Introduction
Ovarian cancer is the second most commonly diagnosed
and most deadly gynecological malignancy worldwide (1). According to 2018 statistics, 225,500
women were diagnosed with ovarian cancer, and 140,200 women died
from this disease worldwide (2).
Etiological studies reported that childbearing, family history of
ovarian cancer, smoking, obesity, and diet may increase the risk of
ovarian cancer (3). At present, the
conventional treatment of ovarian cancer is cytoreductive surgery,
followed by chemotherapy and radiotherapy (4). Cisplatin (DDP) and paclitaxel are the
main chemotherapeutic drugs used in ovarian cancer. However,
>60% of the patients develop advanced-stage relapse or do not
respond to chemotherapy (5,6). Although the majority of patients are
initially sensitive to chemotherapy, they often develop resistance
after multiple relapses. Due to the lack of effective second-line
chemotherapy, the overall survival of patients is short, at only 1
year (7). Therefore, it is crucial
to overcome the drug resistance of patients with ovarian cancer to
improve their prognosis.
MicroRNAs (miRs/miRNAs) are small non-coding RNA
molecules that regulate gene expression by silencing or degrading
their target mRNA molecules, thereby mediating various pathological
processes, including tumorigenesis (8). Various miRNAs are dysregulated in
several cancers. Among them, miR-576-3p was found to be
downregulated in non-melanoma skin cancer (9). In lung adenocarcinoma, miR-576-3p was
significantly reduced, and its overexpression reduced the
expression of mesenchymal markers, and inhibited the migration and
invasion of lung adenocarcinoma cells (10). miR-576-3p downregulation was also
observed in bladder cancer tissues and was found to be correlated
with poor clinical outcome (11).
These findings indicated the negative regulatory effect of
miR-576-3p on cancer development. Moreover, miR-576-3p may also be
closely associated with the chemosensitivity of tumors. Studies
have found that miR-576-3p was downregulated in DDP-resistant human
teratoma cells, as well as Adriamycin-resistant breast cancer
tissues and cells (12,13). However, further investigation is
required to determine whether miR-576-3p plays an important role in
mediating the sensitivity of ovarian cancer cells to DDP-based
chemotherapy.
In present study, TargetScan7.2 (http://www.targetscan.org/vert_72/) predicted
that miR-576-3p may target programmed death-ligand 1 (PD-L1) and
cyclin D1. PD-L1 has been demonstrated to be associated with cancer
prognosis and is highly expressed in gastric, colon, and lung
cancers (14–16). PD-1/PD-L1 signal blockade is
beneficial for the treatment of cancer, including colon and liver
cancer (15,17). Moreover, PD-L1 was found to be
upregulated in DDP-resistant ovarian cancer cells, and
downregulation of PD-L1 increased DDP sensitivity in lung cancer
(18,19). Cyclin D1 is a key regulator of the
G1/S transition and has been reported to play a key role in tumor
cell development and drug resistance (20). Downregulation of cyclin D1 increased
DDP chemosensitivity in gastric cancer (21), whereas overexpression of cyclin D1
was revealed to promote tumor cell growth and resistance of
pancreatic tumor cell lines to DDP-mediated apoptosis (22). Thus, based on the significant role
of PD-L1 and cyclin D1 in cancer development and chemotherapy
sensitivity, the present study investigated whether miR-576-3p
affects the chemosensitivity of ovarian cancer cells to DDP by
regulating PD-L1 and cyclin D1.
Materials and methods
Cell culture, transfection and drug
administration
Cell culture, transfection, and treatment of human
ovarian cancer cells were performed as described previously
(19). Ovarian cancer cells SKOV3
and A2780 were purchased from Procell Life Science & Technology
Co., Ltd. 293T cells were purchased from Shanghai Zhongqiao Xinzhou
Biotechnology Co., Ltd. DDP was purchased from Dalian Meilun
Biology Technology Co., Ltd. Cells were maintained in Minimum
Essential Medium (Invitrogen; Thermo Fisher Scientific, Inc.)
supplemented with 10% fetal bovine serum (Sigma-Aldrich; Merck
KGaA) in a 37°C incubator containing 5% CO2. The
transfection of miR-576-3p mimic, negative control (NC) mimic,
miR-576-3p inhibitor, and NC inhibitor (100 pmol) into cells (at
90% confluence) was performed using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's instructions. At 24 h post-transfection, cells were
used for subsequent experiments. The sequences of the mimics and
inhibitors were as follows: miR-576-3p mimic forward,
5′-AAGAUGUGGAAAAAUUGGAAUC-3′ and reverse,
5′-UUCCAAUUUUUCCACAUCUUUU-3′; NC mimic forward,
5′-UUCUCCGAACGUGUCACGUTT-3′ and reverse,
5′-ACGUGACACGUUCGGAGAATT−3′; miR-576-3p inhibitor,
5′-GAUUCCAAUUUUUCCACAUCUU-3′; NC inhibitor,
5′-UUGUACUACACAAAAGUACUG−3′. The sequences were purchased from JTS
Scientific Biological Technology Co., Ltd.
