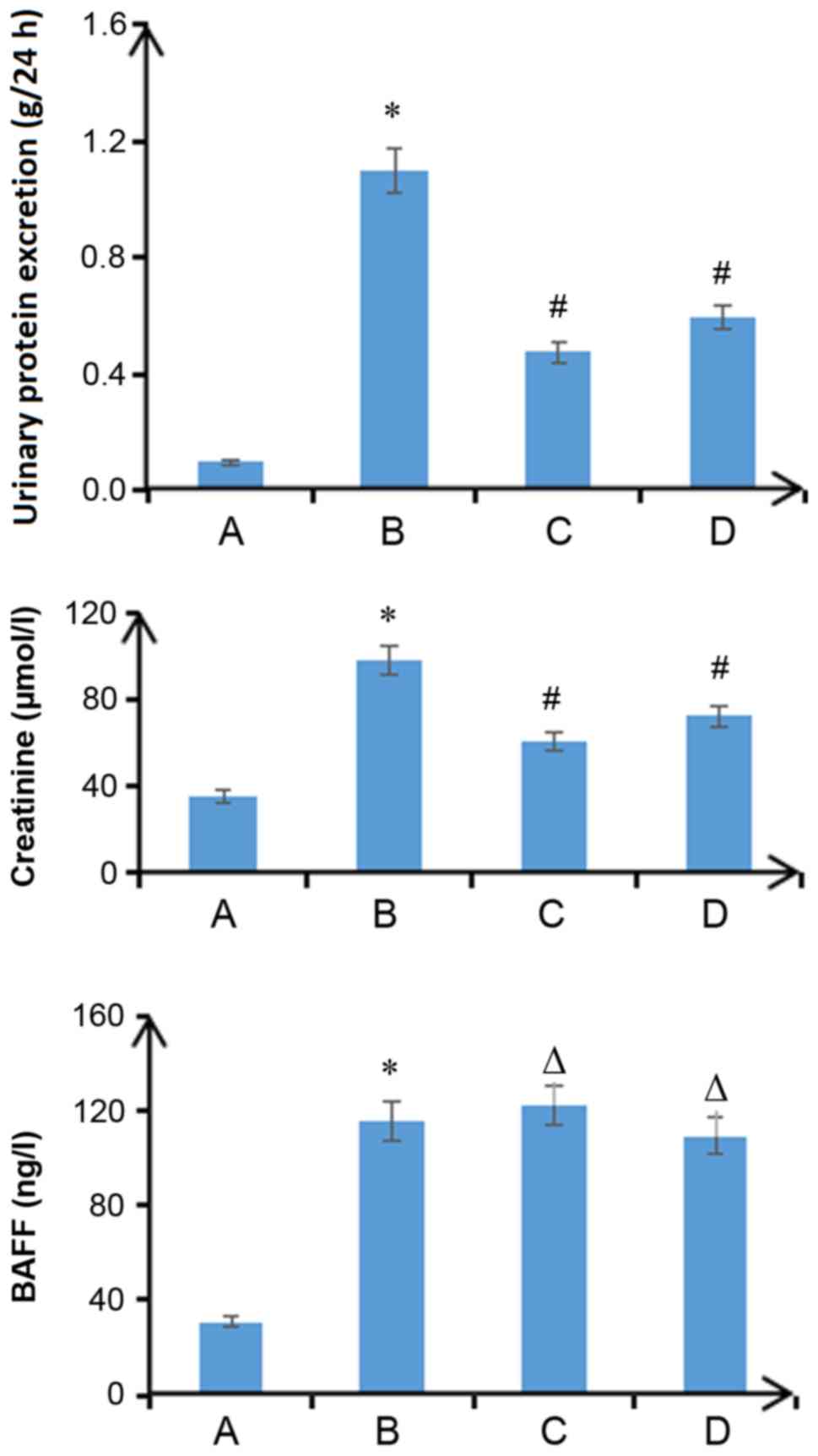
Plasma BAFF, proteinuria and serum
creatinine levels are significantly increased in rats with IgA
nephropathy
Proteinuria, serum creatinine concentration and
plasma BAFF concentration of rats in the IgA nephropathy group were
significantly higher than those in the control group (P<0.05).
In the Fc + IgA and TRAF6-siRNA groups, plasma BAFF concentration
was not significantly different compared with the IgA nephropathy
group (P>0.05); however, the other two markers were
significantly lower than those in the IgA nephropathy group
(P<0.05; Fig. 7).
