Introduction
Osteoporosis is a chronic disease with a heavy
global socioeconomic burden. It is defined as a skeletal disorder,
characterized by decreased bone strength, which in turn predisposes
affected individuals to fractures (1) and damage to the bone microarchitecture
(2). Osteoporosis is diagnosed
based on the presence of fragility fractures in the absence of
other metabolic bone disorders, or a T-score of ≤2.5 in the lumbar
spine (anteroposterior), femoral neck and total hip, and/or 33%
(one-third) radius, even in the absence of a prevalent fracture.
Osteoporosis may also be diagnosed in patients with osteopenia and
increased fracture risk, using FRAX® country-specific
thresholds. Osteopenia is defined as T-score between −1.0 and −2.5,
based on bone mineral density testing (3). There are two major categories of
osteoporosis: Primary and secondary. Sex and age are the primary
influencing factors for primary osteoporosis, whereas secondary
osteoporosis is associated with long-term and high-dose
glucocorticoid treatment (4).
Glucocorticoids such as dexamethasone (Dex) and hydrocortisone are
widely used to treat inflammation and immunological rejection, as
well as autoimmune diseases (5).
These diseases not only include rheumatoid arthritis and systemic
lupus erythematosus, but also asthma, chronic obstructive pulmonary
disease, Crohn's disease and ulcerative colitis (6–12).
Dex-induced osteogenic inhibition has been considered as the most
severe side-effect of this particular treatment type (13), and long-term administration of
glucocorticoids can result in osteoporosis or osteonecrosis
(14). However, the molecular
mechanism underlying glucocorticoid-induced osteogenic inhibition
is not clear.
Nicotinamide mononucleotide (NMN) is an important
NAD+ intermediate whose levels decrease with age. As
such, NMN administration is an effective treatment for age-related
diseases and bone metabolism (Table
I), and can be used to alleviate age-related type 2 diabetes,
ischemia-reperfusion injury and Alzheimer's disease (15). NMN has previously been reported to
improve osteogenesis by regulating sirtuin 1 (SIRT1) (16). The present study aimed to
investigate the role of NMN against the glucocorticoid-induced loss
of bone cell viability via mesenchymal stromal cell (MSC)
regulation. MSCs are non-hematopoietic pluripotent stem cells with
regenerative capacity, and with age, a reduction in the number or
function of MSCs severely limits tissue regeneration (17). However, the mechanism of action of
NMN in glucocorticoid-induced loss of bone cell viability remains
unclear.
 | Table I.Study characteristics. |
Table I.
Study characteristics.
| Study | Type | Primary
findings | (Refs.) |
|---|
| Song et al,
2019 | Research | NMN promotes
osteogenesis via SIRT1 | (16) |
| Zainabadi,
2019 | Review | NMN improve
osteogenesis | (45) |
| Liang et al,
2019 | Research | NMN alleviates
aluminum-induced bone loss by inhibiting the
thioredoxin-interacting protein-NLRP3 inflammasome | (42) |
| Hassan et
al, 2018 | Research | Nicotinamide
phosphoribosyltransferase expression in osteoblasts controls
osteoclast recruitment in alveolar bone remodeling | (46) |
| Mills et al,
2016 | Research | Long-term NMN
administration significantly improves bone density | (47) |
| Baek et al,
2017 | Research | Nicotinamide
phosphoribosyltransferase inhibits receptor activator of nuclear
factor-κB ligand-induced osteoclast differentiation in
vitro | (48) |
| Abed et al,
2014 | Research | Low SIRT1 levels in
human osteoarthritis subchondral osteoblasts lead to abnormal
sclerostin expression which decreases Wnt/β-catenin activity | (49) |
SIRT1, also known as silent mating type information
regulation 2 homolog, was discovered in humans in 1999 (18), and is an NAD-dependent class III
protein deacetylase (19). As a
deacetylase, SIRT1 is closely associated with various other
proteins such as p53, Ku70, forkhead box protein O1, NF-κB,
peroxisome proliferator-activated receptor-γ and p300, and is
involved in the regulation of cell senescence and apoptotic death
under stress conditions, thereby enhancing cellular activity,
self-healing and survival capacity (20–23).
Numerous studies have reported the involvement of SIRT1 in bone
metabolism, and as a mediator of bone mass regulation (24–26).
Conditional knockout of SIRT1 leads to low bone density and mass,
and a significant increase in body weight, skeletal size, bone
volume, osteoblast numbers, alkaline phosphatase (ALP)- and type I
collagen-positive areas in SIRT1 transgenic mice (27).
In the present study, the effects of NMN on
glucocorticoid-induced loss of bone cell viability, and its
underlying mechanisms, underwent preliminary investigation. It was
hypothesized that NMN plays a protective role in
glucocorticoid-induced osteoporosis, and the present study provides
a potential therapeutic method for glucocorticoid-induced
osteoporosis.
Materials and methods
Cell culture and osteogenic
induction
Bone mesenchymal stem cells (BMSCs) at passage 6
were obtained from Cyagen Biosciences, Inc. The cells were cultured
in C57BL/6 Mouse Mesenchymal Stem Cell growth medium (Cyagen
Biosciences, Inc.) containing 10% FBS, 1% glutamine and 1%
penicillin-streptomycin, and incubated at 37°C in a humidified
atmosphere (5% CO2). For osteogenic induction, BMSCs
were seeded into 6-well plates at a density of 2×105
cells per well. The culture medium was substituted for C57BL/6
Mouse Mesenchymal Stem Cell osteogenic differentiation medium
(Cyagen Biosciences, Inc.; 10% FBS, 1% penicillin-streptomycin,
0.2% ascorbate, 1% glutamine, 10−10 M Dex and 10 mM
ß-glycerophosphate), and the cells were incubated until reaching
80% confluence. The negative control group was incubated in
osteogenic differentiation medium supplemented with
10−10 M Dex. For subsequent experiments, BMSCs were used
between passages 7 and 10. The osteogenic induction medium was
replaced every 3 days. After incubation for 7 days, subsequent
experiments were performed.