Construction of DDP-resistant ovarian
cancer cell lines
DDP-resistant ovarian cancer cell lines SKOV3/DDP
and A2780/DDP were established by culturing SKOV3 and A2780 cells
with increasing concentrations of DDP. Briefly, SKOV3 and A2780
cells were resuscitated and cultured in an incubator. At 60%
confluence, cells were treated with DDP at concentrations of 1, 2,
4, and 8 µM. Once the cells were incubated for 48 h, the medium was
discarded and fresh medium was added. After 12 weeks, stable
DDP-resistant SKOV3/DDP and A2780/DDP cells were successfully
established and stored in liquid nitrogen.
Cytotoxicity assay
Cells were seeded into 96-well plates at a cell
count of 5×103 per well. At 24 h after transfection,
medium (200 µl) containing different concentrations of DPP (5, 10,
20, 40, and 80 µM) was added to each well (23,24).
After the cells were incubated for 48 h at 37°C and 5%
CO2 MTT mixture (0.5 mg/ml) was added into each well,
and cells were then incubated at 37°C for 5 h. Subsequently, DMSO
solution (150 µl) was added to dissolve the purple formazan. After
10 min of incubation in the dark, the optical density value of
cells at a wavelength of 570 nm was measured and the half-maximal
inhibitory concentration (IC50) was calculated.
Reverse transcription-quantitative PCR
(RT-qPCR) analysis
Total RNA was extracted from tissues or cells using
a total RNA extraction kit (Tiangen Biotech Co., Ltd.) according to
the manufacturer's instructions. The RNA concentration in each
sample was detected by ultraviolet spectrophotometry (NanoDrop
2000; Thermo Fisher Scientific, Inc.). cDNA was reverse-transcribed
from RNA using RNase inhibitor (BioTeke Corporation) and Super
M-MLV Reverse Transcriptase (BioTeke Corporation). The following
temperature protocol was used for reverse transcription for
miR-576-3p: 37°C for 30 min, 42°C for 30 min and 70°C for 10 min.
The following temperature protocol was used for reverse
transcription for cyclin D1 and PD-L1: 25°C for 10 min, 42°C for 50
min and 80°C for 10 min. Subsequently, RT-qPCR analysis was
performed with primers, SYBR-Green (Sigma-Aldrich; Merck KGaA) and
2X Power Taq PCR Master Mix (BioTeke Corporation) to detect
miR-576-3p expression level. The following thermocycling conditions
were used for qPCR for miR-576-3p: 94°C for 4 min, 40 cycles of
94°C for 15 sec, 60°C for 20 sec and 72°C for 15 sec. The following
thermocycling conditions were used for qPCR for cyclin D1 and
PD-L1: 94°C for 5 min, 40 cycles of 94°C for 15 sec, 60°C for 25
sec and 72°C for 30 sec. The primers used for qPCR were as follows:
miR-576-3p forward, 5′-AAGATGTGGAAAAATTGGAATC-3′ and reverse,
5′-GCAGGGTCCGAGGTATTC-3′; 5S rRNA forward,
5′-GATCTCGGAAGCTAAGCAGG-3′ and reverse, 5′-TGGTGCAGGGTCCGAGGTAT-3′;
cyclin D1 forward, 5′-GCGAGGAACAGAAGTGCG−3′ and reverse,
5′-TGGAGTTGTCGGTGTAGATGC−3′; PD-L1 forward,
5′-AACTACCTCTGGCACATC−3′ and reverse, 5′-ATCCATCATTCTCCCTTT-3′;
β-actin forward, 5′-GGCACCCAGCACAATGAA−3′ and reverse,
5′-TAGAAGCATTTGCGGTGG-3′. The primers were purchased from
GenScript. The expression of miR-576-3p, and cyclin D1 and PD-L1
was calculated and normalized to 5S rRNA and β-actin, respectively.
Gene expression was quantified using the 2−∆∆Cq method
(25).
Western blot analysis
Total protein was extracted from tissues or cells
using IP cell lysis buffer (Beyotime Institute of Biotechnology)
and phenylmethylsulphonyl fluoride protease inhibitor (Beyotime
Institute of Biotechnology). Protein concentration was quantified
using a BCA protein assay kit (Beyotime Institute of
Biotechnology), according to the manufacturer's protocol. Proteins
(20 µl/lane) from each sample were separated via SDS-PAGE (8 and
13% separation gel for different sized proteins) and then
transferred to a PVDF membrane (EMD Millipore). After blocking in
5% skimmed milk powder for 1 h at room temperature the membranes
were incubated with primary antibodies overnight at 4°C, followed
by incubation with horseradish peroxidase-conjugated
anti-rabbit/mouse secondary antibodies for 2 h at room temperature
(1:5,000; cat. nos. A0208 and A0216; Beyotime Institute of
Biotechnology). The primary antibodies used were as follows: PD-L1
(1:1,000; cat. no. DF6526; Affinity Biosciences), cyclin D1
(1:1,000; cat. no. AF0931; Affinity Biosciences), efflux pump
multidrug resistance protein 1 (MDR1; 1:1,000; cat. no. AF5185;
Affinity Biosciences), pro/cleaved caspase-3 (1:1,000; cat. no.
DF6879; Affinity Biosciences), pro/cleaved poly (ADP-ribose)
polymerase (PARP; 1:1,000; cat. no. #9532; Cell Signaling
Technology, Inc.), and β-actin (1:1,000; cat. no. sc-47778; Santa
Cruz Biotechnology, Inc.). Finally, the membranes were visualized
with ECL luminescent reagent (Beyotime Institute of Biotechnology)
and the optical density values of the target bands were analyzed
using Gel-Pro-Analyzer software (version 4; Media Cybernetics,
Inc.). β-actin served as an internal control.