BAFF may enhance the expression of
fibroblast factors in the kidneys by activating the TRAF6/NF-κB
signaling pathway
The expression levels of BAFF, TRAF6, p-NF-κBp65, FN
and CTGF in the kidneys of rats with IgA nephropathy were markedly
increased compared with in the control group (P<0.05), and these
proteins were mainly distributed in the glomerular mesangial area.
The expression levels of BAFF in the TRAF6-siRNA + IgA and Fc
groups were not significantly different from those in the IgA
nephropathy group (P>0.05); however, the expression levels of
TRAF6, p-NF-ΚBP65, FN and CTGF were significantly lower than those
observed in the IgA nephropathy group (P<0.05; Fig. 8).
Inhibition of BAFF-R binding and the
TRAF6/NF-κB signaling pathway may reduce kidney damage in rats with
IgA nephropathy
The expression levels of BAFF, TRAF6, FN, CTGF and
nuclear p-NF-κBP65 proteins in rats with IgA nephropathy were
significantly higher than those in the control group (P<0.05).
Notably, total NF-κB protein expression in the cells did not
change, whereas the proportion of p-NF-κBP65/NF-κBP65 was
significantly higher (P<0.05 vs. the control group). In the
TRAF6-siRNA + IgA and Fc groups, the protein expression levels of
BAFF were not significantly different from those in the IgA
nephropathy group (P>0.05); however, the expression levels of
TRAF6, FN, CTGF and nuclear p-NF-κBP65 proteins were significantly
lower than those in the IgA nephropathy group (P<0.05; Fig. 9).
 | Figure 9.Western blot analysis to detect the
expression levels of marker proteins in kidney tissues. A,
con-siRNA; B, con-siRNA + IgA; C, BAFF-RFc chimera protein + IgA;
D, TRAF6-siRNA + IgA. *P<0.05 vs. group A; ΔP>0.05
vs. group B; #P<0.05 vs. group B. BAFF, B
cell-activating factor; CTGF, connective tissue growth factor; FN,
fibronectin; p-, phosphorylated; siRNA, small interfering RNA;
TRAF, tumor necrosis factor receptor-associated factor. |
Discussion
The pathogenesis of IgA nephropathy is complex and
is a key area of research. The pathogenesis of IgA nephropathy
involves the activation of B cells and the production of abnormal
glycosylated IgA1 (30). BAFF is
an important activator for B-cell activation, which induces B-cell
proliferation, differentiation and the secretion of immunoglobulin;
it also serves an important role in humoral immunity (31). Previous studies have reported that
synthetic double-stranded RNA poly (I) aggravates kidney damage in
rats with IgA nephropathy through the Toll-like receptor 3-BAFF
signaling pathway (32–35). The present study demonstrated that
plasma BAFF levels in patients with IgA nephropathy were
significantly higher than those in the control group, and were
positively correlated with Katafuchi scores, which was consistent
with the results reported by Xin et al (13). These results suggested that BAFF
levels were closely associated with the severity of IgA
nephropathy. In addition, the results revealed that BAFF expression
in the kidneys of patients with IgA nephropathy was significantly
increased and was positively correlated with protein concentrations
in the urine; they were also negatively correlated with the
clearance rate of endogenous creatinine. These results suggested
that BAFF may serve a key role in the pathogenesis of IgA
nephropathy.
It has been reported that BAFF binds to its receptor
to promote the survival, proliferation, differentiation and
maturation of cells by recruiting TRAF and activating JNK,
Rel/NF-κB and MAPK (36). By using
a yeast two-hybrid system, Xu and Shu (37) demonstrated that TRAF3 could bind to
the intracellular domain of BAFF-R and negatively regulate
BAFF-R-mediated NF-κB activation and IL-10 production. Members of
the matrix protein family (TRAF1, −2 and −6), could induce
inflammatory immunity and the apoptotic effect by mediating
different signaling pathways. Pullen et al (38) reported that TRAF6 mediated the
strongest NF-κB activation downstream of CD40 in TRAF family
members, and the CD40-mediated NF-κB signaling pathway was
inactivated in TRAF6-deficient splenocytes. The activation of NF-κB
and downstream inflammatory factors is highly associated with the
occurrence of IgA nephropathy. These findings suggested that
BAFF-mediated activation of B cells and the associated TRAF6/NF-κB
pathway further led to the release of downstream inflammatory
factors, which in turn serve a critical role in the pathogenesis of
immune diseases; however, this mechanism has not been reported in
IgA nephropathy.