Nicotinamide mononucleotide (NMN)
treatment
BMSCs were treated with 1, 5 or 10 mM NMN (cat. No.
1094-61-7) for 7 days. The vehicle group was cultured with
osteogenic differentiation medium for 7 days. The Dex groups were
cultured with osteogenic differentiation medium containing
10−6 M Dex for 7 days. The NMN groups were cultured with
osteogenic differentiation medium containing 10−6 M Dex
and 1, 5 or 10 mM NMN for 7 days.
RNAi and transfection
SIRT1-small interfering (si)RNA and their negative
control (NC) siRNA were purchased from Shanghai GenePharma Co.,
Ltd. BMSCs were transfected with 5 µl SIRT1 siRNA (si-SIRT1:
5′-CCACCUGAGUUGGAUGAUA-3′; 20 µM) or NC siRNA
(5′-ACGUGACACGUUCGGAGAATT-3′; 20 µM) were transfected into BMSCs
using Lipofectamine® RNAi Max reagent (Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. After
48 h, the effect of knockdown was confirmed by reverse
transcription-quantitative PCR (RT-qPCR) and western blotting.
RT-qPCR analysis
Total RNA was isolated from cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) and quantified using an absorbance measurement at a
wavelength of 260 nm. The RNA was reverse-transcribed into cDNA
using a SYBR® PrimeScript™ RT-PCR kit (Takara Bio, Inc.)
The specific primers for mouse ALP, Runt-related transcription
factor 2 (Runx2), osteocalcin (OCN), SIRT1 and peroxisome
proliferator-activated receptor gamma coactivator (PGC)-1α are
listed in Table II. qPCR was
performed using SYBR® Premix Ex Taq (Takara Bio, Inc.)
with the ABI 7500 system (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The thermocycling conditions used for qPCR were
as follows: Initial denaturation at 95°C for 5 min; followed by 40
cycles of denaturation at 95°C for 10 sec, annealing at 60°C for 20
sec and a final extension at 72°C for 20 sec, as previously
described (28). Melting curve
analysis was used to analyze the specificity of transcript
amplification, and target gene expression was quantified using the
2−ΔΔCq method and normalized to the internal reference
gene GAPDH (29).
 | Table II.Primers used for qPCR. |
Table II.
Primers used for qPCR.
|
| Primer sequence
(5′→3′) |
|---|
|
|
|
|---|
| Gene | Forward | Reverse |
|---|
| Runx2 |
GACGAGGCAAGAGTTTCACC |
GGACCGTCCACTGTCACTTT |
| ALP |
TCGGGACTGGTACTCGGATAAC |
GTTCAGTGCGGTTCCAGACATAG |
| OCN |
CAAGCAGGGAGGCAATAAGG |
CGTCACAAGCAGGGTTAAGCQ |
| SIRT1 |
CACATGCCAGAGTCCAAGTT | AAATCCAGA
TCCTCCAGCAC |
| PGC-1α |
AACCACACCCACAGGATCAGA |
TCTTCGCTTTATTGCTCCATGA |
| GAPDH |
AGGAGCGAGACCCCACTAACA |
AGGGGGGCTAAGCAGTTGGT |
Protein extraction and western
blotting
For nuclear and cytoplasmic proteins, the Nuclear
and Cytoplasmic Protein Extraction kit (Beyotime Institute of
Biotechnology) with 1% Triton X-100 (Beyotime Institute of
Biotechnology) was added to cells in order to solubilize plasma
membrane and keep the nuclear membrane intact. The supernatant was
incubated at 4°C for 20 min, and then 500 µl nuclear isolation
buffer was added. Next, homogenates were centrifuged at 600 × g (10
min, 4°C) for separation into the supernatant cytosolic fraction
and pellet nuclear fraction. Proteins were then lysed using RIPA
lysis buffer (Beyotime Institute of Biotechnology) (30). Total protein was extracted from
cells using RIPA lysis buffer containing 10% phenylmethylsulfonyl
fluoride (Beyotime Institute of Biotechnology) according to the
manufacturer's instructions. Protein concentration was assessed
using a bicinchoninic acid kit (Beyotime Institute of
Biotechnology) according to the manufacturer's protocol. Protein
samples (30 µg) were separated by 8–12% SDS-PAGE and
electro-transferred onto PVDF membranes. Membranes were blocked
with 5% non-fat milk for 2 h at room temperature, and then
incubated with primary antibodies against ALP (cat. no. ab229126,
1:1,000), Runx2 (Abcam; cat. no. ab192256, 1:1,000), SIRT1 (Abcam;
cat. no. ab110304, 1:1,000), proliferating cell nuclear antigen
(PCNA; Abcam; cat. no. ab29, 1:1,000), proliferator-activated
receptor gamma coactivator (PGC)-1α (Abcam; cat. no. ab54481,
1:1,000) and GAPDH (Beyotime Institute of Biotechnology; cat. no.
AF5009, 1:1,000) at 4°C overnight. The membranes were washed three
times with TBS-Tween 20 (1% Tween) and incubated with a horseradish
peroxidase (HRP)-labeled goat anti-rabbit IgG (H+L) (Beyotime
Institute of Biotechnology; cat. no. A0208; 1:2,000) or HRP-labeled
goat anti-mouse IgG (H+L) (Beyotime Institute of Biotechnology;
cat. no. A0216; 1:2,000) for 1 h at room temperature. The bands
were visualized using the Western Chemiluminescent HRP Substrate
kit (EMD Millipore), and ImageJ software (version 1.8.0; National
Institutes of Health) was used for densitometric
quantification.