Cell cycle assay
Cells were incubated in 6-well plates at a density
of 2×105 cells/well. After transfection with miR-576-3p
mimic or NC mimic, cells were treated with DDP at 50% of the
IC50 dose at 37°C for 24 h. The treated cells were
harvested and fixed in 70% ethanol at 4°C for 2 h, followed by
incubation with propidium iodide (PI; 25 µl) and RNase A (10 µl) at
37°C for 30 min in the dark. Finally, the cell cycle distribution
was detected by flow cytometry using a NovoCyte flow cytometer
(ACEA Biosciences, Inc.) and analyzed using NovoExpress software
(version 1.2.5; ACEA Biosciences, Inc.).
Cell apoptosis assay
Both early and late apoptotic cells were assessed.
Cells were seeded in 6-well plates at a density of 2×105
cells/well. After transfection, cells were treated with DDP at 50%
of the IC50 dose at 37°C for 24 h. Subsequently, cells
were treated with 5 µl Annexin V-fluorescein isothiocyanate (FITC)
and 10 µl PI for 20 min at room temperature in the dark. Finally,
cells stained with V-FITC and PI were detected by flow cytometry
using a NovoCyte flow cytometer (ACEA Biosciences, Inc.) and
analyzed using NovoExpress (version 1.2.5; ACEA Biosciences,
Inc.).
Dual-luciferase reporter assay
The binding sites between miR-576-3p and PD-L1 and
cyclin D1 were determined using TargetScan (version 7.2; http://www.targetscan.org/vert_72/). PD-L1 and
cyclin D1 fragments containing the target sequence of miR-576-3p
were inserted into pmirGLO vectors (GenScript) to construct
wild-type (wt) and mutant (mut) plasmids. Subsequently, the
plasmids were co-transfected with miR-576-3p mimic, NC mimic,
miR-576-3p inhibitor or NC inhibitor into 293T cells (at 90%
confluence) using Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). After 48 h, luciferase activity was detected
using a Dual-Luciferase Detection kit (Promega Corporation).
Firefly luciferase activities were normalized to Renilla
luciferase activities.
Animal experiments
The present study was approved by the Institutional
Animal Care and Use Committee of Affiliated Yantai Yuhuangding
Hospital (approval no. 2018137; Shandong, China) and performed
according to the Guidelines for the Care and Use of Laboratory
Animals (26). The feeding and
treatment of mice were performed as described in our previous study
(19). Briefly, 12 healthy female
BALB/c nude mice (weight, ~20 g) aged 5–6 weeks were given free
access to food and water and fed adaptively for 1 week. Mice were
randomly divided into two groups: SKOV3/DDP + LV-NC + DDP group and
SKOV3/DDP + LV-miR-576-3p + DDP group (n=6/group). The construction
of SKOV3/DDP cells was performed as aforementioned. The nude mice
were subcutaneously injected (the right side of the back of the
neck) with lentivirus (LV) miR-576-3p infected SKOV3/DDP cells
(2×106 in PBS) or LV control-infected SKOV3/DDP cells
(2×106 in PBS). The tumor was confirmed by a
pathologist. When the tumor volume reached 100 mm3, all
nude mice were intraperitoneally injected with DDP (10 mg/kg) twice
a week for 3 weeks. Tumor size was monitored every 2 days. After 19
days, nude mice were sacrificed by cervical dislocation. Death
verification was confirmed by cessation of heartbeat and
respiration, and absence of reflexes and metabolism. After the mice
stopped breathing, the tumors were resected.
Statistical analysis
All data are presented as the mean ± SD of three
repeats, except for animal experiments (n=6). GraphPad 8.0
(GraphPad Software, Inc.) was used to perform statistical analysis.
An unpaired t-test, one-way ANOVA with Tukey's multiple comparisons
test or two-way ANOVA with Sidak's multiple comparisons test were
used to evaluate statistical significance. P<0.05 was considered
to indicate a statistically significant difference.
Results
miR-576-3p is downregulated whereas
PD-L1 and cyclin D1 are upregulated in DDP-resistant ovarian cancer
cells
To investigate whether miR-576-3p, PD-L1 and cyclin
D1 are involved in DDP resistance of ovarian cancer cells,
DDP-resistant ovarian cancer cell lines SKOV3/DDP and A2780/DDP
were used. The results demonstrated that, as the concentration of
DDP increased, the viability of ovarian cancer cells SKOV3 and
A2780 and DDP-resistant ovarian cancer cells SKOV3/DDP and
A2780/DDP significantly decreased. Furthermore, the cell viability
of ovarian cancer cells was more affected by DDP compared with that
of DDP-resistant cells (Fig. 1A).
In addition, the tolerance of SKOV3/DDP and A2780/DDP cells to DDP
was significantly higher compared with that of SKOV3 and A2780
cells (Fig. 1B). RT-qPCR analysis
was used to detect the mRNA expression of miR-576-3p in
DDP-resistant cells, and the results revealed that the mRNA levels
of miR-576-3p in SKOV3/DDP and A2780/DDP cells were downregulated
compared with those in SKOV3 and A2780 cells (Fig. 1C). Western blotting demonstrated
that the protein expression levels of PD-L1 and cyclin D1 in
DDP-resistant cells were significantly higher compared with those
in non-resistant cells (Fig. 1D and
E). These data indicated that miR-576-3p, PD-L1 and cyclin D1
may be involved in DDP resistance of ovarian cancer cells.
miR-576-3p overexpression increases
DDP sensitivity of DDP-resistant ovarian cancer cells in vitro
The role of miR-576-3p in ovarian cancer cells was
investigated via transfection of miR-576-3p mimic, which
significantly increased the expression of miR-576-3p in ovarian
cancer cells (Fig. S1A).