Notably, our preliminary experiments revealed that
plasma BAFF levels were significantly elevated in patients with IgA
nephropathy compared with in controls, and the expression levels of
TRAF6/NF-κB signaling pathway-related proteins were also markedly
increased in the kidneys of patients with IgA nephropathy. These
findings suggested that BAFF may be involved in the pathogenesis of
IgA nephropathy through the TRAF6/NF-κB signaling pathway. This
hypothesis has been further confirmed in the present cell culture
and animal model experiments. It has previously been reported that
BAFF-R is expressed on the surface of mesangial cells (9). In the present in vitro cell
culture study, the expression of BAFF-R on the cell surface was
confirmed by flow cytometry and western blotting. Following
stimulation with BAFF, BAFF-R expression was increased. In
addition, the results revealed that TRAF6 expression was increased
following BAFF stimulation and the nuclear expression levels of the
transcription factor NF-κBP65 were increased to further activate
the release of downstream cytokines FN and CTGF; this may lead to
proliferation and apoptosis of glomerular mesangial cells.
Furthermore, these markers were significantly reduced following
treatment with the BAFF-R Fc chimera protein, suggesting that BAFF
could be critical in binding to BAFF-R. The expression of
endogenous TRAF6 was significantly inhibited post-transfection with
the shRNA-TRAF6 plasmid, and the expression levels of NF-κBP65 in
the nucleus were reduced, resulting in a reduction in the release
of the corresponding downstream cytokines. These results
demonstrated that BAFF may induce the proliferation of mesangial
cells and the production of fibrotic factors by activating the
TRAF6/NF-KB signaling pathway via binding to BAFF-R.
In the IgA nephropathy model animals, plasma BAFF
levels were significantly increased compared with in animals in the
control group, and the expression levels of TRAF6 and p-NF-κBP65
were increased. These increased expression levels were mainly in
the mesangial regions. These results were consistent with patients
with IgA nephropathy. It was suggested that the high level of BAFF
expression in patients with IgA nephropathy may be involved in the
activation of signaling pathways and development of IgA
nephropathy. Administration of the BAFF-R Fc chimera protein, in
order to interfere with the binding of BAFF and BAFF-R, had no
effect on plasma BAFF levels; however, it did significantly inhibit
activation of the TRAF6/NF-κB signaling pathway. A previous study
reported that siRNA injection into the rat tail vein can
successfully silence related gene expression (27). In this study, administration of
TRAF6-siRNA into the tail vein had no effect on plasma BAFF levels;
however, TRAF6 expression was almost non-existent in rat kidneys in
response to TRAF6-siRNA. TRAF-6 siRNA also resulted in reduced
expression of nuclear p-NF-κBP65 and reduced release of the
corresponding cytokines. The present results revealed that in the
animal model of IgA nephropathy, the plasma levels of BAFF were
increased, and the TRAF6/NF-KB signaling pathway in mesangial cells
was activated by binding to BAFF-R, which may increased fibrogenic
factor release, thus leading to glomerular fibrosis.
In conclusion, the results of the present study
indicated that BAFF may be involved in the pathogenesis of IgA
nephropathy by binding to BAFF-R and activating the TRAF6/NF-κB
signaling pathway. These results provide a novel avenue for
searching for targets for the treatment of IgA nephropathy.
However, BAFF has three receptors; whether the other two receptors
could be involved in the process will be the focus of future
studies.
Acknowledgements
Not applicable.
Funding
This study was supported by the Projects of Science
and Technology Funds of Nantong (grant no. MSZ18086).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YC and GL conceived and coordinated the study, and
designed, performed and analyzed the experiments. YC wrote the
manuscript. XiC, XuC and NG carried out data collection and data
analysis, and revised the paper. WL carried out animal experiments.
All authors reviewed the results and approved the final version of
the manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the medical
ethics committee of the Affiliated Hospital of Nantong University,
and all subjects provide written informed consent. The animal
protocol in the present study was approved by the Medical Ethics
Committee of The Nantong University Affiliated Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
BAFF
|
B cell-activating factor
|
|
TNF
|
tumor necrosis factor
|
|
TRAF
|
TNF receptor-associated factor
|
References
|
1
|
Lee K, Shin J, Park J, Hwang S, Jang HR,
Huh W, Kwon GY, Kim YG, Oh HY, Lee JE and Kim DJ: First-year GFR
slope and long-term renal outcome in IgA nephropathy. Eur J Clin
Invest. 48:e129362018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Roberts IS: Pathology of IgA nephropathy.