Alizarin red and ALP staining
Following osteogenic induction, BMSCs were washed
three times with PBS and fixed with 4% paraformaldehyde for 10 min
at room temperature. Alizarin working solution (1%; 1 g Alizarin
diluted in aqueous solution; Cyagen Biosciences, Inc.) was used to
perform Alizarin staining for 3–5 min, and a 5-bromo, 4-chloro,
3-indolylphosphate/Nitro-Blue Tetrazolium ALP Color Development kit
(Beijing Leagene Biotechnology, Co., Ltd.) was used to perform ALP
staining as previously described (31). After washing three times, images of
the stained cells were immediately captured. Alizarin red staining
was used to determine the effectiveness of osteogenic
differentiation by the number and size of red calcium nodules. ALP
staining was used to determine the ALP of BMSCs according to color
shade. Stained cells were observed using a BX51 light microscope
(Olympus Corporation; magnification, ×100).
Cell viability assay
Cell viability was assessed using a Cell Counting
Kit-8 (CCK-8) assay (Beyotime Institute of Biotechnology),
according to the manufacturer's protocol. BMSCs were cultured and
induced in 96-well plates at density of 5×103 cells/well
for 7 days. Subsequently, 10 µl CCK-8 solution was added to each
well and cultured for 1 h. The optical density of each well at a
wavelength of 450 nm was measured using a microplate reader and
analyzed using GraphPad Prism software (version 8.0; GraphPad
Software, Inc.).
ALP activity assay
After osteogenic induction, BMSCs were harvested and
lysed with RIPA lysis buffer (Beyotime Institute of Biotechnology).
Following centrifugation at 18407 × g at 4°C for 10 min, the lysate
supernatants were collected and added to 96-well plates. ALP
activity was detected with the ALP Assay kit (Beyotime Institute of
Biotechnology) using the p-nitrophenylphosphate method, according
to the manufacturer's instructions. The cells were incubated at
37°C for 30 min, and absorbance was measured with a microplate
reader (Omega Bio-Tek, Inc.) at 405 nm. The ALP level was
normalized to the total protein content, and ALP activity was
demonstrated as a fold change over the corresponding control
group.
Immunofluorescence assay
After osteogenic induction and treatment, BMSCs were
washed with PBS and then fixed in 4% paraformaldehyde for 15 min at
room temperature. The cells were permeabilized with 0.1% Triton-X
100 in PBS for 10 min. After blocking with 5% BSA (Sigma-Aldrich;
Merck KGaA) for 1 h at room temperature, the cells were incubated
with primary antibodies against SIRT1 (Abcam; cat. no. ab110304,
1:2,000) and PCNA (Abcam; cat. no. ab92552, 1:500) at 4°C
overnight. The cells were then incubated with DAPI, Alexa
Fluor® 488-AffiniPure goat anti-mouse IgG [H+L (Jackson
ImmunoResearch Europe, Ltd.; cat. no. 115-545-003, 1:200] and Cy3-
AffiniPure goat anti-rabbit IgG [H+L (Jackson ImmunoResearch
Europe, Ltd.; cat. no. 111-165-003, 1:200)] at room temperature for
1 h. Images of cells were captured using a fluorescence microscope
(Olympus Corporation; magnification, ×1,000).
Statistical analysis
All independent experiments were performed ≥3 times
and the data are presented as the mean ± SEM. Statistical
differences were analyzed using a student's t-test or one-way ANOVA
followed by Tukey's multiple comparison post hoc test. Statistical
analysis was carried out using Prism 8 (GraphPad Software, Inc.),
and P<0.05 was considered to indicate a statistically
significant difference.
Results
Dex inhibits the osteogenic function
of BMSCs
A number of studies have reported that
glucocorticoid treatment can suppress the osteogenic function of
osteoblasts, and lead to osteoporosis and osteonecrosis (13,32–34).
To confirm this hypothesis, BMSCs were treated with Dex (dose
range, 10−9−10−6 M). The mRNA expression
levels of the osteogenic markers ALP, Runx2 and OCN were decreased
with increasing concentrations of Dex (Fig. 1A). Moreover, ALP and Runx2 protein
expression was also reduced with an increasing concentration
gradient of Dex (Fig. 1B). ALP
activity can reflect the osteogenic function of osteoblasts, and a
decrease in ALP activity was observed with an increasing
concentration gradient of Dex (Fig.
1C). Dex also decreased the viability of BMSCs (Fig. 1D). A decrease in ALP activity and
cell viability resulted in a subsequent decrease in the osteogenic
function of osteoblasts. According to these results,
10−6 M Dex was used to treat BMSCs in subsequent
experiments, confirming that the osteogenic ability of BMSCs was
markedly inhibited by 10−6 M Dex (Fig. 1E and F).