Furthermore, miR-576-3p overexpression decreased ovarian cancer
cell viability, and promoted apoptosis and the accumulation of
ovarian cancer cells in the G1 phase (Fig. S2). To study the effects of
miR-576-3p on DDP sensitivity of ovarian cancer cells,
DDP-resistant ovarian cancer cells were transfected with miR-576-3p
mimic or NC mimic. The results demonstrated that miR-576-3p
overexpression significantly increased miR-576-3p mRNA expression
in DDP-resistant cells (Fig. 2A).
miR-576-3p upregulation reduced DDP tolerance in DDP-resistant
cells (Fig. 2B). Moreover, the
viability of DDP-resistant cells decreased with the increase in DDP
concentration, and miR-576-3p overexpression increased the DDP
sensitivity of SKOV3/DDP and A2780/DDP cells (Fig. 2C). Furthermore, DDP treatment had no
significant effect on DDP-resistant cell cycle progression and
apoptosis compared with the control group (Fig. 2D and E). However, overexpression of
miR-576-3p resulted in the accumulation of DDP-resistant cells in
the G1 phase and promotion of cell apoptosis (Fig. 2D and E). Additionally, DDP treatment
reduced the protein levels of PD-L1, cyclin D1 and MDR1, but
increased the cleaved/pro caspase-3 ratio and cleaved/pro PARP
ratio in DDP-treated DDP-resistant cells compared with the control
group (Fig. 3). Moreover,
miR-576-3p overexpression further enhanced the effects of DDP on
PD-L1, cyclin D1, MDR1, cleaved caspase-3 and cleaved PARP protein
levels (Fig. 3). Therefore,
miR-576-3p overexpression increased DDP sensitivity of
DDP-resistant ovarian cancer cells via regulating cell viability
and apoptosis.
 | Figure 3.Effects of miR-576-3p overexpression
on the protein expression levels of PD-L1, cyclin D1, MDR1,
cleaved/pro caspase-3 and cleaved/pro PARP in SKOV3/DDP and
A2780/DDP cells treated with DDP. n=3. *P<0.05, **P<0.01 and
***P<0.001. miR, microRNA; PD-L1, programmed death-ligand 1;
DDP, cisplatin; MDR1, efflux pump multidrug resistance protein 1;
PARP, poly(ADP-ribose) polymerase; NC, negative control. |
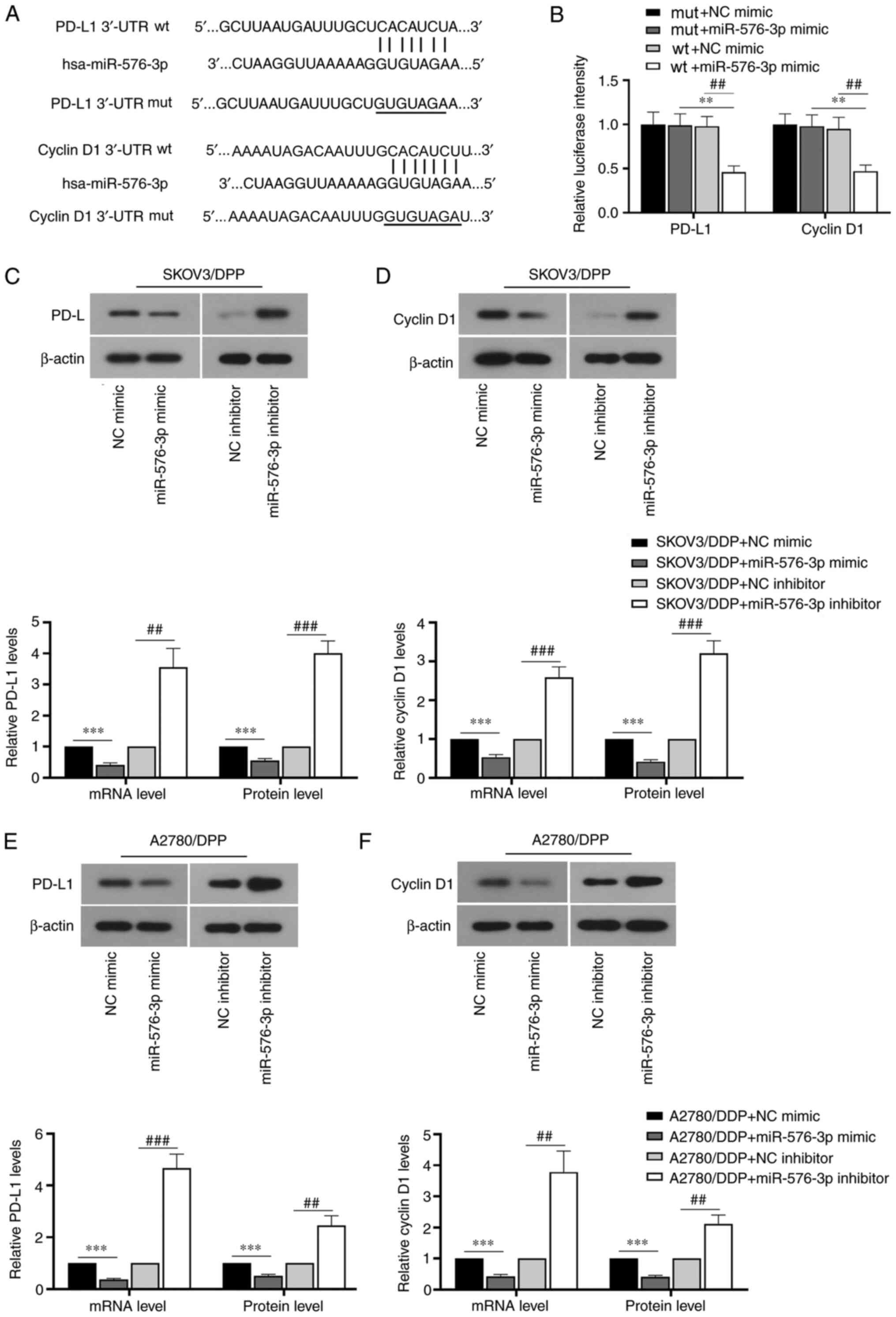
miR-576-3p directly targets PD-L1 and
cyclin D1
A dual-luciferase assay was performed to detect the
targeting relationship between miR-576-3p and PD-L1 and cyclin D1.