Nat Rev Nephrol. 10:445–454. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Coppo R: Treatment of IgA nephropathy:
Recent advances and prospects. Nephrol Ther. 14 (Suppl 1):S13–S21.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rauen T and Floege J: Inflammation in IgA
nephropathy. Pediatr Nephrol. 32:2215–2224. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Coppo R: Biomarkers and targeted new
therapies for IgA nephropathy. Pediatr Nephrol. 32:725–731. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xie YX, He LY, Chen X, Peng XF, Ye MY,
Zhao YJ, Yan WZ, Liu C, Shao J and Peng YM: Potential diagnostic
biomarkers for IgA nephropathy: A comparative study pre- and
post-tonsillectomy. Int Urol Nephrol. 48:1855–1861. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li C, Shi J and Zhao Y: MiR-320 promotes B
cell proliferation and the production of aberrant glycosylated IgA1
in IgA nephropathy. J Cell Biochem. 119:4607–4614. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Park SJ, Oh JY and Shin JI: Could
increased IgA induced by BAFF be the cause of IgA nephropathy
development in Behcet's disease? Comment on: Behcet's disease and
IgA nephropathy (Rheumatol Int. 2012 Jul; 32(7):2227-9). Rheumatol
Int. 34:283–284. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zheng N, Wang D, Ming H, Zhang H and Yu X:
BAFF promotes proliferation of human mesangial cells through
interaction with BAFF-R. BMC Nephrol. 16:722015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ye M, Peng Y, Liu C, Yan W, Peng X, He L,
Liu H and Liu F: Vibration induces BAFF overexpression and aberrant
O-Glycosylation of IgA1 in cultured human tonsillar mononuclear
cells in IgA nephropathy. Biomed Res Int. 2016:91259602016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zheng N, Fan J, Wang B, Wang D, Feng P,
Yang Q and Yu X: Expression profile of BAFF in peripheral blood
from patients of IgA nephropathy: Correlation with clinical
features and Streptococcus pyogenes infection. Mol Med Rep.
15:1925–1935. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shao J, Peng Y, He L, Liu H, Chen X and
Peng X: Capsaicin induces high expression of BAFF and aberrantly
glycosylated IgA1 of tonsillar mononuclear cells in IgA nephropathy
patients. Hum Immunol. 75:1034–1039. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xin G, Shi W, Xu LX, Su Y, Yan LJ and Li
KS: Serum BAFF is elevated in patients with IgA nephropathy and
associated with clinical and histopathological features. J Nephrol.
26:683–690. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
McCarthy DD, Kujawa J, Wilson C, Papandile
A, Poreci U, Porfilio EA, Ward L, Lawson MA, Macpherson AJ, McCoy
KD, et al: Mice overexpressing BAFF develop a commensal
flora-dependent, IgA-associated nephropathy. J Clin Invest.
121:3991–4002. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Walsh MC, Lee J and Choi Y: Tumor necrosis
factor receptor-associated factor 6 (TRAF6) regulation of
development, function, and homeostasis of the immune system.
Immunol Rev. 266:72–92. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kanaya T, Sakakibara S, Jinnohara T,
Hachisuka M, Tachibana N, Hidano S, Kobayashi T, Kimura S, Iwanaga
T, Nakagawa T, et al: Development of intestinal M cells and
follicle-associated epithelium is regulated by TRAF6-mediated NF-KB
signaling. J Exp Med. 215:501–519. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fang J, Muto T, Kleppe M, Bolanos LC,
Hueneman KM, Walker CS, Sampson L, Wellendorf AM, Chetal K, Choi K,
et al: TRAF6 mediates basal activation of NF-KB necessary for
hematopoietic stem cell homeostasis. Cell Rep. 22:1250–1262. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou Y, Jin XH, Jing YX, Song Y, He XX,
Zheng LL, Wang YB, Wei ZY and Zhang GP: Porcine parvovirus
infection activates inflammatory cytokine production through
Toll-like receptor 9 and NF-KB signaling pathways in porcine kidney
cells. Vet Microbiol. 207:56–62. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cai M, Li M, Wang K, Wang S, Lu Q, Yan J,
Mossman KL, Lin R and Zheng C: The herpes simplex virus 1-encoded
envelope glycoprotein B activates NF-KB through the Toll-like
receptor 2 and MyD88/TRAF6-dependent signaling pathway. PLoS One.
8:e545862013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
He X, Zheng Y, Liu S, Shi S, Liu Y, He Y,
Zhang C and Zhou X: MiR-146a protects small intestine against
ischemia/reperfusion injury by down-regulating TLR4/TRAF6/NF-KB
pathway. J Cell Physiol. 233:2476–2488. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang P, Cao J, Liu S, Pan H, Liu X, Sui A,
Wang L, Yao R, Liu Z and Liang J: Upregulated microRNA-429 inhibits
the migration of HCC cells by targeting TRAF6 through the NF-KB
pathway. Oncol Rep. 37:2883–2890. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu J, Zhang Z, Guo Q, Dong Y, Zhao Q and
Ma X: Syringin prevents bone loss in ovariectomized mice via TRAF6
mediated inhibition of NF-KB and stimulation of PI3K/AKT.