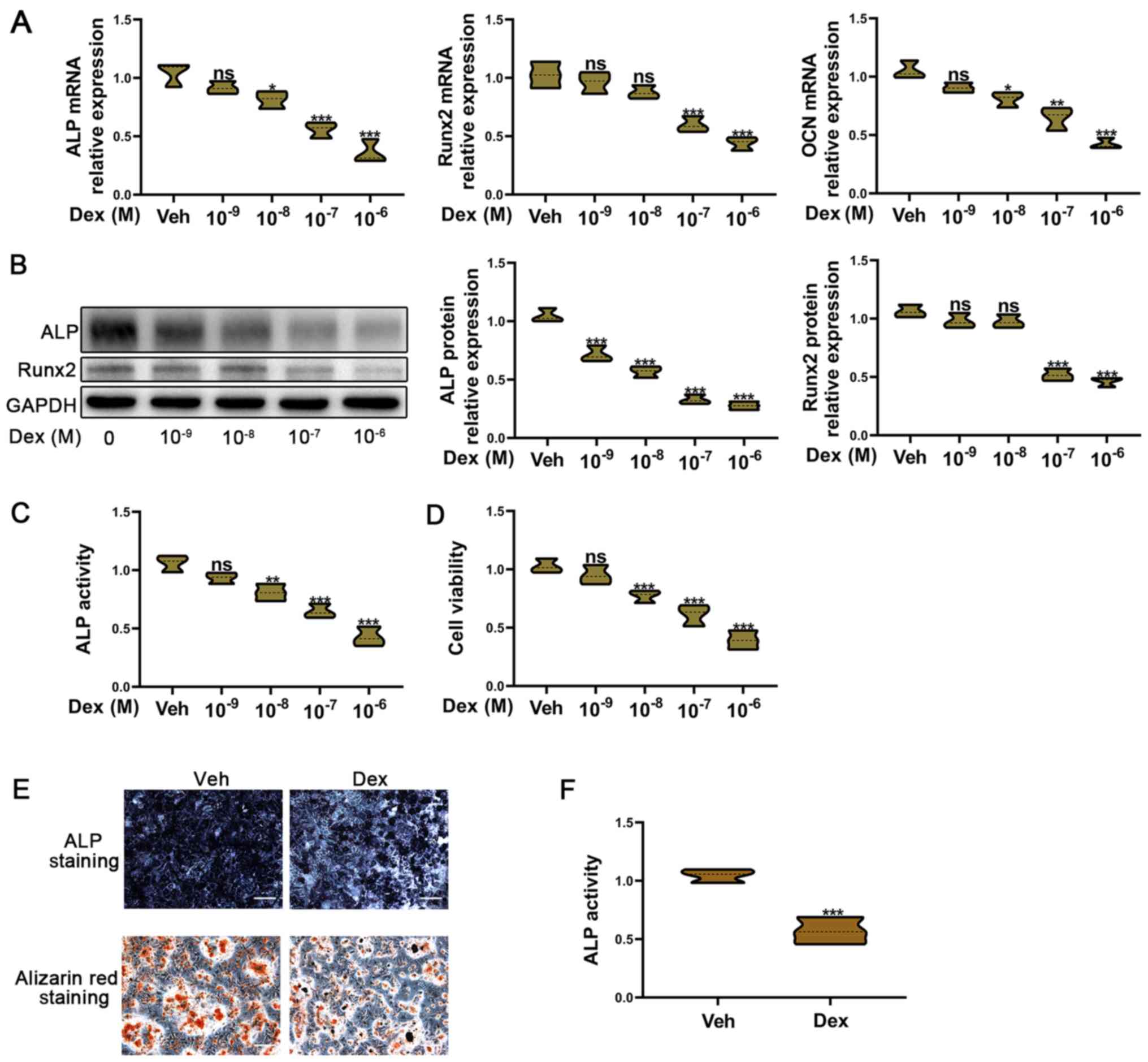 | Figure 1.Dex-induced osteogenic inhibition in
BMSCs. BMSCs were exposed to Dex (range,
10−9−10−6 M) for 7 days. (A) Reverse
transcription-quantitative PCR was used to detect Runx2, ALP and
OCN mRNA expression in BMSCs. (B) Western blotting was used to
determine the Runx2 and ALP protein expression levels in BMSCs. (C)
Relative ALP activity. (D) Cell Counting Kit-8 was used to assess
the viability of BMSCs. (E) Alizarin red and ALP staining assays
were used to measure the osteogenic function of BMSCs treated with
10−6 M Dex for 7 days. Scale bar, 20 µm. (F) Relative
ALP activity of BMSCs treated with 10−6 M Dex for 7
days. All experiments were performed ≥3 times. ***P<0.001,
**P<0.01, *P<0.05 and nsP≥0.05 vs. Veh. Dex,
dexamethasone; BMSCs, bone mesenchymal stem cells; ALP, alkaline
phosphatase; Runx2, Runt-related transcription factor 2; OCN,
osteocalcin; Veh, vehicle. |
NMN attenuates Dex-induced osteogenic
inhibition of BMSCs
A recent study demonstrated that NMN could improve
osteogenesis and reduce the adipogenesis of BMSCs in aging bone
marrow (16). However, the role of
NMN in the glucocorticoid-induced osteogenic inhibition of BMSCs
remains unknown. To investigate whether NMN could promote the
osteogenic ability of glucocorticoid-treated BMSCs, BMSCs were
cultured in medium containing Dex and NMN. The mRNA expression
levels of the osteogenic markers Runx2, ALP and OCN were reduced
following Dex treatment; however, this was attenuated by NMN
(Fig. 2A). The western blotting
results also confirmed that NMN could enhance the osteogenic
ability of BMSCs exposed to Dex, as ALP and Runx2 protein
expression was increased compared with Dex-only cells at 1, 5 and
10 mM NMN (Fig. 2B). The
mineralization ability and ALP activity of BMSCs were also enhanced
by NMN treatment (Fig. 2C). These
results suggest that NMN promotes the osteogenic ability of
glucocorticoid-treated BMSCs.