The binding sites between miR-576-3p and PD-L1 and cyclin D1 were
determined using TargetScan (version 7.2; http://www.targetscan.org/vert_72/). As shown in
Fig. 4A and B, the luciferase
activity in cells transfected with wt-PD-L1 and wt-cyclin D1
plasmids was significantly decreased by miR-576-3p mimic
transfection. The luciferase activity in cells transfected with
mut-PD-L1 and mut-cyclin D1 plasmids showed no significant change.
The results indicated that miR-576-3p can directly target PD-L1 and
cyclin D1. Additionally, the mRNA and protein expression levels of
PD-L1 and cyclin D1 were significantly reduced in SKOV3/DDP and
A2780/DDP cells following miR-576-3p mimic transfection, but were
significantly increased by transfection with a miR-576-3p inhibitor
(Figs. 4C-F and S1B). Therefore, miR-576-3p overexpression
may regulate cell viability and apoptosis via targeting PD-L1 and
cyclin D1.
miR-576-3p overexpression enhances the
efficacy of DDP in inhibiting ovarian tumor growth in vivo
The effects of miR-576-3p on DDP resistance in
ovarian cancer cells were also investigated using a xenograft tumor
model. The results demonstrated that DDP treatment inhibited tumor
growth in the nude mice injected with miR-576-3p
overexpressing-SKOV3/DDP cells compared with the control group
(Fig. 5A and B). The mRNA
expression level of miR-576-3p was upregulated in
miR-576-3p-overexpressing ovarian tumor tissues (Fig. 5C). Western blotting demonstrated
that the protein expression levels of PD-L1, cyclin D1 and MDR1
were downregulated, whereas the cleaved/pro caspase-3 ratio and
cleaved/pro PARP ratio were upregulated in
miR-576-3p-overexpressing tumor tissues treated with DDP (Fig. 5D). Overall, miR-576-3p
overexpression enhanced DDP sensitivity of DDP-resistant ovarian
cancer cells in vivo.
 | Figure 5.miR-576-3p overexpression enhances
the efficacy of DDP in inhibiting ovarian tumor growth in
vivo. (A and B) Changes in the tumor volume in nude mice
injected with miR-576-3p-overexpressing SKOV3 cells treated with
DDP. (C) Effects of miR-576-3p upregulation on mRNA expression
levels of miR-576-3p and (D) the protein expression levels of
PD-L1, cyclin D1, MDR1, cleaved/pro caspase-3 and cleaved/pro PARP
in tumor tissues. n=6. *P<0.05, **P<0.01 and ***P<0.001.
miR, microRNA; PD-L1, programmed death-ligand 1; DDP, cisplatin;
MDR1, efflux pump multidrug resistance protein 1; PARP,
poly(ADP-ribose) polymerase; LV, lentivirus; NC, negative
control. |
Discussion
Ovarian cancer is one of the most malignant tumors
of the female reproductive system. With the widespread application
of chemotherapeutic drugs, patients with ovarian cancer may develop
drug resistance that adversely affects their prognosis. Hence, it
is crucial to identify methods that enhance the sensitivity of
ovarian cancer cells to DPP. Various studies have indicated that
miRNAs have a crucial role in cancer progression and
chemosensitivity due to their important regulatory functions of
targets at both the transcriptional and post-transcriptional
levels. The findings of the present study suggested that miR-576-3p
was downregulated in DDP-resistant ovarian cancer cells, and
miR-576-3p overexpression may enhance DDP sensitivity of ovarian
cancer cells via targeting PD-L1 and cyclin D1.