Phytomedicine. 42:43–50. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhong JH, Li J, Liu CF, Liu N, Bian RX,
Zhao SM, Yan SY and Zhang YB: Effects of microRNA-146a on the
proliferation and apoptosis of human osteoarthritis chondrocytes by
targeting TRAF6 through the NF-KB signalling pathway. Biosci Rep.
37:BSR201605782017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Guobing X, Lili J, Lihua Z and Tiean X:
Application of an improved biuret method to the determination of
total protein in urine and cerebrospinal fluid without
concentration step by use of Hitachi 7170 auto-analyzer. J Clin Lab
Anal. 15:161–164. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shoker A, Hossain MA, Koru-Sengul T, Raju
DL and Cockcroft D: Performance of creatinine clearance equations
on the original Cockcroft-Gault population. Clin Nephrol. 66:89–97.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Katafuchi R, Kiyoshi Y, Oh Y, Uesugi N,
Ikeda K, Yanase T and Fujimi S: Glomerular score as a
prognosticator in IgA nephropathy: Its usefulness and limitation.
Clin Nephrol. 49:1–8. 1998.PubMed/NCBI
|
|
27
|
Lei Y, Tang L, Xie Y, Xianyu Y, Zhang L,
Wang P, Hamada Y, Jiang K, Zheng W and Jiang X: Gold
nanoclusters-assisted delivery of NGF siRNA for effective treatment
of pancreatic cancer. Nat Commun. 8:151302017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang L, Wang Y, Nuerbiye A, Cheng P, Wang
JH, Kasimu R and Li H: Effects of periostracum cicadae on cytokines
and apoptosis regulatory proteins in an IgA nephropathy rat model.
Int J Mol Sci. 19:E15992018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
He L, Peng X, Wang J, Tang C, Zhou X, Liu
H, Liu F, Sun L and Peng Y: Synthetic Double-stranded RNA Poly(I:C)
aggravates IgA nephropathy by triggering IgA class switching
recombination through the TLR3-BAFF Axis. Am J Nephrol. 42:185–197.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mackay F, Figgett WA, Saulep D, Lepage M
and Hibbs ML: B-cell stage and context-dependent requirements for
survival signals from BAFF and the B-cell receptor. Immunol Rev.
237:205–225. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li W, Peng X, Liu Y, Liu H, Liu F, He L,
Liu Y, Zhang F, Guo C, Chen G, et al: TLR9 and BAFF: Their
expression in patients with IgA nephropathy. Mol Med Rep.
10:1469–1474. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tang X, Zhang L and Wei W: Roles of TRAFs
in NF-KB signaling pathways mediated by BAFF. Immunol Lett.
196:113–118. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tian J, Jiao X, Wang X, Geng J, Wang R,
Liu N, Gao X, Griffin N and Shan F: Novel effect of methionine
enkephalin against influenza A virus infection through inhibiting
TLR7-MyD88-TRAF6-NF-KB p65 signaling pathway. Int Immunopharmacol.
55:38–48. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hildebrand JM, Luo Z, Manske MK,
Price-Troska T, Ziesmer SC, Lin W, Hostager BS, Slager SL, Witzig
TE, Ansell SM, et al: A BAFF-R mutation associated with non-Hodgkin
lymphoma alters TRAF recruitment and reveals new insights into
BAFF-R signaling. J Exp Med. 207:2569–2579. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen JX, Shen HH, Niu M, Guo YM, Liu XQ,
Han YZ, Zhang YM, Zhao YL, Bai BK, Zhou WJ and Xiao XH:
Anti-hepatitis B virus effect of matrine-type alkaloid and
involvement of p38 mitogen-activated protein kinase and tumor
necrosis factor receptor-associated factor 6. Virus Res.
215:104–113. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xu LG and Shu HB: TNFR-associated factor-3
is associated with BAFF-R and negatively regulates BAFF-R-mediated
NF-kappa B activation and IL-10 production. J Immunol.
169:6883–6889. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pullen SS, Dang TT, Crute JJ and Kehry MR:
CD40 signaling through tumor necrosis factor receptor-associated
factors (TRAFs). Binding site specificity and activation of
downstream pathways by distinct TRAFs. J Biol Chem.
274:14246–14254. 1999. View Article : Google Scholar : PubMed/NCBI
|