NMN promotes the osteogenic ability of
glucocorticoid-repressed osteoblasts via the SIRT1/PGC-1α signaling
pathway
SIRT1 is considered to be an important regulator of
cellular metabolism. Numerous studies have suggested that NMN
protects cells from stress stimuli, such as oxidative stress, aging
and toxicity stress, by regulating SIRT1 (35). To determine whether NMN represses
glucocorticoid-induced osteogenic inhibition by regulating SIRT1
expression, the mRNA and protein expression of SIRT1 were detected
in BMSCs following Dex administration. SIRT1 mRNA expression was
decreased after Dex treatment; however, NMN treatment restored
these expression levels (Fig. 3A).
PGC-1α, which is downstream of SIRT1, is an important regulator
that is involved in the development of various diseases (36). In the present study, the PGC-1α mRNA
expression level was unchanged by Dex treatment (Fig. 3A); the protein expression of PGC-1α
was decreased following treatment with Dex but increased by Dex +
NMN (Fig. 3B). This trend was also
observed for SIRT1 protein expression, which was increased by Dex +
NMN treatment compared with the Dex-only experiment.
Immunofluorescence assays and western blotting were performed to
further confirm these changes in SIRT1 protein expression (Fig. 3D). SIRT1 is expressed in the
cytoplasm, but not in the nucleus, and was decreased in BMSCs
treated with Dex, while NMN treatment promoted SIRT1 protein
expression (Fig. S1A and B). These
results show that NMN may promote the osteogenic ability of
Dex-treated BMSCs by inducing SIRT1 mRNA and protein expression, as
well as regulating the protein, but not the mRNA expression, of
PGC-1α.
 | Figure 3.SIRT1/PGC-1α signaling is involved in
the protective effects of NMN on Dex-induced osteogenic inhibition.
BMSCs were treated with control, Dex and Dex + NMN for 7 days. (A)
Reverse transcription-quantitative PCR was used to detect the
SIRT1, PGC-1α, Runx2 and ALP mRNA expression levels in BMSCs. (B)
Western blotting was used to determine the SIRT1, PGC-1α, Runx2 and
ALP protein expression levels in BMSCs. (C) Relative ALP activity.
(D) Immunofluorescence was used to detect SIRT1 protein expression
in BMSCs following 2 days of treatment. Scale bar, 5 µm. All
experiments were performed ≥3 times. ***P<0.001 and **P<0.01,
as indicated. SIRT1, sirtuin 1; PGC, peroxisome
proliferator-activated receptor gamma coactivator; NMN,
nicotinamide mononucleotide; Dex, dexamethasone; BMSC, bone
mesenchymal stem cells; ALP, alkaline phosphatase; Runx2,
Runt-related transcription factor 2. |
SIRT1 knockdown reverses the
protective effect of NMN on glucocorticoid-induced osteogenic
inhibition in BMSCs
To further investigate whether NMN enhanced the
osteogenic ability of Dex-treated BMSCs, siRNA was used to inhibit
SIRT1 expression. Firstly, the silencing effects of si-SIRT1 were
determined by RT-qPCR. SIRT1 mRNA expression was decreased in BMSCs
transfected with si-SIRT1, compared with si-NC (Fig. 4A). Secondly, western blotting assays
were used to elucidate the effects of si-SIRT1 on protein
expression. Following knockdown, SIRT1 protein expression was
decreased compared with cells treated with si-NC (Fig. 4B). Finally, SIRT1 mRNA expression
was decreased in Dex + NMN + si-SIRT1 cells (Fig. 4C). The PGC-1α mRNA expression level
was not altered by Dex + NMN + si-SIRT1. Importantly, mRNA
expression of Runx2 was also reduced by Dex + NMN + si-SIRT1.
Furthermore, the protein expression levels of SIRT1, PGC-1α and
Runx2 were promoted by Dex + NMN, and reduced in the Dex + NMN +
si-SIRT1 treatment group (Fig. 2D).
ALP activity and the mineralization ability of BMSCs were enhanced
in the Dex + NMN, but inhibited in the Dex + NMN + si-SIRT1 group.
These results further support that NMN alleviates the
glucocorticoid-induced osteogenic inhibition of osteoblasts by
regulating SIRT1/PGC-1α signaling.
 | Figure 4.SIRT1 knockdown reduces the
protective effects of NMN on Dex-induced osteogenic inhibition.
Si-NC or si-SIRT1 were transfected into BMSCs before Dex and NMN
treatment. SIRT1-knockdown was confirmed by (A) RT-qPCR and (B)
western blotting. (C) RT-qPCR was used to detect the SIRT1, PGC-1α
and Runx2 mRNA expression levels in BMSCs. (D) Western blotting
assays were used to determine the SIRT1, PGC-1α, ALP and Runx2
protein expression levels in BMSCs. (E) Alizarin red and ALP
staining assays were used to assess the osteogenic ability of
BMSCs. Scale bar, 20 µm. All experiments were performed ≥3 times.
***P<0.001, **P<0.01 *P<0.05 and nsP≥0.05, as
indicated. Si, small interfering; NC, negative control; NMN,
nicotinamide mononucleotide; Dex, dexamethasone; BMSC, bone
mesenchymal stem cells; SIRT1, sirtuin 1; PGC, peroxisome
proliferator-activated receptor gamma coactivator; ALP, alkaline
phosphatase; Runx2, runt-related transcription factor 2; RT-qPCR,
reverse transcription-quantitative PCR. |
Discussion
BMSCs possess the potential to differentiate into
osteoblasts, a process that can be suppressed by glucocorticoid use
(32,37,38).
Inhibiting osteogenesis increases the risk of osteoporosis and
osteonecrosis, resulting in bone fracture (39). Due to glucocorticoid-induced
osteogenic inhibition of BMSCs, long-term and high-dose
administration of glucocorticoids can lead to serious side effects,
such as osteoporosis (40). In the
present study, a potential therapeutic method for
glucocorticoid-induced osteogenic inhibition was investigated.