It was observed that DDP-resistant ovarian cancer
cells SKOV3/DDP and A2780/DDP had a higher IC50 value
compared with SKOV3 and A2780 cells, indicating that SKOV3 and
A2780 cells were more sensitive to DDP compared with SKOV3/DDP and
A2780/DDP cells. Furthermore, miR-576-3p overexpression
significantly decreased the IC50 value of DDP-resistant
ovarian cancer cells, indicating that miR-576-3p overexpression
increased the sensitivity of DDP-resistant ovarian cancer cells to
DDP. It was previously demonstrated that miR-576-3p was
downregulated in non-melanoma skin cancer, lung adenocarcinoma and
bladder cancer (9–11). The downregulation of miR-576-3p was
found to be associated with the chemosensitivity of human teratoma
and breast cancer cells (12,13).
The present study demonstrated that miR-576-3p overexpression
inhibited viability and promoted apoptosis of DDP-resistant ovarian
cancer cells. A previous study reported that the antitumor effects
of DDP on drug-resistant ovarian cancer cells may be enhanced by
induction of apoptosis and inhibition of cell migration and
invasion (27). Therefore, the
effects of miR-576-3p overexpression on promoting the sensitivity
of ovarian cancer cells to DDP may be achieved by inhibiting
viability and promoting apoptosis in DDP-resistant ovarian cancer
cells.
In the present study, upregulation of PD-L1, MDR1
and cyclin D1 was observed in DDP-resistant ovarian cancer cells,
whereas miR-576-3p overexpression reduced the expression levels of
PD-L1, MDR1 and cyclin D1. MDR1 is an ATP-binding cassette
transporter that acts as an energy-dependent efflux pump for
multiple drugs and is responsible for reducing drug accumulation in
cells, ultimately causing drug resistance (28). MDR1 efflux is considered to be the
primary mechanism of ovarian cancer resistance to taxanes (29). MDR1 overexpression reduced the
sensitivity of ovarian cancer cells to cisplatin and paclitaxel,
whereas MDR1 knockdown increased chemosensitivity to vincristine,
paclitaxel and docetaxel (30,31).
PD-L1 is an antitumor immune negative regulator. During cancer
development, the increased binding of PD-L1 to the receptor PD-1 on
T cells causes T-cell dysfunction, thereby preventing cytotoxic T
cells from effectively targeting tumor cells, leading to tumor cell
survival (32). Our previous
research found that PD-L1 was highly expressed in DDP-resistant
ovarian cancer cells, and PD-L1 silencing decreased this DDP
resistance (19). Clinical studies
have demonstrated that PD-L1 may be a potential mechanism used by
cancer cells to evade immune defense, and inhibiting the
interaction between PD-1 and PD-L1 may enhance the T cell response
and mediate antitumor activity (33). Currently, PD-L1 inhibitors and
anti-PD-L1 antibodies developed for PD-L1 have been successful in
clinical trials of cancer therapy (34). It was reported that the combined
administration of anti-PD-L1 antibodies and DDP significantly
reduced tumor growth compared with single-agent treatments and
controls (35). These findings
indicated a key role of PD-L1 in regulating DDP resistance of
cancer cells. The survival and proliferation of cancer cells
require the expression of cyclin D1. Cyclin D1 acts as an
allosteric modulator of cellular cyclin-dependent kinase 4 and 6 to
regulate the transition of the cell cycle from the G1 to the S
phase (36). Cyclin D1
overexpression was found to be associated with a high risk of
recurrence of invasive serous ovarian cancer and poor response to
first-line chemotherapy (37).
Consistent with these previous findings, the results of the present
study suggested that miR-576-3p overexpression increased the
chemosensitivity of ovarian cancer cells to DDP via regulating
PD-L1, MDR1 and cyclin D1 levels. Furthermore, in vivo
studies suggested that miR-576-3p overexpression inhibited
tumorigenesis and downregulated the expression of PD-L1, MDR1 and
cyclin D1 in tumor tissues, which was consistent with the in
vitro findings.
In bladder cancer, a targeting relationship between
miR-576-3p and cyclin D1 has already been identified (38), and the present study also further
confirmed this targeting relationship. Additionally, it was
demonstrated that PD-L1 is one of the targets of miR-576-3p.
However, to the best of our knowledge, there is no evidence of
miR-576-3p targeting and regulating the expression of MDR1. The
present study demonstrated that miR-576-3p may indirectly regulate
the expression of MDR1 via PD-L1 and cyclin D1. A previous study
demonstrated that the PD-1/PD-L1 interaction may upregulate the
expression of MDR1 in breast cancer cells (39). In addition, knockdown of cyclin D1
significantly reduced the expression of MDR1 in human glioblastoma
(40). Therefore, the effect of
miR-576-3p overexpression on the chemosensitivity of ovarian cancer
cells may be achieved by targeting PD-L1 and cyclin D1.
Furthermore, it was previously reported that PD-L1 silencing
promoted apoptosis of DDP-resistant ovarian cancer cells and
inhibited cell proliferation, whereas PD-L1 overexpression
inhibited apoptosis and promoted cell proliferation (19). DDP inhibited the proliferation of
human epithelial ovarian cancer cells and promoted apoptosis via
the inhibition of cyclin D1, and the overexpression of cyclin D1
increased the proliferative ability of epithelial ovarian cancer
cells (41,42). Collectively, these findings
demonstrated that miR-576-3p overexpression may promote apoptosis
and inhibit the viability of ovarian cancer cells via decreasing
PD-L1 and cyclin D1 expression.
However, a limitation of the present study is that
there were only six mice in each group for the animal experiments.