The present study suggested NMN as a potential
therapeutic target for Dex-induced inhibition of osteogenesis, and
that SIRT1 was an important downstream target of NMN. The
expression of BMSC osteogenic markers was decreased following
exposure to Dex (range, 10−9−10−6 M); these
included ALP, Runx2 and OCN. ALP staining and alizarin red staining
also confirmed above results. These results suggest that Dex, as a
glucocorticoid, can inhibit the differentiation and osteogenesis of
BMSCs.
Various studies have demonstrated that the
administration of NMN significantly increases the intracellular
levels of NAD+. Much evidence has also confirmed that
intracellular NAD+ is closely associated with bone
diseases. Li et al (41)
demonstrated that intracellular NAD+ levels were
enhanced during osteogenic differentiation. NAD+ is also
involved in the maintenance of osteoblast differentiation, and an
increase in the intracellular levels of NAD+ is a
necessary event for the development of senile osteoporosis. Liang
et al (42) reported that
NMN attenuates aluminum-induced bone loss. However, the protective
effects of NAD+ depletion on glucocorticoid-induced
osteogenic inhibition have yet to be elucidated. In the present
study, NMN was found to alleviate Dex-induced osteogenic
inhibition. NMN treatment was able to promote osteogenic marker
expression in BMSCs pre-treated with Dex. Furthermore, the
mineralization ability and ALP activity of Dex-treated BMSCs was
enhanced by NMN. These results suggest that NMN protects against
Dex-induced osteogenic impairment, although the exact mechanism
requires further investigation.
A previous study found that in aged bone marrow, NMN
improved osteogenesis and reduced adipogenesis by regulating MSCs
via the SIRT1 pathway (17). NMN, a
key NAD+ intermediate, can stimulate BMSC
differentiation and osteogenesis (17). SIRT1 is an NAD+-dependent
deacetylase, which regulates metabolism in a variety of cell types
(21,22). Qu et al (43) revealed that SIRT1 was involved in
osteogenic proliferation and differentiation by regulating
miR-132-3p. Furthermore, Wang et al (44) demonstrated that SIRT1 promotes
osteogenic differentiation and increases alveolar bone mass via B
cell-specific Moloney murine leukemia virus integration site 1. In
the present study, the expression of SIRT1 and its downstream
target PGC-1α was discovered to be decreased in osteoblasts exposed
to Dex. Therefore, it was speculated that the SIRT1/PGC-1α
signaling pathway played an important role in the protective
effects of NMN in Dex-treated BMSCs. In the present study, NMN
treatment was found to increase the mRNA and protein expression
levels of SIRT1. The protein expression of PGC-1α was also enhanced
by NMN treatment, whereas the mRNA levels remained unchanged. Thus,
it was hypothesized that SIRT1 regulates the expression of PGC-1α
protein by deacetylation, while leaving mRNA expression unaltered.
Meanwhile, the results of immunofluorescence also indicated that
Dex treatment decreased protein expression of SIRT1 in the
cytoplasm, while NMN promoted the SIRT1 expression in BMSCs treated
with Dex.
To further confirm the role of SIRT1 in this
process, si-SIRT1 was used to knock down SIRT1, which inhibited the
protective effect of NMN in glucocorticoid-induced osteogenic
inhibition. Knockdown of SIRT1 was found to reduce the expression
of the osteogenic markers that were increased with NMN treatment.
Alizarin red and ALP staining also confirmed the importance of
SIRT1 in the protective effect of NMN in Dex-treated BMSCs.
Importantly, SIRT1 knockdown was able to reduce the protein
expression of PGC-1α improved by NMN treatment in BMSCs exposed to
Dex. Together, these results suggest that NMN attenuates
Dex-induced osteogenic inhibition by regulating the SIRT1/PGC-1α
signaling pathway, and that SIRT1 regulates this process through
improving the protein expression of PGC-1α rather than PGC-1α mRNA
(Fig. 5).
Song et al (16) reported that NMN could promote
osteogenesis in aged bone marrow. However, the effect of NMN in
glucocorticoid-induced osteoporosis was unknown. Our work confirmed
the effect of NMN in glucocorticoid-induced osteogenic inhibition.
Together, these two studies suggest a therapeutic role of NMN in
osteoporosis caused by age and glucocorticoids. However, as the
present study was performed in vitro, future in vivo
studies will be required to validate the findings.
In conclusion, the results of the present study show
that Dex is capable of inhibiting the differentiation and
mineralization of BMSCs. Moreover, NMN can alleviate Dex-induced
osteogenic inhibition by regulating SIRT1/PGC-1α expression. These
findings provide a novel mechanism to improve the understanding of
glucocorticoid-induced osteogenic inhibition, and indicate that NMN
may be a potential therapeutic target.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Professor Yan Jiang
(The Second Affiliated Hospital of Nanchang University) for
provided suggestions regarding experimental design and article
writing.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
RH performed the experiments and collected the data.
JT designed the study, analyzed the data and drafted the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Baccaro LF, Conde DM, Costa-Paiva L and
Pinto-Neto AM: The epidemiology and management of postmenopausal
osteoporosis: A viewpoint from Brazil. Clin Interv Aging.
10:583–591. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen Q, Shou P, Zhang L, Xu C, Zheng C,
Han Y, Li W, Huang Y, Zhang X, Shao C, et al: An
osteopontin-integrin interaction plays a critical role in directing
adipogenesis and osteogenesis by mesenchymal stem cells. Stem
Cells. 32:327–337. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Camacho PM, Petak SM, Binkley N, Clarke
BL, Harris ST, Hurley DL, Kleerekoper M, Lewiecki EM, Miller PD,
Narula HS, et al: American association of clinical endocrinologists
and American college of endocrinology clinical practice guidelines
for the diagnosis and treatment of postmenopausal
osteoporosis-2016-executive summary. Endocr Pract. 22:1111–1118.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xu D, Gao Y, Hu N, Wu L and Chen Q:
miR-365 ameliorates dexamethasone-induced suppression of
osteogenesis in MC3T3-E1 cells by targeting HDAC4. Int J Mol Sci.
18:E9772017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yuasa M, Yamada T, Taniyama T, Masaoka T,
Xuetao W, Yoshii T, Horie M, Yasuda H, Uemura T, Okawa A and Sotome
S: Dexamethasone enhances osteogenic differentiation of bone
marrow- and muscle-derived stromal cells and augments ectopic bone
formation induced by bone morphogenetic protein-2. PLoS One.
10:e01164622015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fardet L, Petersen I and Nazareth I:
Prevalence of long-term oral glucocorticoid prescriptions in the UK
over the past 20 years. Rheumatology (Oxford). 50:1982–1990. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hoes JN, Bultink IE and Lems WF:
Management of osteoporosis in rheumatoid arthritis patients. Expert
Opin Pharmacother. 16:559–571. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Edens C and Robinson AB: Systemic lupus
erythematosus, bone health, and osteoporosis. Curr Opin Endocrinol
Diabetes Obes. 22:422–431. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Monadi M, Javadian Y, Cheraghi M, Heidari
B and Amiri M: Impact of treatment with inhaled corticosteroids on
bone mineral density of patients with asthma: Related with age.
Osteoporos Int. 26:2013–2018. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Caramori G, Ruggeri P, Arpinelli F, Salvi
L and Girbino G: Long-term use of inhaled glucocorticoids in
patients with stable chronic obstructive pulmonary disease and risk
of bone fractures: A narrative review of the literature. Int J
Chron Obstruct Pulmon Dis. 14:1085–1097. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Krela-Kaźmierczak I, Szymczak A,
Łykowska-Szuber L, Eder P and Linke K: Osteoporosis in
gastrointestinal diseases. Adv Clin Exp Med. 25:185–190. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kitazaki S, Mitsuyama K, Masuda J, Harada
K, Yamasaki H, Kuwaki K, Takedatsu H, Sugiyama G, Tsuruta O and
Sata M: Clinical trial: Comparison of alendronate and alfacalcidol
in glucocorticoid-associated osteoporosis in patients with
ulcerative colitis. Aliment Pharmacol Ther. 29:424–430. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li J, Zhang N, Huang X, Xu J, Fernandes
JC, Dai K and Zhang X: Dexamethasone shifts bone marrow stromal
cells from osteoblasts to adipocytes by C/EBPalpha promoter
methylation. Cell Death Dis. 4:e8322013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sosa M and Gomez de Tejada MJ:
Glucocorticoid-Induced osteoporosis. N Engl J Med. 380:1378–1379.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang X, Hu X, Yang Y, Takata T and Sakurai
T: Nicotinamide mononucleotide protects against β-amyloid
oligomer-induced cognitive impairment and neuronal death. Brain
Res. 1643:1–9. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Song J, Li J, Yang F, Ning G, Zhen L, Wu
L, Zheng Y, Zhang Q, Lin D, Xie C and Peng L: Nicotinamide
mononucleotide promotes osteogenesis and reduces adipogenesis by
regulating mesenchymal stromal cells via the SIRT1 pathway in aged
bone marrow. Cell Death Dis. 10:3362019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Signer RA and Morrison SJ: Mechanisms that
regulate stem cell aging and life span. Cell Stem Cell. 12:152–165.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Frye RA: Characterization of five human
cDNAs with homology to the yeast SIR2 gene: Sir2-like proteins
(sirtuins) metabolize NAD and may have protein
ADP-ribosyltransferase activity. Biochem Biophys Res Commun.
260:273–279. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sherman JM, Stone EM, Freeman-Cook LL,
Brachmann CB, Boeke JD and Pillus L: The conserved core of a human
SIR2 homologue functions in yeast silencing. Mol Biol Cell.
10:3045–3059. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liang F, Kume S and Koya D: SIRT1 and
insulin resistance. Nat Rev Endocrinol. 5:367–373. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chua KF, Mostoslavsky R, Lombard DB, Pang
WW, Saito S, Franco S, Kaushal D, Cheng HL, Fischer MR, Stokes N,
et al: Mammalian SIRT1 limits replicative life span in response to
chronic genotoxic stress. Cell Metab. 2:67–76. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Haigis MC and Guarente LP: Mammalian
sirtuins-emerging roles in physiology, aging, and calorie
restriction. Genes Dev. 20:2913–2921. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Leibiger IB and Berggren PO: Sirt1: A
metabolic master switch that modulates lifespan. Nat Med. 12:34–36;
discussion 36. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee HW, Suh JH, Kim AY, Lee YS, Park SY
and Kim JB: Histone deacetylase 1-mediated histone modification
regulates osteoblast differentiation. Mol Endocrinol. 20:2432–2443.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cohen-Kfir E, Artsi H, Levin A, Abramowitz
E, Bajayo A, Gurt I, Zhong L, D'Urso A, Toiber D, Mostoslavsky R
and Dresner-Pollak R: Sirt1 is a regulator of bone mass and a
repressor of Sost encoding for sclerostin, a bone formation
inhibitor. Endocrinology. 152:4514–4524. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Iyer S, Han L, Bartell SM, Kim HN, Gubrij
I, de Cabo R, O'Brien CA, Manolagas SC and Almeida M: Sirtuin1
(Sirt1) promotes cortical bone formation by preventing beta-catenin
sequestration by FoxO transcription factors in osteoblast
progenitors. J Biol Chem. 289:24069–24078. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sun W, Qiao W, Zhou B, Hu Z, Yan Q, Wu J,
Wang R, Zhang Q and Miao D: Overexpression of Sirt1 in mesenchymal
stem cells protects against bone loss in mice by FOXO3a
deacetylation and oxidative stress inhibition. Metabolism.