Moreover, further studies are needed to explore whether miR-576-3p
can regulate cisplatin sensitivity of ovarian cancer cells by
directly regulating PD-L1 and cyclin D1 in vivo.
In conclusion, previous findings have indicated that
both PD-L1 and cyclin D1 are important in the modulation of ovarian
tumor progression. Combined with the findings of the present study,
it was shown that that miR-576-3p played a key role in ovarian
cancer development and DDP resistance via negatively regulating
PD-L1 and cyclin D1 expression.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZ and WZ performed the experiments, contributed to
data analysis and made substantial contributions to the conception
and design. QT, JL and SW contributed to data analysis and the
experimental materials. CX made substantial contributions to the
conception and design of the work, gave final approval of the
version to be published, agreed to be accountable for all aspects
of the work in ensuring that questions related to the accuracy or
integrity of any part of the work are appropriately investigated
and resolved, drafted the manuscript and critically revised the
manuscript for important intellectual content. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Animal Care and Use Committee of Affiliated Yantai Yuhuangding
Hospital (approval no. 2018137; Shandong, China) and performed
according to the Guidelines for the Care and Use of Laboratory
Animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Salehi F, Dunfield L, Phillips KP, Krewski
D and Vanderhyden BC: Risk factors for ovarian cancer: An overview
with emphasis on hormonal factors. J Toxicol Environ Health B Crit
Rev. 11:301–321. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bristow RE and Chi DS: Platinum-based
neoadjuvant chemotherapy and interval surgical cytoreduction for
advanced ovarian cancer: A meta-analysis. Gynecol Oncol.
103:1070–1076. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pignata S, C Cecere S, Du Bois A, Harter P
and Heitz F: Treatment of recurrent ovarian cancer. Ann Oncol. 28
(Suppl 8):viii51–viii6. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bookman MA: Optimal primary therapy of
ovarian cancer. Ann Oncol. 27 (Suppl 1):i58–i62. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jayson GC, Kohn EC, Kitchener HC and
Ledermann JA: Ovarian cancer. Lancet. 384:1376–1388. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hayes J, Peruzzi PP and Lawler S:
MicroRNAs in cancer: Biomarkers, functions and therapy. Trends Mol
Med. 20:460–469. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Balci S, Ayaz L, Gorur A, Yildirim Yaroglu
H, Akbayir S, Dogruer Unal N, Bulut B, Tursen U and Tamer L:
microRNA profiling for early detection of nonmelanoma skin cancer.
Clin Exp Dermatol. 41:346–351. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Greenawalt EJ, Edmonds MD, Jain N, Adams
CM, Mitra R and Eischen CM: Targeting of SGK1 by miR-576-3p
Inhibits Lung Adenocarcinoma Migration and Invasion. Mol Cancer
Res. 17:289–298. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Meng FM, Meng FM and Song XL: MiR-576-3p
is a novel marker correlated with poor clinical outcome in bladder
cancer. Eur Rev Med Pharmacol Sci. 21:973–977. 2017.PubMed/NCBI
|
|
12
|
Port M, Glaesener S, Ruf C, Riecke A,
Bokemeyer C, Meineke V, Honecker F and Abend M: Micro-RNA
expression in cisplatin resistant germ cell tumor cell lines. Mol
Cancer. 10:522011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lv J, Xia K, Xu P, Sun E, Ma J, Gao S,
Zhou Q, Zhang M, Wang F, Chen F, et al: miRNA expression patterns
in chemoresistant breast cancer tissues. Biomed Pharmacother.
68:935–942. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang X, Wu WKK, Gao J, Li Z, Dong B, Lin
X, Li Y, Li Y, Gong J, Qi C, et al: Autophagy inhibition enhances
PD-L1 expression in gastric cancer. J Exp Clin Cancer Res.
38:1402019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wyss J, Dislich B, Koelzer VH, Galván JA,
Dawson H, Hädrich M, Inderbitzin D, Lugli A, Zlobec I and Berger
MD: Stromal PD-1/PD-L1 expression predicts outcome in colon cancer
patients. Clin Colorectal Cancer. 18:e20–e38. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rojkó L, Reiniger L, Téglási V, Fábián K,
Pipek O, Vágvölgyi A, Agócs L, Fillinger J, Kajdácsi Z, Tímár J, et
al: Chemotherapy treatment is associated with altered PD-L1
expression in lung cancer patients. J Cancer Res Clin Oncol.
144:1219–1226. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cai J, Qi Q, Qian X, Han J, Zhu X, Zhang Q
and Xia R: The role of PD-1/PD-L1 axis and macrophage in the
progression and treatment of cancer. J Cancer Res Clin Oncol.
145:1377–1385. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jiang Z, Yang Y, Yang Y, Zhang Y, Yue Z,
Pan Z and Ren X: Ginsenoside Rg3 attenuates cisplatin resistance in
lung cancer by downregulating PD-L1 and resuming immune. Biomed
Pharmacother. 96:378–383. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zuo Y, Zheng W, Liu J, Tang Q, Wang SS and
Yang XS: MiR-34a-5p/PD-L1 axis regulates cisplatin chemoresistance
of ovarian cancer cells. Neoplasma. 67:93–101. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sherr CJ: G1 phase progression: Cycling on
cue. Cell. 79:551–555. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hu J, Fang Y, Cao Y, Qin R and Chen Q:
miR-449a Regulates proliferation and chemosensitivity to cisplatin
by targeting cyclin D1 and BCL2 in SGC7901 cells. Dig Dis Sci.