88:61–71. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yu W, Zhu C, Xu W, Jiang L and Jiang S:
Neuropeptide Y1 receptor regulates glucocorticoid-induced
inhibition of osteoblast differentiation in murine MC3T3-E1 cells
via ERK signaling. Int J Mol Sci. 17:E21502016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tang Z, Hu B, Zang F, Wang J, Zhang X and
Chen H: Nrf2 drives oxidative stress-induced autophagy in nucleus
pulposus cells via a Keap1/Nrf2/p62 feedback loop to protect
intervertebral disc from degeneration. Cell Death Dis. 10:5102019.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shi GX, Zheng XF, Zhu C, Li B, Wang YR,
Jiang SD and Jiang LS: Evidence of the role of R-Spondin 1 and its
receptor Lgr4 in the transmission of mechanical stimuli to
biological signals for bone formation. Int J Mol Sci. 18:E5642017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lane NE: Glucocorticoid-Induced
osteoporosis: New insights into the pathophysiology and treatments.
Curr Osteoporos Rep. 17:1–7. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ohnaka K, Tanabe M, Kawate H, Nawata H and
Takayanagi R: Glucocorticoid suppresses the canonical Wnt signal in
cultured human osteoblasts. Biochem Biophys Res Commun.
329:177–181. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Briot K and Roux C: Glucocorticoid-induced
osteoporosis. RMD Open. 1:e0000142015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nogueiras R, Habegger KM, Chaudhary N,
Finan B, Banks AS, Dietrich MO, Horvath TL, Sinclair DA, Pfluger PT
and Tschöp MH: Sirtuin 1 and sirtuin 3: Physiological modulators of
metabolism. Physiol Rev. 92:1479–1514. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jung HY, Lee D, Ryu HG, Choi BH, Go Y, Lee
N, Lee D, Son HG, Jeon J, Kim SH, et al: Myricetin improves
endurance capacity and mitochondrial density by activating SIRT1
and PGC-1α. Sci Rep. 7:62372017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Henneicke H, Gasparini SJ,
Brennan-Speranza TC, Zhou H and Seibel MJ: Glucocorticoids and
bone: Local effects and systemic implications. Trends Endocrinol
Metab. 25:197–211. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Canalis E, Mazziotti G, Giustina A and
Bilezikian JP: Glucocorticoid-induced osteoporosis: Pathophysiology
and therapy. Osteoporos Int. 18:1319–1328. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Infante A and Rodriguez CI: Osteogenesis
and aging: Lessons from mesenchymal stem cells. Stem Cell Res Ther.
9:2442018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chen TL: Inhibition of growth and
differentiation of osteoprogenitors in mouse bone marrow stromal
cell cultures by increased donor age and glucocorticoid treatment.
Bone. 35:83–95. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li Y, He J, He X, Li Y and Lindgren U:
Nampt expression increases during osteogenic differentiation of
multi- and omnipotent progenitors. Biochem Biophys Res Commun.
434:117–123. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liang H, Gao J, Zhang C, Li C and Wang Q,
Fan J, Wu Z and Wang Q: Nicotinamide mononucleotide alleviates
Aluminum induced bone loss by inhibiting the TXNIP-NLRP3
inflammasome. Toxicol Appl Pharmacol. 362:20–27. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Qu H, Li T, Jin H, Zhang S and He B:
Silent mating type information regulation 2 homolog (SIRT1)
influences osteogenic proliferation and differentiation of MC3T3-E1
cells via regulation of miR-132-3p. Med Sci Monit. 25:2289–2295.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang H, Hu Z, Wu J, Mei Y, Zhang Q, Zhang
H, Miao D and Sun W: Sirt1 promotes osteogenic differentiation and
increases alveolar bone mass via Bmi1 activation in mice. J Bone
Miner Res. 34:1169–1181. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zainabadi K: Drugs targeting SIRT1, a new
generation of therapeutics for osteoporosis and other bone related
disorders? Pharmacol Res. 143:97–105. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hassan B, Baroukh B, Llorens A, Lesieur J,
Ribbes S, Chaussain C, Saffar JL and Gosset M: NAMPT expression in
osteoblasts controls osteoclast recruitment in alveolar bone
remodeling. J Cell Physiol. 233:7402–7414. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Mills KF, Yoshida S, Stein LR, Grozio A,
Kubota S, Sasaki Y, Redpath P, Migaud ME, Apte RS, Uchida K, et al:
Long-term administration of nicotinamide mononucleotide mitigates
age-associated physiological decline in mice. Cell Metab.
24:795–806. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Baek JM, Ahn SJ, Cheon YH, Lee MS, Oh J
and Kim JY: Nicotinamide phosphoribosyltransferase inhibits
receptor activator of nuclear factor-kB ligand-induced osteoclast
differentiation in vitro. Mol Med Rep. 15:784–792. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Abed É, Couchourel D, Delalandre A, Duval
N, Pelletier JP, Martel-Pelletier J and Lajeunesse D: Low sirtuin 1
levels in human osteoarthritis subchondral osteoblasts lead to
abnormal sclerostin expression which decreases Wnt/β-catenin
activity. Bone. 59:28–36. 2014. View Article : Google Scholar : PubMed/NCBI
|



