59:336–345. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Biliran H Jr, Wang Y, Banerjee S, Xu H,
Heng H, Thakur A, Bollig A, Sarkar FH and Liao JD: Overexpression
of cyclin D1 promotes tumor cell growth and confers resistance to
cisplatin-mediated apoptosis in an elastase-myc
transgene-expressing pancreatic tumor cell line. Clin Cancer Res.
11:6075–6086. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu R, Guo H and Lu S: MiR-335-5p restores
cisplatin sensitivity in ovarian cancer cells through targeting
BCL2L2. Cancer Med. 7:4598–4609. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zou J, Liu L, Wang Q, Yin F, Yang Z, Zhang
W and Li L: Downregulation of miR-429 contributes to the
development of drug resistance in epithelial ovarian cancer by
targeting ZEB1. Am J Transl Res. 9:1357–1368. 2017.PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals. Guide for the Care and Use of Laboratory Animals. 8th
edition. National Academies Press (US); Washington, DC: 2011,
PubMed/NCBI
|
|
27
|
Wang H, Luo Y, Qiao T, Wu Z and Huang Z:
Luteolin sensitizes the antitumor effect of cisplatin in
drug-resistant ovarian cancer via induction of apoptosis and
inhibition of cell migration and invasion. J Ovarian Res.
11:932018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Joncourt F, Buser K, Altermatt H, Bacchi
M, Oberli A and Cerny T: Multiple drug resistance parameter
expression in ovarian cancer. Gynecol Oncol. 70:176–182. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kavallaris M, Kuo DY, Burkhart CA, Regl
DL, Norris MD, Haber M and Horwitz SB: Taxol-resistant epithelial
ovarian tumors are associated with altered expression of specific
beta-tubulin isotypes. J Clin Invest. 100:1282–1293. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wu DD, Li XS, Meng XN, Yan J and Zong ZH:
MicroRNA-873 mediates multidrug resistance in ovarian cancer cells
by targeting ABCB1. Tumour Biol. 37:10499–10506. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang H, Wang J, Cai K, Jiang L, Zhou D,
Yang C, Chen J, Chen D and Dou J: Downregulation of gene MDR1 by
shRNA to reverse multidrug-resistance of ovarian cancer A2780
cells. J Cancer Res Ther. 8:226–231. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang J, Dang F, Ren J and Wei W:
Biochemical aspects of PD-L1 regulation in cancer immunotherapy.
Trends Biochem Sci. 43:1014–1032. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Iwai Y, Ishida M, Tanaka Y, Okazaki T,
Honjo T and Minato N: Involvement of PD-L1 on tumor cells in the
escape from host immune system and tumor immunotherapy by PD-L1
blockade. Proc Natl Acad Sci USA. 99:12293–12297. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hamanishi J, Mandai M, Matsumura N, Abiko
K, Baba T and Konishi I: PD-1/PD-L1 blockade in cancer treatment:
Perspectives and issues. Int J Clin Oncol. 21:462–473. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fournel L, Wu Z, Stadler N, Damotte D,
Lococo F, Boulle G, Ségal-Bendirdjian E, Bobbio A, Icard P,
Trédaniel J, et al: Cisplatin increases PD-L1 expression and
optimizes immune check-point blockade in non-small cell lung
cancer. Cancer Lett. 464:5–14. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Qie S and Diehl JA: Cyclin D1, cancer
progression, and opportunities in cancer treatment. J Mol Med
(Berl). 94:1313–1326. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Abdelrahman AE, Fathy A, Elsebai EA, Nawar
N and Etman WM: Prognostic impact of Apaf-1, Cyclin D1, and AQP-5
in serous ovarian carcinoma treated with the first-line
chemotherapy. Ann Diagn Pathol. 35:27–37. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liang Z, Li S, Xu X, Xu X, Wang X, Wu J,
Zhu Y, Hu Z, Lin Y, Mao Y, et al: MicroRNA-576-3p inhibits
proliferation in bladder cancer cells by targeting cyclin D1. Mol
Cells. 38:130–137. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu S, Chen S, Yuan W, Wang H, Chen K and
Li D and Li D: PD-1/PD-L1 interaction up-regulates MDR1/P-gp
expression in breast cancer cells via PI3K/AKT and MAPK/ERK
pathways. Oncotarget. 8:99901–99912. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang J, Wang Q, Cui Y, Liu ZY, Zhao W,
Wang CL, Dong Y, Hou L, Hu G, Luo C, et al: Knockdown of cyclin D1
inhibits proliferation, induces apoptosis, and attenuates the
invasive capacity of human glioblastoma cells. J Neurooncol.
106:473–484. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Dai J, Wei RJ, Li R, Feng JB, Yu YL and
Liu PS: A study of CCND1 with epithelial ovarian cancer cell
proliferation and apoptosis. Eur Rev Med Pharmacol Sci.
20:4230–4235. 2016.PubMed/NCBI
|
|
42
|
Xia B, Yang S, Liu T and Lou G: miR-211
suppresses epithelial ovarian cancer proliferation and cell-cycle
progression by targeting Cyclin D1 and CDK6. Mol Cancer. 14:572015.
View Article : Google Scholar : PubMed/NCBI
|